Abstract
Background
There is substantial evidence of an inverse association between birth weight and later blood pressure (BP) in populations from high-income countries, but whether this applies in low-income countries, where causes of low birth weight are different, is not certain.
Objective
We conducted a review of the evidence on the relationship between birth weight and BP among African children and adolescents.
Methods
Medline, EMBASE, Global Health and Web of Science databases were searched for publications to October 2016. Papers reporting the relationship between birth weight and BP among African children and adolescents were assessed. Bibliographies were searched for further relevant publications. Selected papers were summarized following the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines.
Results
Sixteen papers from 13 studies conducted in nine African countries (Nigeria, Republic of Seychelles, Gambia, Democratic Republic of Congo, Cameroon, South Africa, Algeria, Zimbabwe and Angola) were reviewed. Eight studies were cohorts, while five were cross-sectional. The relationship between birth weight and later BP varied with age of the participants. Studies in neonates showed a consistently positive association, while predominantly inverse associations were seen among children, and studies in adolescents were inconsistent.
Conclusion
Based on the limited number of studies identified, the relationship between birth weight and later BP may vary with age in African children and adolescents. Not all studies adequately controlled for confounding, notably gender or age. Whether the inverse relationship between birth weight and BP in later life observed in Western settings is also seen in Africa remains unclear.
Keywords: birth weight, blood pressure, Africa, systematic review
Introduction
A strong geographical correlation between infant mortality (from 1921 to 1925) and adult ischaemic heart disease (IHD) mortality (from 1968 to 1978) was observed by Barker and Osmond 1. They postulated that factors that increased the risk of death during infancy also increased susceptibility to IHD among those who survived infancy, and later, showed that blood pressure (BP) in adulthood was positively related to placenta weight but inversely associated with birth weight 2. They suggested that poor fetal nutrition indicated by intrauterine growth restriction (IUGR) and low birth weight was associated with this increased susceptibility to IHD.
Subsequently, several studies (mainly from high-income countries [HICs]) have investigated the relationship between birth size parameters (for example birth weight, head circumference, placenta size) and later cardiovascular disease (CVD) risk (mainly BP), with birth weight the most widely studied parameter. Results from several of these studies have shown an inverse association between birth weight and BP later in life 3–7. A smaller number of studies have reported positive or no association between birth weight and later BP. For example, positive associations have been reported among UK neonates 8 and Chinese children 9 while birth weight was not associated with BP among American adolescents 10. The relationship between birth weight and later BP differed by gender among UK adolescents: a negative association was seen in the males but a positive association in the females 11. Systematic reviews have reported that, on average, systolic blood pressure (SBP) drops by 2-4 mmHg for every kilogram increase in birth weight 12,13. These reviews have predominantly comprised of studies among adults from HIC.
In HICs the prevalence of low birth weight varies between 5% and 8% 14. Low birth weight is more common in Africa (7% in Nigeria 15, 8% Uganda 16, 11% Zambia 17, 17% Zimbabwe and Benin 18,19 and 28% Ethiopia 18) and on average African population have lower birth weights when compared with European populations 20. In HICs, low birth weight is predominantly due to prematurity (most commonly as a result of maternal smoking in pregnancy 21), whereas, in developing countries, low birth weight for gestational age constitutes most of the low birth weight infants22. The causes of low birth weight differ between rural, tropical Africa and developed or non-tropical settings: for example, malaria (an important cause of low birth weight) is restricted to the tropics 23 and prophylactic antimalarial drugs in pregnancy reduce the risk of low birth weight 24,25. We hypothesised that the relationship between birth weight and BP in African settings might differ from that commonly observed in HICs.
The role of birth weight in the later development of BP is important to African countries: these have a high burden of malnutrition 26–28, low birth weight 18,19 and raised BP 29–32. Early life interventions that reduce maternal malnutrition and extremes of birth weight (both low and high) may thus control childhood BP (before clinical manifestation of disease) and could be vital in the prevention of high BP in adulthood.
The absence of birth weight records for adults in many African countries and the low accuracy of maternally recalled birth weight 33 limits prospects for studying the relationship between birth weight and BP in adulthood in this setting. However, the emergence of a number of birth cohorts (with birth records) in Africa provides opportunities to investigate the relationship between birth weight and BP among African children and adolescents. Childhood BP predicts BP in early adulthood 34,35, thus studies of the relationship between birth weight and BP among children are important in the identification of at-risk groups for targeted interventions early in life. We conducted a qualitative assessment of the direction and consistency of the relationship between birth weight and BP among African children and adolescents using a systematic review of existing literature.
Methods
A literature search covering publications up to 15th October 2016 with no restriction on start date was performed using Medline, EMBASE, Global Health and Web of Science databases. The search was performed on combinations of the keywords: (hypertension OR blood pressure) AND (birth weight) AND (paediatric OR child OR young people OR youth OR juvenile OR adolescent OR youngster OR pubescent OR teenage OR new-born OR minor OR infant) AND (Africa OR individual names of countries in Africa).
Original papers on the relationship between birth weight and BP among children and or adolescents, between ages 0 to 19 years and resident in Africa were reviewed. No restrictions on language or publication dates were applied. Publications on children and or adolescents of African ancestry not residing in Africa were excluded. Papers on the same participants were considered as one study. If more than one paper reported on the same participants at the same age, the most complete paper was included in the review. Papers reporting on the same participants were included and reviewed separately if they reported on the relationship between birth weight and BP at different ages. No additional information was sought from authors. Reference lists of the included papers were searched for additional relevant publications.
Search results were exported to Endnote reference management software (Thomson Reuters, version X7) and duplicates removed. Two independent authors (SL and EW) assessed titles and abstracts for inclusion in the full-text review and then assessed full-text articles for inclusion in the data synthesis. Inconsistencies were discussed and consensus reached at each stage of the selection process.
