Summary
Objective
To assess the prevalence of and characteristics associated with current smoking in an Asian HIV-positive cohort, calculate the predictive risks of cardiovascular disease (CVD), coronary heart disease (CHD), and myocardial infarction (MI), and identify the impact that simulated interventions may have.
Methods
Logistic regression analysis distinguished associated characteristics. Five-year predictive risks of CVD, CHD, MIs and the impact of simulated interventions were calculated utilizing the Data Collection on Adverse Effects of Anti-HIV Drugs Study (D:A:D) algorithm.
Results
Smoking status data were collected from 4,274 participants and 1,496 of these had sufficient data for simulated intervention calculations. Current smoking prevalence in these two groups was similar (23.2% vs. 19.9%). Characteristics associated with current smoking included being ≥50 years compared to 30–39 years (OR, 0.65; 95% confidence interval [CI], 0.51–0.83), HIV exposure through injection drug use compared to heterosexual exposure (OR, 3.03; CI, 2.25–4.07), and receiving ART at study sites in Singapore, South Korea, Malaysia, Japan, and Vietnamese sites in comparison to Thailand (all odds ratios >2). Alternatively, females were less likely to smoke than males (OR, 0.11; CI, 0.08–0.14). In simulated interventions smoking cessation demonstrated the greatest impact in reducing CVD and CHD risk and closely approximated the impact of switching from abacavir to an alternate antiretroviral in the reduction of five-year MI risk.
Conclusion
Multiple interventions could reduce CVD, CHD, and MI risk in Asian HIV-positive patients, with smoking cessation potentially being the most influential.
Keywords: smoking prevalence, Asia, cardiovascular risk, HIV
Introduction
Individually, tobacco use, cardiovascular disease (CVD), and HIV all contribute to morbidity and mortality in Asia. However, limited studies have focused on how these epidemics are intertwined within resource limited settings (1–5). As antiretroviral therapy (ART) access expands, there is a greater need to better understand long-term treatment outcomes and the emergence of non-communicable diseases in the complex context of HIV infection (6–14). Within Asia, smoking is considered the third leading cause of death (10, 15, 16). When comparing the number of CVD-related deaths in resource-limited to resource-rich settings, the ratio is more than four to one (5, 17, 18). This has significant implications for people living with HIV (PLWH) on ART who carry a higher risk of CVD than the general population (19).
We sought to assess smoking prevalence in a regional cohort of Asian PLWH on ART, predictors of current smoking and associated demographic factors. Simulated interventions utilizing The Data Collection on Adverse Effects of Anti-HIV Drugs Study (D:A:D) risk algorithm were applied as a predictive tool to demonstrate how smoking may influence future CVD, coronary heart disease (CHD), and myocardial infarction (MI) risk outcomes and how simulated interventions may mitigate risk.
Methods
The study population consisted of participants enrolled in the TREAT Asia HIV Observational Database (TAHOD). The cohort has previously been described (20, 21). Briefly, TAHOD is an observational study of PLWH involving 21 adult treatment sites in 12 countries and territories in Asia with recruitment that began in September 2003. The primary objective of the cohort study is to monitor and evaluate HIV disease and treatment outcomes. Each site contributes data from 100–450 participants that are biannually transferred to the data management and biostatical analysis center at the Kirby Institute, Sydney, Australia. At the September 2013 data transfer the entire cohort was comprised of 8,694 participants, 5691 (70.4%) were male, median age was 40.8 years (interquartile range [IQR], 34.7–47.8), and 5,220 (64.6%) were exposed to HIV via heterosexual contact. Of the total cohort 8,079 (93%) were on ART. Institution Review Board approval was obtained at the participating sites, the analysis center, and the coordinating center (TREAT Asia/amfAR, Bangkok). Informed consent was waived in this study unless locally required by a site’s institutional review board.
