Significance
The hypothalamic-pituitary-adrenal axis is a critical neurohormonal network regulating homeostasis and coordinating stress responses. Here we demonstrate that an oscillating pattern of plasma cortisol is important for maintenance of healthy brain responses as measured by functional neuroimaging and behavioral testing. Our data highlight the crucial role of glucocorticoid rhythmicity in (i) modulating sleep behavior and working memory performance, and (ii) regulating the human brain’s responses under emotional stimulation. Current optimal cortisol replacement therapies for patients with primary or secondary adrenal insufficiently are associated with poor psychological status, and these results suggest that closer attention to aspects of chronotherapy will benefit these patients and may also have major implications for improved glucocorticoid dynamics in stress and psychiatric disease.
Keywords: glucocorticoid rhythmicity, human brain, emotional processing, fMRI study
Abstract
Glucocorticoids (GCs) are secreted in an ultradian, pulsatile pattern that emerges from delays in the feedforward-feedback interaction between the anterior pituitary and adrenal glands. Dynamic oscillations of GCs are critical for normal cognitive and metabolic function in the rat and have been shown to modulate the pattern of GC-sensitive gene expression, modify synaptic activity, and maintain stress responsiveness. In man, current cortisol replacement therapy does not reproduce physiological hormone pulses and is associated with psychopathological symptoms, especially apathy and attenuated motivation in engaging with daily activities. In this work, we tested the hypothesis that the pattern of GC dynamics in the brain is of crucial importance for regulating cognitive and behavioral processes. We provide evidence that exactly the same dose of cortisol administered in different patterns alters the neural processing underlying the response to emotional stimulation, the accuracy in recognition and attentional bias toward/away from emotional faces, the quality of sleep, and the working memory performance of healthy male volunteers. These data indicate that the pattern of the GC rhythm differentially impacts human cognition and behavior under physiological, nonstressful conditions and has major implications for the improvement of cortisol replacement therapy.
Glucocorticoids (GCs), predominantly cortisol in man, are critical for life and are key regulators of cognitive, metabolic, and immunologic homeostasis (1). The lipophilic nature of these hormones allows them easy access to all tissues of the body, including the central nervous system, where they can cross the blood–brain barrier to act on GC receptors and mineralocorticoid receptors (MRs) found in both neuronal and glial populations (2). In the rat, there is an extensive literature on the importance of GCs for short- and long-term neurocognitive adaptation to stressful conditions (3–6), and clinical studies in healthy human subjects, using functional neuroimaging techniques, have clearly demonstrate the importance of GCs in the neural response to stress (7–16).
During the basal “unstressed” physiological state, plasma cortisol levels vary across the 24-h cycle with a circadian rhythm that reaches a peak soon after awakening and falls to low levels late at night. This circadian rhythmicity is important for synaptic function (17, 18) and is made up from an underlying ultradian rhythm, which can be modified by internal or external stressors (19). This ultradian rhythm of GC secretion has been observed in all mammalian species studied, including rodents (20), sheep (21), horse (22), deer (23), cow (24, 25), monkey (26, 27), and man (28–37). Mathematical biomodeling also provides a very strong rationale for the importance of this oscillating rhythm (38–41).
Current regimens of cortisol replacement therapy for patients lacking adrenal function do not replace ultradian rhythmicity and result in considerable morbidity. Several centers have confirmed that these treatment regimens result in impaired health-related quality of life (42–47), adverse metabolic and cardiovascular risk profiles (48), increased levels of proinflammatory cytokines (49), and reduced activity, low motivation, and mental fatigue, with associated high levels of unemployment and disability benefits (43, 50–52). It was initially assumed that this was a direct consequence of the inability of oral medication to provide the normal circadian variation of cortisol, and that this could be improved by continuous s.c. infusions of the hormone. This has proven to not be the case, however, and Gagliardi et al. (53) have convincingly shown that “nonpulsatile” continuous s.c. infusion does not improve subjective health status, while Oksnes et al. (54), in an open-label study, showed only small changes in a limited number of domains in a quality of life questionnaire and suggested that this might be due to the lack of ultradian pulses in their infusion protocol.
Pulsatility is a key feature of many hormone systems, and to date biomedicine has failed to mimic this important facet of physiological regulation (36). With respect to cortisol, there are clear theoretical reasons why pulsatile replacement might be important. We know that GC is pulsed not only in the blood, but also in peripheral tissues, such as the brain (55). Furthermore, the brain responds dynamically to these oscillations (56) with differing genomic (57) and rapid nongenomic responses, including the accumulation of glutamatergic receptors into synapses and induction of long-term potentiation (58). Even the behavioral responses of rodents to a mild stressor are dependent on endogenous pulses (59).
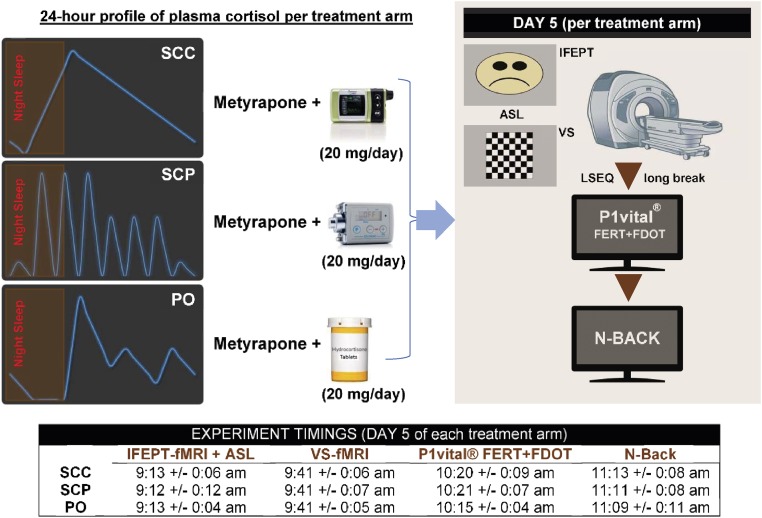
Motivated by the clinical need for improved GC-based therapeutics and the preclinical evidence, we hypothesized that under normal, nonstressful, nonpathological conditions, different ultradian GC rhythms might be translated differently in relevant GC-responsive human brain regions, and that this differential processing should be detectable using well-designed experimental protocols. We have developed a “block and replace” protocol (i.e., combined metyrapone administration with hydrocortisone infusion) in which we can reliably impose definitive patterns of plasma hydrocortisone (60). This has allowed us to provide three predetermined patterns of cortisol replacement therapy: (i) normal circadian rhythmicity lacking any physiological ultradian rhythm [s.c.-continuous hydrocortisone infusion (SCC)], (ii) normal circadian and ultradian rhythmicity [s.c.-pulsatile hydrocortisone infusion (SCP)], and (iii) current optimal oral replacement therapy (PO), characterized by suboptimal circadian and ultradian rhythms. We have used these three treatment regimens in a double-blind, placebo-controlled, crossover study in healthy male volunteers to assess the importance of cortisol rhythmicity for normal brain activity in man, using a combination of functional magnetic resonance imaging (fMRI) and psychological tasks (Fig. 1), based on stimulation with emotionally valenced cues [implicit facial expression processing task (IFEPT) and parts of the P1vital emotional test battery, respectively], that recruit GC responsive brain regions previously shown to be sensitive to changes in GC infusion patterns in our preclinical studies (58, 59). We also gathered dynamic measurements of affective state throughout these interventions.
Fig. 1.
Study design. Each healthy male volunteer took part in three 5-d, randomized-order, block and replace studies. In each arm of the study, endogenous hydrocortisone was suppressed with metyrapone, and hydrocortisone was replaced at a dose of 20 mg/d via (i) s.c. with a normal circadian rhythm provided by continuous s.c. infusion with an Animas Vibe pump (SCC), (ii) pulsatile s.c. infusion providing both circadian and ultradian rhythms with a Canè CRONO P pump (SCP), or (iii) oral treatment (PO), using a three times daily regimen, resulting in just three pulses during the day and a prolonged low level at night. During each pharmacologic intervention, participants were given hydrocortisone/placebo tablets and were connected to one of the pumps (infusing placebo/hydrocortisone). Between pharmacologic interventions, there was a washout period of at least 2 wk. In the morning of the fifth day of each pharmacologic intervention, participants were attending the clinical facility and undergoing time-controlled functional (fMRI) and perfusion (ASL) magnetic resonance imaging experiments, completing an LSEQ and engaging with various computerized behavioral and cognitive tests. VS, visual stimulation task (flashing checkerboard). The mean timing (and the corresponding SD) per outcome measure per group is displayed.
