Abstract
In Alzheimer’s disease (AD) the blood-brain barrier (BBB) is compromised, thus therapeutic targeting of the BBB to enhance its integrity and function could be a unique approach to treat, slow or hold the progression of AD. Recently, we have developed an in vitro high-throughput screening assay to screen for compounds that increase the integrity of a cell-based BBB model. Results from primary screen identified multiple hit compounds that enhanced the monolayer integrity. Herein, further characterization of selected hit compounds, namely 8-bromoguanosine cyclic monophosphate, JW74, 1,10-phenanthroline monohydrate, SB216763 and α-tocopherol was performed. Compounds were subjected to concentration-dependent studies to determine their EC50 and potency to enhance the cell-based model integrity by the Lucifer Yellow permeability and amyloid-beta (Aβ) transport across the monolayer. The compounds demonstrated different EC50s to enhance the monolayer integrity ranging from 0.4 to 12.8 μM, and different effect on enhancing Aβ transport with highest transport observed for α-tocopherol (2.2-fold increase). Such effects were associated with increased levels of tight junction proteins such as claudin-5 and/or ZO-1, and Aβ major transport proteins LRP1 and P-glycoprotein. In vivo studies for α-tocopherol were performed in AD mouse model; consistent with the in vitro results α-tocopherol significantly increased BBB integrity measured by IgG extravasation, and reduced brain Aβ levels. In conclusion, findings support our developed cell-based BBB model as a functional predictive in vivo tool to select hit compounds, and suggest that enhancing BBB tightness and function has the potential to reduce Aβ pathology associated with AD.
Keywords: High-throughput screening, Blood-brain Barrier, Amyloid-β clearance, α-Tocopherol, Brain endothelial cells, IgG extravasation
Graphical Abstract
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive dementia that cause cognitive and behavioral impairment. AD is the sixth-highest cause of death across all ages in the United States but most individuals with the disease are 65 and older (Hebert et al., 2012). The number of patients with AD is continually increasing along with increased economic burden to healthcare systems worldwide (Alzheimer’s Association, 2017). While the exact cause(s) of AD is unknown, it may incorporate both environmental and genetic factors affecting many cellular and molecular processes in the brain (Selkoe et al., 2001). Several suggested hallmarks or disease pathways have been reported, however, two main pathological hallmarks are associated with AD, the accumulation of extracellular deposition of amyloid-β (Aβ) peptide derived from the proteolytic cleavage of amyloid precursor protein (APP) to form insoluble amyloid plaques, and neurofibrillary tangles (NFTs) composed of insoluble intracellular twisted fibers of hyper-phosphorylated tau protein aggregated in the neurons (Bancher et al. 1989; Selkoe, 2001).
The blood-brain barrier (BBB) provides a unique microenvironment for the brain, allows communication with the systemic compartment and prevents toxins and unwanted substances to invade into the CNS (Abbott et al., 2010). The BBB consists of a multi-layered unit composed of a thick continuous, non-fenestrated endothelial cells connected by tight junction proteins to control paracellular permeability of soluble substances (Abbott et al., 2010). In addition to endothelial cells, the BBB is composed of basement membrane, pericytes and astrocytic end-feet (Rubin and Staddon, 1999; Abbott et al., 2010). Several transporters and receptors are expressed on the endothelial cells of the BBB including P-glycoprotein (P-gp) and low-density lipoprotein receptor related protein-1 (LRP1), which regulate traffic across the BBB and play a key role in the clearance of Aβ (Shibata et al. 2000; Sagare et al., 2012; Qosa et al., 2014a). In AD, several reports indicated the BBB integrity and functional activity is compromised that correlated well with dysfunctional clearance of Aβ across the BBB and formation of Aβ plaques (Sagare et al., 2012), neuroinflammation, oxidative stress, and neuronal damage (Bowman et al., 2007).
To date, there is no single drug available for the treatment of AD. While non-drug treatments and available drugs in the markets may help with cognitive and behavioral symptoms, they neither cure underlying mechanisms of the disease nor capable of slowing the disease progression (Aisen et al., 2012; Schneider, 2013). Recently, we have developed a high-throughput screening (HTS) assay to identify hit compounds that have the potential to treat AD pathology (Qosa et al., 2016). Our HTS assay is based on screening for compounds that enhance or protect the integrity of a cell-based BBB model with cerebral amyloid angiopathy (CAA) characteristics. Results of this screen identified several hit compounds that improved the cell-based BBB model integrity. Among these hits, five investigational compounds reported to have a neuroprotective effect and/or affect pathways associated with AD were selected for further evaluation in the current study. These hit compounds are 8-bromoguanosine 3,5-cyclic monophosphate sodium salt (Fernández-Tomé et al., 1999; Wirtz-Brugger and Giovanni, 2000), JW74 (Hu et al., 2016), 1,10-phenanthroline monohydrate (Lim et al., 1997), SB216763 (Pena-Ortega et al., 2012), and α-tocopherol (Mangialasche et al., 2012).
The aim of this study was first to in vitro characterize the hit compounds and confirm their potency to enhance the cell-based BBB model integrity and function to increase Aβ transport, followed by mechanistic studies. Then, in vivo studies were performed for α-tocopherol, selected due to its highest potency, to investigate its effect on BBB integrity and brain Aβ load in the AD mouse model 5XFAD.
