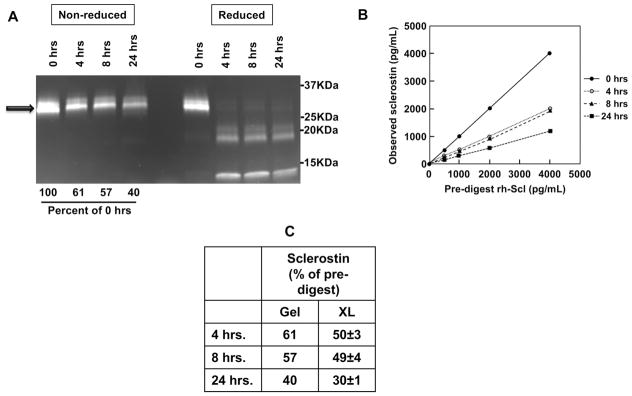
Figure 3. The LIAISON sclerostin CLIA does not detect sclerostin fragments.
(A) rh-Sclerostin samples were digested using thrombin for 0, 4, 8, 24 hour time-points. Half of the digests from each time point were run on a SDS-PAGE gradient gel (4–12%), under either non-reducing conditions or reducing conditions (β-mercaptoethanol and boiling), followed by quantitation of band intensities on an imager. Arrow denotes full-length rh-sclerostin prior to thrombin digestion. (B) Digests were diluted to multiple concentrations spanning the measuring range of the assay, based on initial rh-sclerostin concentrations, using charcoal stripped serum, and assayed by the LIAISON sclerostin CLIA. (C) Gel assessments of intact rh-sclerostin and LIAISON sclerostin CLIA measurements are compared and expressed as percent of pre-digested rh-sclerostin.