Data was extracted independently by two authors (SL and EW) using standardized data extraction sheets on the year of publication, year of birth, location, age of participants, study design, number of participants, exclusion criteria, study aim, mean birth weight, source of birth weight data, BP measurement procedure, mean BP (SBP and, or, diastolic blood pressure [DBP]), relationship between birth weight and BP and how this was assessed, and whether there was adjustment for confounders. Information was recorded as presented in the original publication, except where the overall mean BP or birth weight was missing; in this case, where possible the overall mean was calculated from any stratum-specific means presented. Studies were assessed for selection bias and adjustment for confounding. Meta-analysis was not performed due to diversity in studies included in the review, in terms of their design, analysis, source population and covariates controlled for in the analysis. Guidelines from the preferred reporting items for systematic review and meta-analysis (PRISMA) 36 were followed.
Results
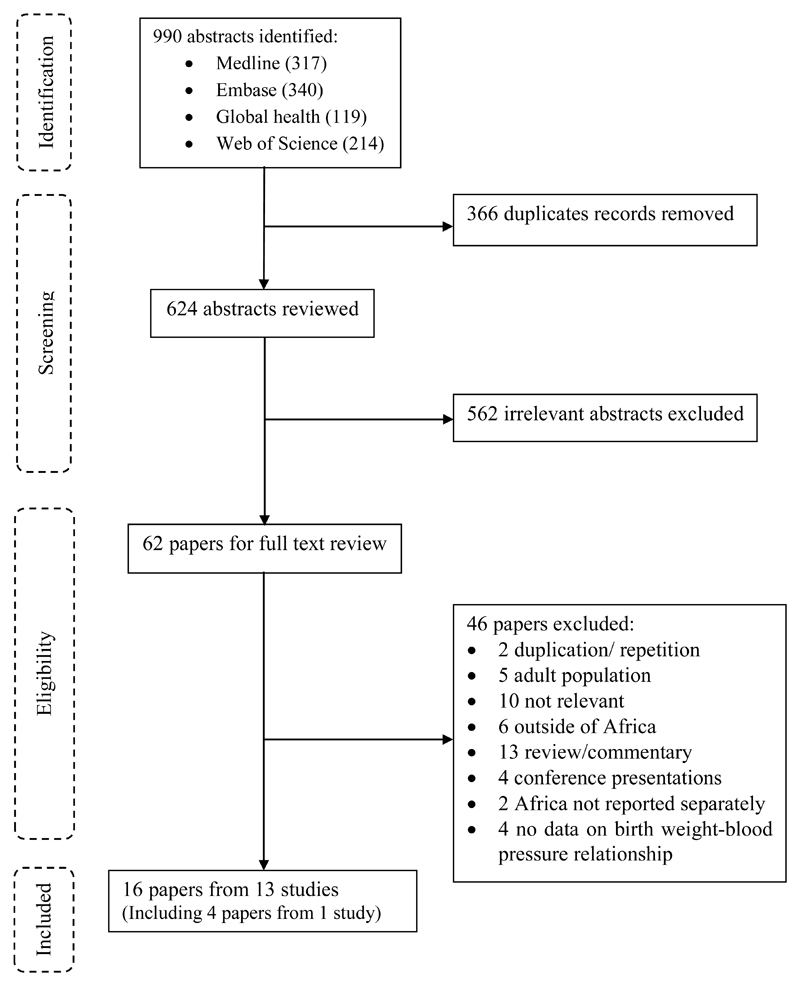
A total of 990 published abstracts were retrieved from four databases, of these 366 duplicates were removed, leaving 624 abstracts for review (Figure 1). Of these, 562 were excluded and of the remaining 62 papers that were subjected to full-text review, 46 were excluded. Of the 46 papers excluded, two papers (Steyn et al 37 and Sadoh et al 38) were duplicates (reported on the same participants at the same age) of one of the included papers. Thus, 16 papers from 13 studies describing, but not necessarily focusing on, the relationship between birth weight and BP were included in the final review and data extraction (Figure 1).
Figure 1.
Flow Diagram for systematic review.
Of the 16 papers reviewed, 6 were from West Africa 39–44, 6 Southern Africa 45–50, 2 Central Africa 51,52, 1 East Africa 53 and 1 North Africa 54. Four papers from Southern Africa were from the same cohort but presented data on BP at different ages of follow-up46–49. Four papers reported results in neonates (0-28 days) 41,43,44,51, four in children (1-9 years) 39,40,49,55, four in adolescents (10-19 years) 42,47,48,54 and four in both children and adolescents 45,46,52,53. The papers were published between 1989 and 2016.
The main characteristics of the reviewed papers are shown in table 1. Briefly, all papers included both males and females. The number of participants ranged from 157 to 2743 individuals per paper, with five papers reporting on more than 1000 participants. Seven papers had less than 500 participants. Two of the reviewed papers did not present quantitative information on the relationship between birth weight and BP. Eleven of the papers (from eight studies) described results from cohorts, while five papers reported results from cross-sectional studies.
