Abstract
Background
Gender differences vary across geographical settings and are poorly reported in the literature. The aim of this study was to evaluate demographics and clinical characteristics of participants from the Australian HIV Observational Database (AHOD), and to explore any differences between females and males in the rate of new clinical outcomes, as well as initial immunological and virological response to antiretroviral therapy.
Methods
Time to a new clinical end-point, all-cause mortality and/or AIDS illness was analysed using standard survival methods. Univariate and covariate adjusted Cox proportional hazard models were used to evaluate the time to plasma viral load suppression in all patients that initiated antiretroviral therapy (ART) and time to switching from a first-line ART to a second-line ART regimen.
Results
There was no significant difference between females and males for the hazard of all-cause mortality [adjusted hazard ratio: 0.98 (0.51, 1.55), P = 0.67], new AIDS illness [adjusted hazard ratio: 0.75 (0.38, 1.48), P = 0.41] or a composite end-point [adjusted hazard ratio: 0.74 (0.45, 1.21), P = 0.23]. Incident rates of all-cause mortality were similar between females and males; 1.14 (0.61, 1.95) vs 1.28 (1.12, 1.45) per 100 person years. Virological response to ART was similar for females and males when measured as time to viral suppression and/or time to virological failure.
Conclusion
This study supports current Australian HIV clinical care as providing equivalent standards of care for male and female HIV-positive patients. Future studies should compare ART-associated toxicity differences between ART-associated toxicity differences between men and women living with HIV in Australia.
Keywords: AIDS, gender, treatment
Introduction
Approximately 9% of people living with HIV in Australia are women, and the majority likely acquired their infection as a result of heterosexual contact, either in Australia or overseas.1 Over the past decade (2001–2010), the number of women newly diagnosed with HIV in Australia has increased.1 Forty-four per cent more women were diagnosed in the period from 2006 to 2010 than in the period from 2001 to 2005.1
Women are underrepresented in HIV clinical trials2,3 and sex specific analyses relating to outcome and toxicity are then often limited by a lack of statistical power. Researchers have attempted to overcome this by either pooling several studies4 or designing studies to recruit a greater proportion of women to men.5 Most of these studies have not demonstrated a difference in efficacy of antiretroviral therapy (ART) when higher discontinuation rates among women are taken into account.4–6 Previous cohort studies from countries outside of Australia have reported differences in outcomes among women,7,8 although without direct comparison to men. When comparisons in outcomes according to sex have been made, results are conflicting.4,9–11 However, sex-specific differences in toxicity remain largely unknown. To date, there have been no studies analysing outcomes in relation to ART for HIV-infected Australian women compared with HIV-infected men.
The aim of this study is to evaluate gender differences in terms of demographics and clinical characteristics of participants from the Australian HIV Observational Database (AHOD), and to explore any differences between females and males in the rates of new clinical outcomes, as well as initial immunological and virological response to antiretroviral therapy.
Methods
The study population includes all patients enrolled in the AHOD, with at least one follow-up visit. The AHOD is an observational cohort study of HIV-positive individuals attending specialised GP sites, sexual health clinics and tertiary referral centres throughout Australia. Details regarding the cohort and data collected have previously been described.12 In summary, this longitudinal observational cohort study has been ongoing since 1999, with 29 active sites contributing information for a total of 3495 enrolled participants. Core data collected includes typical patient demographics, HIV disease stage (CD4 T-cell counts, HIV viral load and AIDS defining illness), as well as antiretroviral drug treatment histories and laboratory data (including total cholesterol, high-density lipoprotein, haemoglobin, alanine transaminase and aspartate transaminase). Data are contributed in a standardised electronic form extracted from computerised patient management systems every 6 months. Data are merged, collated and summarised on behalf of contributing sites at The Kirby Institute, UNSW, Sydney.