EXPERIMENTAL PROCEDURES
Materials
Fibronectin from bovine plasma and Lucifer Yellow (LY) were purchased from Sigma-Aldrich (St. Louis, MO). HTS 3μm polycarbonate membrane transwell 96-well plates and 0.45 μm polyester membrane transwell 24 well plates were purchased from Corning (Corning, NY). Rat-tail collagen type-I, Dulbecco’s modi ed Eagle’s medium (DMEM), sterile PBS, and penicillin/streptomycin antibiotics were obtained from Gibco (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Atlanta biologicals (Flowery Branch, GA). 14C-inulin ([carboxyl-14C]-inulin, M.W. 5000 Da) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Synthetic mono-iodinated and nonoxidized Aβ40 (125I-Aβ40; human, 2200 Ci/mmol) was purchased from PerkinElmer (Boston, MA). Total protein measurement’s reagents with the bicinchoninic acid (BCA) method were obtained from Pierce (Rockford, IL). Antibodies used were mouse monoclonal antibody against light chain LRP1 (Abcam, Cambridge, MA), mouse monoclonal antibody C-219 against P-gp from BioLegend (San Diego, CA), monoclonal antibody for claudin-5 and ZO-1 were purchased from Invitrogen (Carlsbad, CA), goat polyclonal antibodies against actin (C-11), and HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). All other chemicals, reagents and supplies were purchased from VWR (West Chester, PA) and Sigma-Aldrich.
Screened compounds
From the primary screen (Qosa et al., 2016), we identified several hit compounds from which the following compounds were selected for further characterization due to available studies reporting their potential neuroprotective effects (Table 1) (Lim et al., 1997; Fernández-Tomé et al., 1999; Wirtz-Brugger and Giovanni, 2000; Mangialasche et al., 2012; Pena-Ortega et al., 2012; Hu et al., 2016). These compounds are 8-bromoguanosine 3,5-cyclic monophosphate sodium salt (BrG), JW74, 1,10-phenanthroline monohydrate (Phen), SB216763 (SB2) and α-tocopherol (Tph).
Table 1.
EC50 values, fold change and potency of hit compounds obtained from primary and secondary screening that showed significant enhancement in the barrier intactness of bEnd3 monolayer.
| Compound | Pharmacological activity# | EC50 (μM) | Fold ↓ in Pc | Potency |
|---|---|---|---|---|
| BrG | Activator of cGMP-dependent protein kinases | 4.9 | 0.14 | 0.03 |
| JW74 | Inhibitor of canonical Wnt signaling | 3.6 | 0.27 | 0.08 |
| Phen | Metalloprotease inhibitor | 0.4 | 0.15 | 0.38 |
| SB2 | GSK-3β inhibitor | 12.8 | 0.43 | 0.03 |
| Tph | Potent antioxidant | 2.4 | 0.37 | 0.15 |
Cell culture
The immortalized mouse brain endothelial cell line, bEnd3, was obtained from ATCC (Manassas, VA). bEnd3 cells, passage 25–35, were cultured in DMEM supplemented with 10% FBS, penicillin G (100 IU/ml), streptomycin (100 g/ml), 1% w/v nonessential amino acids, and glutamine 2 mM. Cultures were maintained in a humidified atmosphere (5% CO2/95% air) at 37°C and media was changed every other day.
bEnd3 cells based-BBB model for HTS
Cells seeding was performed as reported previously (Qosa et al., 2016). In brief, bEnd3 cells were plated onto HTS transwell 96-well plate with polycarbonate membrane inserts, 4.26 mm diameter with 3 μm pores size, at a seeding density of 50,000 cells/cm2. Inserts were coated with 50 μl of fibronectin solution (30 μg/ml in PBS). Two hours later bEnd3 cells were loaded to the apical side (A) at a density of 50,000 cells/cm2, and 200 μl of fresh media were added to the basolateral side (B). Cells were incubated and cultured at 37°C, 5%CO2/95% air for five days to achieve optimal barrier integrity of bEnd3 cells. At the end of incubation period, effect of the screened compounds on the barrier function of bEnd3 cells were evaluated by measurement of LY permeation coefficient as described below.
Concentration-dependent permeability studies for EC50 and potency determination
On day six of cell culture, cells were treated with 50 μl of hit compounds in the concentration range 0.078–20 μM, added to upper chamber (A side), for 24 h at 37°C, 5% CO2/95% air. At the end of treatment period (day 6 post-seeding), the integrity of the endothelial barrier was evaluated by measuring the permeability of LY across the monolayer as we reported previously (Qosa et al., 2016). LY transport was initiated by adding 50 μl of LY (100 μM) diluted in transport buffer (141 mM NaCl, 4 mM KCl, 2.8 mM CaCl2, 1 mM MgSO4, 10 mM HEPES, and 10 mM D-glucose, pH 7.4) to A side, and 200 μl of warmed freshly prepared transport buffer added to lower chamber (B side) of each transwell. One hour later, LY concentrations in A and B sides were read at excitation and emission wavelengths of 485 and 530 nm, respectively, using Synergy 2 microplate reader (Biotek, VT) supported with Gene5 software (Biotek) for data acquisition. Apparent permeation coefficient (Pc) was calculated from the following equation (Qosa et al. 2016):
| Equation 1 |
Where, Vb is the volume of B side (200 μl), Cb is the concentration of LY (μM) in the B side, Ca is the concentration of LY (μM) in the A side, A is the membrane area (0.143 cm2), and T is the time of transport (3600 sec). EC50 values and Curve fittings were performed using GraphPad Prism (Version7.03, CA) software.
Aβ Transport across bEnd3 cells-based BBB model
Aβ transport studies were performed as we previously reported (Qosa et al., 2014a; Qosa et al., 2014b). bEnd3 cells were plated on 0.45 μm polyester membrane transwell 24-well plates, then treated with the compounds (10 μM) on day five for 24 h. Next day, Aβ40 transport assay was initiated by addition of a mixture of 0.1 nM 125I-Aβ40 and 0.05 mM 14C-inulin in media to the B-side. At the end of incubation period, 30 min, aliquots from both sides were separately collected for radioactivity analysis using Wallac 1470 Wizard Gamma and Wallac 1414 WinSpectral Liquid Scintillation Beta Counter (PerkinElmer; Waltham, MA). The transport quotients of 125I-Aβ40 (TQB→A) was calculated using the following equation (Qosa et al., 2014a; Qosa et al., 2014b):
| Equation 2 |
where Aβ40 (A) is the cpm of 125I-Aβ40 in the upper side, and Aβ40 (B) is the cpm of 125I-Aβ40 in the lower side, inulin (A) is the dpm of 14C-inulin in the upper side, and inulin (B) is the dpm of 14C-inulin in the lower side. Three independent experiments were carried out for each compound.