Given the data required for the D:A:D algorithm, predicted risks could only be generated for patients with an available baseline smoking assessment, cholesterol, HDL cholesterol, diabetes history, systolic blood pressure, and history of ART. Data analysis was limited to those with documented smoking data between March 2012 and September 2013. Self-reported smoking status included a yes/no response to current smoking, former smoking, or never smoking cigarettes. Comorbidities and their definitions included: being overweight as a body mass index >25 kg/m2 and obesity as a body mass index of >30 kg/m2 (22), hypertension being a systolic blood pressure >140 mmHg (22), diabetes when two consecutive fasting blood glucose values ≥7 mmol/L were observed (23), dyslipidemia when total cholesterol >6.2 mmol/L or HDL cholesterol <1 mmol/L or triglyceride >2.26 mmol/L were observed (24), and hepatitis B and C infection confirmed with a positive hepatitis B surface antigen test or positive hepatitis C antibody test, respectively.
Five-year predicted risks of CVD, CAD and MIs were based on the D:A:D risk algorithm, which delineates low (<1%), moderate (1–5%), high (5–10%) and very high (>10%) risk (25). The D:A:D algorithm more accurately ascertains cardiovascular risk in PLWH compared to the Framingham score and has been validated in PLWH in high-income countries as well as successfully applied in Thailand and Brazil (26, 27). Poisson regression models with prospective follow up of participants from baseline to time to event were calculated. Covariates were included in the model where the association with the outcome was significant at a p-value of <0.05.
Baseline was defined by the most recent smoking assessment date. CD4 cell count and viral load were within a window period of three months on either side of the smoking assessment and other laboratory and physical parameters were within six months. Patients with prior documented evidence of hepatitis B, hepatitis C, or diabetes were considered to have that condition at the time of their latest smoking assessment.
Statistical analysis
Logistic regression evaluated baseline characteristics predictive of current smoking. Multivariate models selected variables based on a significance level of ≤0.10 in the univariate analyses. Predictors were retained in the multivariate model with a p-value of ≤0.05. Chi-squared test compared prevalence of comorbidities by prevalence of reported smoking status. The impact of different interventions on the predicted risk of CVD, CHD, and MI were investigated by creating the following hypothetical scenarios: 1) current smokers became former smokers; 2) patients with low HDL cholesterol increased their level to 1mmol/L; 3) patients with high cholesterol reduced their level to 5.2mmol/L; 4) patients with high systolic blood pressure reduced their level to 130mmHg; 5) patients using lopinavir switched to an alternate antiretroviral (ARV); 6) patients using abacavir switched to an alternate ARV. The target values defined for scenarios 2), 3) and 4) were based on recommendations from the U.S. National Heart, Blood, and Lung Institute (24).
Stata version 12 (College Station, TX, USA) was used for all statistical analysis.
Results
Smoking Prevalence
We identified 4,274 participants with a recorded smoking status. Of those, 2,278 patients were excluded from the prediction analysis due to missing data required for D:A:D calculations, leaving 1,496 patients. The prevalence rates of current, former, and never smoking between the total and the number of participants with sufficient data for analyses were similar (23.2 vs. 19.9, 20.3 vs. 22.5, and 56.5 vs. 57.6; Table 1).
Table 1.
