Introduction
Federally mandated assessments of nursing home (NH) residents drive individualized care planning through a process depicted in Figure 1.1 Whenever possible, residents and their family members should be involved in this process by: participating in resident assessments; working with staff to understand the severity and scope of the problems detected by these assessments; and providing input as to the appropriate course of action in response to identified problems.
Figure 1. Assessment and Care Planning Process in Nursing Homes (Adapted from Resident Assessment Instrument Minimum Data Set Version 3.0).

*Assessment—Taking stock of all observations, information, and knowledge about a resident from all available sources (e.g., medical records, the resident, resident’s family, and/or guardian or other legally authorized representative).
†Decision-making — Determining with the resident (resident’s family and/or guardian or other legally authorized representative), the resident’s physician and the interdisciplinary team, the severity, functional impact, and scope of a resident’s clinical issues and needs.
‡Care Planning—Establishing a course of action with input from the resident (resident’s family and/or guardian or other legally authorized representative), resident’s physician and interdisciplinary team that moves a resident toward resident-specific goals utilizing individual resident strengths and interdisciplinary expertise; crafting the “how” of resident care
Overall, resident participation in their own care planning assessments is high; over 80 percent of all NH residents are able to respond to assessment items screening for pain, cognition, and depression.2 However, NH residents with dementia were 40% less likely to be able to participate in their care planning assessments compared to cognitively intact NH residents.2 Involving family members in the care planning assessment process for NH residents with dementia may improve the quality of remaining life and the end-of-life care received by this vulnerable population.3–6
The concept of involving family members or representatives in the NH care planning process is not new. In 1986, the Institute of Medicine’s “Improving the Quality of Care in Nursing Homes” report recommended NHs be required to notify a resident’s representative in the event of a care conference or other changes in the resident’s status (Recommendation 3-7D).7 The resulting Omnibus Budget Reconciliation Act of 1987 mandated written individualized care plans “prepared with the participation to the extent practicable of the resident or the resident’s family or legal representative” (H.R. 3545, SEC. 4201).8
Yet, 30 years later, little is known about the prevalence of family and representative involvement in care planning for long-stay NH residents. The best available evidence comes from an Office of the Inspector General chart audit of 375 residents being prescribed atypical antipsychotics.9 The audit revealed that 91 percent of the time there was no evidence that the resident or the family member participated in the care planning process, and almost 60 percent of the time there was no documentation as to why resident and family member participation was impractical.9
To our knowledge, this report is the first of its size to consider family participation in the care planning process for long-stay NH residents with and without dementia. The objectives of this report were to: 1. Describe the prevalence of family participation in care planning for long-stay NH residents with varying degrees of cognitive impairment; and 2. Identify other resident and NH characteristics associated with family participation in care planning.
Methods
We conducted a secondary analysis of data provided directly to researchers by a large, for-profit NH organization participating in PROVEN, a PRagmatic trial Of Video Education in Nursing homes. Details of this pragmatic, cluster-randomized trial have been published elsewhere.10 Briefly, the goal of the trial is to test a video intervention to improve advance care planning in NHs, particularly among long-stay residents with advanced dementia and other life limiting illness.
Sample
The sample for this study consisted of long-stay nursing home residents who had a quarterly or annual assessment in the last quarter of 2015 (October 1 through December 31, 2015), and at least one quarterly, annual or change in status assessment in 2016. We further limited the analytic sample to NHs in which at least 25 residents met our criteria for prevalent long-stay residents to facilitate multi-level analyses.
Data
Long-stay nursing home residents in CMS-certified nursing homes are assessed at least once per quarter. The Resident Assessment Instrument (RAI) is the standardized tool used to conduct these assessments. The data from these assessments is referred to as the Minimum Data Set (MDS). MDS data include information on resident preferences, activities of daily living, diagnoses, cognition, and overall health status. MDS data were linked to NH-level data from LTCfocus (LTCfocus.org) and the Certification and Survey Provider Enhanced Reporting (CASPER) system (Medicare.gov). The Brown Institutional Review Board approved the study by expedited review.