We compared differences between gender and standard patient characteristics using a two-tailed χ2 test significant at the α = 0.05 level. The difference in patient characteristics evaluated included age, mode of HIV exposure, country of birth, hepatitis co-infection, history of ART including type of initial and subsequent regimens, pre- and post-ART CD4 count and plasma HIV RNA viral load (pVL). Categorisation levels for each factor are outlined in Table 1.
Table 1.
Patient demographics and characteristics by sex
| Factor | Level | Female (n = 210) N (%) |
Male (n = 3285) N (%) |
P-valueA |
|---|---|---|---|---|
| Age at enrolment (years) | Mean | 36.4 | 40.7 | <0.0001 |
| Median (Q1–Q3) | 34.1 (30.0–42.8) | 39.5 (33.3–46.9) | ||
| Mode of exposure | MSM | n/a | 2586 (79) | <0.0001 |
| Heterosexual | 154 (73) | 191 (6) | ||
| IDU | 18 (9) | 183 (6) | ||
| Blood product/other | 32 (15) | 289 (9) | ||
| Unknown | 6 (3) | 36 (1) | ||
| Country of birth | Australia | 174 (83) | 2994 (91) | <0.0001 |
| Asia–Pacific | 19 (9) | 85 (3) | ||
| Americas | 3 (1) | 39 (1) | ||
| Africa | 6 (3) | 22 (1) | ||
| Europe | 8 (4) | 145 (4) | ||
| Patient care setting | General practice | 53 (25) | 1240 (38) | 0.001 |
| Hospital | 53 (25) | 733 (22) | ||
| Sexual health clinic | 104 (50) | 1312 (40) | ||
| Year of cohort enrolment | 1999–2004 | 135 (64) | 2235 (68) | 0.26 |
| 2005–2012 | 75 (36) | 1050 (32) | ||
| Hepatitis B surface antigen | Positive | 10 (5) | 138 (4) | 0.17 |
| Negative | 175 (83) | 2593 (79) | ||
| Not tested | 25 (12) | 554 (17) | ||
| Hepatitis C antibody | Positive | 33 (16) | 349 (11) | 0.01 |
| Negative | 164 (78) | 2580 (79) | ||
| Not tested | 13 (6) | 356 (11) | ||
| AIDS illness at cohort enrolment | Yes | 32 (15) | 714 (22) | 0.03 |
| No | 178 (85) | 2571 (78) | ||
| Year of ART | 1996–2004 | 127 (60) | 2216 (67) | 0.11 |
| 2005–2011 | 53 (25) | 675 (21) | ||
| ART naïve | 30 (15) | 394 (12) | ||
| Initial ART anchor agent | NNRTI | 76 (36) | 1173 (36) | 0.71 |
| [2 N(t)RTI + _ ] | PI | 89 (42) | 1344 (41) | |
| II/other | 15 (7) | 374 (11) | ||
| ART naïve | 30 (14) | 394 (12) | ||
| Number of ART regimen changes since initiation | Mean | 5.02 | 4.99 | 0.94 |
| Median (Q1–Q3) | 3 (2–7) | 4 (2–7) | ||
| Total treatment interruption time (%) | Mean | 11.5 | 9.9 | 0.29 |
| Median (Q1–Q3) | 0.2 (0.1–12.3) | 0.1 (0–10.6) | ||
| CD4 cell count (cells µL−1) | ||||
| Nadir | Mean | 277 | 274 | 0.86 |
| Median (Q1–Q3) | 257 (170–356) | 250 (132–377) | ||
| CD4 at ART initiation | Mean | 337 | 345 | 0.72 |
| Median (Q1–Q3) | 285 (190–420) | 310 (170–475) | ||
| CD4 at enrolment | Mean | 494 | 508 | 0.50 |
| Median (Q1–Q3) | 480 (294–666) | 470 (315–660) | ||
| Recent CD4 | Mean | 620 | 576 | 0.05 |
| Median (Q1–Q3) | 584 (375–810) | 550 (380–740) | ||
| Plasma viral load (copies mL−1) | ||||
| Peak pVL | Mean | 177 337 | 283 201 | <0.0001 |
| Median (Q1–Q3) | 46 350 (8690–132519) | 100 000 (26 800–306 472) | ||
| pVL at ART initiation | Mean | 75 757 | 147 152 | 0.006 |
| Median (Q1–Q3) | 4295 (400–60 511) | 14 665 (500–100 000) | ||
| pVL at enrolment | Mean | 22 666 | 32609 | 0.64 |
| Median (Q1–Q3) | 399 (50–5830) | 399 (50–8000) | ||
| Recent pVL | Mean | 10 711 | 21 971 | 0.94 |
| Median (Q1–Q3) | 40 (39–125) | 49 (39–119) |
MSM, men who have sex with men; n/a, not applicable; IDU, injecting drug use; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; pVL, plasma viral load
Categorical factor: two-tailed χ2 test significant at the α = 0.05 level; Continuous factor: Analysis of Variance.