Results
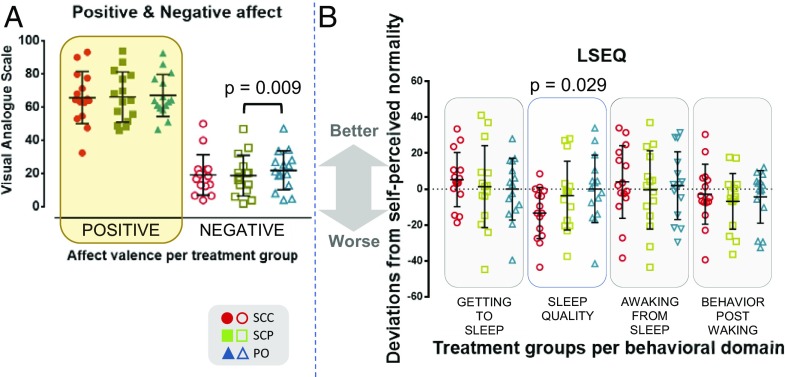
Ecological momentary assessment (EMA) data obtained throughout the study showed no difference in either positive or negative affect between the SCC and SCP groups [F(1.587, 22.221) = 0.196; P = 0.773 and F(1.321, 18.493) = 2.303; P = 0.141, respectively]. However, individuals in the PO group showed higher negative mood ratings compared with the SCP group (mean difference of 3.154; 95% CI, 0.754–5.554; P = 0.009) (Fig. 2A).
Fig. 2.
(A) Mean positive and negative affect of individuals per treatment arm, as assessed by EMA techniques. The higher the value (0–100), the stronger the corresponding (self-perceived) mood valence. Across treatments, subjects scored high for positive affect (65.7 ± 15.7 for the SCC group, 66.1 ± 15.2 for the SCP group, and 67 ± 12.7 for the PO group) and low for the negative affect (19.1 ± 12.2 for the SCC group, 18.7 ± 12.2 for the SCP group, and 21.9 ± 11.7 for the PO group). No major variations were observed in the positive affect scores across treatments. In contrast, there was a notable difference in the negative affect scores between the SCP and PO groups (P < 0.01). (B) Self-perceived sleep behavior. Deviations from the self-perceived normality of night sleep, as assessed by the LSEQ, across the three treatment groups. LSEQ evaluates four domains of sleep behavior: ease of falling asleep, quality of sleep, ease of awakening, and behavior after awakening. The sleep that participants were asked to evaluate was the one they experienced during the night of the fourth day/early morning of the fifth (last) day after starting each pharmacologic intervention. The values displayed in the scatterplot have been transformed [50 − (LSEQ score)] to more clearly demonstrate any improvements (positive values) or deteriorations (negative values) in sleep behavior compared with what each participant subjectively considered “normal” or “usual” (value 0). A notable interaction between cortisol dynamics and quality of sleep was observed (P < 0.05). The raw values of the LSEQ are presented in the SI Appendix, Fig. S1.
The Nonpulsatile GC Rhythm Is Associated with Poorer Quality of Sleep.
An interaction of cortisol dynamics was found in one of the four domains of sleeping behavior assessed by the Leeds sleep evaluation questionnaire (LSEQ) [F(1.914, 26.801) = 4.137; P = 0.029; ω2 = 0.12]. Based on the volunteers’ responses on a visual analog scale (VAS), the quality of sleep is poorer (i.e., more and/or longer periods of restlessness and wakefulness) when undergoing the s.c.-continuous hydrocortisone infusion compared with the other two modes of hydrocortisone replacement (Fig. 2B and SI Appendix, Fig. S1). Post hoc analysis with a Bonferroni adjustment did not reveal any pairwise differences between any combinations of groups, however.
The N-Back Task Reveals an Effect of Optimal Pulsatile GC Replacement in Retention of Working Memory Capacity Under Increased Cognitive Demands.
The n-back task is considered to reflect working memory processes, when n equals 2 or more; in the latter cases, the working memory buffer must be updated continuously to keep track of what the current stimulus must be compared with, necessitating maintenance and manipulation of information in working memory. Zero- and one-back tasks are used as control sessions. Two subjects performed poorly (i.e., significantly low %accuracy scores, with values of studentized residuals below −3) in these control sessions in at least one of the study arms, which indicates a systematic bias; therefore, these subjects were excluded from further analysis for this task (n = 13 per study group).
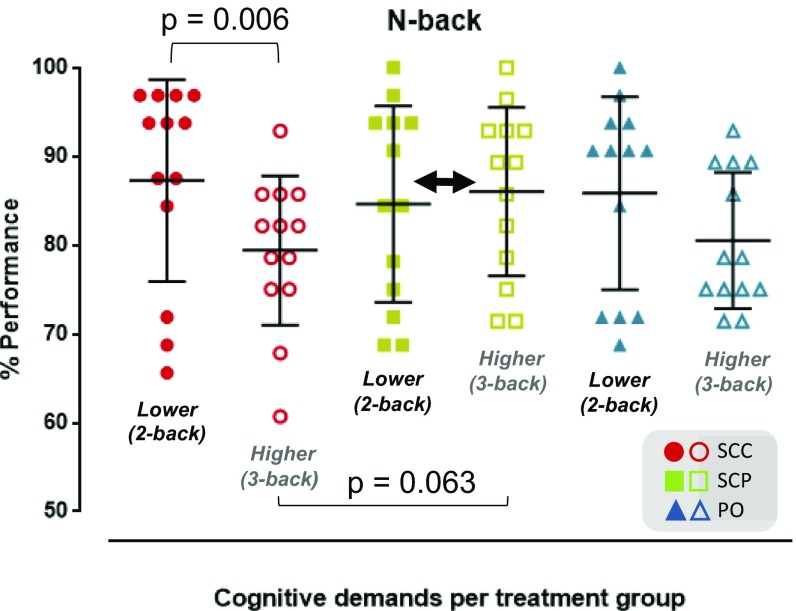
ANOVA elicited a two-way interaction of [cognitive load] × [cortisol dynamics] on participants’ performance in the n-back task [F(1.525, 18,304) = 4.437; P = 0.035]. Volunteers in the SCP group retained the same performance across the two- and three-back sessions, in contrast to the performance in the other two treatment modes, where performance in three-back sessions was poorer compared with that in the two-back sessions, especially in the SCC group (mean drop in %accuracy score from two- to three-back sessions, 7.9%; 95% CI, 2.7–13%; P = 0.006; ω2 = 0.28; mean difference in the %accuracy in three-back sessions between the SCP and SCC groups, 6.6%; 95% CI, −0.3 to 13.5%; P = 0.063; ω2 = 0.10) (Fig. 3).
Fig. 3.
Cognitive performance. The marginal mean estimates of %accuracy indicate the three treatment groups’ performance in the working memory task (n-back), and how they change when going from the two-back test (87.3 ± 11.4 for the SCC group, 84.6 ± 11.1 for the SCP group, and 85.8 ± 10.9 for the PO group) to the more challenging three-back test (79.4 ± 8.4 for the SCC group, 86 ± 9.5 for the SCP group, and 80.5 ± 7.7 for the PO group). The working memory performance in the SCC group drops notably when the cognitive demands increase (P < 0.01). In contrast to the other two treatment modes, the SCP group retains its working memory capacity when the cognitive demands increase (double-headed black arrow), especially compared with the SCC group (P = 0.063).
ANOVA did not demonstrate any two-way interaction of [cognitive load] × [cortisol dynamics] on participants’ reaction times for correct responses [F(1.403, 16.835) = 0.663; P = 0.477], or any significant interactions for the main effects of each factor. Therefore, the differences in %accuracy reported above have not been confounded by differences in reaction times.
The SCP Group Shows Reduced Accuracy in Recognizing Negatively Valenced Emotional Input, as Assessed by P1vital Face Expression Recognition Task.