Variables
Cognitive Functioning
We used the Cognitive Function Scale (CFS), to classify long stay residents’ cognitive functioning as: cognitively intact, mildly impaired, moderately impaired, or severely impaired. The CFS is created using two measures: the Brief Interview for Mental Status (BIMS) and the Cognitive Performance Scale (CPS).11 Briefly, the BIMS is a resident screening tool with three cognitive tasks: immediate recall of three words, delayed recall of three words, and orientation to year, month, and day. The BIMS is not completed on residents who are “rarely or never understood.” The CFS uses the CPS to account for missing data on the BIMS. The CPS is based on several items available in the MDS that do not require resident feedback including staff members’ assessment of resident: short term memory, cognitive skills for daily decision making, ability to make oneself understood, and eating dependence.
Participation in Assessment
MDS, v.3.0, Section Q, Items Q0100A-C, ask the MDS assessor to record whether or not the resident, family member, or legal authorized representative participated in the assessment process (Yes or No). There is also a place to indicate that no family members or legal representatives exist for the resident. The instructions for completing this item describe the importance of resident and family participation in assessment interviews and care planning meetings, described collectively as the assessment process. According to the RAI, participation should be assessed by reviewing the resident’s medical record; asking the resident, family member, or legal representative directly; and asking staff members who completed the assessment.
Other Resident-Level Characteristics
Although we were primarily interested in family participation by degree of resident cognitive impairment, we explored several other resident-level characteristics that we hypothesized might be associated with family participation including: age (years); gender; marital status (currently married versus windowed, divorced, or never married); race (only African American versus all other groups which was predominately Caucasian) and ethnicity (Hispanic); presence of a Alzheimer’s or non-Alzheimer’s dementia diagnosis (active diagnosis items I4800,I4200, or I800A-J); number of activities of daily living (ADLs) for which a resident required extensive assistance or was totally dependent, out of 10 possible ADLs; communication barriers to expressing own wishes; and presence of other conditions that may affect a resident’s capacity for decision-making, including stroke, intellectual disability, severe mental illness (bipolar disorder or schizophrenia). We also examined the relationship between family or legal representative involvement and the presence of certain concerning symptoms, including: behavioral symptoms (verbal or physical aggression toward self or others, or rejection of care); psychotic symptoms (delusions or hallucinations); or physical symptoms (fever, vomiting, dehydration, or significant weight loss of 5% in last month or 10% in last 6 months).
NH-Level Characteristics
Based on the existing NH quality literature, we hypothesized the following NH-level characteristics may be associated with family participation in the care planning process: percent of residents whose primary payer is Medicare or other/private pay; percent of residents within a NH who are African American; percent of residents within a NH who are Hispanic; average activities of daily living (ADL) dependency within the NH; CMS quality star rating; and percent of nonpsychotic residents receiving antipsychotics. We also considered social work staffing ratios (number of social work staff per 100 resident beds), with the hypothesis that social workers may be charged with getting family members involved in the care planning process.
Analyses
All of our analyses are based on one assessment for each eligible long-stay resident in the analytic sample. For each eligible resident, we included the first assessment for each resident in which a family member or representative participated, or the first available assessment for residents without any family or legal representative involvement during 2016. Thus, we had one observation per resident representing whether or not the resident had any family/representative participation over the course of one year (0 if no participation, 1 if any participation).
Resident characteristics were described using frequencies for categorical variables and means for continuous variables, stratified by degree of cognitive impairment. The proportion of residents who had any family representation was calculated for the entire sample and at each level of cognitive impairment. We ran multivariable regression models to consider the association between resident- and NH-level characteristics and any family or representative participation in care planning assessments during 2016. To account for the clustering of residents in NHs, we employed random effects logistic regression modelling that accounts for the hierarchical data structure. Resident and NH characteristics were entered into the fixed part of the model, with a random intercept for each NH. The random effects model assumes variation across NHs is not correlated with the independent variables included in the model (resident factors). We tested this assumption using the Hausman test which confirmed it was an appropriate model. All analyses were conducted using STATA, SE Version 14.
