Significance
Ambrosia beetles are among the true fungus-farming insects and cultivate fungal gardens on which the larvae and adults feed. After invading new habitats, some species destructively attack living or weakened trees growing in managed and unmanaged settings. Ambrosia beetles adapted to weakened trees tunnel into stem tissues containing ethanol to farm their symbiotic fungi, even though ethanol is a potent antimicrobial agent that inhibits the growth of various fungi, yeasts, and bacteria. Here we demonstrate that ambrosia beetles rely on ethanol for host tree colonization because it promotes the growth of their fungal gardens while inhibiting the growth of “weedy” fungal competitors. We propose that ambrosia beetles use ethanol to optimize their food production.
Keywords: fungus-farming insects, plant–insect–microbe interactions, symbiosis, insect–fungus mutualism, host screening
Abstract
Animal–microbe mutualisms are typically maintained by vertical symbiont transmission or partner choice. A third mechanism, screening of high-quality symbionts, has been predicted in theory, but empirical examples are rare. Here we demonstrate that ambrosia beetles rely on ethanol within host trees for promoting gardens of their fungal symbiont and producing offspring. Ethanol has long been known as the main attractant for many of these fungus-farming beetles as they select host trees in which they excavate tunnels and cultivate fungal gardens. More than 300 attacks by Xylosandrus germanus and other species were triggered by baiting trees with ethanol lures, but none of the foundresses established fungal gardens or produced broods unless tree tissues contained in vivo ethanol resulting from irrigation with ethanol solutions. More X. germanus brood were also produced in a rearing substrate containing ethanol. These benefits are a result of increased food supply via the positive effects of ethanol on food-fungus biomass. Selected Ambrosiella and Raffaelea fungal isolates from ethanol-responsive ambrosia beetles profited directly and indirectly by (i) a higher biomass on medium containing ethanol, (ii) strong alcohol dehydrogenase enzymatic activity, and (iii) a competitive advantage over weedy fungal garden competitors (Aspergillus, Penicillium) that are inhibited by ethanol. As ambrosia fungi both detoxify and produce ethanol, they may maintain the selectivity of their alcohol-rich habitat for their own purpose and that of other ethanol-resistant/producing microbes. This resembles biological screening of beneficial symbionts and a potentially widespread, unstudied benefit of alcohol-producing symbionts (e.g., yeasts) in other microbial symbioses.
Hosts evolve to facilitate their beneficial symbionts selectively (1), while symbionts commonly compete within the host-provided environment (2). The best-studied mechanisms by which hosts maintain their association with beneficial symbionts are partner choice and partner fidelity (i.e., vertical transmission of symbionts through generations). Competition-based screening is a third theoretical mechanism that empirically is hardly studied (3, 4). It states that hosts can maintain mutualistic associations with beneficial symbionts by creating a demanding environment that is demanding in such a way that the host-preferred symbiont is better able to endure the demands. To our knowledge, the only examples come from squid–bacteria, ant–bacteria, and ant–plant mutualisms, but screening is likely to be much more widespread (3).
Fungus farming as a source of nutrition arose in ants, termites, and beetles between 40‒100 My before humans began domesticating plants for agriculture (5). Members of these three insect lineages are true fungus farmers that propagate, cultivate, and sustainably harvest their fungal gardens (6). This lifestyle evolved only once in ants and termites but originated more than 10 times in the bark beetles (Scolytinae) and once in the pinhole borers (Platypodinae) (Coleoptera: Curculionidae) (5–8). About 3,400 scolytine and 1,400 platypodine species are collectively known as “ambrosia beetles” for their obligate mutualism with nutritional fungal symbionts (9). Notably, larvae and adult ambrosia beetles obtain all their nutrition solely by consuming their symbiont(s), which is necessary to properly develop and reproduce (10–13).
Ambrosia beetles vertically transmit their fungal symbionts to host trees using specialized structures called “mycangia” that range from simple pits and grooves to comparatively large and complex pouches (9, 14, 15). Fungal symbionts carried within the mycangia are mostly of the ascomycete genera Ambrosiella and Raffaelea and have not been found as free-living species, but their ancestors were free-living and relied on arthropods for dispersal (16, 17). A tight coevolutionary pattern and specific beetle–fungal associations have been documented, particularly for beetle genera with large, elaborate mycangia (16–19).