Demographic Variables and Smoking Status
| All patients on ART with smoking status available (n=4274) |
Patients on ART with all cardiovascular disease risk data available (n=1496) |
|
|---|---|---|
| Median (IQR) age, years | 40.7 (34.5 – 47.6) | 44.0 (38.5 – 50.4) |
| Age, years | ||
| <30 | 348 (8.1%) | 40 (2.7%) |
| 30 – 39 | 1480 (34.6%) | 370 (24.7%) |
| 40 – 49 | 1540 (36.0%) | 646 (43.2%) |
| ≥50 | 906 (21.2%) | 440 (29.4%) |
| Sex | ||
| Female | 1253 (29.3%) | 482 (32.2%) |
| Male | 3021 (70.7%) | 1014 (67.8%) |
| HIV exposure | ||
| Heterosexual | 2776 (65.0%) | 1178 (78.7%) |
| Homosexual | 900 (21.1%) | 223 (14.9%) |
| Injecting drug use | 348 (8.1%) | 32 (2.1%) |
| Other/unknown | 250 (5.8%) | 63 (4.2%) |
| Family history of MI or stroke | ||
| Yes | 165 (3.9%) | 56 (3.7%) |
| No | 2296 (53.7%) | 888 (59.4%) |
| Unknown | 1813 (42.4%) | 552 (36.9%) |
| Location of TAHOD site(s) | ||
| Thailand | 1291 (30.2%) | 695 (46.5%) |
| Vietnam | 536 (12.5%) | 3 (0.2%) |
| Indonesia | 454 (10.6%) | 47 (3.1%) |
| India | 406 (9.5%) | 197 (13.2%) |
| Hong Kong SAR | 357 (8.4%) | 350 (23.4%) |
| Philippines | 339 (7.9%) | 5 (0.3%) |
| Taiwan | 301 (7.0%) | 6 (0.4%) |
| Malaysia | 224 (5.2%) | 91 (6.1%) |
| Japan | 190 (4.4%) | 32 (2.1%) |
| South Korea | 92 (2.2%) | 22 (1.5%) |
| Singapore | 69 (1.6%) | 48 (3.2%) |
| China | 15 (0.4%) | 0 (0.0%) |
| Smoking status | ||
| Current | 992 (23.2%) | 298 (19.9%) |
| Former | 867 (20.3%) | 337 (22.5%) |
| Never | 2415 (56.5%) | 861 (57.6%) |
ART, antiretroviral therapy; IQR, interquartile range; MI, myocardial infarction; TAHOD, TREAT Asia HIV Observational Database; SAR, special administrative regions of China
Demographic Variables
Table 1 outlines the demographic data of the 4,274 participants with documented smoking status. The median age was 40.7 years (IQR, 34.5–47.6) with the majority being male (70.7%) and exposed to HIV through heterosexual intercourse (65%). The age categories and percentages included >50 years (21.2 %), 40–49 years (36%), 30–39 years (34.6%) and <30 years (8.1%). Of the 1,496 participants with sufficient data for D:A:D calculations, the median age was older by 3.3 years (IQR, 38.5–50.4) and had a history of longer ART treatment by 2.3 years (IQR, 3.2–9.9). However, differences between the median age and duration of ART treatment were not statistically significant.
Table 2 outlines the percentage exposed to ART that may contribute to CVD, CAD and MI risk as identified under the D:A:D algorithm. In the 1,496 participants, 1.2% were exposed to indinavir, 12.7% to lopinavir, and 14.2% to abacavir. The median CD4 cell count was 460 cells/mm3 (IQR 336–616 cells/mm3) and 66.7% of participants had a viral load <400 copies/ml.
Table 2.
Comorbidities and HIV Care and Treatment Variables
| Patients on ART with all cardiovascular disease risk data available (n=1496) |
|
|---|---|
| Median (IQR) body mass index | 22.3 (20.3 – 24.6) |
| Body mass index | |