Results
A total of 21,660 residents in 297 nursing homes had a quarterly or annual assessment in the fourth quarter of 2015, among whom, 18,714 (86%) had a quarterly, annual or change in status assessment in the same NH during 2016. Five NHs had less than 25 eligible prevalent long-stay residents and were excluded from analysis. 82 prevalent long-stay residents were excluded due to not having complete cognitive status information or not having any existing family members or legal representatives for the nursing home to contact. The final analytic sample consisted of 18,552 prevalent long-stay residents in 292 NHs. The inclusion flow diagram is provided as Figure 2.
Figure 2.
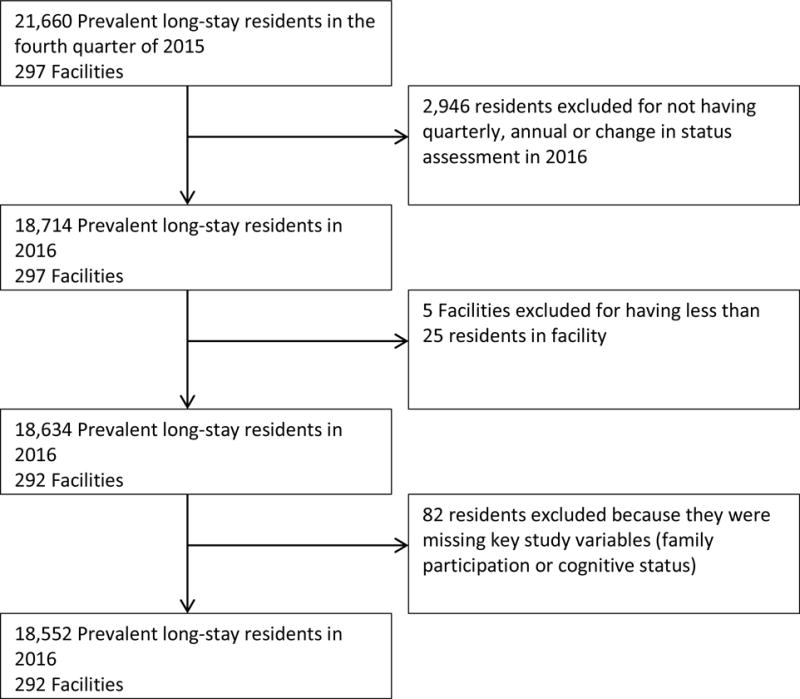
Study Inclusion Flow Diagram
Consistent with national averages,11 a total 31% of long-stay residents were cognitively intact; 22% were mildly impaired; 34% were moderately impaired; and 13% had severe cognitive impairment (Table 1). Greater cognitive impairment was associated with: older age (p<.001), Black race (p=0.005) or Hispanic ethnicity (p<.001), a greater likelihood of having an Alzheimer’s disease dementia diagnosis (p<.001), an increased dependence performing activities of daily living (ADLs) (p<.001), a decrease in the prevalence of other conditions that may affect decision-making capacity (intellectual disability, p<.001; schizophrenia or bipolar disorder, p<.001), a greater likelihood of displaying any agitated or aggressive behaviors (p<.001), increased presence of psychotic behaviors (delusions or hallucinations) (p<.001), and an increased likelihood of significant weight loss (5% in last month or 10% in last six months) (p<.001).
Table 1.