As with other ambrosia beetles in the tribe Xyleborini (Scolytinae), male Xylosandrus germanus are flightless, and host selection is made by female foundresses (20). Adult females tunnel within the sapwood of trees, where they create galleries to farm their symbionts and rear offspring. Conceivably, this environment is created in a way that selectively facilitates vertically transmitted food fungi over parasitic and pathogenic fungi. Choice of a selective substrate for growing the fungus gardens may be a potent mechanism to do so. An initial competitive advantage to the food cultivars is given by the inoculation of host tunnels with masses of spores overflowing from the mycangium or present in the feces of the founding female (15). A white fungal layer of Ambrosiella or Raffaelea conidia or conidiophores is produced only in the beetles’ presence (10–12). During initial excavation, host tunnels can become inoculated not only with the fungal symbionts but also with a variety of microbial competitors that can hitchhike on the integument of the foundresses. These “weedy” microbial competitors and pathogens include fungi (e.g., Aspergillus, Paecilomyces, and Penicillium) and bacteria (11, 12, 21, 22). If unmanaged, these microbes can disrupt the establishment of the gardens of ambrosia beetles and other fungus-farming insects (23) and eventually dominate the gallery system (24, 25). It is therefore important for foundresses to ensure that their fungal cultivars become established within freshly excavated tunnels promptly and that they keep dominating other weedy microorganisms (24, 25). Furthermore, ambrosia beetles begin ovipositing only after their fungal gardens are established and flourishing (10–12, 24); otherwise the foundress will die or abandon the tunnel, and colonization will be unsuccessful (26).
Most species of ambrosia beetles attack dying or dead trees, but some exotic species introduced to new habitats destructively attack living trees growing in managed and unmanaged systems (27–30). Ethanol has long been known to attract many ambrosia beetles (31), and its emission from living but weakened trees in the early stages of physiological stress is used by some of the more aggressive beetle species to locate new hosts for establishing fungal gardens and producing offspring (26, 27). A byproduct of anaerobic respiration, ethanol is a ubiquitous component of the sapwood, phloem, and cambium of many living tree species (32, 33). Ethanol is present in some tissues of healthy trees, but in weakened trees it increases dramatically due to limited oxygen availability resulting from a variety of physiological stressors (32–34). When given free choice, X. germanus preferentially attacked flood-intolerant tree species with elevated ethanol levels over flood-tolerant tree species containing little to no ethanol (26). When confined without choice to stem tissues, X. germanus excavated galleries, established fungal gardens, and produced offspring in flood-stressed trees but created only superficial tunnels absent of fungal growth and offspring in nonflooded trees (26). A strong preference for host tissues containing ethanol was decisively demonstrated by Kelsey et al. (35): More than four times more ambrosia beetle attacks occurred above ethanol-infused sapwood tissues than in the opposite side of the same log that did not receive ethanol treatment.
While ethanol is highly attractive to many ambrosia beetles, it is also a well-known antimicrobial agent that has been used as a preservative by humans since prehistory (36). Ethanol reduces the postharvest decay of fruits and extends the shelf-life of food products by inhibiting various fungi, including Aspergillus spp. and Penicillium spp. (37). Ambrosia beetles’ specific selection of host tissues containing a potent antimicrobial agent to cultivate their fungal gardens would therefore seem counterproductive. Here we present evidence that X. germanus, and probably many other ambrosia beetles, rely on the ethanol within tissues of living trees to optimize the production of their fungal gardens and therefore successful offspring production. We propose that this is a measure to achieve improved growth and to create a competitive advantage for Ambrosiella and Raffaelea fungus cultivars.
Results
Ethanol and Host Tree Colonization Success.
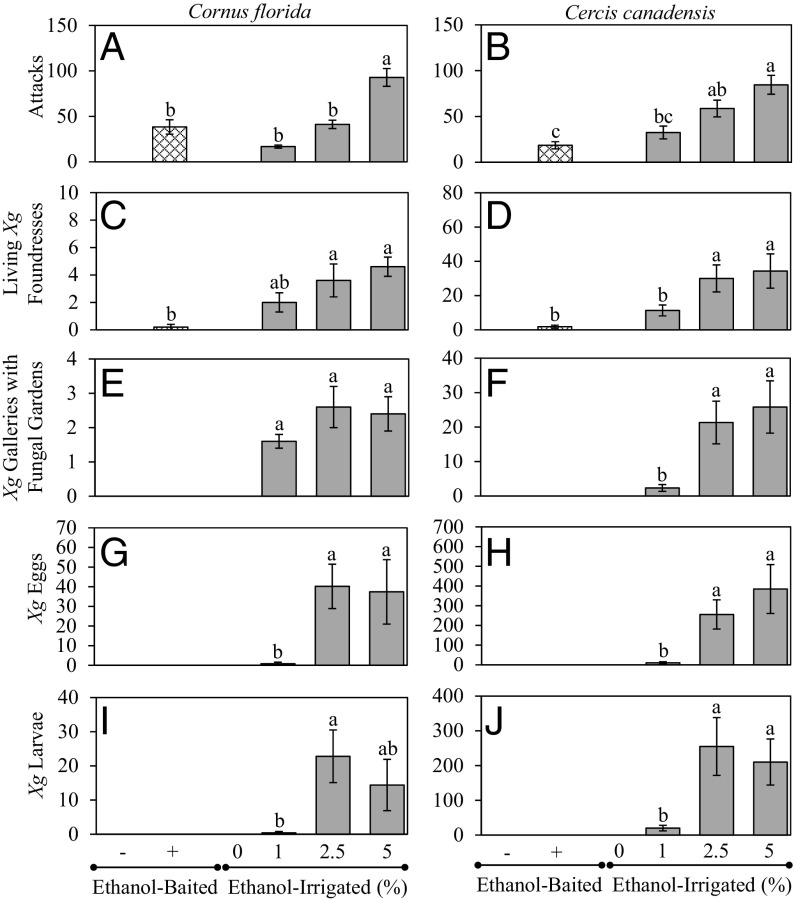
Field-based experiments compared the absence or presence of ethanol within stems of dogwood (Cornus florida) and redbud (Cercis canadensis) trees with the colonization success of X. germanus. Ethanol sachet lures attracted beetles and induced attacks on stems, but X. germanus failed to establish fungal gardens or produce broods in the absence of in vivo ethanol. Irrigating the roots of trees with ethanol solutions [0%, 1%, 2.5%, or 5% (vol/vol)] also attracted beetles and induced attacks on stems, and X. germanus foundresses established fungal gardens and produced brood in the presence of in vivo ethanol within these tissues.