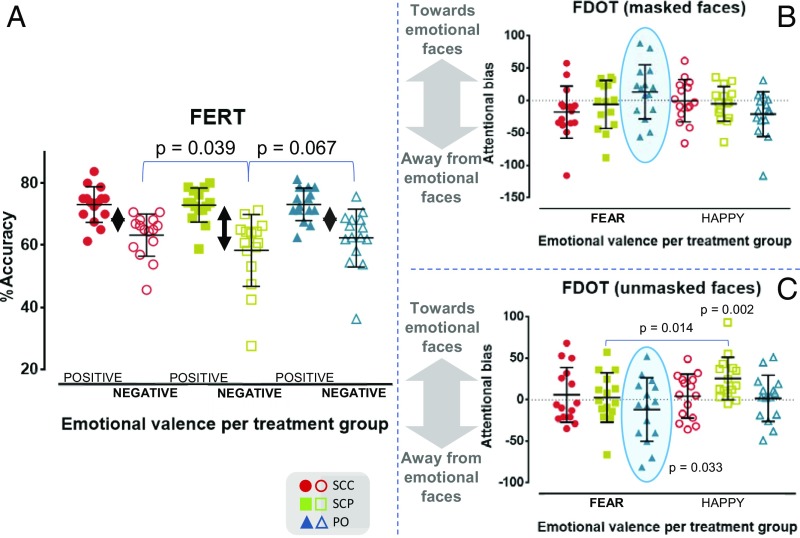
The Face Expression Recognition Task (FERT) measures individuals’ accuracy and speed in interpreting facial expressions. After each brief exposure to a human face, subjects need to make one of seven choices, indicating that they encountered a neutral expression, a positive expression (i.e., happy, surprise), or a negative expression (i.e., angry, fear, disgust, sad). The %accuracy scores for recognizing neutral faces did not differ across the treatment groups [F(1.780, 24.914) = 0.463; P = 0.612] and were higher than those for recognizing faces with an emotional valence. ANOVA elicited a two-way interaction of [valence] × [cortisol dynamics] on the %accuracy [F(1.629, 22.809) = 3.747; P = 0.047]; across the treatment groups, participants showed greater accuracy in recognizing positive emotions compared with negative emotions, in agreement with earlier research (61, 62). While the accuracy in recognizing positive emotions did not change between treatments, this was not the case with negative emotions [F(1.480, 20.714) = 6.492; P = 0.011; ω2 = 0.20]. The perception of negative facial expressions was reduced in the SCP group compared with the SCC group (mean difference of 4.9%; 95% CI, 0.2–9.6%; P = 0.039) and the PO group (mean difference of 4%; 95% CI, −0.2 to 8.2%; P = 0.067) (Fig. 4A), consistent with a reduction in the processing of negative information.
Fig. 4.
(A) Behavioral data on emotional face recognition. The scatterplot shows the %accuracy scores for recognizing positively valenced (happy, surprise) and negatively valenced facial expressions (sad, angry, fearful, disgusted) per treatment group. Similar to what has been observed as an effect of established antidepressant and anxiolytic agents on healthy male volunteers, the pulsatile GC rhythm approximating physiology (SCP) increases the within-group difference in the accuracy scores between correctly identifying positively valenced from negatively valenced human faces (double-headed black arrows). This phenomenon occurs as the result of the comparatively reduced accuracy of subjects in the SCP group in correctly identifying faces expressing negative emotions (on average by 4.9% vs. SCC; P = 0.029 and on average by 4% vs. PO; P = 0.067). More details are provided in SI Appendix, Fig. S2. (B) Behavioral data on emotion-related attentional bias at the subliminal level (masked faces). The scatterplot presents each treatment group’s vigilance scores toward (positive values) or away (negative values) from masked fearful and happy faces. (C) Scatterplot presenting each treatment group’s vigilance scores toward (positive values) or away (negative values) from unmasked fearful and happy faces. In the SCP group, there is a significant difference (P = 0.014) in the vigilance score between unmasked fearful and happy faces. Moreover, in the PO group, there is a strong trend for changing the direction of the attentional bias when going from masked to unmasked fearful faces (light-blue ellipsoidal frame in B and C; P = 0.067 not shown in figure). Moreover, the PO group showed a statistically significant absolute bias away from unmasked fearful faces (P = 0.033), and the SCP group showed a statistically significant absolute bias toward unmasked happy faces (P = 0.002). Overall, fast-acting neural processing of fearful faces in the SCP group and of happy faces in the SCC group led to minimal attentional bias. In all other cases, the transition from subliminal to more conscious attentional mechanisms led to changes in the direction of the attentional bias, either from stimulus avoidance to vigilance or, only for the processing of fearful faces in the PO group, vice versa.
These treatment-related perceptual variations were confirmed by ANOVA on the %origin of misclassifications [F(1.637, 22.916) = 4.120; P = 0.036; ω2 = 0.12], since subjects receiving the optimal pulsatile hydrocortisone replacement are more likely to misclassify negative emotional faces compared with those receiving the other two modes of hydrocortisone replacement, as well as by the main effect of treatment on the %destination of misclassifications [F(1.665, 23.312) = 6.522; P = 0.008; ω2 = 0.20], since the SCP group misclassified toward emotional faces with a significantly higher frequency compared with the other two groups (SI Appendix, Fig. S2). Overall, the optimal pulsatile treatment resulted in lower accuracy for recognizing negatively valenced faces and, consequently, more misclassifications of negatively valenced faces.
There was no two-way interaction of [valence] × [cortisol dynamics] by ANOVA on the reaction time of subjects’ responses [F(1.927, 26.971) = 1.959; P = 0.162]. There was also no main effect of treatment on reaction time [F(1.668, 23.351) = 1.026; P = 0.361]. However, emotional valence showed a main effect [F(1, 14) = 48.857; P < 0.001], similar to that found in earlier studies (61, 62); independent of treatment group and on average, participants tended to respond faster to faces with a positive emotional valence compared with faces with a negative emotional valence (mean difference, 211 ms; 95% CI, 164–257 ms; P < 0.001).
The P1vital Emotional Face-Related Attentional Bias Task Indicated That the SCP Group Shows Facilitation of Attention Deployment Toward Positive Stimuli.
The Facial Dot-Probe Task (FDOT) assesses attention to positive and negative stimuli using a reaction time measure, the vigilance score. While subjects are asked to maintain their attention to the middle of a screen, emotionally valenced and neutral stimuli are presented above and below this central point. After presentation, one of the stimuli is replaced by two dots, vertically or horizontally oriented. The subjects are instructed to guide their attention and correctly identify the orientation of the two dots as quickly as possible. The facial stimuli are presented for either 100 ms (unmasked) or 16 ms and then replaced for the remaining 84 ms with a jumbled face (masked) (63). In the three treatment groups, the mean %accuracy scores for identifying the orientation of the two dots were very high and very similar (97.5%, 97.1%, and 96.7%). ANOVA elicited a three-way interaction of [masking] × [valence] × [cortisol dynamics] on the vigilance score of the subjects’ responses [F(1.771, 24.778) = 4.039; P = 0.035], driven by the differential, treatment-dependent deployment of attention toward or away from emotional faces when moving from the subliminal level (masked faces; Fig. 4B) to the brief presentation level (unmasked faces; Fig. 4C).
Across treatments and emotional valences (happy and fearful faces), we can observe a pattern in which subjects showed a negative vigilance score (i.e., attentional bias away from emotional faces) at a subliminal level, transformed to a positive one (i.e., attentional bias toward emotional faces) at a brief presentation level. This pattern not only was reversed for fearful faces in subjects receiving oral hydrocortisone replacement, but the difference in the vigilance scores between the two perceptual levels was notable [F(1.879, 26.301) = 3.456; P = 0.049; ω2 = 0.08; mean difference, 25.1; 95% CI, −2 to 52.3; P = 0.067], especially due to the deployment of these subjects’ attention away from unmasked fearful faces [absolute attentional bias: t(14) = −2.363; P = 0.033; d = 0.31]. At a preconscious level, subjects receiving the optimal pulsatile treatment tended to be strongly attracted by happy faces, not only per se [absolute attentional bias: t(14) = 3.872; P = 0.002; d = 1.00], but also in comparison with fearful faces [F(1, 14) = 5.874; P = 0.030; ω2 = 0.14; mean difference in vigilance score, 23.1; 95% CI, 5.5–40.7; P = 0.014]. Overall, GC rhythmicity seems to interact with the fast-acting neural pathways that coordinate the deployment of attention in the presence of emotional stimuli.