Characteristics of Prevalent Long-Stay Nursing Home Residents by Degree of Cognitive Impairment
| No Cognitive Impairment (N=5,786, 31.2%) |
Mild Cognitive Impairment (N=4,002, 21.6%) |
Moderate Cognitive Impairment (N=6,397, 34.5%) |
Severe Cognitive Impairment (N=2,367, 12.8%) | p | Entire Sample (N=18,552, 100%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Demographics | |||||||||||
| Male | 0.360 | 0.480 | 0.331 | 0.471 | 0.277 | 0.448 | 0.238 | 0.426 | *** | 0.310 | 0.462 |
| Age (Years) | 74.040 | 13.522 | 80.082 | 12.322 | 84.261 | 11.128 | 80.651 | 14.002 | *** | 79.711 | 13.220 |
| Married | 0.151 | 0.358 | 0.173 | 0.378 | 0.174 | 0.379 | 0.232 | 0.422 | *** | 0.174 | 0.379 |
| Black | 0.128 | 0.334 | 0.141 | 0.349 | 0.133 | 0.339 | 0.156 | 0.363 | ** | 0.136 | 0.343 |
| Hispanic | 0.025 | 0.156 | 0.037 | 0.189 | 0.041 | 0.199 | 0.046 | 0.211 | *** | 0.036 | 0.186 |
| Needs Interpreter | 0.010 | 0.100 | 0.024 | 0.154 | 0.032 | 0.175 | 0.046 | 0.209 | *** | 0.025 | 0.157 |
| Dementia Diagnosis | 0.327 | 0.469 | 0.638 | 0.481 | 0.836 | 0.370 | 0.828 | 0.378 | *** | 0.634 | 0.482 |
| ADL Dependencies (#) | 3.803 | 2.908 | 4.592 | 2.891 | 5.549 | 2.614 | 6.990 | 1.581 | *** | 4.982 | 2.865 |
| Other conditions potentially affecting decision-making | |||||||||||
| Intellectual Disability | 0.003 | 0.054 | 0.003 | 0.055 | 0.003 | 0.056 | 0.012 | 0.110 | *** | 0.004 | 0.065 |
| Stroke | 0.067 | 0.250 | 0.067 | 0.250 | 0.060 | 0.237 | 0.064 | 0.245 | 0.064 | 0.245 | |
| Severe Mental Illness | 0.147 | 0.354 | 0.123 | 0.329 | 0.094 | 0.292 | 0.063 | 0.244 | *** | 0.113 | 0.316 |
| Symptoms that may prompt facilities to involve families | |||||||||||
| Any Agitated or Aggressive Behaviors | 0.096 | 0.295 | 0.122 | 0.327 | 0.194 | 0.395 | 0.179 | 0.383 | *** | 0.146 | 0.353 |
| Delusions or Hallucinations | 0.037 | 0.188 | 0.062 | 0.241 | 0.111 | 0.314 | 0.061 | 0.240 | *** | 0.071 | 0.256 |
| Physical Symptoms | |||||||||||
| Fever | 0.009 | 0.095 | 0.007 | 0.083 | 0.011 | 0.105 | 0.016 | 0.124 | ** | 0.010 | 0.100 |
| Vomiting | 0.011 | 0.103 | 0.009 | 0.096 | 0.011 | 0.106 | 0.009 | 0.094 | 0.010 | 0.101 | |
| Dehydration | 0.001 | 0.026 | 0.001 | 0.035 | 0.003 | 0.050 | 0.003 | 0.058 | * | 0.002 | 0.042 |
| Weight Loss | 0.035 | 0.185 | 0.053 | 0.224 | 0.076 | 0.265 | 0.088 | 0.283 | *** | 0.060 | 0.237 |
p<.05,
p<.01,
p<.001
Differences in Care Plan Participation by Degree of Cognitive Impairment
For the entire sample of long-stay nursing home residents, 16.0% had a family member, significant other, or legal representative participate in at least one 2016 care planning assessment in 2016. Family and representative participation differed by degree of resident cognitive impairment (Figure 3). 8.0% of prevalent long-stay nursing home residents with no cognitive impairment had any family or representative participation in 2016, compared to 14.7% of residents with mild impairment, 20.3% of residents with moderate impairment, and 26.3% of residents with severe impairment. However, only 17.6% of residents with severe cognitive impairment were able to participate in care planning about their own assessment (in absence of family or representative participation). Over half (56%) of long-stay NH residents with severe cognitive impairment had no one representing them in their care planning assessments during 2016.
Figure 3.