| <18.5 | 139 (9.3%) |
| 18.5 – 25 | 952 (63.6%) |
| 25 – 30 | 266 (17.8%) |
| >30 | 48 (3.2%) |
| Unknown | 91 (6.1%) |
| Median (IQR) blood pressure, mmHg | 128 (116 – 139) / 79 (70 – 85) |
| Hypertension (systolic >140 mmHg) | 304 (20.3%) |
| Diabetes mellitus (fasting ≥7mmol/L) | 124 (8.3%) |
| Median (IQR) total cholesterol, mmol/L | 5.1 (4.4 – 5.8) |
| Total cholesterol (>6.2 mmol/L) | 686 (45.9%) |
| Median (IQR) HDL cholesterol, mmol/L | 1.2 (1.0 – 1.5) |
| HDL cholesterol (<1 mmol/L) | 322 (21.5%) |
| Median (IQR) triglycerides, mmol/L | 1.7 (1.1 – 2.6) |
| Triglycerides (>2.26 mmol/L) | 584 (39.0%) |
| HBV co-infection | |
| Positive | 130 (8.7%) |
| Negative | 1168 (78.1%) |
| Not tested | 198 (13.2%) |
| HCV co-infection | |
| Positive | 83 (5.5%) |
| Negative | 1178 (78.7%) |
| Not tested | 235 (15.7%) |
| Median (IQR) CD4 cell count, cells/mm3 | 460 (336 – 616) |
| CD4 count, cells/mm3 | |
| <200 | 85 (5.7%) |
| ≥200 | 1149 (76.8%) |
| Unknown | 262 (17.5%) |
| Viral load, copies/mL | |
| ≥400 | 46 (3.1%) |
| <400 | 998 (66.7%) |
| Unknown | 452 (30.2%) |
| Prior AIDS diagnosis | |
| Yes | 721 (48.2%) |
| None known | 775 (51.8%) |
| Current indinavir use | |
| Yes | 18 (1.2%) |
| No | 1478 (98.8%) |
| Current lopinavir use | |
| Yes | 190 (12.7%) |
| No | 1306 (87.3%) |
| Current abacavir use | |
| Yes | 213 (14.2%) |
| No | 1283 (85.8%) |
| Median (IQR) time since ART initiation, years | 6.0 (3.2 – 9.9) |
| Time since ART initiation, years | |
| <2 | 140 (9.4%) |
| 2 – 4 | 413 (27.6%) |
| 4 – 8 | 366 (24.5%) |
| >8 | 577 (38.6%) |
| Year of smoking assessment | |
| Before 2011 | 43 (2.9%) |
| 2011 | 165 (11.0%) |
| 2012 | 690 (46.1%) |
| 2013 | 598 (40.0%) |
| Smoking status | |
| Current | 298 (19.9%) |
| Former | 337 (22.5%) |
| Never | 861 (57.6%) |
ART, antiretroviral therapy; IQR, interquartile range; MI, myocardial infarction; TAHOD, TREAT Asia HIV Observational Database
The median time since ART initiation was 6.0 years (IQR, 3.2–9.9). The duration of ART treatment categories included >8 years (38.6%), 4–8 years (24.5%), 2–4 years (27.6%), <2 years (9.4%
Predictors of Current Smoking
Predictive factors of smoking in the multivariate analysis were: age, sex, HIV exposure category, and location of treatment site. Participants aged ≥50 years were less likely to smoke than those aged 30–39 years (OR 0.65; 95% confidence interval [CI], 0.51–0.83), females were less likely to smoke than males (OR 0.11; CI, 0.08–0.14), MSM were less likely to smoke than heterosexuals (0.51; CI 0.38–0.67), and HIV exposure via injection drug use were more likely to currently smoke compared to heterosexual sex exposure (3.03; CI, 2.25 – 4.07). In comparison to sites in Thailand, participants in Singapore (OR 5.37; CI, 3.16–9.11), South Korea (OR 3.94; 2.43–6.39), Malaysia (OR 3.32; 2.39–4.63), Japan (OR 3.09; 2.06–4.65), and Vietnam (OR 2.35; 1.77–3.12; Table 3) were more likely to smoke.
Table 3.