Different GC Rhythms Change the Neural Processing of Emotional Input.
Our fMRI protocol assessing the processing of emotional faces (i.e., IFEPT) provoked significant activations from most of the predefined regions of interest (ROIs; SI Appendix, Table S3) and the combined evaluation of the fMRI experiments (on emotional face processing and nonemotional visual stimulation) with the ROI analysis on the regional perfusion data revealed the presence of GC-susceptible brain areas in which the underlying neural processing of emotional input is rhythm-sensitive. These rhythm-dependent neural responses specifically relate to facial expression processing rather than to nonspecific differences due to neural reactivity or neural coupling or due to differences in the resting perfusion of these brain areas between the groups.
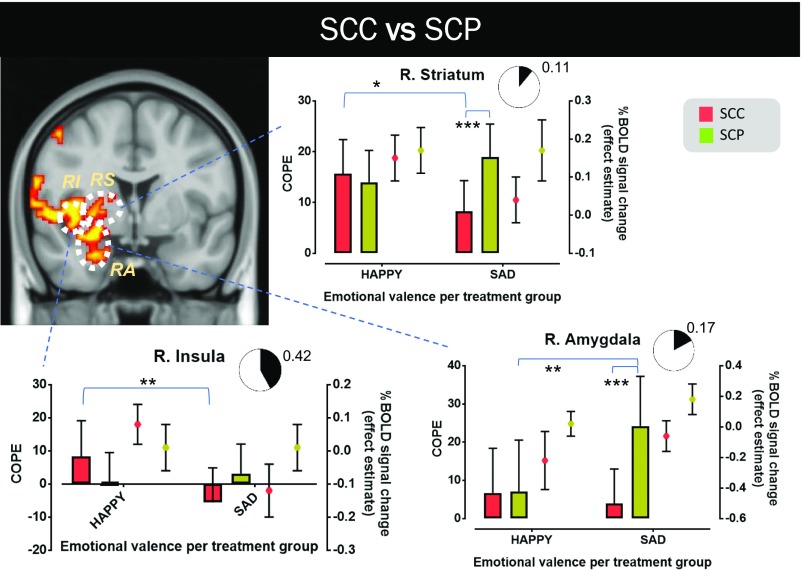
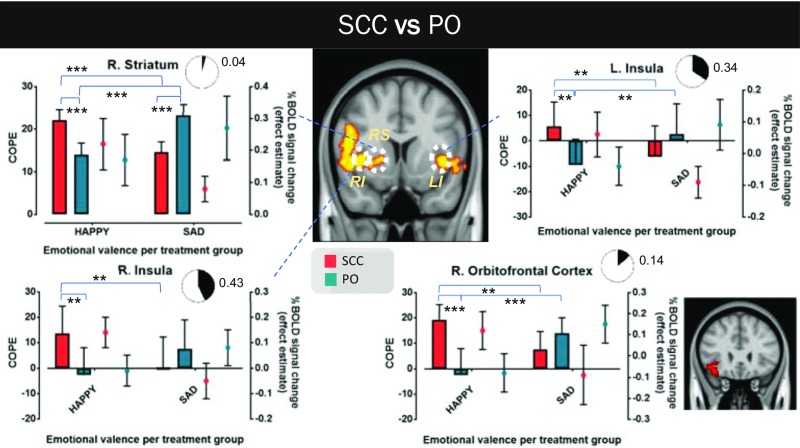
Whole-brain analysis of the fMRI data acquired during the IFEPT identified differential patterns of activation in brain region clusters (familywise error-corrected; Z-threshold = 2.3; P < 0.05) in which blood oxygen level-dependent (BOLD) signal activity patterns corresponding to emotional face discrimination showed notable variations as a function of emotional expression and group. These included some of our predefined ROIs and were observed in the differential processing of happy and sad faces, and fearful and sad faces, between the SCC and SCP groups (SI Appendix, Fig. S4) and in the differential processing of happy and sad faces between the SCC and PO groups (SI Appendix, Fig. S5). The five ROIs specified were parts of the right amygdala, right striatum, right orbitofrontal cortex, and right and left insula; the corresponding data are presented in Figs. 5 and 6 and SI Appendix, Fig. S6.
Fig. 5.
Neural processing differences in emotional discrimination in GC-sensitive brain regions between the SCC and SCP groups. The coronal view of MNI152 standard space (spatial resolution of 0.5 mm) shows these brain regions in yellow-red, in which BOLD signal changes in response to happy and sad faces differ notably between the two treatment groups. Three ROIs are identified in this view (RA, RI, and RS). For each ROI, the mean value of the corresponding contrast of parameter estimate (COPE; happy vs. baseline and sad vs. baseline) and its mean across-subject effect estimate (%BOLD signal change vs. baseline) are presented. A measure of the effect size of each mode of cortisol dynamics on the differential processing between happy and sad faces is also shown (pie charts depicting the extent of the involvement of the corresponding brain region in the differential processing). In the SCP group, the right amygdala (“R. Amygdala” graph) is activated significantly more strongly when subjects encounter sad faces compared with happy faces (P < 0.01), and this activation intensity in response to viewing sad faces is notably higher compared with that in subjects in the SCC group (P < 0.001). Similarly, the right striatum (“R. Striatum” graph) is also activated significantly more strongly in response to viewing sad faces in the SCP group compared with the SCC group (P < 0.001). Moreover, in the SCC group, this brain structure is more responsive to happy faces compared with sad faces (P < 0.05). The same applies for the right insula (“R. Insula” graph; P < 0.01). The results reported here are not confounded by nonspecific differences in neural reactivity, neural coupling, or resting perfusion in these brain regions. The black lines represent mean ± SD. More details on the cluster-corrected results of the whole-brain analysis are provided in SI Appendix, Fig. S4. RA, right amygdala; RI, right insula; RS, right striatum. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 6.
Neural processing differences on emotional discrimination in GC-sensitive brain regions between the SCC and PO groups. The coronal view of MNI152 standard space (spatial resolution of 0.5 mm) shows these brain regions in yellow-red, in which BOLD signal changes in response to happy and sad faces differ notably between the two treatment groups. Three ROIs are identified in this view (LI, RI, and RS). A forth ROI (right orbitofrontal cortex), not shown in the previous coronal view, is illustrated independently in another, smaller coronal view of the standard space (in red) next to the corresponding graph. For each ROI, the mean value of the corresponding COPE (happy vs. baseline and sad vs. baseline) and its mean across-subject effect estimate (%BOLD signal change vs. baseline) are presented. A measure of the effect size of each mode of cortisol dynamics on the differential processing between happy and sad faces is also shown (pie charts depicting the extent of the involvement of the corresponding brain region in the differential processing). All ROIs show an almost opposite response to happy and sad faces between the two treatment groups. In the SCC group (similar to what is illustrated in Fig. 5), happy face presentation provokes a much stronger activation compared with sad face presentation, which in some cases leaves the ROI irresponsive or even deactivates it. In contrast, in the PO group, sad face presentation leads to higher activations compared with happy face presentation, which in some cases leaves the ROI irresponsive or even deactivates it. The results reported here are not confounded by nonspecific differences in neural reactivity, neural coupling, or resting perfusion in these brain regions. Graph bars represent mean ± SD. More details about the cluster-corrected results of the whole-brain analysis are provided in SI Appendix, Fig. S5. L, left; LI, left insula; R, right; RI, right insula; RS, right striatum. **P < 0.01; ***P < 0.001.
During our control task, visual stimulation (i.e., flashing checkerboard) whole-brain analysis did not reveal any notable BOLD signal variations between the treatment groups in areas responsible for visual processing (occipital and temporal lobes) or any of the ROIs predefined for this study (SI Appendix, Fig. S7). Thus, the variations detected in those five ROIs in the IFEPT fMRI experiment should reflect differences in the processing of facial traits and emotion rather than nonspecific differences due to neural reactivity or neural coupling.