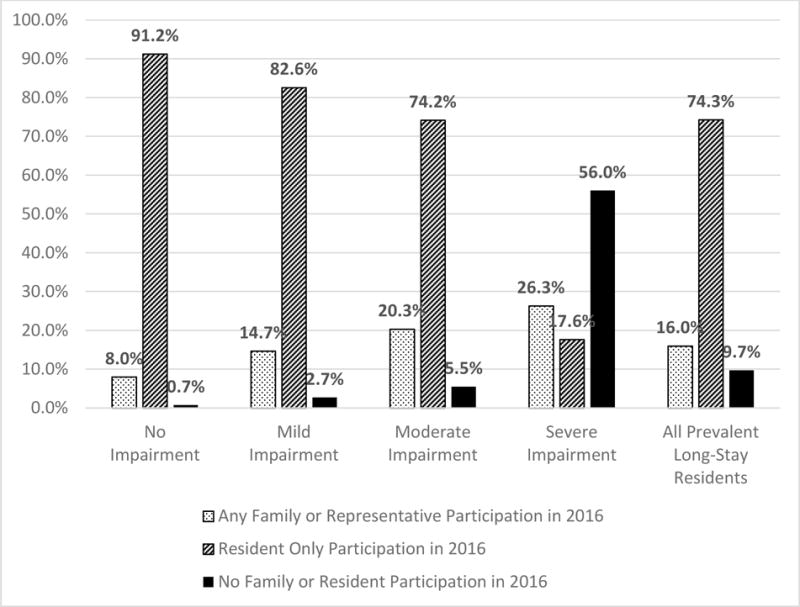
Family and Resident Participation by Degree of Cognitive Impairment (Unadjusted)
The relationship between cognitive impairment and any family/representative participation in 2016 was also found in the full multiple regression model (Table 2, Model 1). After controlling for potentially relevant resident characteristics, long-stay residents with mild cognitive impairment were 4.5 percentage points more likely to have any family participation than those without cognitive impairment (95% CI: 3.1, 5.9); those with moderate impairment were 11.3 percentage points more likely to have any participation than those without cognitive impairment (95% CI: 9.7, 12.9); and those with severe cognitive impairment were 18.0 percentage points more likely to have any family participation compared to long-stay residents without cognitive impairment (95% CI: 15.6, 20.3).
Table 2.
Association Between Resident and Facility Characteristics of Probability of Any Family Involvement in 2016
| Model 1. Resident characteristics | Model 2. Resident and facility characteristics | |||||
|---|---|---|---|---|---|---|
| Percentage Point | SE | P>z | Percentage Point | SE | P>z | |
| Resident Characteristics | ||||||
| Cognitive Function (reference=no impairment) | ||||||
| Mild Impairment | 4.52 | 0.70 | 0.00 | 4.54 | 0.70 | 0.00 |
| Moderate Impairment | 11.30 | 0.84 | 0.00 | 11.35 | 0.84 | 0.00 |
| Severe Impairment | 17.95 | 1.18 | 0.00 | 18.08 | 1.19 | 0.00 |
| Demographics | ||||||
| Male | −0.13 | 0.54 | 0.82 | −0.13 | 0.54 | 0.81 |
| Age (years) | 0.00 | 0.02 | 0.85 | 0.00 | 0.02 | 0.83 |
| Black | 3.83 | 0.61 | 0.00 | 3.86 | 0.61 | 0.00 |
| Hispanic | −0.07 | 0.76 | 0.93 | −0.25 | 0.77 | 0.74 |
| Married | −0.68 | 1.26 | 0.59 | −0.84 | 1.28 | 0.51 |
| Interpreter Needed | 7.09 | 1.36 | 0.00 | 7.14 | 1.37 | 0.00 |
| Severity of Cognitive and Physical Impairment | ||||||
| MDS Dementia Diagnosis | 2.48 | 0.62 | 0.00 | 2.50 | 0.63 | 0.00 |
| ADL Dependencies (#) | 0.26 | 0.10 | 0.01 | 0.27 | 0.10 | 0.01 |
| Other conditions potentially affecting decision-making | ||||||
| Intellectual Disability | 9.45 | 3.01 | 0.00 | 9.57 | 3.04 | 0.00 |
| Stroke | 2.28 | 0.95 | 0.02 | 2.32 | 0.96 | 0.02 |
| Severe Mental Illness | −0.55 | 0.79 | 0.49 | −0.57 | 0.80 | 0.47 |
| Symptoms that may prompt facilities to involve families | ||||||
| Any Agitated or Aggressive Behaviors | 1.23 | 0.66 | 0.06 | 1.24 | 0.66 | 0.06 |
| Delusions or Hallucinations | 2.97 | 0.85 | 0.00 | 3.01 | 0.86 | 0.00 |
| Physical Symptoms | ||||||
| Fever | 2.88 | 2.16 | 0.18 | 2.97 | 2.18 | 0.17 |
| Vomiting | 1.11 | 2.25 | 0.62 | 1.13 | 2.27 | 0.62 |
| Dehydration | −0.01 | 4.47 | 1.00 | −0.06 | 4.52 | 0.99 |
| Weight Loss | 2.72 | 0.87 | 0.00 | 2.74 | 0.88 | 0.00 |
| Facility Characteristics | ||||||
| Residents by Primary Payer (reference=Medicaid) | ||||||
| Medicare Primary Payer (%) | −0.44 | 0.16 | 0.01 | |||
| Other/Private Primary Payer (%) | 0.12 | 0.13 | 0.36 | |||
| Facility Demographics | ||||||
| Under 65 (%) | −0.12 | 0.14 | 0.39 | |||
| Black (%) | 0.18 | 0.08 | 0.03 | |||
| Hispanic (%) | 0.17 | 0.16 | 0.29 | |||
| Staffing | ||||||
| Social workers per 100 beds | 4.71 | 2.20 | 0.03 | |||