Concentration-dependent studies for Aβ transport across the monolayer was performed for Tph in the concentration range between 1–10 μM as described above.
In vivo evaluation of Tph in AD mouse model
In vivo evaluation of α-tocopherol for its effect on BBB integrity and Aβ brain load was performed in 5XFAD mouse model of AD. 5XFAD female mice (Jackson laboratory, Bar Harbor, ME) were housed in plastic containers under the conditions of 12 h light/dark cycle, 22°C, 35% relative humidity, with ad libitum access to water and food. 5XFAD mutations include: APP KM670/671NL (Swedish), APP I716V (Florida), APP V717I (London), PSEN1M146L, PSEN1 L286V, leading to early and aggressive Aβ accumulation associated with in ammatory astrocytes activation and cognitive decline (Oakley et al., 2006). All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisiana at Monroe according to the National Institutes of Health guidelines. Surgical and treatment procedures were consistent with the IACUC policies and procedures.
Animals’ treatment
5XFAD mice were divided into two groups (n = 5/group), a control group fed for one month with regular diet (Teklad Laboratory diets, Harlan Laboratories, Madison, WI). Instead of providing the mice with municipal tap water, they were supplemented with Aqua-Jel (Ancare Corporation; Bellmore, NY), which is a non-wetting sterile water gel for animal hydration (Moccia et al., 2010). Treatment group was fed with regular diet and provided with 10 mg/kg/day of Tph mixed with hydrogel for one month. All treatments were started at the age of 4 months for a period of one month ending the experiment at the age of 5 months. Diet and Aqu-Jel were changed every day to maintain freshness. During the treatment period, animals’ body weights were measured every 2 weeks and health status and normal behavior were checked daily. Mice body weights were not significantly different between the treatment and control groups, and were in the range of 22.5 ± 2.5 to 25 ± 2.1 g, respectively. At the end of the treatment period, mice were killed for brains collection.
Brain microvessels isolation
Brain microvessels were isolated as described previously (Cirrito et al., 2005). Each brain hemisphere was homogenized in ice-cold DPBS followed by the addition of one volume of 30% Ficoll 400 (Sigma-Aldrich). Homogenates were centrifuged at 8000 g for 10 min, and the resulting pellets were suspended in ice-cold DPBS containing 1% BSA and passed over a glass bead column to collect microvessels adhering to the glass beads. Isolated microvessels were lysed in RIPA buffer containing 1xcomplete mammalian protease inhibitor mixture followed by centrifugation at 21,000 g for 1 h at 4°C. The supernatant was collected as protein extract and stored at −80°C until the time of analysis by Western blot.
Western blot for cells lysates and isolated microvessels
Cells were seeded in 10 mm cell culture dishes (Corning, NY) at a density of 1×106 cells per dish and allowed to grow in a humidified incubator (5%CO2/95% air) at 37°C. At 80–90% confluence, cells were treated with media containing 0.1% DMSO as control or 10 μM of each compound for 24 h. At the end of treatment period, cells were washed twice with ice-cold PBS, scraped, collected, and lysed in RIPA buffer containing 1xcomplete mammalian protease inhibitor mixture. Total protein was determined using the BCA protein assay. Samples were stored at −80°C until time of analysis.
Protein extracts (25μg) from cell lysates and brain microvessels were loaded and resolved using 7.5% SDS-polyacrylamide gel, then transferred electrophoretically onto nitrocellulose membranes. Membranes were blocked with 2% BSA and incubated overnight with monoclonal antibodies for P-gp (C-219), LRP1, claudin-5 and ZO-1. Actin was used for normalization of the samples. For detection, the membranes were washed free of primary antibody and incubated with HRP-labeled secondary IgG anti-mouse antibody for P-gp and claudin-5, anti-rabbit IgG antibody for LRP1 and ZO-1. Proteins’ blots were developed using a chemiluminescence detection kit (Thermo Fisher Scientific; Waltham, MA). Bands were visualized using ChemiDoc system (Bio Rad; Hercules, CA), and bands intensities were measured by densitometric analysis.
Immunohistochemical analyses
For each treatment group (n=5 mice per group), image acquisition was performed in six groups of tissue sections spanning the cortex and hippocampus, each separated by 150 μm and each containing three 15-μm sections (total of 18 sections per mouse). All cryostat brain slices were acetone-fixed then blocked for 1 h with 10% normal donkey serum in PBS. Double immunostaining of capillaries with Aβ was performed using rabbit polyclonal collagen IV antibody (Millipore; Temecula, CA) at 1:200 dilution followed by IgG-CFL 594 conjugated donkey anti-rabbit (Santa Cruz Biotechnology) as a secondary antibody, and Alexa Fluor-488 conjugated anti-Aβ antibody (6E10) (BioLegend) for Aβ detection at 1:200 dilution. To assess IgG extravasation from brain microvessels, brain sections were xed and blocked, as described above, then probed by dual immunohistochemical staining for collagen IV and mouse IgG using uorescein-conjugated donkey anti-IgG (Santa Cruz Biotechnology), both at 1:200 dilution. All images were captured using Nikon Eclipse Ti-S inverted uorescence microscope (Melville, NY) at a total magnification of 20X. Quanti cation of total Aβ load and IgG extravasation in brain hippocampus and cortex was performed using ImageJ version 1.44 software after adjusting for threshold (National Institutes of Health, Bethesda, MD).
Statistical analysis
Data were expressed as mean ± SD for in vitro studies and mean ± SEM for in vivo studies. The experimental results were statistically analyzed for significant difference using two-tailed unpaired Student’s t-test for two groups and using one-way ANOVA with post hoc test for more than two groups. A P-value of less than 0.05 was considered statistically significant. Data analyses were done using GraphPad Prism version 7.03.