Predictors of Current Smoking Status
| Predictor | Number of current smokers |
Number of current non- smokers |
Univariate OR (95%CI) |
p | p overall | Multivariate¥ OR (95%CI) | p | p overall |
|---|---|---|---|---|---|---|---|---|
| Age¤, years | ||||||||
| <30 | 78 | 270 | 0.81 (0.61 – 1.07) | 0.130 | 1.11 (0.80 – 1.53) | 0.528 | ||
| 30 – 39 | 390 | 1090 | 1.00 | 1.00 | ||||
| 40 – 49 | 351 | 1189 | 0.83 (0.70 – 0.97) | 0.023 | 1.02 (0.83 – 1.25) | 0.862 | ||
| ≥50 | 173 | 733 | 0.66 (0.54 – 0.81) | <0.001 | 0.002† | 0.65 (0.51 – 0.83) | 0.001 | 0.001† |
| Sex¤ | ||||||||
| Male | 932 | 2089 | 1.00 | 1.00 | ||||
| Female | 60 | 1193 | 0.11 (0.09 – 0.15) | <0.001 | 0.11 (0.08 – 0.14) | <0.001 | ||
| HIV exposure¤ | ||||||||
| Heterosexual | 516 | 2260 | 1.00 | 1.00 | ||||
| Homosexual | 200 | 700 | 1.25 (1.04 – 1.50) | 0.017 | 0.51 (0.38 – 0.67) | <0.001 | ||
| Injecting drug use | 224 | 124 | 7.91 (6.23 – 10.05) | <0.001 | 3.03 (2.25 – 4.07) | <0.001 | ||
| Other/unknown | 52 | 198 | 1.15 (0.84 – 1.58) | 0.391 | <0.001‡ | 0.62 (0.42 – 0.89) | 0.011 | <0.001‡ |
| Location of TAHOD site(s)¤ | ||||||||
| Thailand | 186 | 1105 | 1.00 | 1.00 | ||||
| Vietnam | 209 | 327 | 3.80 (3.01 – 4.79) | <0.001 | 2.35 (1.77 – 3.12) | <0.001 | ||
| Indonesia | 159 | 295 | 3.20 (2.50 – 4.10) | <0.001 | 1.53 (1.12 – 2.08) | 0.007 | ||
| India | 20 | 386 | 0.31 (0.19 – 0.50) | <0.001 | 0.20 (0.12 – 0.33) | <0.001 | ||
| Hong Kong | 82 | 275 | 1.77 (1.32 – 2.37) | <0.001 | 1.50 (1.09 – 2.06) | 0.012 | ||
| Philippines | 25 | 314 | 0.47 (0.31 – 0.73) | 0.001 | 0.40 (0.25 – 0.64) | <0.001 | ||
| Taiwan | 72 | 229 | 1.87 (1.37 – 2.54) | <0.001 | 1.71 (1.16 – 2.51) | 0.006 | ||
| Malaysia | 97 | 127 | 4.54 (3.34 – 6.17) | <0.001 | 3.32 (2.39 – 4.63) | <0.001 | ||
| Japan | 64 | 126 | 3.02 (2.15 – 4.23) | <0.001 | 3.09 (2.06 – 4.65) | <0.001 | ||
| South Korea | 40 | 52 | 4.57 (2.94 – 7.10) | <0.001 | 3.94 (2.43 – 6.39) | <0.001 | ||
| Singapore | 37 | 32 | 6.87 (4.17 – 11.30) | <0.001 | 5.37 (3.16 – 9.11) | <0.001 | ||
| China | 1 | 14 | 0.42 (0.06 – 3.25) | 0.409 | <0.001‡ | 0.41 (0.05 – 3.22) | 0.394 | <0.001‡ |
| Family history of MI or stroke | ||||||||
| No | 548 | 1748 | 1.00 | |||||
| Yes | 36 | 129 | 0.89 (0.61 – 1.30) | 0.550 | ||||
| Unknown | 408 | 1405 | - | |||||
| CD4 cell count, cells/mm3 | ||||||||
| ≥200 | 540 | 2146 | 1.00 | |||||
| <200 | 150 | 263 | 2.27 (1.82 – 2.83) | <0.001 | ||||
| Unknown | 302 | 873 | - | |||||
| Time since ART initiation, years | ||||||||
| <2 | 248 | 681 | 1.00 | |||||
| 2 – 4 | 285 | 958 | 0.82 (0.67 – 0.99) | 0.044 | ||||
| 4 – 8 | 215 | 822 | 0.72 (0.58 – 0.89) | 0.002 | ||||
| >8 | 244 | 821 | 0.82 (0.67 – 1.00) | 0.051 | 0.032† |
ART, antiretroviral therapy; IQR, interquartile range; MI, myocardial infarction; TAHOD, TREAT Asia HIV Observational Database;
Included in final model;
Adjusted for predictors of any failure included in the final model;
Time updated;
p overall for linear trend;
p overall for heterogeneity
Five-year Predicted Risk Using the D:A:D Risk Algorithm
Figure 1 illustrates percentages of participants with high or very high five-year predicted risk of CVD (15.5%), CHD (11.1%), and MI (5.6%). In the hypothetical intervention scenarios smoking cessation demonstrated the greatest impact followed by switching from abacavir to an alternate antiretroviral (ARV). This equated to a reduction of 4.15% and 2.54% in CVD, and 3.15% and 2.41% in CHD. With regards to MI, switching from abacavir to an alternate ARV demonstrated the greatest reduction at 2.13% followed by smoking cessation at 1.73%. These interventions were followed by normalizing dyslipidemia values, normalizing blood pressure values, switching from lopinavir to an alternate ARV, and increasing HDL.