| Physical Acuity | ||||||
| Average ADL Score | −0.33 | 0.60 | 0.58 | |||
| Quality Indicators | ||||||
| Star Rating for Quality | −0.98 | 0.94 | 0.30 | |||
| Off-label antipsychotic use (%) | −0.17 | 0.21 | 0.41 | |||
Other Resident and NH Characteristics Associated with Any Family/Representative Participation
Other resident characteristics associated with any family/representative participation during 2016 include: being African American; requiring an interpreter to communicate with a doctor or healthcare staff; having a dementia diagnosis in the MDS; having a greater number of ADL dependencies; having an intellectual disability or previous stroke; displaying agitated, aggressive, or psychotic behaviors; and experiencing significant weight loss.
Adding NH characteristics to the model did not change the resident-level findings (Table 2, Model 2). NHs with a greater percentage of residents with Medicare as a primary payer (rehabilitation-focused NHs) were less likely to have any family involvement for their long-stay population (p=.01). NHs with more on-staff social workers per 100 resident beds were more likely to have family involvement (p=.03). NHs with greater concentrations of African American residents were more likely to have family involvement (p=.03). NH-level physical acuity and NH-level quality were not associated with family participation. Average NH-level family participation in resident care planning assessments over the course of one year ranged from 2.3% to 97.9% (mean 24.4%). Most of the between-NH variation remains unexplained in our current model (residual intraclass correlation= .57).
Discussion
This paper has three main findings. First, rates of family participation in care planning assessments for long-stay nursing home residents are low. Second, although family participation increases with degree of resident cognitive impairment, over half of all long-stay residents with severe cognitive impairment do not have anyone participating in their assessments and are unable to participate in their own assessments. Finally, in this sample of nearly 300 NHs drawn from a large for-profit chain, there is considerable NH-level variation in the degree of family involvement in care planning.
NH-level participation may be a measure of quality improvement capacity. Quality improvement capacity refers to having the right number of people engaged and ready to act.12 The strongest NH-level predictor in this sample appears to be social work staffing. Having more on-staff social workers per resident bed increases the probability of family involvement. It is likely that social workers play a key role in contacting family members and coordinating their involvement in the assessment and care planning process.13 However, regulations for social work staff in nursing homes varies greatly by state14 with federal regulation requiring only 1 social worker per 120 nursing home beds (a much larger caseload than most social workers think is feasible).15 As a NH-level quality capacity measure, family involvement may be a particularly important metric for spouses and caregivers trying to choose the best NH in which to place their loved ones.