RESULTS
Effect of screened hit compounds on LY paracellular permeation across the cell-based BBB model and on tight junction proteins levels
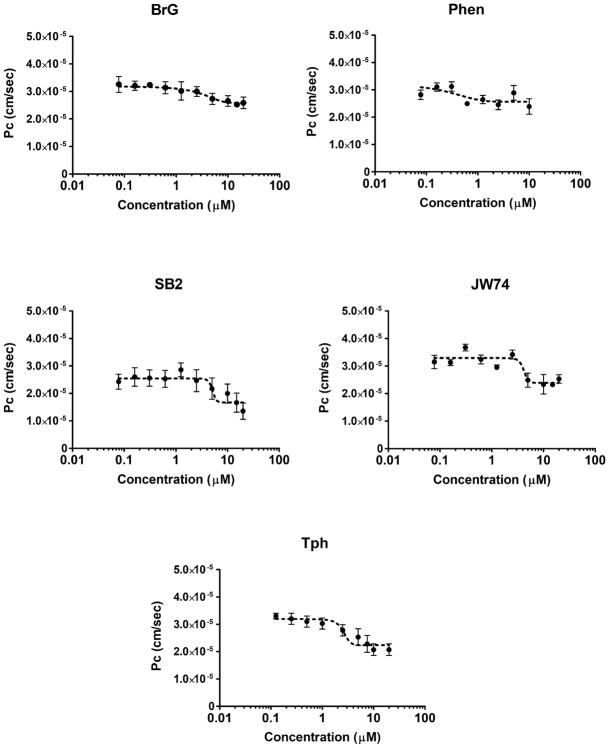
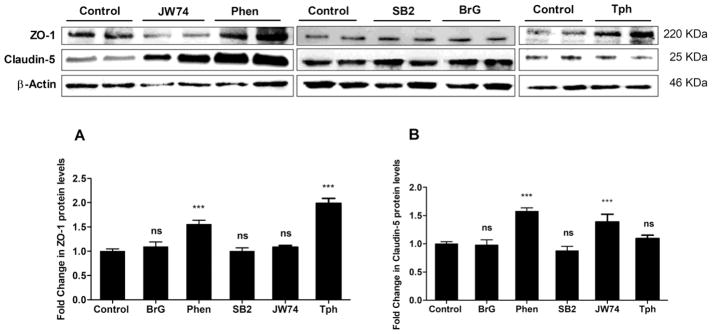
The purpose of this secondary screen was to confirm the observed effect from our primary screen previously reported (Qosa et al., 2016), and to estimate compounds potencies to enhance the monolayer tightness. Tested compounds were validated in 10-point concentration-response studies in the range 0.078–20 μM. As shown in Figure 1 and Table 1, all compounds demonstrated a sigmoidal concentration-response reduction in LY permeation across the bEnd3 monolayer up to 20 μM except the compound Phen. Phen showed toxicity above 10 μM as determined by significant increase in LY permeation suggesting a leaky monolayer possibly caused by cells death and thus de-attachment. The potency of each compound was determined as the ratio of fold change in the permeation coefficient (Pc; equation 1) and EC50 values (i.e. fold change/EC50), the higher the ratio the higher is the potency. According to obtained values, Phen and Tph showed to be most potent compounds among the tested compounds. Results from Western blot analysis revealed Phen and Tph significantly induced ZO-1 by 1.6 and 2-fold (p < 0.001, Figure 2a), respectively, and JW74 and Phen induced claudin-5 protein levels by 1.4 and 1.6-fold, respectively, (p < 0.001, Figure 2b). On the other hand, BrG and SB2 have no effect on tested tight junctions.
Figure 1.
Hit compounds secondary screen. 10-Point concentration-response curves of hit compounds showed significant enhancement in the barrier function of the bEnd3 cells-based BBB model. The compounds were tested in the concentration range 0.078–20 μM to calculate compounds EC50s. Data represent mean±SD, n = 6 for each concentration.
Figure 2.
Representative western blot and densitometry analysis of ZO-1 (a), claudin-5 (b) and β-actin, as loading control, proteins levels in bEnd3 cells treated for 24 h with 10 μM of JW74, Phen, SB2, BrG and Tph. Data are presented as mean ± SD of 3 independent experiments. ***P<0.001 compared to control group, ns=not significant.
Effect of hit compounds on 125I-Aβ40 transport across the cell-based BBB model and on Aβ major transport proteins levels
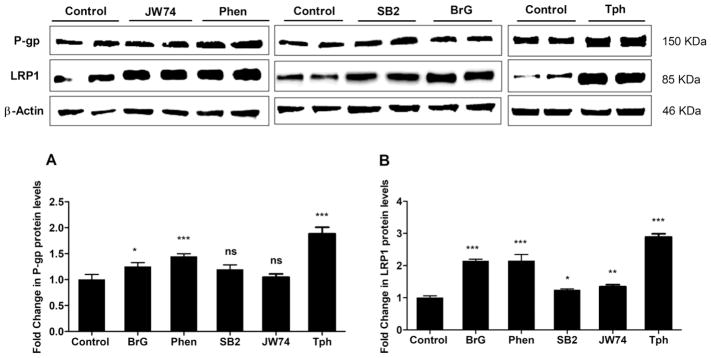
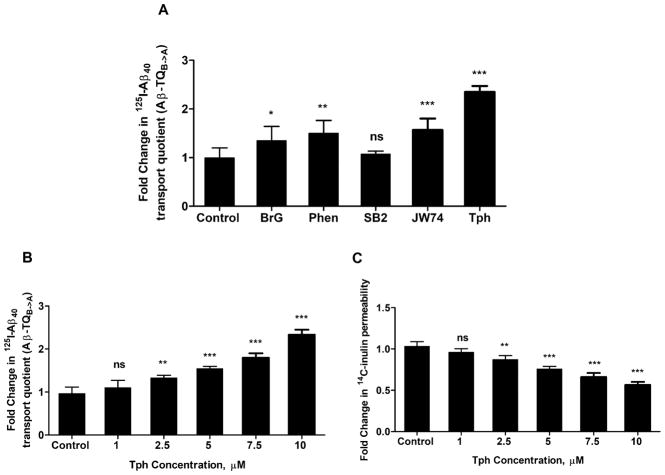
The effect of hit compounds on the transport of Aβ across the cell-based BBB model was evaluated by monitoring the transport of 125I-Aβ40 from basolateral side (mimics the brain side) to apical side (mimics the blood side), which was then used to calculate 125I-Aβ40 transport quotient (TQB→A) according to equation 2. As shown in Fig. 3a, cells treatment with compounds, tested at 10 μM concentration, significantly increased 125I-Aβ40 transport by 1.35 to 2.4-fold compared to control, except SB2 that has no effect on 125I-Aβ40 transport across the monolayer. Tph treatment demonstrated highest effect. Western blot analysis revealed all tested compounds significantly up-regulated the protein levels of P-gp and LRP1, except SB2 which only increased LRP1 by 25% (P<0.05), and JW74 by 34% (P<0.01), however both compounds have no significant effect on P-gp protein levels (Fig. 4).