Table 1. Description of the studies included in the systematic review.
| Author | Year of Publication | Year of birth | Place, Country | Age at BP assessment | Study size | Study type | Source of subjects | Reason for exclusion | Study aim |
|---|---|---|---|---|---|---|---|---|---|
| Ayoola | 2011 | NM | Ibadan, Nigeria | 1-3 days | 436 | Cohort | Hospital | Preterm, twins, metabolic defects, congenital abnormalities or sever birth trauma, babies of women with HIV, STDs, hypertension or diabetes | Evaluate the impact of maternal malaria on new-born BP |
| Chiolero | 2011 | 1984 to 1997 | Republic of Seychelles | 5.5, 9.1, 12.5, 15.5 years | 2743 | Cohort | School | Not mentioned | Assess the association between BW, weight change, and current BP across the entire age-span of childhood and adolescence |
| Hawkesworth | 2009 | 1989 to 1994 | West Kiang, Gambia | 11-17 years | 1267 | Cohort | Community | Preterm, implausible BP reading, ambiguity on treatment allocation | Investigate the effect of maternal protein-energy supplementation on BP in adolescence |
| Longo-Mbenza | 1999 | 1980 to 1991 | Kinshasa, DRC | 5-16 years, mean 11 years |
2409 | CS | School | Preterm birth, small for gestational age | Examine the possible association between LBW and hypertension later in life |
| Margetts | 1991 | 1980 to 1988 | Rural, Gambia | 1-9 years | 675 | Cohort | Community | Febrile illnesses | Relate BP levels in children to their mother’s weight in pregnancy |
| Sadoh | 2010 | NM | Benin City, Nigeria | 1-4 days | 473 | CS | Hospital | Preterm, abnormal APGAR score, congenital abnormality, admission to neonatal unit, babies of mothers with pre-eclampsia or diabetes | Determine the association between maternal and neonatal factors with BP at birth |
| Salvi | 2010 | 1986 to 1990 | Metlili, Algeria | 15-19 years | 568 | CS | School | Major disabilities, significant heart disease, renal or liver disease | Assess the association of current body weight and birth weight with BP values in school children living in Algerian Sahara |
| Law | 2000 | 1989 to 1993 | Sagamu Nigeria | 3-6 years, mean 4.4 years |
293 | Cohort | NM | Preterm, weighing less than 2.5 kg | Determine how reduced fetal growth is related to raised BP in countries where chronic malnutrition is common |
| Woelk | 1998 | 1987 to 1989 | Harare, Zimbabwe | Mean 6.5 years | 583 | Cohort | School | Twins, not born in Harare | Determine whether poor uterine growth may be associated with increased BP and subsequent hypertension in adulthood |
| Silva | 2016 | 2000 to 2006 | Luanda, Angola | 7-12 years, mean 9.4 years |
157 | CS | School | Not classified as Tanner stage I, completed 12 months between recruitment and examinations, high BP, obesity | Determine the factors associated with pulse wave velocity values and propose preliminary reference values in pre-pubertal Angolan school children |
| Nwokoye | 2015 | NM | Enugu, Nigeria | 1-2 days | 310 | CS | Hospital | Birth asphyxia, preterm, post term, sick babies, not weighing 2.5 to 4.0 kg, babies of mothers on antihypertensive drugs or illicit drugs | Determine BP values in apparently healthy term newborns in the first 48 hours of life and evaluate the factors affecting BP at birth |
| Youmbissi | 1989 | NM | Yaounde, Cameroon | At birth | 202 | Cohort | Hospital | Preterm, sick neonates, babies of mothers on diuretics or anti-hypertensive therapy during pregnancy or labour | Evaluate SBP variations in new-borns |
| Levitt* | 1999 | 1990 | Soweto, South Africa | 5 years | 818 | Cohort | Community | Twins | Examine the relationship between BW and BP at 5 years in a cohort of South African children |
| Kagura* | 2016 | 1990 | Soweto South Africa | 5, 8, 10, 13, 14, 16 and 18 years | 1937 | Cohort | Community | Twins, non-black, pregnancy during adolescence | Examine the association between early growth and BP trajectories and assess the influence of height on the association between early growth and BP trajectories |
| Griffiths* | 2012 | 1990 | Soweto, South Africa | 16 years | 358 | Cohort | Community | Twins, non-black | Understand the relationship between household and neighbourhood SES with SBP |
| Adair* | 2013 | 1990 | Soweto, South Africa | 18 years | 1222 | Cohort | Community | Twins | Investigate how linear growth and weight gain relative to linear growth in childhood and adulthood are related to health and human capital outcomes in young adults |
Papers from the same cohort (Birth to twenty cohort)
BP; Blood pressure, SBP; Systolic blood pressure, NM; Not mentioned, CS; Cross-sectional study, DRC; Democratic Republic of Congo, BW; birth weight, LBW; low birth weight
Except for one paper, in which the source of participants was unclear, participants were recruited from schools (n=5) 45,50,52–54, hospitals (n=4) 41,43,44,51 and communities (n=6; representing three studies) 40,42,46–49. Preterm children were excluded in seven papers: all four of the hospital-based studies41,43,44,51, one school-based 52 and one community based42. Twins were excluded in six papers (representing three studies) 41,46–48,50. Children who were small for gestational age or who weighed less than 2.5kg at birth were excluded from three papers; one in neonates 44, one in children39 and one in children and adolescents52. There was variability in the study aims of the papers included: only six described assessing the association between birth weight and BP as one of their main aims 39,50,52–54,56.
Table 2 summarizes birth weight and BP measurements and values, statistical analysis methods and the relationship between birth weight and BP in the reviewed papers.