Time to a new clinical end-point, all-cause mortality and/or AIDS illness was analysed by using standard survival methods. Models were adjusted a priori for the following factors: age, sex, HIV exposure, care setting, year of initiation of ART, country of birth, hepatitis B, hepatitis C co-infection, time-updated CD4 count and time-updated pVL. Time origin was the date of AHOD enrolment and records were censored at event data, lost-to-follow-up or after 10 years of follow up (administration censor), whichever occurred first.
We used univariate and covariate adjusted Cox proportional hazard models to evaluate the time to pVL suppression in all patients who had ART initiated. We defined first instance of viral load suppression where pVL was <400 copies mL−1. Time to virological failure was defined as the first occurrence of pVL being >1000 copies mL−1 from the first instance of pVL suppression. Patient follow-up time was censored at date of death, or date of last clinical visit. Patients were considered lost-to-follow-up if duration between pVL measures was >12 months.
Time to switching from a first-line ART to a second-line ART regimen was investigated using univariate- and covariate-adjusted Cox proportional hazard models. We defined first-line ART as a combination of two nucleotide reverse transcriptase inhibitors (NRTI) anchored with at least one of the following antiviral agents: protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase inhibitor (II) or NRTI (e.g. triple NTRI). A switch to second-line ART was defined as a change of the anchor agent; for example, PI → NNRTI. We excluded minor switches within agent class substitutions from our definition of switching to capture major modification to treatment only.
All statistical calculations were computed using StataCorp 2011 (Stata statistical software: Release 12; College Station, TX, USA). Tabulation and data summaries were constructed using SAS (SAS Institute Inc 2011. Base SAS ® 9.3 NC).
Results
Of the 3495 patients enrolled in the AHOD, 210 (6%) were women and 3285 (94%) were men. The median age at enrolment was 34.1 [interquartile range (IQR): 30.0–42.8] years for women and 39.5 (IQR: 33.3–46.9) years for men (statistically significantly different, P < 0.0001). The majority of participants were born in Australia; 174 (83%) women and 2994 (91%) men. Of the women in the AHOD, 50% received their HIV care through a sexual health clinic, 25% through general practice and 25% through a hospital (Table 1).
The prevalence of viral hepatitis co-infection between males and females was similar, with 4% of women and 5% of men co-infected with hepatitis B virus (positive Hepatitis B surface antigen) and 11% of women and 16% of men co-infected with hepatitis C virus (defined as hepatitis C virus antibody positive).
Most patients initiated ART between 1999 and 2004 (Table 1); however, 15% of women and 12% of men remained antiretroviral naïve. The initial antiretroviral regimen was similar between females and males, with protease-inhibitor anchored regimens the most common (42% of females and 41% males). The median CD4 T-cell count at time of treatment initiation was 285 cells µL−1 (IQR: 190–420) in females and 310 cells µL−1 (IQR: 170–475) in males. The immunological responses to ART appeared similar, with substantial increases in mean CD4 cell counts for females and males; the most recent CD4 cell count was 584 (IQR: 375–810) for women and 550 (IQR: 380–740) and was recorded as the most recent CD4 cell count (Table 1). We evaluated differences between rates of CD4 cell count change after ART initiation and found no statistically significant differences (data not shown).