At another level, resting perfusion has been shown to have an inverse relationship with the BOLD responses and thus is an important confounding factor in fMRI studies (64). Our arterial spin labeling (ASL) data showed comparable regional resting perfusion (pairwise comparisons presented in SI Appendix, Fig. S6 and Table S8) across the five GC-sensitive ROIs responding differently to emotional faces in the IFEPT fMRI experiment, further strengthening the concept that these variations reflect existing differences in the underlying neural processing of facial traits and emotion.
Different GC Rhythms Change the Functional Connectivity of GC-Sensitive Brain Regions and Their Functional Roles in Processing of Emotional Input.
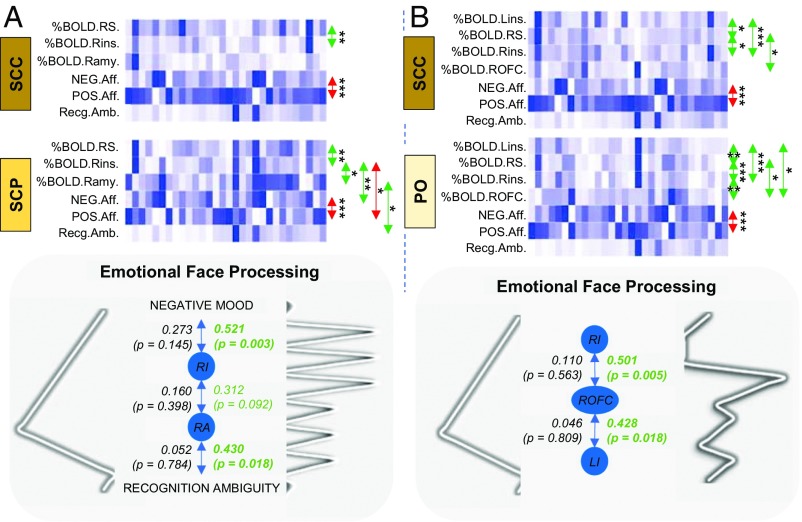
Pursuant to our fMRI data assessing the neural processing of happy and sad faces, and our behavioral data from FERT on the recognition of happy and sad faces, we investigated whether there is a functional link between these GC rhythm-sensitive neural and psychological processes, and how different GC rhythms might alter that link. We conducted a multiple post hoc correlation analysis between the absolute values of the effect estimates (absolute %BOLD signal changes) of the brain regions, which were differentially responsive to viewing happy and sad faces across the treatment groups, an index of ambiguity in recognizing emotion corresponding to happy and sad faces, derived from the FERT data, and the EMA data illustrating the positive and negative affective state of individuals presented earlier (Fig. 7).
Fig. 7.
Correlation analysis between the absolute values of the effect estimates of the brain regions, which were differentially responsive to viewing happy and sad faces across the treatment groups, an index of ambiguity in recognizing emotion corresponding to happy and sad faces, and the positive and negative affective states of individuals. The values of each outcome measure are illustrated on a white to dark-blue scale (with white indicating the lowest value and dark-blue indicating the highest value). Spearman’s rank-order correlation test was used; positive correlations (between the corresponding datasets) are indicated with a green arrow, and negative correlations are indicated with a red arrow. The scales of the negative and positive effects are (as expected) strongly anticorrelated with each other. A positive functional connectivity between the right insula and the right striatum (during emotional processing) is observed. (A) The differences in the neural processing of emotional input observed in the right amygdala and the right insula of the SCP group compared with the SCC group seem to (at least partially) reflect changes in their functional role (like monitoring ambiguity in recognizing facial expressions or the affective state) during the processing of emotional input. (B) The differences in neural processing of emotional input observed in the bilateral insula and the right orbitofrontal cortex of the PO group compared with the SCC group seem to (at least partially) reflect changes in their in-between functional connectivity during the processing of emotional input. %BOLD, absolute %BOLD signal change (compared with baseline) during exposure to the corresponding emotional face (happy and sad); LI/Lins, left insula; NEG.Aff., negative affect score; POS.Aff., positive affect score; RA/Ramy, right amygdala; Recg.Amb., index of recognition ambiguity when engaging with the corresponding emotional faces (happy and sad); RI/Rins, right insula; ROFC, right orbitofrontal cortex; RS, right striatum. *P < 0.1; **P < 0.01; ***P < 0.001.
This post hoc analysis was decided in the light of the existing strong evidence about the role of the amygdala in the detection of ambiguous, emotion-relevant cues (65, 66) and the insular cortex in the integration of internal cues, like affective state (67) and empathic processing (68, 69), to the cognitive evaluation of emotional input, as well as the involvement of the orbitofrontal cortex and striatum in the neural systems mediating emotional processing (70, 71).
To define the ambiguity in recognizing emotional faces, we created an index derived from the data of the FERT. We divided the number of misclassifications from or toward each valence (sad and happy faces) by the number of emotional faces of that valence correctly recognized for each mode of hydrocortisone replacement such that the higher the index, the greater the degree of ambiguity or difficulty in specifically recognizing each emotion. This index was chosen because of its concurrent very high correlation coefficient with both, the %accuracy scores (for correctly identifying), and the number of misclassifications involving the corresponding emotional valence (SI Appendix, Fig. S9).
In the SCC and SCP groups, only in the latter were the %BOLD signal changes of the right amygdala and right insula highly correlated with the monitoring of ambiguity in recognizing happy and sad faces [r(30) = 0.430; P = 0.018] and the affective state [r(30) = 0.521; P = 0.003], respectively. Moreover, in the SCP group, these two brain structures showed an increased in-between functional connectivity compared with the SCC group (Fig. 7A). Furthermore, subjects on the oral replacement showed an increased functional connectivity between the right orbitofrontal cortex with bilateral insulae [rPO(30) = 0.501; P = 0.005 for the right insula and rPO(30) = 0.428; P = 0.018, for the left insula] during the neural processing of the happy and sad faces, not present in the SCC group (Fig. 7B).
Relationship of Sleep Quality with Outcomes of the Study.
Since cortisol impacts sleep physiology, and the latter influences emotional and memory processing in man (72–75), we plotted our LSEQ-derived sleep evaluations against the rest of our data (SI Appendix, Fig. S6 and Table S10). Quality of sleep had a moderate positive correlation with the %BOLD signal changes in the pulsatile and continuous infusion groups reported in the right insula in the context of discriminating between fearful and sad faces [r(30) = 0.344; P = 0.063, accounting for the 11.8% of the statistical variance of these BOLD signal responses], and a moderate negative correlation with the number of misclassifications originating from negatively valenced faces [r(45) = −0.366; P = 0.014, accounting for the 13.4% of the statistical variance on the number of misclassifications]. In all other cases, no significant correlations were specified.
Discussion
We have investigated the neural processing and behavioral responses related to emotional perception and cognitive performance in healthy male volunteers on three patterns of the same total dose hydrocortisone replacement therapy. The SCP group was characterized by physiological circadian and ultradian GC rhythms (SI Appendix, Fig. S11), the SCC group was characterized by a normal circadian but no ultradian GC variation, and the PO group (in which we used current optimal GC replacement recommendations) was characterized by a delayed circadian peak of cortisol, two further pulses during the day, and very low levels at awakening. It is the low quality of life, reduced activity, low motivation, and mental fatigue reported in patients on oral replacement therapy or continuous infusion therapy that motivated the present study.
The pattern of GC replacement differentially modulates processes related to working memory and sleep physiology. In particular, a lack of physiological cortisol pulsatility is associated with poorer working memory performance at times of increased cognitive demands. Moreover, the absence of ultradian rhythmicity correlates with a poorer self-perceived quality of sleep. Our data demonstrate that different patterns of plasma cortisol oscillations have a differential impact on the GC-sensitive brain regions underlying emotional processing, with distinct consequences for (i) the accuracy of recognizing emotional faces (particularly the negatively valenced ones), (ii) the direction of this perceptual bias, and (iii) the attentional bias toward or away from emotional faces. These GC rhythm-dependent changes could reflect functional modifications among corticolimbic brain regions underlying the differential recognition of negatively valenced faces as observed in the FERT, or the differential deployment of attention across perceptual levels, in the presence of emotional stimuli, as observed in the FDOT.