Table 2. Main results from the studies included in the systematic review.
| Author | Mean BW | Source of BW data | Mean BP (mm Hg) | Procedure for BP measurement | Relationship between BW and BP | Adjusting factors |
|---|---|---|---|---|---|---|
| Ayoola | 2.9 kg | Hospital birth records | SBP=71.0 DBP=36.1 |
With the child comfortable on the mother’s lap for five minutes or asleep, three BP measurements with a minute’s interval between successive measurements were taken on the left arm using a Datascope monitor. Mean of the last two readings was used for analysis. | Positive BW-BP association. SBP increased by 8.35 mmHg per Kg increase in BW (95% CI: 4.36, 12.35, p<0.001). DBP increased by 3.07mmHg per Kg increase in BW (95% CI: 0.26, 5.88, p=0.032) | Gestational age, baby length, maternal malaria, age, weight, height, BP, gravidity, antenatal visits |
| Chiolero | 3.1 kg | Medical records | Could not be determined | After five minutes’ rest, two BP measurements were taken on the right arm with a minute’s interval between each using automated devices (OmronM5; Omron). Mean of the two measurements was used for analysis. | No overall BW-BP association. BW was not associated with SBP or with DBP, with exception of girls at 12.5 years among whom BW was inversely associated with SBP (β=0.9, 95% CI: -1.6, -0.1, p=0.026) and DBP (β=-0.7, 95% CI: -1.3, -0.1, p=0.028) | Current weight |
| Hawkesworth | 2.9 kg | Birth records | SBP=110.5 DBP=64.7 |
Measured in triplicate using the automated Omron 7051T device (Omron, UK), following manufacturer’s instruction. Mean of the three measurements was used for analysis | No association between BW and BP, β=-0.001, 95% CI: -0.002, 0.000, P=0.06 | Age, current body size, sex, gestational age, birth season, |
| Longo-Mbenza | 2.4 kg | Parental recall, medical records | Could not be determined | With child in a sitting position and relaxed for 20 minutes, five BP measurements were obtained using an automatic device (HEM-705 CP; Omron, Tokyo, Japan). Not clear which measurements were used in the analysis | BW inversely correlated with BP. With r=-0.1, p<0.001 for SBP and r=-0.1, p<0.05 for DBP. LBW had twice the odds of hypertension OR=2.0, 95% CI: 0.9, 8.2, p<0.01 for SBP and OR=2.3, 95% CI: 0.6, -11.5, p<0.01 for DBP1 | NM |
| Margetts | 3.0 kg | Birth records | 1 year: SBP= 89.3 DBP= 56.2, 9 years: SBP=102.7 DBP=63.9 |
After five minutes in a sitting position or on the mother’s knee in the young children, BP was measured twice on the right arm using an automated device (Dinamap model: 18465X). Not clear which measurements were used in the analysis | BW not associated with SBP at any age | NM |
| Sadoh | 3.2 kg | Hospital birth records | SBP=69.2 | Measured one hour after feeds between 11:00 and 13:30 using a Dinamap 8100 monitor (Critikon, Tampa Fla) device, when the baby was asleep or awake and calm. Three BP measurements were obtained within three minutes of each other on the right arm. Mean of three BP readings was used in the analysis | Positive BW-BP association BW was correlated with SBP (r=0.235, p=0.001), SBP rose by 3.61 mmHg per 0.5 kg increase in birth weight | NM |
| Salvi | 3.4 kg | Obstetric records in local hospitals | SBP=118.1 DBP=69.9 |
Three BP measurements with 3 minutes’ interval between successive measurements were taken on the left arm, after 10 minutes’ rest in a sitting position. An automated oscillmetric device (Omron 705T; Omron, Kyoto Japan) was used. Average of the three measurements was used for the analysis | No correlation between BW and SBP | Multivariate analysis was not done |
| Law | 3.2 kg | Birth records | SBP=101.6 | After five minutes’ rest, three BP measurements were taken on the left arm using automated BP machines (Dinamap model 8100) with a one-minute interval between consecutive measurements. Mean of the three measurements was used for the analysis | No association between BW and SBP (β=0.4, 95% CI: -2.1, 2.9) | Gender, observer, child's status, (crying or not) current weight and cuff size. |
| Woelk | 3.0 kg | Birth records | SBP=108.3 DBP= 62.1 |
With child sitting quietly, BP was measured in the morning on the right arm. Three measurements were taken two minutes apart using a Dinamap model 8100 BP. The average of the last two BP readings was used for analysis | Inverse relationship between BW and SBP. SBP rose by 1.73 mmHg per kg decrease in BW (95% CI: 0.18, 3.28), P=0.0286. No association between BW and DBP (β=-1.06, 95% CI: -2.57, 0.45) | Current weight |
| Silva | 3.2 kg | - | SBP=102 DBP=62 |
After resting for 5-10 minutes in a sitting position, three consecutive BP measurements were taken on the left arm with a two minutes’ interval using an automatic sphygmomanometer (OMRON, model HEM-742, Nanjing, Chine). Mean of last two readings was used for analysis. | No correlation between BW and SBP, r=-0.016, weak correction between DBP and BW r=0.09, p <0.05. Categorising birth weight into 4 groups, no association between birth weight category and SBP (p=0.991) or DBP (p=0.059). | NM |
| Nwokoye2 | 2.5-4.0 kg | Hospital birth records | Day 1, SBP=63.3 DBP=36.8 Day 2, SBP=65.6 DBP=40.0 |
After 10-15 minutes’ rest, a single BP measurement was taken on the right arm when the infant was awake and quiet or asleep and in spine position using an oscillometic machine (Dinamap 8100) |
Positive correlation between BW and SBP, r=0.37, p<0.01 between 0-24 hours and r=0.29, p<0.01 between 25-48 hours. No correlation between BW and DBP |
NM |
| Youmbissi | 3.2 kg | Hospital birth records | SBP=65.1 | Measured in the morning, on the right arm of a quiet and awake child. Three measurements were taken using a zero sphygmomanometer. Average of the three measurements used for the analysis | No association between BW and SBP, r=0.12 | NM |
| Levitt* | 3.1 kg | Birth records | SBP=108.0 DBP=62.6 |
After 10 minutes’ rest, BP was measured in triplicate using a Dinamap vital signs Monitor (1846SX). The lowest DBP with its matching SBP were used in the analysis |
Inverse association between BW and SBP (r=-0.05, p<0.001), SBP fell by 3.4mmHg, (95% CI: 1.4, 5.3) per kg increase in BW. No association between BW and DBP |
Current weight and height |
| Kagura* | 3.1 kg | Birth records | Could not be determined | After five minutes’ rest, BP was measured in triplicate with two-minute intervals between successive measurements, using the Dinamap Signs monitor 1846SX (Critikon) at 5 years and Omron M6 (Omron, Kyoto, Japan) at 8 to 18 years. Not clear which measurements were used in the analysis | Inverse association between BW and middle BP trajectory among boys (OR=0.75, 95% CI: 0.58, 0.96, p=0.0223). No other associations seen | Height, SES, maternal age, parity and gestational age |