Figure 1.
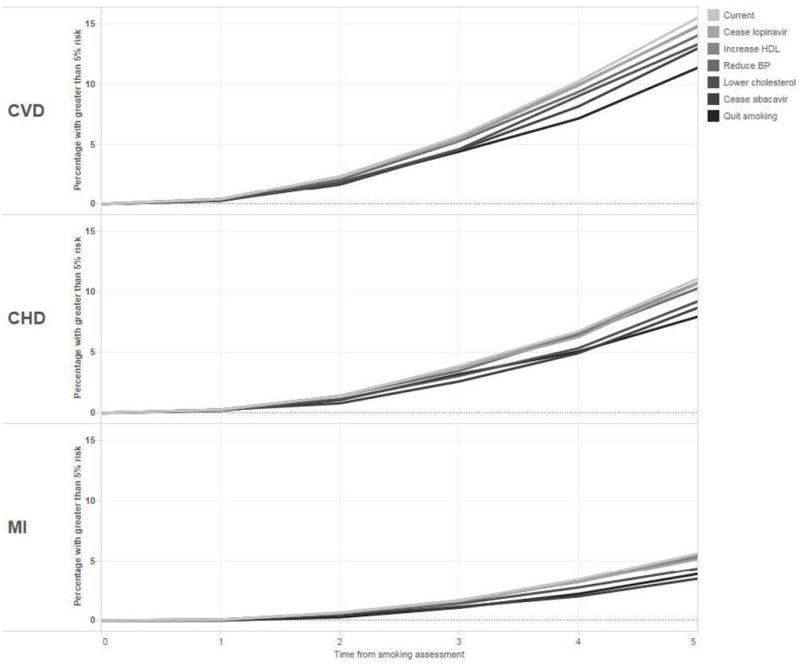
Percentage of patients with ≥5% predicted risk of cardiovascular disease (CVD), coronary heart disease (CHD), and myocardial infarction (MI) over time by simulated interventions
Discussion
To our knowledge, this is the first study to address predictors associated with current smoking in an Asian HIV-positive regional cohort. Resource-rich countries have reported current smoking prevalence in PLWH approximately doubles that in HIV-negative populations (18, 28–31). However, our findings suggest a lower smoking prevalence than that of resource-rich countries and the general male Asian population (34.7–61.1%) (32, 33). Furthermore, the prevalence of ever smoking in our cohort (43.5%) is lower than that found in a pooled Asian cohort (65.1% in men and 7.1% in women) (34). This discrepancy may be due to the lower percentage of women and relatively higher percentage of MSM in our cohort, who we identified as less likely to have ever smoked. Another explanation may be an overrepresentation of participants residing in settings with a smoking prevalence rate of <40% (i.e., India and Hong Kong SAR) and an underrepresentation in settings with a prevalence rate of >40% (i.e., China, South Korea, Japan, and Malaysia) (35).
Our analyses suggest that HIV exposure through injection drug use is a predictor of current smoking and being female and >50 years of age are negatively associated with current smoking. This corresponds with some studies in resource-rich settings (27, 31).