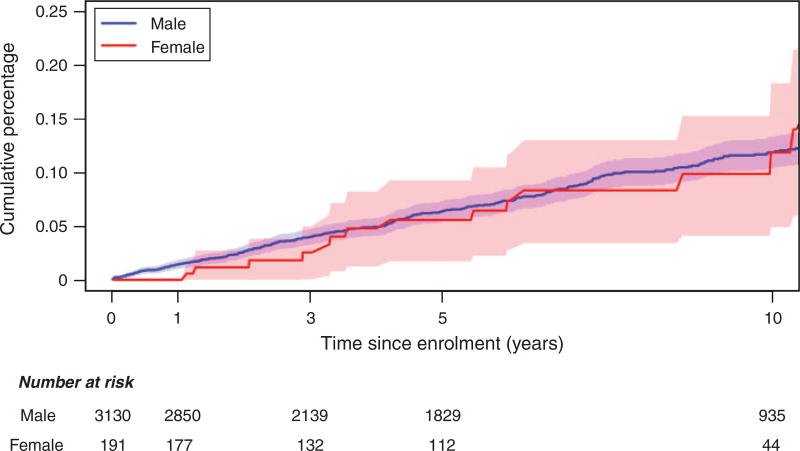
There was no significant difference between females and males for the hazard of all-cause mortality [adjusted hazard ratio: 0.98 (0.51, 1.55), P = 0.67], new AIDS illness [adjusted hazard ratio: 0.75 (0.38, 1.48), P = 0.41] or a composite end-point [adjusted hazard ratio: 0.74 (0.45, 1.21), P = 0.23; Table 2]. Incident rates of all-cause mortality were similar between females and males; 1.14 (0.61, 1.95) vs 1.28 (1.12, 1.45) per 100 person years, respectively; Table 3. Kaplan–Meier cumulative incidence curves further illustrate the minimal difference in hazard ratios (Fig. 1).
Table 2.
Clinical end-point univariate- and covariate-adjusted Cox proportional hazard ratios
| End-point | Hazard ratio (female vs male) |
95% confidence intervals |
P-value |
|---|---|---|---|
| All-cause mortality | |||
| Univariate | 0.89 | 0.51–1.55 | 0.67 |
| MultivariableA | 0.98 | 0.56–1.75 | 0.97 |
| AIDS illness | |||
| Univariate | 0.76 | 0.39–1.48 | 0.42 |
| MultivariableA | 0.75 | 0.38–1.48 | 0.41 |
| All-cause mortality & AIDS illness (combined) | |||
| Univariate | 0.70 | 0.43–1.14 | 0.16 |
| MultivariableA | 0.74 | 0.45–1.21 | 0.23 |
Adjusted for age at cohort enrolment, injecting drug use (IDU) HIV exposure, hepatitis C virus (HCV), hepatitis B virus (HBV), recent CD4 cell count, recent HIV viral load ≥100 000 copies mL−1, year of cohort enrolment, number antiretroviral therapy (ART) modifications and clinical care setting.
Table 3.
Clinical end-point incidence rates by gender
| End-point | Female incidence rate (per 100 person-years) (95% CI) |
Male incidence rate (per 100 person-years) (95% CI) |
|---|---|---|
| All-cause mortality | 1.14 (0.61–1.95) | 1.28 (1.12–1.45) |
| AIDS illness | 0.81 (0.37–1.54) | 1.06 (0.92–1.22) |
| All-cause mortality & AIDS illness (combined) | 1.54 (0.90–2.47) | 2.18 (2.01–2.40) |
Fig. 1.
Cumulative incidence of all-cause mortality comparing females and males participating in the Australian HIV Observational Database (AHOD).