While the %BOLD signal change in the right amygdala of the SCP group in response to emotional faces relates to the difficulty of the individuals in correctly recognizing emotions, the same brain region becomes dissociated from monitoring emotional ambiguity in the SCC group. In this context, it is worth noting that amygdala %BOLD signal changes in the SCP group were significantly higher when subjects were viewing sad (negatively valenced) faces compared with happy (positively valenced) faces. This is in line with the FERT data, supporting the notion that the decreased accuracy (i.e., increased uncertainty) of subjects on the optimal pulsatile infusion in recognizing emotional faces is negative valence-specific. Similarly, while in the SCP group the right insula seems to integrate internal cues, like the affective state, into the process of encoding facial expressions, it becomes dissociated from that process in the SCC group. Finally, the functional connectivity between the right orbitofrontal cortex and insular cortices during emotional face presentation appears to be very strong in the PO group, which is not the case in the SCC group.
Previous studies using the FERT and FDOT for assessing neuropsychological processes involved in depression and anxiety (76) have shown similar responses of healthy subjects receiving antidepressant and anxiolytic regimens to the responses that we find in our patients on the pulsatile SCP regimen. This implies an involvement of GC rhythmicity in the psychophysiological mechanisms regulating mood and anxiety.
Previous neuroimaging experiments have provided evidence that high levels of exogenous GCs, mimicking stress-associated states, alter both the neural processing in response to emotional or cognitive stimulation and the resting state functional connectivity (7–14). These studies suggest a specific role for MRs in some of these stress-related changes in both neural processing and functional connectivity (15, 16), while MR or GC receptor antagonism impacts the amygdala-dependent processing of emotional faces (77). Our study findings now provide evidence that even in the absence of a stressful stimulus, the ultradian GC rhythm is critical in regulating neural dynamics and, consequently, behavioral and cognitive phenotypes. Future studies in patients with adrenocortical insufficiency are now needed, not only to help reduce the morbidity of current replacement regimens, but also to provide evidence from longer-term modification of replacement cortisol rhythmicity for improved brain function and a more personalized approach to GC therapeutics.
Materials and Methods
Study Design.
This randomized, double-blind, placebo-controlled crossover study of three different modes of hydrocortisone replacement in healthy subjects was registered with the United Kingdom Clinical Research Network (IRAS reference 106181, UKCRN-ID-15236; October 23, 2013). The study followed the CONSORT guidelines for randomized controlled trials (SI Appendix, Fig. S12).
Participants.
Fifteen right-handed, healthy male volunteers age 20–33 y were included in the study (SI Appendix, Table S13). The subjects had no history of neuropsychiatric disease as confirmed by clinical assessment and were excluded if they had received a diagnosis or had a family history of a psychiatric disorder. The Ethics Committee of the University of Bristol approved the study, and all participants provided informed written consent. Each volunteers passed a detailed screening session, part of which was the acquisition of a high-resolution anatomic MRI scan.
Pharmacologic Interventions.
The cortisol biosynthesis blocking agent (metyrapone) was taken orally in all three arms of the study, together with three different methods for replacing GCs with a fixed daily dose of 20 mg of hydrocortisone: (i) s.c. via a pump, delivering normal circadian but no ultradian rhythmicity (SCP group), (ii) normal circadian and ultradian rhythmicity (SCC group), or (iii) per os, three times daily (after waking up, during lunch and dinner) (PO group) (60).
Randomization and Blinding Procedures.
Block randomization schedules were generated by staff members not directly involved in data collection for this study. Each subject was randomly assigned to one of the six possible orders of treatment, with the limitation that that the difference in the final number of subjects between any order of treatments should not exceed 1, when the total number of participants reaches 15. Dispensing and processing of all medication/placebo was managed by a third party (Bristol Royal Infirmary University Hospital Pharmacy). In all treatment arms, subjects were required to take the same daily regimen of tablets and remain connected to an s.c. pump. Anonymized data from all outcome measures were stored in the University of Bristol central servers, were cleaned at an individual level without knowledge of which session corresponds to which subject, and, consequently, further postprocessed and compared statistically at a group level, without knowledge of which group corresponds to which mode of hydrocortisone substitution.
EMA.
Throughout each 5-d treatment period, subjects had to reply (via a provided android phone) to a fixed set of nine questions about self-perceived reactivity and feelings of well-being at multiple time points during each day (SI Appendix, Fig. S14). A total of 1,018 assessments were collected. Principal component analysis was used to reduce the nine items of the questionnaire and identified two factors: positive and negative affect.
Neuroimaging Study.
Prior to the fMRI experiments, shimming along the longitudinal axis of the main magnetic field was performed to reduce the impact of geometric distortions and improve signal acquisition from the areas of the orbitofrontal cortices. Moreover, field maps were acquired and used during the preprocessing stage of the functional image analysis, improving the coregistration of the functional images to the corresponding high-resolution anatomic images (SI Appendix, Table S15). This study follows the guidelines for reporting fMRI data suggested by Poldrack et al. (78). The ROIs for the fMRI studies have been defined a priori (60). Functional and perfusion neuroimaging data analyses were carried out using FMRIB software library 5.0 (79) and Statistical Parametric Mapping version 8, and involved a number of preprocessing and postprocessing steps (SI Appendix, Figs. S16 and S17) (80–83).
LSEQ.
Ten self-rating questions examined four domains of sleeping behavior: (i) getting to sleep, (ii) quality of sleep, (iii) awakening from sleep, and (iv) behavior following waking. Rating was done on a 100-mm VAS, with a higher score indicating poorer sleep. The score for each of the four domains (per study arm and participant) was derived from the average of the scores for the corresponding questions.
FERT.
Two hundred and fifty images of artificially created human faces were displayed (one at a time) on the computer screen for 500 ms and each time replaced by a blank screen until the subject responded. The facial characteristics of each image were developed based on the Pictures of Affect Series (84) but morphed between each prototype emotion and neutral in such a way as to produce images depicting the full emotion (100%) and images presenting the emotion with gradually increasing degrees of ambiguity. For each emotion, 40 images were displayed (equally weighted for other nonemotional features, such as sex, skin color, eye color, etc.). In addition, 10 faces with a neutral expression were included. Images were presented in a random order. More details on the dependent variables constructed are provided in SI Appendix, Fig. S18.
FDOT.
One hundred and ninety-two pairs of images depicting facial expressions were displayed on the screen (one pair at a time) for 100 ms. The images used in this task were taken from the JACFEE/JACNeuF sets of facial expressions (85). Each pair of faces comprised one emotional expression and one neutral expression of the same individual (in 128 of the trials) or two neutral expressions of the same individual (in 64 of the trials). One-half of the emotional faces were fearful, and the other half were happy. In the fearful–neutral and happy–neutral face trials, the emotional faces appeared above and below the central fixation position with equal frequency. The task design involved congruent trials (dots replace an emotional face) and incongruent trials (dots replace a neutral face while an emotional face is present). Incorrect trials were excluded from the data analysis. Attentional vigilance scores were calculated for each participant by subtracting the mean reaction time of congruent trials from incongruent trials.
N-Back.
This test comprised 16 sessions of letter presentation (one letter at a time during each session) and four levels of difficulty (types of n-back). Subjects were asked to make a response to the letters seen based on the type of n-back. In the zero-back type, the subjects were instructed to press SAME every time the predefined target letter (X) appeared on the screen, and DIFFERENT for all other letters. In the one-, two-, and three-back types, subjects needed to pay attention to the letters as they changed and press SAME if the letter on the screen was the same as the letter one trial, two trials, or three trials back, respectively. If it was not the same, then they needed to press DIFFERENT. The 16 n-back sessions were displayed in a pseudorandom order (four of each kind), and each contained 10 letters, each of which was presented for 500 ms. The interstimulus interval was 1,517 ms. Performance in all types of n-back sessions was measured as %accuracy ([1 − [(no. of wrong + no. of nonresponses)/total no. of trials]] × 100) and response time (across correct trials) for each of the task conditions. The accuracy of responses in the cognitively very-low-demanding zero-back and one-back sessions were used to assess the presence of any systematic bias in subjects’ performance, while the accuracy of responses and reaction times in the cognitively high-demanding two-back and three-back sessions were used as indicators of the working memory performance, according to latest psychometric theoretical schemes and practices (86).
Statistical Analysis of the Behavioral Data.