In simulated interventions, smoking cessation demonstrated the greatest potential impact on reducing five-year predicted risks of CVD and CHD, and closely approximated the impact of switching from abacavir to an alternate ARV in the reduction of five-year MI risk. This is consistent with Helleberg et al., who concluded that conditions attributed to smoking doubled the risk of mortality in PLWH vs. the general population (36) and Petoumenos et al., who concluded CVD, CHD and MI incidence rate ratios deceased with an increase in time since smoking cessation (37).
This study has multiple limitations that affect generalizability. Data analyses were restricted to those with a recorded smoking status, which is subject to reporting bias. As our criteria for comorbidities were based on cross-sectional data, our prevalence may be overestimated due to selective testing of patients most at risk of those comorbidities. In addition, missing data prevented the application of the D:A:D algorithm uniformly with simulations primarily completed in Thai, Hong Kong SAR, and Indian participants. Variability in the number of cohort sites located within each country may also impact generalizability due to over or under representation. Thai participants were chosen as the reference group for analysis due to the larger number of participants with the full complement of data and a smoking history (43.5%) that approximated the regional mean of male smokers (47.9%) (36). Consequently, results would differ had another country been chosen. Moving forward, longitudinal studies that quantify smoking exposure would better characterize the degree of risk and associated health outcomes in Asian PLWH, allowing for epidemiological tracking of emerging trends. Primary and secondary intervention studies focused on CVD would guide future care as non-communicable diseases become more common among aging Asian PLWH.
Acknowledgments
The authors thank the TAHOD study members for their significant contribution in the generation of this manuscript:
CV Mean, V Saphonn* and V Khol, National Center for HIV/AIDS, Dermatology & STDs, and University of Health Sciences, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee*, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
S Pujari*, K Joshi and A Makane, Institute of Infectious Diseases, Pune, India;
TP Merati* ‡, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University &Sanglah Hospital, Bali, Indonesia;
E Yunihastuti* †, D Imran and A Widhani, Working Group on AIDS Faculty of Medicine, University of Indonesia/ CiptoMangunkusumo Hospital, Jakarta, Indonesia;
S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
BLH Sim*, YM Gani and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines;
WW Wong*, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore;
P Phanuphak*, K Ruxrungtham, A Avihingsanon and P Chusut, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul*, S Sungkanuparph, L Chumla and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai, Thailand;
P Kantipong* and P Kambua, ChiangraiPrachanukroh Hospital, Chiang Rai, Thailand;
W Ratanasuwan* and R Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand;
VK Nguyen*, VH Bui and TT Cao, National Hospital for Tropical Diseases, Hanoi, Vietnam;
TT Pham*, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn*, N Durier* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, A Jiamsakul* and DC Boettiger, The Kirby Institute, UNSW Australia, Sydney, Australia.
* TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair
Grant Number : U01AI069907.National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute.
References
- 1.Mills EJ, Barnighausen T, Negin J. HIV and aging--preparing for the challenges ahead. The New England journal of medicine. 2012;366(14):1270–3. doi: 10.1056/NEJMp1113643. [DOI] [PubMed] [Google Scholar]
- 2.Furber AS, Maheswaran R, Newell JN, Carroll C. Is smoking tobacco an independent risk factor for HIV infection and progression to AIDS? A systemic review. Sexually transmitted infections. 2007;83(1):41–6. doi: 10.1136/sti.2005.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SR, Swaminathan S, Flanigan T, Mayer KH, Niaura R. HIV & smoking in India. The Indian journal of medical research. 2009;130(1):15–22. [PubMed] [Google Scholar]
- 4.Hearps AC, Martin GE, Rajasuriar R, Crowe SM. Inflammatory Co-morbidities in HIV+ Individuals: Learning Lessons from Healthy Ageing. Current HIV/AIDS reports. 2014;11(1):20–34. doi: 10.1007/s11904-013-0190-8. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. Journal of the American College of Cardiology. 2012;60(14):1207–16. doi: 10.1016/j.jacc.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 6.HIV and aging: A special supplement to the UNAIDS report on the global AIDS epidemic 2013 [press release] Geneva, Switzerland: 2013. 2013. [Google Scholar]