Virological response to ART was similar for females and males when measured as time to viral suppression and/or time to virological failure (Table 4). There was also no difference in the time to second-line ART switch between females and males (Table 4). These findings were consistent for both univariate- and covariate-adjusted models.
Table 4.
HIV treatment-related outcomes for males and females: time to virological suppression; time to virological failure after first instance of viral suppression; and time to switch of first-line ART anchor agent
| End-point | Hazard ratio (Female vs Male) |
95% confidence intervals |
P-value |
|---|---|---|---|
| Time to pVL suppression | |||
| Univariate | 1.18 | 0.99–1.39 | 0.06 |
| MultivariableA | 1.08 | 0.91–1.28 | 0.37 |
| Time from pVL suppression to pVL failure | |||
| Univariate | 1.10 | 0.81–1.26 | 0.95 |
| MultivariableA | 0.97 | 0.77–1.21 | 0.76 |
| Time to switch of first-line ART anchor agent | |||
| Univariate | 1.03 | 0.81–1.30 | 0.82 |
| MultivariableA | 1.05 | 0.83–1.34 | 0.67 |
pVL, plasma viral load; ART, antiretroviral therapy
Adjusted for age at ART initiation, injecting drug use HIV exposure, hepatitis C virus, hepatitis B virus, CD4 cell count at ART initiation, HIV viral load ≥100 000 copies mL−1 at ART initiation, year of ART initiation and clinical care setting.
Discussion
This study is the first to compare and report HIV-specific outcomes and responses to treatment by HIV-infected adults in Australia according to sex. The main findings are that females and males respond equally well to ART in terms of CD4 cell response and viral suppression, and have similar incidence of all-cause mortality and time to new AIDS illness. First-line regimens between sexes are similar and durability does not appear significantly different, as similar rates of switching were reported.
Previous prospective cohort studies, from other resource-rich settings, have reported a decrease in AIDS-related deaths in the post highly active antiretroviral therapy era among women.7,8 These analyses, however, are limited by no direct comparison with HIV-infected males receiving treatment over the same time period, from the same geographical region, making assessment of any difference in magnitude of the reduction in mortality between males and females difficult. Findings from our study support the idea that there are minimal differences in patient and HIV treatment outcomes in a contemporary cohort of male and female HIV-positive persons living and receiving care in Australia.
When comparisons between men and women have been attempted, the findings have differed according to region and study. In a large cohort study from Asia, male sex was significantly associated with a higher mortality rate compared with women (male vs female; hazard ratio 2.21, 95% CI 1.31–3.43; P = 0.002) within the same cohort after ART initiation.9 In another cohort study with participants from Europe and Canada, women had improved survival and lower risk of progression to AIDS and non-AIDS mortality following initiation of treatment compared with men.10 However, a more recent meta-analysis including data from 40 randomised controlled trials did not find a difference in outcome at 48 weeks according to gender.4 In contrast, a meta-analysis of seven randomised controlled trials in treatment-naïve participants reported a statistically significant difference in virological suppression, with women less likely to achieve this compared with men.13 The conflicting nature of these findings highlights the importance of analysing and reporting outcomes according to gender, in different locations and settings. It is reassuring that in our cohort we did not find significant differences in treatment outcomes for women compared with men.
To date, gender differences in choice of initial regimen, indications for switching, time to switching and choice of second-line regimens have not been well described. Reported gender differences in duration of therapy before interruption or change are most pronounced in the first 12 months of therapy.14 In a recent study by Emery et al.,14 which included only 39 women, all of whom were co-infected with hepatitis C, the primary reasons for therapy interruption differed between gender, with mental health significantly more common in women. Interestingly, in the study by Emery et al., men were more likely to switch to a new regimen immediately whereas women were more likely to have a break before resumption of therapy. The explanation for this is not clear, nor is the generalisability given the small number of participants. In a larger cohort analysis of factors associated with modification to the first ART regimen in the Asia–Pacific region (major modification defined as starting a new class or substituting two or more antiretrovirals from within the same class) no significant difference according to gender was reported.15 Similarly, a retrospective study from sub-Saharan Africa reporting on 3749 participants from three African countries found that male sex was a predictor of mortality after starting ART, but that gender was not a predictor of the switch to a second-line combination.16 In contrast, a recent study from Canada examined outcomes and factors associated with switching for non-virological failure and reported that switching at least twice was less likely if you were male (adjusted odds ratio 0.53 95% CI 0.39–0.71).17 This study did not analyse reasons for switching such as gender-specific issues including pregnancy. The results from our study confirm no difference between males and females in terms of time to switching or number of switches. However, no assessment of reason for switching can be made based on the current AHOD data. In the future, it would be useful to collect and analyse this data to inform strategies to minimise switching among men and women.