| Griffiths* | 3.1 kg | Birth records | SBP=114.8 | In participants in a sitting position, three measurements were taken using a digital (Omron M6; Omron Kyoto, Japan) device with a rest of several minutes between successive measurements. The average of the last two measurements was used for analysis |
Inverse association between BW and SBP among boys (β=-0.003, p<0.1). No association between BW and SBP among girls |
Current height |
| Adair* | 3.1 kg | Hospital birth records | SBP=117.5 DBP=71.4 |
After 5-10 minutes’ rest, three BP measurements were taken using a digital device (Omron M6). The average of the last two measurements was used for analysis | No association between BW and BP. For SBP β= 0.05, 95% CI: -.79, 0.90 in males and 0.08 (-0.69, 0.84) in females, and for DBP β=-0.05 (-0.78, 0.68) among males and 0.17 (-0.51, 0.85) in females | NM |
Papers from the same study group (Birth to twenty cohort)
BW; birth weight, BP; blood pressure, SES; Socioeconomic status, NM; Not mentioned, r; correlation coefficient, β: linear regression coefficient, OR; Odds ratio, CI; Confidence interval
95% CI and p-value reported as in paper but are inconsistent
Mean birth weight not reported and could not be calculated (determined)
Birth weight ascertainment
Birth weight was either measured and recorded immediately after birth (predominantly in the cohort studies), or extracted from birth or child health card records. In one study 52, parentally recalled birth weight was used when birth records were missing (in an unknown number of participants). Mean birth weight varied from 2.4 to 3.4 kg.
Blood pressure assessment
Blood pressure procedures were relatively similar across studies. All studies used automated devices, except for one study, which used the sphygmomanometer machine. Eleven papers reported a resting period (from 5 to 20 minutes) before proceeding with measuring the BP. In four studies, measurements were taken on the left arm while in six studies measurements were on the right arm; the remaining studies did not include this information.
Blood pressure was measured in triplicates in the majority of papers (n=12) with one paper reporting single measurement, two reporting double measurement and one reporting five measurements. The rest period between consecutive BP measurements varied from 1 to 3 minutes.
Of the 12 papers that measured BP in triplicate, five used the mean of all three measurements in data analysis, five used the mean of the last two measurements, one used the lowest DBP (with matching SBP) and one was unclear. For the paper where BP was measured five times and one of the two papers where BP was measured in duplicate, it was unclear which measurements or combination thereof were used for data analysis. In the other paper where BP was measured in duplicate, the mean of the two measurements was used in the analysis.
Among neonates, mean SBP varied from 65.1 to 71.0 mmHg and in children and adolescents from 89.3 to 118.1 mmHg. Mean DBP varied from 36.1 to 63.9 mmHg in neonates and between 56.2 and 71.4 mmHg among children and adolescents. Mean SBP and DBP generally increased with age over the course of childhood and adolescence, this was especially apparent in the four papers that reported results from the same cohort study at different ages.
Birth weight and blood pressure relationship
The relationship between birth weight and SBP varied across papers; seven papers reported no association 39,40,42,45,47,51,54, six an inverse association46,48–50,52,53 and three a positive association 41,43,44. Among the neonates, three out of four papers reported a positive association while one reported no association. Of the four papers in children, two reported inverse associations and two no association. The papers on children and adolescents predominately found inverse associations (three out of four papers) while among adolescents, three papers found no association and one an inverse association.
Of the seven papers with participant size less than 500 individuals39,41,43–45,48,51, three papers reported no association between birth weight and SBP, one an inverse association and three a positive association 43–45,51. Studies with larger participant sizes (greater than 500 individuals) were more likely to report inverse associations. Of the nine studies with participant size over 500, three reported no association between birth weight and SBP, five an inverse association and one reported a positive association.
In three of the six papers reporting inverse associations 46,48,53, analyses were conducted at different ages and, or, separately for males and females, with inverse associations only seen among particular subgroups (girls at 12.5 years 53, boys only 46,48) and analyses from other subgroups showing no evidence of association.
Analysis approaches used to assess the relationship between birth weight and BP were diverse, varying from simple correlation analysis with no adjustment for potential confounders, to more complex group-based trajectory modelling approaches. Multivariable analysis, adjusting for potential confounder(s) (often including age, sex or body size [weight or height]) was conducted in eight papers, of these five reported an inverse association, two no association and one a positive association. In comparison, of the eight papers that did not undertake adjustment for confounders, one reported an inverse association between birth weight and SBP, five reported no association and two a positive association.
The relationship between birth weight and DBP was described in eight papers; a positive association was seen in two papers, inverse association in two papers and no association was reported in four papers. Of these eight papers, two were in neonates, two in children, three in children and adolescents, and one in adolescents only.
Discussion
Overall, this systematic review of existing literature showed varied results. We identified 16 papers from 13 studies addressing the question of whether the inverse relationship between birth weight and BP in later life seen in Western settings is also present in Africa. The relatively small number of studies and their heterogeneity in design and analysis prohibits definitive conclusions. However, we found some evidence to suggest that the relationship between birth weight and SBP in Africa varies with the participants’ age: positive associations were seen in neonates and inverse associations mainly in children. Among adolescents, the relationship was either inverse or showed no evidence of association. Only a few papers reported on the relationship between birth weight and DBP, with most papers reporting no relationship between birth weight and DBP.