Figure 3.
(a) Effect of bEnd3 cells treatment for 24 h with 10 μM of JW74, Phen, SB2, BrG and Tph on the transport of 125I-Aβ40, added to the lower chamber of the inserts, across bEnd3 monolayer. 125I-Aβ40 transport is determined as transport quotient (Aβ-TQB→A). (b) Effect of increasing concentration of Tph on the transport of 125I-Aβ40, and (c) permeability of 14C-inulin. Data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ns=not significant.
Figure 4.
Representative western blot and densitometry analyses of (a) P-gp and (b) LRP1 proteins levels in bEnd3 cells treated for 24 h with 10 μM of JW74, Phen, SB2, BrG and Tph. Data are presented as mean ± SD of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001, ns=not significant.
Concentration-dependent effect of Tph on bEnd3 monolayer tightness and 125I-Aβ40 transport across the cell-based BBB model
Next, we performed concentration-dependent studies to evaluate the effect of Tph added to the apical side at different concentrations ranging from 1.0 to 10.0 μM on 125I-Aβ40 transport and bEnd3 monolayer tightness. As shown in Figure 3b and c, results demonstrated Tph significantly increased 125I-Aβ40 transport and reduced 14C-inulin, used as a paracellular permeability marker, in a concentration-dependent manner.
In vivo effect of Tph treatment on the protein levels of Aβ clearance proteins and BBB tightness in 5XFAD mice
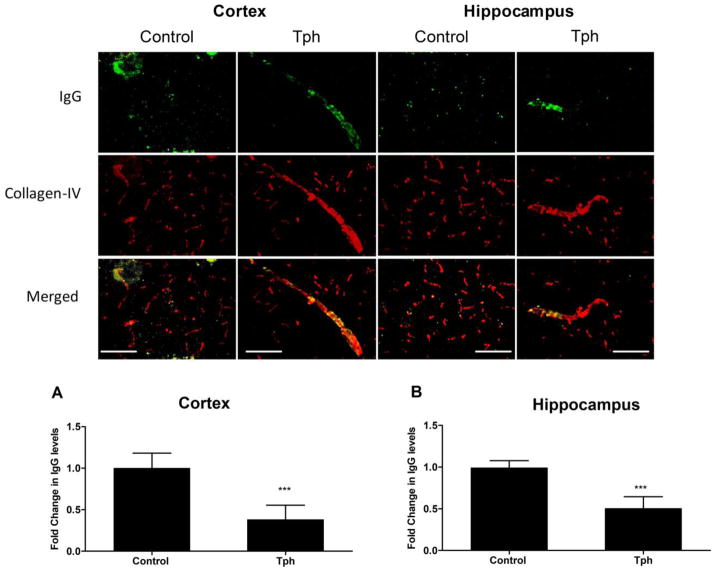
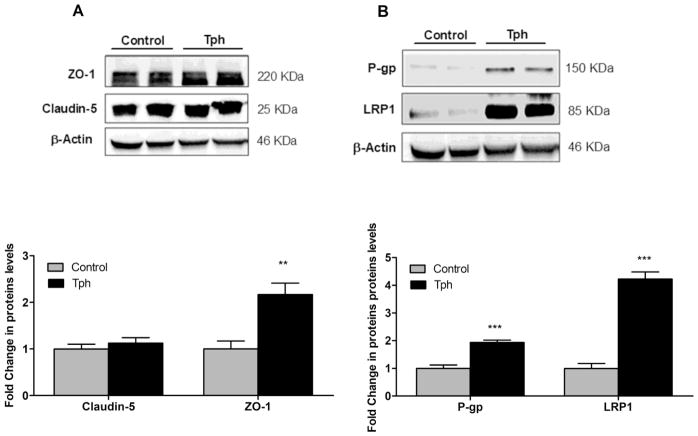
To investigate the effect of one-month treatment with Tph on BBB tightness, level of IgG extravasation in mice brains was evaluated and compared to vehicle treated mice. Immunostaining results demonstrated Tph signi cantly reduced IgG extravasation by 65 and 55% in the cortexes and hippocampi of 5XFAD mice brains, and retained IgG inside blood microvessels when compared to control group (Fig. 5, P<0.001). This result suggests Tph enhanced tightness of the BBB in 5XFAD mice brains. In addition, and consistent with the in vitro data, Tph treatment significantly increased the levels of ZO-1 by 2.1-fold but not claudin-5 in isolated microvessels from mice brains (Fig. 6A). Moreover, 5XFAD mice treatment with Tph significantly induced Aβ major transport proteins P-gp and LRP1 levels by 1.95 and 4.2-fold, respectively, compared to control group.
Figure 5.
Effect of Tph treatment (10 mg/kg/day for one month) on BBB tightness in 5XFAD mice. The figure shows representative brain sections stained with anti-IgG antibody to detect IgG extravasation (green) and collagen antibody (red) to detect microvessels, and their optical density quantitation in cortex (a) and hippocampus (b) regions. Data are presented as mean ± SEM of five mice in each group. ***P<0.001 compared to control group. Scale bar, 50 μm.
Figure 6.