Statistical analysis was performed using SPSS version 23, and corresponding graphs were created in GraphPad Prism version 5.03. The influence of the different cortisol rhythms on subjects’ responses as assessed by the EMA, LSEQ, FERT, FDOT and n-back was evaluated with repeated-measures mixed-model ANOVA, either three-way (FDOT), two-way (FERT, n-back), or one-way (EMA, LSEQ), depending on the psychological test. In all cases, one of the within-subject factors was the treatment group (three levels: SCC, SCP, and PO). In the case of FDOT, one-sample t tests were also used to compare attentional bias scores to zero within each group to clarify where an absolute bias was present. Tests for detecting outliers, normality in the distribution of data (Shapiro–Wilk test), and sphericity have been used and taken into consideration for the data analysis. Two genuinely unusual values were detected in the FERT dataset referring to reaction time; in this case, repeated-measures ANOVA was performed with and without the outliers, and in both cases no significant differences were found between the treatment groups. The datasets derived from the FERT and the negative affect scores were not normally distributed. Although the assumption of sphericity (Mauchly’s test) was not violated in any case, we used Greenhouse–Geisser correction regardless. Two-tailed tests were performed for all analyses, and P was set to 0.05. All results shown in the corresponding table are mean ± SD. CIs refer to ±2 SD. As a measure of the effect size, ω2 was used for the ANOVA, and Cohen’s d was used in the Student’s t test. Pairwise comparisons with Bonferroni adjustment were performed to investigate any (simple) main effects of treatment across the three study groups. Pearson’s product-moment and Spearman’s rank-order correlation were used for correlation analyses (SI Appendix, Tables S10 and S19).
All study-related procedures not directly available in the main text are provided in SI Appendix. Additional information is available in Kalafatakis et al. (60).
Supplementary Material
Acknowledgments
We thank the clinicians from the Pharmacy of the Bristol Royal Infirmary for their crucial contribution in the randomization process of the study and for realizing its double-blind, placebo-controlled nature. We also thank all the scientists at the Henry Wellcome Laboratories for Integrative Neuroscience and Endocrinology and the Clinical Research and Imaging Centre of the University of Bristol for their support and assistance in completing this work. We especially appreciate the assistance of Dr. Kristin Schmidt (University of Oxford) in optimizing the technicalities of the neurobehavioral investigation. Funding for this work was provided by the Medical Research Council (DCS Grant MR/J0125481/1) and the Wellcome Trust (Grant 089647/Z/09/Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714239115/-/DCSupplemental.
References
- 1.Kalafatakis K, Russell G-M, Zarros A, Lightman S-L. Temporal control of glucocorticoid neurodynamics and its relevance for brain homeostasis, neuropathology and glucocorticoid-based therapeutics. Neurosci Biobehav Rev. 2016;61:12–25. doi: 10.1016/j.neubiorev.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B-S, De Kloet E-R, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 3.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee B, et al. Brain-specific homeobox factor as a target selector for glucocorticoid receptor in energy balance. Mol Cell Biol. 2013;33:2650–2658. doi: 10.1128/MCB.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lussier A-L, Romay-Tallón R, Caruncho H-J, Kalynchuk L-E. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Joëls M, Baram T-Z. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Symonds C-S, McKie S, Elliott R, William Deakin J-F, Anderson I-M. Detection of the acute effects of hydrocortisone in the hippocampus using pharmacological fMRI. Eur Neuropsychopharmacol. 2012;22:867–874. doi: 10.1016/j.euroneuro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Henckens M-J, van Wingen G-A, Joëls M, Fernández G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc Natl Acad Sci USA. 2011;108:5801–5806. doi: 10.1073/pnas.1019128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veer I-M, et al. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Vaisvaser S, et al. Neural traces of stress: Cortisol-related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henckens M-J, van Wingen G-A, Joëls M, Fernández G. Corticosteroid-induced decoupling of the amygdala in men. Cereb Cortex. 2012;22:2336–2345. doi: 10.1093/cercor/bhr313. [DOI] [PubMed] [Google Scholar]
- 12.Henckens M-J, van Wingen G-A, Joëls M, Fernández G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henckens M-J, et al. Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Hum Brain Mapp. 2012;33:2885–2897. doi: 10.1002/hbm.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henckens M-J, van Wingen G-A, Joëls M, Fernández G. Time-dependent effects of cortisol on selective attention and emotional interference: A functional MRI study. Front Integr Nuerosci. 2012;6:66. doi: 10.3389/fnint.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel S, et al. Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology. 2015;40:947–956. doi: 10.1038/npp.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel S, et al. A stress-induced shift from trace to delay conditioning depends on the mineralocorticoid receptor. Biol Psychiatry. 2015;78:830–839. doi: 10.1016/j.biopsych.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Liston C, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liston C, Gan W-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell G-M, Kalafatakis K, Lightman S-L. The importance of biological oscillators for hypothalamic-pituitary-adrenal activity and tissue glucocorticoid response: Coordinating stress and neurobehavioural adaptation. J Neuroendocrinol. 2015;27:378–388. doi: 10.1111/jne.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiga F, et al. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology. 2011;152:1448–1457. doi: 10.1210/en.2010-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulkerson W-J, Tang B-Y. Ultradian and circadian rhythms in the plasma concentration of cortisol in sheep. J Endocrinol. 1979;81:135–141. doi: 10.1677/joe.0.0810135. [DOI] [PubMed] [Google Scholar]
- 22.Cudd T-A, et al. Ontogeny and ultradian rhythms of adrenocorticotropin and cortisol in the late-gestation fetal horse. J Endocrinol. 1995;144:271–283. doi: 10.1677/joe.0.1440271. [DOI] [PubMed] [Google Scholar]
- 23.Ingram J-R, Crockford J-N, Matthews L-R. Ultradian, circadian and seasonal rhythms in cortisol secretion and adrenal responsiveness to ACTH and yarding in unrestrained red deer (Cervus elaphus) stags. J Endocrinol. 1999;162:289–300. doi: 10.1677/joe.0.1620289. [DOI] [PubMed] [Google Scholar]
- 24.Fulkerson W-J, Sawyer G-J, Gow C-B. Investigations of ultradian and circadian rhythms in the concentration of cortisol and prolactin in the plasma of dairy cattle. Aust J Biol Sci. 1980;33:557–561. doi: 10.1071/bi9800557. [DOI] [PubMed] [Google Scholar]
- 25.Lefcourt A-M, Bitman J, Kahl S, Wood D-L. Circadian and ultradian rhythms of peripheral cortisol concentrations in lactating dairy cows. J Dairy Sci. 1993;76:2607–2612. doi: 10.3168/jds.S0022-0302(93)77595-5. [DOI] [PubMed] [Google Scholar]
- 26.Sarnyai Z, et al. The concordance of pulsatile ultradian release of adrenocorticotropin and cortisol in male rhesus monkeys. J Clin Endocrinol Metab. 1995;80:54–59. doi: 10.1210/jcem.80.1.7829639. [DOI] [PubMed] [Google Scholar]
- 27.Holaday J-W, Martinez H-M, Natelson B-H. Synchronized ultradian cortisol rhythms in monkeys: Persistence during corticotropin infusion. Science. 1977;198:56–58. doi: 10.1126/science.197603. [DOI] [PubMed] [Google Scholar]
- 28.Follenius M, Simon C, Brandenberger G, Lenzi P. Ultradian plasma corticotropin and cortisol rhythms: Time-series analyses. J Endocrinol Invest. 1987;10:261–266. doi: 10.1007/BF03348128. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann A, Veldhuis J-D, Deuschle M, Standhardt H, Heuser I. Twenty-four-hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 30.Pasquali R, et al. Pulsatile secretion of ACTH and cortisol in premenopausal women: Effect of obesity and body fat distribution. Clin Endocrinol (Oxf) 1998;48:603–612. doi: 10.1046/j.1365-2265.1998.00458.x. [DOI] [PubMed] [Google Scholar]
- 31.Young E-A, Carlson N-E, Brown M-B. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 32.Gavrila A, et al. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 33.Crofford L-J, et al. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Henley D-E, et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: The effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2009;94:4234–4242. doi: 10.1210/jc.2009-1174. [DOI] [PubMed] [Google Scholar]