This review supports an earlier review by Law 12 that did not include any of the papers reviewed herein (only two of the papers included in the present review had been published at the time of the Law review, and of these Margetts et al 40 was excluded for missing quantitative information while Youmbissi et al 51 was not mentioned). The Law review reported inconsistencies in the relationship between birth weight and BP, especially among adolescents. Generally, inverse associations were among children and positive associations in neonates, inconsistencies could be due to differences in age, sample sizes and statistical analysis approaches. Interestingly, results from the cohort studies included in our review, that measured BP at more than one-time point (different ages), did not show evidence of increasing strength of the association between birth weight and BP with age as reported by the earlier review 12. Studies in neonates consisted mainly of less than 500 participants and reported positive associations. Studies with smaller participant numbers are more likely to be underpowered to detect real associations, but also to produce spurious positive or negative associations 57.However, this may not be a factor for the results among neonates, which are consistent.
The relationship between birth weight and BP among adolescents has been reported in previous reviews as either inconsistent 12 or inverse with smaller effects than observed among (prepubescent) children 13. Similarly, this review found an inconsistent relationship between birth weight and BP among adolescents, while the relationship among younger children was generally inverse. The positive relationship observed in neonates is as expected, with the duration between birth and BP assessment too short to allow for any impact of subsequent weight trajectory. Explanations for the changing relationships among children and adolescents are uncertain, but could possibly relate to different growth patterns, for example, catch-up growth among those of low birth-weight, and hormonal changes occurring at adolescence 12,58.
Adjustment for possible confounding factors varied and was often incomplete. Consistent with previous reviews, which mainly included papers from high-income countries, studies adjusting for current body size (weight and, or, height) were more likely to report an inverse relationship 59,60 than those that did not make such an adjustment. Adjusting for current size has been noted to lead to a stronger inverse relationship between birth weight and BP compared to results without such adjustments 6. In most of the reviewed papers, that reported estimates adjusted for current size, unadjusted estimates for the effect of birth weight on BP were not reported. Therefore, we were unable to establish whether adjusting for current weight leads to stronger inverse relationships in these populations. The interpretation of findings adjusting for current weight is complex 6,59 because current weight may be seen as a confounder or mediator of the effect of birth weight BP 6.
Several mechanisms such as obesity, salt-sensitivity, renin-angiotensin system, and endothelial activation are important in the pathophysiology of hypertension. None of the reviewed papers investigated the role of these factors and their impact on BP. Recent evidence suggests that the relationship between birth weight and BP could be U shaped 61, highlighting the importance of both reduced and excessive nutrition in utero. It was not possible to examine this hypothesis from the papers reviewed. Compared to birth weight, other measures such as birth BMI or pendular index may more accurately reflect the birth size, but none of the studies reviewed included these measures.
Studies were subject to selection bias as individuals most likely to be low birth weight (such as preterm and twins) were excluded in many studies. This could have led to an underestimation of the effect of birth weight on BP. Furthermore, characteristics (such as maternal hypertension, parasitic infections, socioeconomic status [past or current]) that influence birth weight and may also be associated with BP in offspring were not adjusted for in these studies. Hence, estimates were subject to residual confounding. It remains uncertain what role (if any) such factors have in the relationship between birth weight and subsequent BP in children or adolescents.
Inconsistencies seen between reviewed papers are less likely to be due to differences in BP measurement procedures, as studies followed a similar approach. For nearly all papers, there was an initial rest period (before starting BP procedure) and between successive BP measurements, automated devices were used and analysis was based on the average of two or three measurements. In the majority of studies, early life information including birth weight was prospectively collected thus studies were less prone to misclassification and recall bias.
Generally, studies reviewed came from all the African regions but with a strong representation of West Africa and Southern Africa. East Africa and North Africa had the least number of papers (one each) reviewed. The only paper from East Africa reported on an island population, thus there were no results on the mainland population of East Africa.
In conclusion, relatively few studies have investigated the relationship between birth weight and BP later in life in Africa. The relationship between birth weight and blood pressure varied depending on the age of the participants. Our review emphasises the need for larger studies on the relationship between birth weight and later BP from Africa, applying appropriate control of potential confounding factors. Accumulating evidence on raised BP in Africa and understanding the impact of growth in early life and of prenatal exposures on BP later in life is key in identifying at-risk groups and developing early life interventions to reduce BP risk in later life.
Acknowledgements
None
Financial Support
This work was supported with funding from the Commonwealth Scholarship Commission (S.L., Ph.D. funding at the LSHTM); the Wellcome Trust (A.E., grant number 095778), (L.S., grant number 098504/Z/12/Z); and the UK Medical Research Council (E.W., grant number MR/K012126/1).