Low family involvement in care planning may affect the quality of end-of-life care received by residents with advanced dementia. The rationale for having patients and their families participate in the creation and updating of individualized care plans is that their preferences and desires should be the principal drivers of a plan that is consistent with residents’ rights. If patients no longer have the cognitive or physical capacity to participate in the care planning process, families are assumed to be their advocates who are best able to express patients’ preferences for how care and treatment are to be provided. We know documenting preferences for less aggressive care, such as do not hospitalize (DNH) and do not resuscitate (DNR) orders, reduces hospitalizations and burdensome transitions at the end-of-life.16–18 Yet, the default status in the NH is full code, or “do everything.” Lack of family involvement in care planning may partially explain why 20 percent of NH residents with advanced dementia experience a burdensome transition in the last 90 days of life,18 and 14 percent still die in the hospital.19
While not the main focus of our analyses, we did observe large racial differences in family participation. Compared to families of Caucasian residents, families of African-American residents were more likely to be involved in care planning. This finding is consistent with the literature suggesting racial and ethnic differences in the frequency and intensity of community-based caregiving.20 Cultural drivers of racial and/or ethnic differences in caregiving in the NH setting is an area ripe for further research.
The major limitation of this work is generalizability. We only have data from one large nursing home corporation and, as such, we are unable to look at variation across chains or across ownership types (e.g., for-profit, not-for-profit). The intra-NH variation suggests policy and practices at the corporate level are not having a consistent effect on family involvement. At this point, we do not know what is driving this variation or the best policy options to improve family participation. Exploring this variation qualitatively (as well as quantitatively) is an important next step for this research, and a potential quality improvement project for the corporation.
It is also important to look at variation in family participation across a larger number of NHs. Although having data for 18,552 residents in 292 NHs is a significant improvement over the previous estimates based on an audit of 375 individual records,9 the data exists to look at the effect of participation on resident outcomes on a national level. As part of the MDS, CMS collects resident, family member, and representative participation information for every care planning assessment. To date, CMS has not made the MDS participation data used in this study widely available for research purposes. Another limitation is the ambiguity of the participation measure itself. It is unclear whether the item refers only to the care planning assessment, or to the larger care planning process which includes a care planning meeting and the development of a care plan. If NHs are interpreting the item differently, or participation is being documented at different points in the assessment and care planning process, that could account for part of the large intra-NH variation we observed. In time, releasing participation data would also likely standardize use of the measure.
Conclusion
Understanding if and how family members actively and meaningfully participate in care planning for their loved ones living in NHs is an important and understudied topic.21 To make a case for the increased use of limited nursing home resources to involve family members in care, we must be able to: 1. Identify who benefits from having a representative involved in care and what outcomes are sensitive to representative participation; and 2. Determine whether or not the benefits of having a family member involved in care planning consistent across NHs. Releasing participation data with the other sections of the Minimum Data Set (MDS, 3.0), would allow for these questions to be considered more systematically for the over 3 million people living in US nursing homes, in particular for the most vulnerable population – residents with severe cognitive impairment.
Acknowledgments
Support: This work is supported by the National Institutes of Health (NIH) Common Fund, through a cooperative agreement (NIA 4UH3AG049619-02) from the Office of Strategic Coordination within the Office of the NIH Director. The views presented here are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Dr. McCreedy is supported by 4T32 HS000011-30 AHRQ “National Research Service Award”
Dr. Mitchell is supported by NIH-NIA K24AG033640
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting and Competing Interests: We have no conflicts of interest to report.