Gender-based differences in toxicity have been reported for nucleoside analogues, non-nucleoside analogues and protease inhibitors.5,6,18–22 With higher rates of toxicity reported according to gender for many of the antiretroviral agents, it is not surprising that some studies have also reported that women are more likely to change treatment regimens due to toxicity.5,22 The AHOD does not formally collect toxicity data, but given the importance of this in terms of durability and the potential impact on switching, it is an issue worth considering for future studies.
The other limitations of this study include its cohort study design, its limited power (able only to detect large differences in hazards ratio >2) and its generalisability to other populations.
In recognition of the disparity between female representation in clinical studies and the fact that globally women comprise more than 50% of adults living with HIV, the Joint United Nations Programme on HIV/AIDS has stated that ‘researchers and trial sponsors should recruit women in to clinical trials in order to verify safety and efficacy from their standpoint’.22 Although this is from a document pertaining to biomedical research in HIV, the principles apply to other clinical and social research in HIV. Several editorials have been published highlighting the importance of developing policies for Journal Editors23 and gender-sensitive reporting in medical research.24 In 2012, the European Association of Science Editors developed a committee working to advance gender- and sex-sensitive reporting and communication in science.25 Gender differences vary across geographical settings and are poorly reported in the literature. This study supports current Australian HIV clinical care as providing equivalent standards of care for male and female HIV-positive patients. Future studies should compare ART-associated toxicity differences and reasons for switching between Australian men and women.
Acknowledgments
The Australian HIV Observational Database is funded as part of the Asia–Pacific HIV Observational Database, a program of the American Foundation for AIDS Research (amfAR), and is supported in part by a grant from the USA National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID) (Grant No. U01-AI069907) and by unconditional grants from Merck Sharp & Dohme; Gilead; Bristol-Myers Squibb; Boehringer Ingelheim; Roche; Pfizer; GlaxoSmithKline; and Janssen-Cilag. The Kirby Institute is funded by The Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Australian HIV Observational Database contributors (Asterisks indicate steering committee members in 2013)
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, S Agrawal, T Vincent, Holdsworth House, Darlinghurst; D Allen, JL Little, Holden Street Clinic, Gosford; D Smith, C Mincham, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, V Ieroklis, East Sydney Doctors, Surry Hills; DJ Templeton*, CC O’Connor, S Phan, RPA Sexual Health Clinic, Camperdown; E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Grotowski, S Taylor, Tamworth Sexual Health Service, Tamworth; D Cooper, A Carr, F Lee, K Hesse, K Sinn, R Norris, St Vincent’s Hospital, Darlinghurst; R Finlayson, I Prone, A Patel, Taylor Square Private Clinic, Darlinghurst; R Varma, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, C McGrath, V McGrath, S Halligan, Illawarra Sexual Health Service, Warrawong; L Wray, P Read, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, Parramatta Sexual Health Clinic, Parramatta; D Smith*, V Furner, Albion Street Centre, Sydney; Clinic 16 – Royal North Shore Hospital, Sydney; S Fernando, Dubbo Sexual Health Centre, Dubbo; Holdsworth House Medical Practice, Byron Bay, J Chuah*; J Watson*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney, Sydney; M Law*, K Petoumenos*, S Wright*, H McManus*, C Bendall*, M Boyd*, The Kirby Institute, UNSW Australia, Sydney. Northern Territory: N Ryder, R Payne, Communicable Disease Centre, Royal Darwin Hospital, Darwin. Queensland: D Russell, S Doyle-Adams, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, K McGill, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, A Gibson, H Magon, Brisbane Sexual Health and HIV Service, Brisbane; B Dickson*, CaraData. South Australia: W Donohue, O’Brien Street General Practice, Adelaide. Victoria: R Moore, S Edwards, R Liddle, P Locke, Northside Clinic, North Fitzroy; NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, MGiles*, T Korman, J Williams*, Monash Medical Centre, Clayton. Western Australia: D Nolan, J Robinson, Department of Clinical Immunology, Royal Perth Hospital, Perth.