- 7.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR, et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. American journal of public health. 2010;100(10):1896–903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS care. 2013;25(4):451–8. doi: 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuckler D, Basu S, McKee M. Drivers of inequality in Millennium Development Goal progress: a statistical analysis. PLoS medicine. 2010;7(3):e1000241. doi: 10.1371/journal.pmed.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon PK, Jeemon P, Arora NK, Mathur P, Maskey M, Sukirna RD, et al. Status of epidemiology in the WHO South-East Asia region: burden of disease, determinants of health and epidemiological research, workforce and training capacity. International journal of epidemiology. 2012;41(3):847–60. doi: 10.1093/ije/dys046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigo C, Rajapakse S. Current Status of HIV/AIDS in South Asia. Journal of global infectious diseases. 2009;1(2):93–101. doi: 10.4103/0974-777X.56249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. Journal of the American Geriatrics Society. 2014;62(3):447–53. doi: 10.1111/jgs.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Tobacco Fact Sheet No 339. 2014 [updated May 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs339/en/
- 16.World Health Organization. WHO Report on the global tobacco epidemic, 2008. Geneva: 2008. Available from: http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf. [Google Scholar]
- 17.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Current problems in cardiology. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medicine Io. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 19.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30(Suppl 2):S171–6. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. Journal of acquired immune deficiency syndromes. 2005;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamers RL, Oyomopito R, Kityo C, Phanuphak P, Siwale M, Sungkanuparph S, et al. Cohort profile: The PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) monitoring studies to evaluate resistance--HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. International journal of epidemiology. 2012;41(1):43–54. doi: 10.1093/ije/dyq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. Reducing risk in heart disease: An expert guide to clinical practice for secondary prevention of coronary heart disease Melbourne. 2012 Available from: http://www.heartfoundation.org.au/SiteCollectionDocuments/Reducing-risk-in-heart-disease.pdf.
- 23.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva: 2006. [Google Scholar]
- 24.National Heart, Blood, and Lung Institute. Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) 2002 Available from: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf.
- 25.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 26.Edwards-Jackson N, Kerr S, Tieu H, Ananworanich J, Hammer S, Ruxrungtham K, et al. Cardiovascular risk assessment in persons with HIV infection in the developing world: comparing three risk equations in a cohort of HIV-infected Thais. HIV medicine. 2011;12(8):510–5. doi: 10.1111/j.1468-1293.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 27.Nery MW, Martelli CM, Silveira EA, de Sousa CA, Falco Mde O, de Castro Ade C, et al. Cardiovascular risk assessment: a comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. TheScientificWorldJournal. 2013;2013:969281. doi: 10.1155/2013/969281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrosillo N, Cicalini S. Smoking and HIV: time for a change? BMC medicine. 2013;11:16. doi: 10.1186/1741-7015-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS and behavior. 2010;14(4):824–35. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 30.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proceedings of the American Thoracic Society. 2011;8(3):313–9. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Oursler KA, Goetz MB, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS education and prevention : official publication of the International Society for AIDS Education. 2009;21(3 Suppl):40–53. doi: 10.1521/aeap.2009.21.3_supp.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA : the journal of the American Medical Association. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 33.Scollo MaW, MH . Tobacco in Australia: Facts and issues. Melbourne: Cancer Council Victoria; 2012. [Google Scholar]
- 34.Zheng W, McLerran DF, Rolland BA, Fu Z, Boffetta P, He J, et al. Burden of total and cause-specific mortality related to tobacco smoking among adults aged >/= 45 years in Asia: a pooled analysis of 21 cohorts. PLoS medicine. 2014;11(4):e1001631. doi: 10.1371/journal.pmed.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. World Health Statistics. Geneva: 2010. 2010. Available from: http://www.who.int/gho/publications/world_health_statistics/EN_WHS10_Full.pdf. [Google Scholar]
- 36.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(5):727–34. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 37.Petoumenos K, Worm S, Reiss P, de Wit S, d'Arminio Monforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV medicine. 2011;12(7):412–21. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