Footnotes
Conflicts of Interest
None
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301(6746):259–62. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schack-Nielsen L, Holst C, Sorensen TI. Blood pressure in relation to relative weight at birth through childhood and youth in obese and non-obese adult men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(12):1539–46. doi: 10.1038/sj.ijo.0802166. [DOI] [PubMed] [Google Scholar]
- 4.Adair LS, Martorell R, Stein AD, et al. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter? The American journal of clinical nutrition. 2009;89(5):1383–92. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law CM, de Swiet M, Osmond C, et al. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306(6869):24–7. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake KV, Gurrin LC, Evans SF, et al. Adjustment for current weight and the relationship between birth weight and blood pressure in childhood. Journal of hypertension. 2000;18(8):1007–12. doi: 10.1097/00004872-200018080-00003. [DOI] [PubMed] [Google Scholar]
- 7.Pharoah PO, Stevenson CJ, West CR. Association of blood pressure in adolescence with birthweight. Archives of disease in childhood Fetal and neonatal edition. 1998;79(2):F114–8. doi: 10.1136/fn.79.2.f114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Sullivan MJ, Kearney PJ, Crowley MJ. The influence of some perinatal variables on neonatal blood pressure. Acta Paediatr. 1996;85(7):849–53. doi: 10.1111/j.1651-2227.1996.tb14166.x. [DOI] [PubMed] [Google Scholar]
- 9.Perng W, Rifas-Shiman SL, Kramer MS, et al. Early Weight Gain, Linear Growth, and Mid-Childhood Blood Pressure: A Prospective Study in Project Viva. Hypertension. 2016;67(2):301–8. doi: 10.1161/HYPERTENSIONAHA.115.06635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkner B, Hulman S, Kushner H. Effect of birth weight on blood pressure and body size in early adolescence. Hypertension. 2004;43(2):203–7. doi: 10.1161/01.HYP.0000109322.72948.24. [DOI] [PubMed] [Google Scholar]
- 11.Macintyre S, Watt G, West P, Ecob R. Correlates of blood pressure in 15 year olds in the west of Scotland. Journal of epidemiology and community health. 1991;45(2):143–7. doi: 10.1136/jech.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. Journal of hypertension. 1996;14(8):935–41. [PubMed] [Google Scholar]
- 13.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. Journal of hypertension. 2000;18(7):815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Saada A. The social determinants of infant mortality and birth outcomes in Western developed nations: a cross-country systematic review. Int J Environ Res Public Health. 2013;10(6):2296–335. doi: 10.3390/ijerph10062296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlui M, Azahar N, Oche OM, Aziz NA. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Glob Health Action. 2016;9:28822. doi: 10.3402/gha.v9.28822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndibazza J, Muhangi L, Akishule D, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(4):531–40. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chibwesha CJ, Zanolini A, Smid M, et al. Predictors and outcomes of low birth weight in Lusaka, Zambia. Int J Gynaecol Obstet. 2016;134(3):309–14. doi: 10.1016/j.ijgo.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assefa N, Berhane Y, Worku A. Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PloS one. 2012;7(6) doi: 10.1371/journal.pone.0039957. e39957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denoeud L, Fievet N, Aubouy A, et al. Is chloroquine chemoprophylaxis still effective to prevent low birth weight? Results of a study in Benin. Malar J. 2007;6:27. doi: 10.1186/1475-2875-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiono PH, Klebanoff MA, Graubard BI, Berendes HW, Rhoads GG. Birth weight among women of different ethnic groups. Jama. 1986;255(1):48–52. [PubMed] [Google Scholar]
- 21.Delnord M, Blondel B, Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Curr Opin Obstet Gynecol. 2015;27(2):133–42. doi: 10.1097/GCO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garner P, Kramer MS, Chalmers I. Might efforts to increase birthweight in undernourished women do more harm than good? Lancet. 1992;340(8826):1021–3. doi: 10.1016/0140-6736(92)93022-f. [DOI] [PubMed] [Google Scholar]
- 23.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(1):30–5. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 24.Muanda FT, Chaabane S, Boukhris T, et al. Antimalarial drugs for preventing malaria during pregnancy and the risk of low birth weight: a systematic review and meta-analysis of randomized and quasi-randomized trials. BMC medicine. 2015;13:193. doi: 10.1186/s12916-015-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cot M, Le Hesran JY, Miailhes P, Esveld M, Etya'ale D, Breart G. Increase of birth weight following chloroquine chemoprophylaxis during the first pregnancy: results of a randomized trial in Cameroon. Am J Trop Med Hyg. 1995;53(6):581–5. doi: 10.4269/ajtmh.1995.53.581. [DOI] [PubMed] [Google Scholar]
- 26.Akombi BJ, Agho KE, Merom D, Renzaho AM, Hall JJ. Child malnutrition in sub-Saharan Africa: A meta-analysis of demographic and health surveys (2006-2016) PloS one. 2017;12(5) doi: 10.1371/journal.pone.0177338. e0177338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vorster HH, Kruger A. Poverty, malnutrition, underdevelopment and cardiovascular disease: a South African perspective. Cardiovascular journal of Africa. 2007;18(5):321–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Benzekri NA, Sambou J, Diaw B, et al. High Prevalence of Severe Food Insecurity and Malnutrition among HIV-Infected Adults in Senegal, West Africa. PloS one. 2015;10(11) doi: 10.1371/journal.pone.0141819. e0141819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guwatudde D, Mutungi G, Wesonga R, et al. The Epidemiology of Hypertension in Uganda: Findings from the National Non-Communicable Diseases Risk Factor Survey. PloS one. 2015;10(9) doi: 10.1371/journal.pone.0138991. e0138991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavishe B, Biraro S, Baisley K, et al. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC medicine. 2015;13:126. doi: 10.1186/s12916-015-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65(2):291–8. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 32.Mbewu A, Mbanya JC. Cardiovascular Disease. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd ed. Washington (DC): 2006. [Google Scholar]