References
- 1.Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual, version 1.15. Centers for Medicare & Medicaid Services; Oct, 2017. [Google Scholar]
- 2.Thomas KS, Wysocki A, Intrator O, Mor V. Finding Gertrude: The resident’s voice in Minimum Data Set 3.0. J Am Med Dir Assoc. 2014;15(11):802–806. doi: 10.1016/j.jamda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilly J, Reed P. Dementia care practice recommendations for assisted living residences and nursing homes. Alzheimer’s Association; 2006. [Google Scholar]
- 4.Tilly J, Reed P, Gould E, Fok A. Phase 3: End-of-Life Care. Alzheimer’s Association; 2007. Dementia Care Practice Recommendations for Assisted Living Residences and Nursing Homes. [Google Scholar]
- 5.Reinhardt JP, Chichin E, Posner L, Kassabian S. Vital conversations with family in the nursing home: preparation for end-stage dementia care. J Soc Work End Life Palliat Care. 2014;10(2):112–126. doi: 10.1080/15524256.2014.906371. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KH, Hobson A, Steiner P, Rodel B. Patients with dementia involving families to maximize nursing care. J Gerontol Nurs. 1992;18(7):19–25. doi: 10.3928/0098-9134-19920701-07. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine Committee on Nursing Home R. Improving the Quality of Care in Nursing Homes. Washington (DC): National Academies Press (US) National Academy of Sciences; 1986. [Google Scholar]
- 8.Omnibus Budget Reconciliation Act 1987 (OBRA 87) Public Law. 1987:100–203. [PubMed] [Google Scholar]
- 9.Levinson D. Nursing Facility Assessments and Care Plans for Residents Receiving Atypical Antipsychotic Drugs. 2012 [Google Scholar]
- 10.Mor V, Volandes AE, Gutman R, Gatsonis C, Mitchell SL. PRagmatic trial Of Video Education in Nursing homes: The design and rationale for a pragmatic cluster randomized trial in the nursing home setting. Clinical trials. 2017;14(2):140–151. doi: 10.1177/1740774516685298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68–e72. doi: 10.1097/MLR.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevan H. How can we build skills to transform the healthcare system? J Res Nurs. 2010;15(2):139–148. [Google Scholar]
- 13.Bern-Klug M, Kramer KW. Core functions of nursing home social services departments in the United States. J Am Med Dir Assoc. 2013;14(1):75 e71–77. doi: 10.1016/j.jamda.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Bern-Klug M. State variations in nursing home social worker qualifications. J Gerontol Soc Work. 2008;51(3–4):379–409. doi: 10.1080/01634370802039734. [DOI] [PubMed] [Google Scholar]
- 15.Bern-Klug M, Kramer KW, Sharr P, Cruz I. Nursing home social services directors’ opinions about the number of residents they can serve. J Aging Soc Policy. 2010;22(1):33–52. doi: 10.1080/08959420903396426. [DOI] [PubMed] [Google Scholar]
- 16.Grabowski DC, Stewart KA, Broderick SM, Coots LA. Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39. doi: 10.1177/1077558707308754. [DOI] [PubMed] [Google Scholar]
- 17.Zweig SC, Kruse RL, Binder EF, Szafara KL, Mehr DR. Effect of do-not-resuscitate orders on hospitalization of nursing home residents evaluated for lower respiratory infections. J Am Geriatr Soc. 2004;52(1):51–58. doi: 10.1111/j.1532-5415.2004.52010.x. [DOI] [PubMed] [Google Scholar]
- 18.Gozalo P, Teno JM, Mitchell SL, Skinner J, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212–1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Zheng NT, Temkin-Greener H. Quality of end-of-life care of long-term nursing home residents with and without dementia. J Am Geriatr Soc. 2013;61(7):1066–1073. doi: 10.1111/jgs.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rote SM, Moon H. Racial/Ethnic Differences in Caregiving Frequency: Does Immigrant Status Matter? J Gerontol B Psychol Sci Soc Sci. 2016 Aug 29; doi: 10.1093/geronb/gbw106. [DOI] [PubMed] [Google Scholar]
- 21.Gaugler JE. Family involvement in residential long-term care: a synthesis and critical review. Aging Ment Health. 2005;9(2):105–118. doi: 10.1080/13607860412331310245. [DOI] [PMC free article] [PubMed] [Google Scholar]