Coding of Death Form (CoDe) reviewers: AHOD reviewers: D Sowden, DJ Templeton, J Hoy, L Wray, K Morwood, T Read, N Roth, I Woolley, MKelly, K Choong, CC O’Connor. Treat Asia HIV Observational Database (TAHOD) reviewers: PCK Li, MP Lee, S Vanar, S Faridah, A Kamarulzaman, JY Choi, B Vannary, R Ditangco, K Tsukada, S Pujari, A Makane, OT Ng, AJ Sasisopin. Independent reviewers: M Boyd.
References
- 1.The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2013. The University of New South Wales, Sydney: The Kirby Institute; 2013. Available online at: http://www.kirby.unsw.edu.au [verified 18 June 2015] [Google Scholar]
- 2.Johnston R, Curno MJ, Etya-ale H, Hodges-Mameletzis I, Price M, Rossi S, et al. Female participation in HIV Studies; 7th IAS Conference on HIV Pathogenesis treatment and prevention; 30 June–3 July 2013; Kuala Lumpur, Malaysia. [Google Scholar]
- 3.Westreich D, Rosenberg M, Schwartz S, Swamy G. Representation of women and pregnant women in HIV research: a limited systematic review. PLoS One. 2013;8:e73398. doi: 10.1371/journal.pone.0073398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soon GG, Min M, Struble KA, Chan-Tack KM, Hammerstrom T, Qi K, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008) AIDS Patient Care STDS. 2012;26:444–53. doi: 10.1089/apc.2011.0278. [DOI] [PubMed] [Google Scholar]
- 5.Currier J, Averitt Bridge D, Hagins D, Zorrilla CD, Feinberg J, Ryan R, Falcon R, Tennenberg A, Mrus J, Squires K. GRACE (Gender, Race, And Clinical Experience) Study Group. Sex-based outcomes of darunavir–ritonavir therapy: a single-group trial. Ann Intern Med. 2010;153:349–57. doi: 10.7326/0003-4819-153-6-201009210-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squires KE, Johnson M, Yang R, Uy J, Sheppard L, Absalon J, McGrath D. Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir and lopinavir/ritonavir at 96 weeks in the CASTLE study. J Antimicrob Chemother. 2011;66:363–70. doi: 10.1093/jac/dkq457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith DK, Gardner LI, Phelps R, Hamburger ME, Carpenter C, Klein RS, et al. Mortality rates and causes of death in a cohort of HIV-infected and uninfected women, 1993–1999. J Urban Health. 2003;80:676–88. doi: 10.1093/jurban/jtg074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–8. doi: 10.1016/S0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiertiburanakul S, Boettiger D, Lee MP, Omar SF, Tanuma J, Ng OT, Durier N, et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J Int AIDS Soc. 2014;17:18804. doi: 10.7448/IAS.17.1.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarrin I, Geskus R, Bhaskaran K, Prins M, Perez-Hoyos S, Muga R, et al. CASCADE Collaboration Gender differences in HIV progression to AIDS and death in industrialized countries: slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168:532–40. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 11.Kwakwa H, Spencer D, Evans C, Garner W, Walker I, Temme L. Gender difference in virologic outcomes in a meta-analysis of randomized controlled trials in HIV1-infected patients on antiretroviral therapy; XIX International AIDS conference; 22–27 July 2012; Washington DC, USA. Abstract THPE041. [Google Scholar]