Effect of Tph treatment (10 mg/kg/day for one month) on tight junction proteins and Aβ transport proteins in microvessels isolated from 5XFAD mice brain. (a) Representative blots and densitometry analysis of claudin-5 and ZO-1. (b) Representative blots and densitometry analysis of P-gp and LRP1. Data are presented as mean ± SEM of five mice in each group. **P<0.01, ***P<0.001.
In vivo effect of Tph treatment on Aβ load in in 5XFAD mice brains
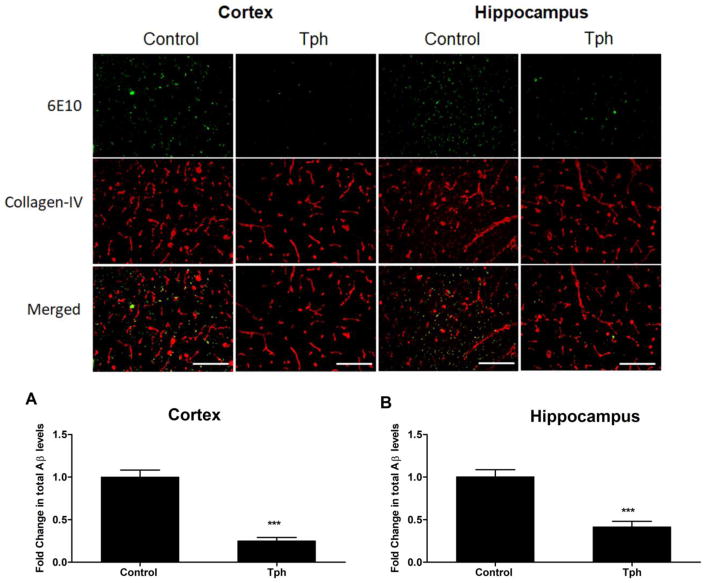
To investigate the effect of one-month treatment of Tph on total Aβ load, immunohistochemical analysis of Aβ colocalization with collagen-IV (a marker of microvessels) in the brains of 5XFAD mice was performed. Compared to vehicle traeted mice, Tph treatment demonstrated a significant reduction in total Aβ immunostaining (Fig. 7). Semiquantitative analysis for total Aβ showed Tph treatment caused a significant reduction by 75 and 59% in total Aβ levels in the cortexes and hippocampi of mice brains when compared to the control group (Fig. 7, P<0.001).
Figure 7.
Effect of Tph treatment (10 mg/kg/day for one month) on total Aβ brain levels in 5XFAD mice. Representative brain sections stained with 6E10 antibody against Aβ to detect total Aβ load (green) and collagen antibody (red) to detect microvessels, and their optical density quantitation in cortex (a) and hippocampus (b) regions demonstrate significant reduction in Aβ load. Data are presented as mean ± SEM of five mice in each group. ***P<0.001 compared to control group. Scale bar, 50 μm.
DISCUSSION
Several studies suggested disruption of the BBB as a causal factor in AD where its function is compromised during aging and neurodegenerative disorders (Sagare et al., 2012; Marques et al., 2013; Erdő et al., 2017). Recently, we have developed and reported a cell-based BBB model, which provided a successful, efficient, and large scale HTS assay in identifying potential therapeutic compounds to enhance the monolayer intactness (Qosa et al., 2016). The primary screen of LOPAC®1280 library from Sigma-Aldrich identified several hit compounds, among which are 8-bromoguanosine 3,5-cyclic monophosphate sodium salt (BrG), JW74, 1,10- phenanthroline monohydrate (Phen), SB216763 (SB2) and α-tocopherol (Tph). These compounds were selected for secondary screening based on literature studies demonstrating their pharmacological activities as summarized in Table 1. The purpose of this work was to perform secondary screen studies to confirm the observed effect from the primary screen, estimate compounds potencies to enhance the monolayer tightness, evaluate effect on Aβ transport, to mechanistically characterize the observed effect, and finally to test most potent compound in vivo. Selected compounds were validated in 10-point concentration-response studies in the range 0.078–20 μM. All compounds showed a sigmoidal concentration-response reduction in LY permeation across the bEnd3 monolayer up to 20 μM except the compound Phen that showed toxicity above 10 μM. Compounds potency to enhance tightness were determined and ranked. Potency ranged from 0.03–0.38 in the following order: Phen > Tph > JW74 > BrG=SB2. To understand the mechanism by which the compounds increased bEnd3 monolayer intactness, we studied their effect on the levels of key proteins that play important role in determining the permeability properties of endothelial cells by Western blot. Tested proteins were claudin-5, a transmembrane protein, and ZO-1, a cytoplasmic protein interacts with transmembrane proteins in multi-protein complexes linked to the actin cytoskeleton (Wolburg et al., 2002). ZO-1 interacts with the C-terminal domain of claudins (Itoh et al., 1999), JAMs (Bazzoni et al., 2000), and occludin (Furuse et al., 1994). With the exception of compounds BrG and SB2, all compounds substantially induced the proteins levels of ZO-1 and/or claudin-5, which could explain, at least in part, the observed reduction in paracellular permeability of LY. Phen, Tph and JW74 significantly induced ZO-1 and/or claudin-5; BrG and SB2 on the other hand did not alter tested tight junctions, yet, their effect on other tight junction proteins cannot be excluded as an explanation for reduced LY permeability across the monolayer.