- 33.Lule SA, Webb EL, Ndibazza J, et al. Maternal recall of birthweight and birth size in Entebbe, Uganda. Tropical medicine & international health : TM & IH. 2012;17(12):1465–9. doi: 10.1111/j.1365-3156.2012.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA. Blood pressure tracking in urban black South African children: birth to twenty cohort. BMC pediatrics. 2015;15:78. doi: 10.1186/s12887-015-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood Pressure Trajectories From Childhood to Young Adulthood Associated With Cardiovascular Risk: Results From the 23-Year Longitudinal Georgia Stress and Heart Study. Hypertension. 2017;69(3):435–42. doi: 10.1161/HYPERTENSIONAHA.116.08312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyn K, de Wet T, Richter L, Cameron N, Levitt NS, Morrell C. Cardiovascular disease risk factors in 5-year-old urban South African children--the Birth to Ten Study. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2000;90(7):719–26. [PubMed] [Google Scholar]
- 38.Sadoh WE, I SE. Oscillometric blood pressure reference values of African full-term neonates in their first days postpartum. Cardiovascular journal of Africa. 2009;20(6):344–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Law CM, Egger P, Dada O, et al. Body size at birth and blood pressure among children in developing countries. International journal of epidemiology. 2000;30(1):52–7. doi: 10.1093/ije/30.1.52. [DOI] [PubMed] [Google Scholar]
- 40.Margetts BM, Rowland MG, Foord FA, Cruddas AM, Cole TJ, Barker DJ. The relation of maternal weight to the blood pressures of Gambian children. International journal of epidemiology. 1991;20(4):938–43. doi: 10.1093/ije/20.4.938. [DOI] [PubMed] [Google Scholar]
- 41.Ayoola OO, Gemmell I, Omotade OO, Adeyanju OA, Cruickshank JK, Clayton PE. Maternal malaria, birth size and blood pressure in Nigerian newborns: insights into the developmental origins of hypertension from the Ibadan growth cohort. PloS one. 2011;6(9) doi: 10.1371/journal.pone.0024548. e24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawkesworth S, Prentice AM, Fulford AJ, Moore SE. Maternal protein-energy supplementation does not affect adolescent blood pressure in The Gambia. International journal of epidemiology. 2009;38(1):119–27. doi: 10.1093/ije/dyn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadoh WE, Ibhanesehbor SE, Monguno AM, Gubler DJ. Predictors of newborn systolic blood pressure. West African journal of medicine. 2010;29(2):86–90. [PubMed] [Google Scholar]
- 44.Nwokoye I, Uleanya N, Ibeziako N, Ikefuna A, Eze J, Ibe J. Blood pressure values in healthy term newborns at a tertiary health facility in Enugu, Nigeria. Nigerian Journal of Clinical Practice. 2015;18(5):584–8. doi: 10.4103/1119-3077.158944. [DOI] [PubMed] [Google Scholar]
- 45.Silva ABT, Capingana DP, Magalhaes P, Molina MDB, Baldo MP, Mill JG. Predictors and Reference Values of Pulse Wave Velocity in Prepubertal Angolan Children. Journal of Clinical Hypertension. 2016;18(8):725–32. doi: 10.1111/jch.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagura J, A LS, Munthali RJ, Pettifor JM, Norris SA. Association between early life growth and blood pressure trajectories in black South African children. Hypertension. 2016;68(5):1123–31. doi: 10.1161/HYPERTENSIONAHA.116.08046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths PL, Sheppard ZA, Johnson W, Cameron N, Pettifor JM, Norris SA. Associations between household and neighbourhood socioeconomic status and systolic blood pressure among urban South African adolescents. Journal of biosocial science. 2012;44(4):433–58. doi: 10.1017/S0021932012000107. [DOI] [PubMed] [Google Scholar]
- 49.Levitt NS, Steyn K, De Wet T, et al. An inverse relation between blood pressure and birth weight among 5 year old children from Soweto, South Africa. Journal of epidemiology and community health. 1999;53(5):264–8. doi: 10.1136/jech.53.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woelk G, Emanuel I, Weiss NS, Psaty BM. Birthweight and blood pressure among children in Harare, Zimbabwe. Archives of disease in childhood Fetal and neonatal edition. 1998;79(2):F119–22. doi: 10.1136/fn.79.2.f119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youmbissi TJ, O N, Mbede J, Nasah BT. Blood pressure profiles of a group of African children in the first year of life. Journal of Tropical Pediatrics. 1989;35(5):245–6. doi: 10.1093/tropej/35.5.245. [DOI] [PubMed] [Google Scholar]
- 52.Longo-Mbenza B, Ngiyulu R, Bayekula M, et al. Low birth weight and risk of hypertension in African school children. Journal of cardiovascular risk. 1999;6(5):311–4. doi: 10.1177/204748739900600507. [DOI] [PubMed] [Google Scholar]
- 53.Chiolero A, Paradis G, Madeleine G, Hanley JA, Paccaud F, Bovet P. Birth weight, weight change, and blood pressure during childhood and adolescence: a school-based multiple cohort study. Journal of hypertension. 2011;29(10):1871–9. doi: 10.1097/HJH.0b013e32834ae396. [DOI] [PubMed] [Google Scholar]
- 54.Salvi P, Meriem C, Temmar M, et al. Association of current weight and birth weight with blood pressure levels in Saharan and European teenager populations. American journal of hypertension. 2010;23(4):379–86. doi: 10.1038/ajh.2009.275. [DOI] [PubMed] [Google Scholar]
- 55.Woelk GB. Is low birth weight a risk factor for adult hypertension? A literature review with particular reference to Africa. South African Medical Journal Suid-Afrikaanse Tydskrif Vir Geneeskunde. 1995;85(12 Pt 2):1348–9. 52-3. [PubMed] [Google Scholar]
- 56.Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: early programming of cortisol axis. Journal of Clinical Endocrinology & Metabolism. 2000;85(12):4611–8. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- 57.Berlin JA, Begg CB, Louis TA. An Assessment of Publication Bias Using a Sample of Published Clinical Trials. Journal of the American Statistical Association. 1989;84(406):381–92. [Google Scholar]
- 58.Ewald DR, Haldeman L., PhD Risk Factors in Adolescent Hypertension. Glob Pediatr Health. 2016;3 doi: 10.1177/2333794X15625159. 2333794X15625159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardy R, Sovio U, King VJ, et al. Birthweight and blood pressure in five European birth cohort studies: an investigation of confounding factors. European journal of public health. 2006;16(1):21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- 60.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360(9334):659–65. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 61.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–50. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]