- 12.The Australian HIV Observational Database Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3:28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 13.Emery J, Pick N, Mills EJ, Cooper CL. Gender differences in clinical, immunological and virological outcomes in highly active antiretroviral-treated HIV-HCV coinfected patients. Patient Prefer Adherence. 2010;4:97–103. doi: 10.2147/ppa.s9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright S, Boyd MA, Yunihastuti E, Law M, Sirisanthana T, Hoy J, Pujari S, Lee MP, Petoumenos K. International Epidemiologic Databases to Evaluate AIDS (IeDEA) Asia-Pacific HIV Observational Database (APHOD). Rates and factors associated with major modifications to first-line combination antiretroviral therapy: results from the Asia-Pacific region. PLoS One. 2013;8:e64902. doi: 10.1371/journal.pone.0064902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, Doro Altan A, Zimba Ida V, San Lio MM, De Luca A. DREAM Program Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–22. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 16.Hull M, Cescon A, Raboud J, Klein MB, Walmsley S, Ding E, Shurgold S, et al. CANOC Collaboration. Switching from first antiretroviral therapy regimen while virologically suppressed is associated with increased risk of subsequent virologic failure; 20th International AIDS Conference; 20–25 July 2014; Melbourne, Australia. Abstract TUAB0103. [Google Scholar]
- 17.Mazhude C, Jones S, Murad S, Taylor C, Easterbrook PJ. Female sex but not ethnicity is a strong predictor of non-nucleoside reverse transcriptase inhibitor-induced rash. AIDS. 2002;16:1566–8. doi: 10.1097/00002030-200207260-00020. [DOI] [PubMed] [Google Scholar]
- 18.Galli M, Ridolfo AL, Adorni F, Gervasoni C, Ravasio L, Corsico L, et al. Body habitus changes and metabolic alterations in protease inhibitor naïve HIV-1-infected patients treated with two nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2002;29:21–31. doi: 10.1097/00042560-200201010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal D, Chakravarty J, Chaube L, Rai M, Agrawal NR, Sundar S, et al. High incidence of zidovudine induced anemia in HIV infected patients in eastern India. Indian J Med Res. 2010;132:386–9. [PubMed] [Google Scholar]
- 20.Bersoff-Matcha SJ, Miller WC, Aberg JA, van Der Horst C, Hamrick HJ, Jr, Powderly WG, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124–9. doi: 10.1086/317536. [DOI] [PubMed] [Google Scholar]
- 21.Spire B, Carrieri P, Garzot MA, L’henaff M, Obadia Y. TRT-5 Group Factors associated with efavirenz discontinuation in a large community-based sample of patients. AIDS Care. 2004;16:558–64. doi: 10.1080/09540120410001716342. [DOI] [PubMed] [Google Scholar]
- 22.UNAIDS/WHO. Ethical considerations for biomedical HIV prevention trials: guidance document. 2007 Available online at: http://data.unaids.org/pub/manual/2007/jc1349_ethics_2_11_07_en.pdf [verified 1 September 2014]
- 23.Heidari S, Eckert MJ, Kippax S, Karim QA, Sow PS, Wainberg MA. Time for gender mainstreaming in editorial policies. J Int AIDS Soc. 2011;14:11. doi: 10.1186/1758-2652-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidari S, Abdool Karim Q, Auerbach JD, Buitendijk SE, Cahn P, Curno MJ, et al. Gender-sensitive reporting in medical research. J Int AIDS Soc. 2012;15:11. doi: 10.1186/1758-2652-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidari S, Babor T. Science editors: evaluate gender equality in journals. Nature. 2013;495:47. doi: 10.1038/495047e. [DOI] [PubMed] [Google Scholar]