AD is associated with increased accumulation and reduced clearance of Aβ peptide, which leads to the formation of extracellular Aβ plaques, composed mainly of 40 and 42 amino acids (Selkoe, 2001; Sagare et al., 2012). While both Aβ peptide species, Aβ40 and Aβ42, are implicated in the pathogenesis of AD (Chang et al., 2013), Aβ40 was used in this work for practicality reasons as it has much faster clearance rate than Aβ42 with less tendency to aggregate in the experimental settings (Ito et al., 2006). The effect of selected compounds on the transport of Aβ across the cell-based BBB model was evaluated by monitoring 125I-Aβ40 transport across the monolayer. The results showed all tested compounds, except SB2, increased 125I- Aβ40 transport by 1.35 to 2.4-fold compared to control, with Tph showing the highest effect. Aβ removal from the brain across the BBB is mainly facilitated by LRP1 and P-gp (Shibata et al., 2000; Cirrito et al., 2005; Qosa et al., 2014a). Several studies including ours have demonstrated a role of both proteins in Aβ transport across the BBB and that their up-regulation enhances Aβ clearance (Abuznait et al., 2013; Mohamed et al., 2016). Thus, to explain the transport increase, the proteins levels of P-gp and LRP1 were evaluated by Western blot. Our findings showed Phen, Tph and BrG significantly up-regulated the levels of both P-gp and LRP1, which could explain, at least in part, the increased transport of 125I-Aβ40.
Findings from in vitro data suggested Phen and Tph have the highest potency to enhance the endothelium tightness using LY assay compared to other investigated compounds, however, Phen showed toxicity at concentrations higher than 10 μM, and its effect on 125I-Aβ40 was lower than Tph. Accordingly, Tph was selected for in vivo proof-of-concept evaluation in an AD mouse model for its effect on BBB tightness and Aβ brain levels. Before initiating the in vivo studies, however, concentration-dependent studies for the effect of Tph on 125I-Aβ40 and 14C-inulin transport across the monolayer were performed and confirmed Tph effect on the BBB model function to clear 125I-Aβ40, and tightness using inulin as a permeability marker in a concentration-dependent manner.
Several studies have reported the benefits of Tph in the treatment of AD. Tph is a major lipid-soluble antioxidant that maintains cell integrity by preventing lipid peroxidation in cellular membranes (Burton et al., 1982); it also acts by scavenging reactive oxygen species (ROS) before they damage the cells (Burton et al., 1982; Herrera and Barbas, 2001). In addition, Tph demonstrated a protective effect against AD pathogenesis. Tph treatment of neuronal cells isolated from rat brains prevented Aβ-associated ROS from forming and decreased oxidative stress markers (Yatin et al., 2000). Furthermore, treatment of mild and moderate AD patients with vitamin E and C for three months decreased plasma lipoperoxidation susceptibility by 60% (Galbusera et al., 2004). It has also been reported that low levels of Tph in plasma increased the risk for mild cognitive impairment and AD (Mangialasche et al., 2012). This finding was supported by another study reported the alteration in Tph status in AD where increased brain and reduced plasma concentrations of Tph in AD patients have been observed, suggesting a possible compensatory response to oxidative damage (Aisen et al., 2012). Beside these reported effects, findings from our study support the positive effect of Tph by enhancing the BBB integrity and function in vivo in 5XFAD mice, a model of AD.
Available evidence suggests that AD brains have leaky BBB manifested by increased IgG extravasation to the brain (Ryu and McLarnon, 2009). Our data demonstrated that one-month treatment of Tph enhanced BBB tightness as evident by reduced levels of IgG extravasation, and retained IgG inside the blood capillaries when compared to the control group. The significant reduction in IgG extravasation could be explained by increased levels of tight junction proteins mainly ZO-1, which subsequently increased the BBB tightness and retained IgG in microvessels’ lumen. Consistent with the in vitro data, Tph, however, has no effect on claudin-5 (Fig. 6a). Furthermore, our findings demonstrated Tph significantly reduced total Aβ load in the cortex and hippocampus of 5xFAD mice brains. This reduction in Aβ load was associated with significant increase in Aβ major transport proteins P-gp and LRP1 expressed in the endothelial cells of the BBB. While further studies are necessary to evaluate overall effect of Tph on Aβ pathology, these findings could explain, at least in part, the reduced total load of Aβ in 5XFAD mice brains caused by enhanced clearance of Aβ across the BBB via P-gp and LRP1 activation as reported previously (Abuznait et al., 2013; Qosa et al., 2015; Mohamed et al., 2016; Batarseh et al., 2017; Pereira et al., 2018). In AD, several mechanisms have been proposed for BBB increased permeability, reduced levels of tight junction and Aβ transport proteins, among which is increased reactive oxygen species (ROS) associated with oxidative stress (Abdul-Muneer et al., 2015; Ronaldson and Davis, 2015; Milczarek et al., 2017), which induce BBB disruption. This disruptive effect could be directly or indirectly influenced by accumulation of Aβ (Sita et al., 2017). Thus, Tph effect on enhancing the BBB integrity and function could be explained, at least in part, by its antioxidant effect to scavenge ROS. Additional studies are on going to investigate the molecular mechanism(s) by which Tph increased tight junction protein ZO-1 and Aβ transport major proteins P-gp and LRP1.
Collectively, findings from the in vivo data of increased BBB tightness, reduced Aβ load, and increased levels of ZO-1 and transport proteins determined in isolated microvessels from 5XFAD mice brains treated with Tph demonstrated a considerable consistency when compared with the in vitro data. These results support our developed cell-based BBB model as a functional predictive in vivo tool to select lead compounds. Studying each hit compound separately provides a great opportunity to examine the potential of these hits to hold or slow progression, or treat AD. Both the in vitro and in vivo results demonstrated Tph as a promising candidate to prevent, hold or slow the progression of AD pathology. Further in vivo preclinical studies combined with cognitive function testing are required to confirm current findings.
Acknowledgments
This research work was funded by the National Institute of Neurological Disorders and Stroke (NIH/NINDS) under grant number R15NS091934, and Louisiana Board of Reagent’s Research Competitive Program under grant number LEQSF (2013–16)-RD-A-16.