- 35.Russell G-M, et al. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci. 2010;30:6106–6115. doi: 10.1523/JNEUROSCI.5332-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lightman S, Terry J-R. The importance of dynamic signalling for endocrine regulation and drug development: Relevance for glucocorticoid hormones. Lancet Diabetes Endocrinol. 2014;2:593–599. doi: 10.1016/S2213-8587(13)70182-7. [DOI] [PubMed] [Google Scholar]
- 37.Gibbison B, et al. Dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit Care Med. 2015;43:791–800. doi: 10.1097/CCM.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Aslakson E, Gurbaxani B-M, Vernon S-D. Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor Biol Med Model. 2007;4:8. doi: 10.1186/1742-4682-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker J-J, Terry J-R, Lightman S-L. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277:1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rankin J, Walker J-J, Windle R, Lightman S-L, Terry J-R. Characterizing dynamic interactions between ultradian glucocorticoid rhythmicity and acute stress using the phase-response curve. PLoS One. 2012;7:e30978. doi: 10.1371/journal.pone.0030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker J-J, et al. The origin of glucocorticoid hormone oscillations. PLoS Biol. 2012;10:e1001341. doi: 10.1371/journal.pbio.1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleicken B, et al. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2010;72:297–304. doi: 10.1111/j.1365-2265.2009.03596.x. [DOI] [PubMed] [Google Scholar]
- 43.Tiemensma J, et al. Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: Cross-sectional study and review of the literature. Eur J Endocrinol. 2014;171:171–182. doi: 10.1530/EJE-14-0023. [DOI] [PubMed] [Google Scholar]
- 44.Løvås K, Loge J-H, Husebye E-S. Subjective health status in Norwegian patients with Addison’s disease. Clin Endocrinol (Oxf) 2002;56:581–588. doi: 10.1046/j.1365-2265.2002.01466.x. [DOI] [PubMed] [Google Scholar]
- 45.Hahner S, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92:3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- 46.Erichsen M-M, et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: Observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94:4882–4890. doi: 10.1210/jc.2009-1368. [DOI] [PubMed] [Google Scholar]
- 47.De Bucy C, Guignat L, Niati T, Bertherat J, Coste J. Health-related quality of life of patients with hypothalamic-pituitary-adrenal axis dysregulations: A cohort study. Eur J Endocrinol. 2017;177:1–8. doi: 10.1530/EJE-17-0048. [DOI] [PubMed] [Google Scholar]
- 48.Filipsson H, Monson J-P, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91:3954–3961. doi: 10.1210/jc.2006-0524. [DOI] [PubMed] [Google Scholar]
- 49.Bergthorsdottir R, et al. Visceral fat and novel biomarkers of cardiovascular disease in patients with Addison’s disease: A case-control study. J Clin Endocrinol Metab. 2017;102:4264–4272. doi: 10.1210/jc.2017-01324. [DOI] [PubMed] [Google Scholar]
- 50.Løvås K, et al. Replacement of dehydroepiandrosterone in adrenal failure: No benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88:1112–1118. doi: 10.1210/jc.2002-020769. [DOI] [PubMed] [Google Scholar]
- 51.van der Valk E-S, et al. Decreased physical activity, reduced QoL and presence of debilitating fatigue in patients with Addison’s disease. Clin Endocrinol (Oxf) 2016;85:354–360. doi: 10.1111/cen.13059. [DOI] [PubMed] [Google Scholar]
- 52.Ricoux A, et al. [Oral glucocorticoid-induced psychiatric side effects: Focus on clinical specificities, incidence, risk factors and treatment] Rev Med Interne. 2013;34:293–302. French. doi: 10.1016/j.revmed.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Gagliardi L, et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison’s disease: A randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99:4149–4157. doi: 10.1210/jc.2014-2433. [DOI] [PubMed] [Google Scholar]
- 54.Oksnes M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison’s disease: A randomized clinical trial. J Clin Endocrinol Metab. 2014;99:1665–1674. doi: 10.1210/jc.2013-4253. [DOI] [PubMed] [Google Scholar]
- 55.Qian X, Droste S-K, Lightman S-L, Reul J-M, Linthorst A-C. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–4353. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joëls M, Karst H, DeRijk R, de Kloet E-R. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Stavreva D-A, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarabdjitsingh R-A, et al. Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proc Natl Acad Sci USA. 2014;111:14265–14270. doi: 10.1073/pnas.1411216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarabdjitsingh R-A, et al. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain region-specific manner. Endocrinology. 2010;151:5369–5379. doi: 10.1210/en.2010-0832. [DOI] [PubMed] [Google Scholar]
- 60.Kalafatakis K, et al. Effects of the pattern of glucocorticoid replacement on neural processing, emotional reactivity and well-being in healthy male individuals: Study protocol for a randomised controlled trial. Trials. 2016;17:44. doi: 10.1186/s13063-016-1159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harmer C-J, et al. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- 62.Pringle A, Browning M, Cowen P-J, Harmer C-J. A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1586–1592. doi: 10.1016/j.pnpbp.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Dehaene S, Changeux J-P, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Brown G-G, et al. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: Differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- 65.Spunt R-P, Adolphs R. The neuroscience of understanding the emotions of others. Neurosci Lett. 2017 doi: 10.1016/j.neulet.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer C-F. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 67.Craig A-D. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 68.Hennenlotter A, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 69.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 70.Bechara A, Damasio H, Damasio A-R. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 71.Hare T-A, Tottenham N, Davidson M-C, Glover G-H, Casey B-J. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 72.Born J, Späth-Schwalbe E, Schwakenhofer H, Kern W, Fehm H-L. Influences of corticotropin-releasing hormone, adrenocorticotropin, and cortisol on sleep in normal man. J Clin Endocrinol Metab. 1989;68:904–911. doi: 10.1210/jcem-68-5-904. [DOI] [PubMed] [Google Scholar]
- 73.Vandekerckhove M, Cluydts R. The emotional brain and sleep: An intimate relationship. Sleep Med Rev. 2010;14:219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Deliens G, Gilson M, Peigneux P. Sleep and the processing of emotions. Exp Brain Res. 2014;232:1403–1414. doi: 10.1007/s00221-014-3832-1. [DOI] [PubMed] [Google Scholar]
- 75.Payne J-D, Nadel L. Sleep, dreams, and memory consolidation: The role of the stress hormone cortisol. Learn Mem. 2004;11:671–678. doi: 10.1101/lm.77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harmer C-J, Duman R-S, Cowen P-J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young K-D, et al. The effect of mineralocorticoid and glucocorticoid receptor antagonism on autobiographical memory recall and amygdala response to implicit emotional stimuli. Int J Neuropsychopharmacol. 2016;19:pyw036. doi: 10.1093/ijnp/pyw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poldrack R-A, et al. Guidelines for reporting an fMRI study. Neuroimage. 2008;40:409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jenkinson M, Beckmann C-F, Behrens T-E, Woolrich M-W, Smith S-M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Chen G, Taylor P-A, Cox R-W. Is the statistic value all we should care about in neuroimaging? Neuroimage. 2017;147:952–959. doi: 10.1016/j.neuroimage.2016.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okell T-W, Chappell M-A, Kelly M-E, Jezzard P. Cerebral blood flow quantification using vessel-encoded arterial spin labeling. J Cereb Blood Flow Metab. 2013;33:1716–1724. doi: 10.1038/jcbfm.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herscovitch P, Raichle M-E, Kilbourn M-R, Welch M-J. Positron emission tomographic measurement of cerebral blood flow and permeability-surface area product of water using [15O]water and [11C]butanol. J Cereb Blood Flow Metab. 1987;7:527–542. doi: 10.1038/jcbfm.1987.102. [DOI] [PubMed] [Google Scholar]
- 83.Parkes L-M, Tofts P-S. Improved accuracy of human cerebral blood perfusion measurements using arterial spin labeling: Accounting for capillary water permeability. Magn Reson Med. 2002;48:27–41. doi: 10.1002/mrm.10180. [DOI] [PubMed] [Google Scholar]
- 84.Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- 85.Matsumoto D, Ekman P. Japanese and Caucasian Facial Expressions of Emotion (JACFEE) Intercultural and Emotion Research Laboratory, Department of Psychology, San Francisco State University; San Francisco: 1988. [Google Scholar]
- 86.Jaeggi S-M, Buschkuehl M, Perrig W-J, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.