Footnotes
Notes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2015;51:966–979. doi: 10.1007/s12035-014-8752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS Chem Neurosci. 2013;4:973–982. doi: 10.1021/cn400024q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012:a006395. doi: 10.1101/cshperspect. pii:a006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Batarseh YS, Bharate SS, Kumar V, Kumar A, Vishwakarma RA, Bharate SB, Kaddoumi A. Crocus sativus Extract Tightens the Blood-Brain Barrier, Reduces Amyloid β Load and Related Toxicity in 5XFAD Mice. ACS Chem Neurosci. 2017;8:1756–1766. doi: 10.1021/acschemneuro.7b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer’s disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GW, Joyce A, Ingold KU. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Chang CC, Althaus JC, Carruthers CJ, Sutton MA, Steel DG, Gafni A. Synergistic interactions between Alzheimer’s Aβ40 and Aβ42 on the surface of primary neurons revealed by single molecule microscopy. PLoS One. 2013;8:e82139. doi: 10.1371/journal.pone.0082139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier. J Cereb Blood Flow Metab. 2017;37:4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tomé P, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Neuroprotective effects of DETA-NONOate, a nitric oxide donor, on hydrogen peroxide-induced neurotoxicity in cortical neurons. Neuropharmacology. 1999;38:1307–1315. doi: 10.1016/s0028-3908(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbusera C, Facheris M, Magni F, Galimberti G, Sala G, Tremolada L, Isella V, Guerini FR, Appollonio I, Galli-Kienle M, Ferrarese C. Increased susceptibility to plasma lipid peroxidation in Alzheimer disease patients. Curr Alzheimer Res. 2004;1:103–109. doi: 10.2174/1567205043332171. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 Census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43–56. https://www.alz.org/documents_custom/2017-facts-and-figures.pdf. [PubMed] [Google Scholar]
- Hu H, Liu B, Zuo Y, Liu D, Xie R, Cui W. dl-3-n-butylphthalide suppresses PDGF-BB-stimulated vascular smooth muscle cells proliferation via induction of autophagy. Life Sci. 2016;151:182–188. doi: 10.1016/j.lfs.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-beta peptide (1–40) across the rat blood-brain barrier. Neurosci Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Russell MJ, Cullen MJ, Tökés ZA. Matrix metalloproteinases in dog brains exhibiting Alzheimer-like characteristics. J Neurochem. 1997;68:1606–1611. doi: 10.1046/j.1471-4159.1997.68041606.x. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, Xu W, Kivipelto M, Costanzi E, Ercolani S, Pigliautile M, Cecchetti R, Baglioni M, Simmons A, Soininen H, Tsolaki M, Kloszewska I, Vellas B, Lovestone S, Mecocci P NeuroMed Consortium. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging. 2012;33:2282–2290. doi: 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener. 2013;8:38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczarek A, Starzyński RR, Styś A, Jończy A, Staroń R, Grzelak A, Lipiński P. A drastic superoxide-dependent oxidative stress is prerequisite for the down-regulation of IRP1: Insights from studies on SOD1-deficient mice and macrophages treated with paraquat. PLoS One. 2017;12:e0176800. doi: 10.1371/journal.pone.0176800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia KD, Olsen CH, Mitchell JM, Landauer MR. Evaluation of Hydration and Nutritional Gels as Supportive Care after Total-Body Irradiation in Mice (Mus musculus) J Am Assoc Lab Anim Sci. 2010;49:323–328. [PMC free article] [PubMed] [Google Scholar]
- Mohamed LA, Keller JN, Kaddoumi A. Role of P-glycoprotein in mediating rivastigmine effect on amyloid-β brain load and related pathology in Alzheimer’s disease mouse model. Biochim Biophys Acta. 2016;1862:778–787. doi: 10.1016/j.bbadis.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Ortega F, Solis-Cisneros A, Ordaz B, Balleza-Tapia H, Javier Lopez-Guerrero J. Amyloid beta 1-42 inhibits entorhinal cortex activity in the beta-gamma range: role of GSK-3. Curr Alzheimer Res. 2012;9:857–863. doi: 10.2174/156720512802455403. [DOI] [PubMed] [Google Scholar]
- Pereira CD, Martins F, Wiltfang J, da Cruz E, Silva OAB, Rebelo S. ABC Transporters Are Key Players in Alzheimer’s Disease. J Alzheimers Dis. 2018;61:463–485. doi: 10.3233/JAD-170639. [DOI] [PubMed] [Google Scholar]
- Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in amyloid-β clearance across mouse and human blood-brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology. 2014a;79:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Batarseh YS, Mohyeldin MM, El Sayed KA, Keller JN, Kaddoumi A. Oleocanthal enhances amyloid-β clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem Neurosci. 2015;6:1849–1859. doi: 10.1021/acschemneuro.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, LeVine H, 3rd, Keller JN, Kaddoumi A. Mixed oligomers and monomeric amyloid-β disrupts endothelial cells integrity and reduces monomeric amyloid-β transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta. 2014b;1842:1806–1815. doi: 10.1016/j.bbadis.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Mohamed LA, Al Rihani SB, Batarseh YS, Duong QV, Keller JN, Kaddoumi A. High-throughput screening for identification of blood-brain barrier integrity enhancers: a drug repurposing opportunity to rectify vascular amyloid toxicity. J Alzheimers Dis. 2016;53:1499–1516. doi: 10.3233/JAD-151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeting transporters: promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res. 2015;1623:39–52. doi: 10.1016/j.brainres.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012:a011452. doi: 10.1101/cshperspect. pii: a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS. Alzheimer disease pharmacologic treatment and treatment research. Continuum (Minneap Minn) 2013;19:339–357. doi: 10.1212/01.CON.0000429180.60095.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz-Brugger F, Giovanni A. Guanosine 3′,5′-cyclic monophosphate mediated inhibition of cell death induced by nerve growth factor withdrawal and beta-amyloid: protective effects of propentofylline. Neuroscience. 2000;99:737–750. doi: 10.1016/s0306-4522(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Sita G, Hrelia P, Tarozzi A, Morroni F. P-glycoprotein (ABCB1) and Oxidative Stress: Focus on Alzheimer’s Disease. Oxid Med Cell Longev. 2017;2017:7905486. doi: 10.1155/2017/7905486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Butterfield DA. Vitamin E Prevents Alzheimer’s Amyloid beta-Peptide (1-42)-Induced Neuronal Protein Oxidation and Reactive Oxygen Species Production. J Alzheimers Dis. 2000;2:123–131. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]