Dogwood trees baited with ethanol lures sustained a total of 192 attacks, while trees irrigated with 1%, 2.5%, or 5% ethanol solutions sustained 84, 206, and 464 total attacks, respectively (Fig. 1A). There was no difference in cumulative attacks on ethanol-baited dogwoods compared with trees irrigated with 1% or 2.5% ethanol solutions (Fig. 1A). Similarly, redbud trees baited with ethanol lures sustained a total of 111 attacks, while trees irrigated with 1%, 2.5%, or 5% ethanol solutions sustained 195, 352, and 507 total attacks, respectively (Fig. 1B). There was no difference in cumulative attacks on ethanol-baited redbud trees compared with trees irrigated with 1% ethanol (Fig. 1B).
Fig. 1.
(A and B) Cumulative ambrosia beetle attacks per tree on C. florida (A) and C. canadensis (B) trees that were ethanol-baited or irrigated with ethanol solutions containing 0%, 1%, 2.5%, or 5% ethanol (vol/vol). (C–J) Trees were dissected to determine the mean number per tree of X. germanus living foundresses (C and D), tunnels containing living X. germanus foundresses and fungal gardens (E and F), X. germanus eggs (G and H), and X. germanus larvae (I and J). Different letters denote significant differences in mean values using one-way ANOVA and Tukey’s HSD at P < 0.05 (n = 5 and 6 trees per treatment for C. florida and C. canadensis, respectively). (A) F19 = 22.65; P < 0.0001. (B) F23 = 13.53; P < 0.0001. (C) F19 = 4.11; P = 0.024. (D) F23 = 8.88; P = 0.0006. (E) F14 = 1.14; P = 0.35. (F) F17 = 6.17; P = 0.011. (G) F14 = 7.03; P = 0.01. (H) F17 = 8.10; P = 0.0041. (I) F14 = 4.75; P = 0.03. (J) F17 = 6.66; P = 0.009. Error bars represent ± SE.
Following dissection of the stems, a comparable number of living X. germanus foundresses were recovered from ethanol-baited dogwoods and from trees irrigated with 1% ethanol (Fig. 1C). A comparable number of X. germanus foundresses were also recovered from ethanol-baited redbuds and from 1% ethanol-irrigated trees (Fig. 1D). However, despite 192 and 111 total ambrosia beetle attacks, respectively, no tunnels created in ethanol-baited dogwoods or redbuds contained living X. germanus foundresses with established fungal gardens. In contrast, tunnels with foundresses and fungal gardens occurred in dogwoods (Fig. 1E) and redbuds (Fig. 1F) irrigated with 1%, 2.5%, or 5% ethanol. No tunnels containing an X. germanus foundress and oviposited eggs or larvae occurred in ethanol-baited dogwoods or redbuds (Fig. 1 G–J). In contrast, tunnels/galleries containing an X. germanus foundress and eggs and larvae were found in dogwood and redbud trees irrigated with 1%, 2.5%, or 5% ethanol (Fig. 1 G–J). No species of Scolytinae established fungal gardens or produced brood in the ethanol-baited dogwoods or redbuds.
Ethanol and Ambrosia Beetle Offspring Production.
Bioassays using an artificial rearing substrate infused with 0%, 0.1%, 0.5%, 1%, 2.5%, or 5% (vol/vol) ethanol characterized the effects of ethanol on fungal garden establishment and offspring production by X. germanus. The presence of X. germanus’ greyish-white ambrosial gardens peaked in tubes containing 2.5% ethanol; in particular, fungus was present in 12.5% of tubes containing 0% and 0.1% ethanol, in 87.5% of tubes containing 0.5% and 1% ethanol, in 100% of tubes containing 2.5% ethanol, and in 0% of tubes containing 5% ethanol. The occurrence of larvae and pupae within the artificial rearing substrate were observed only in the presence of X. germanus’ greyish-white fungal gardens. Thus, the number of larvae and pupae per substrate tube also increased and then decreased in response to increasing amounts of ethanol from 0 to 5% (Fig. 2 A and B).
Fig. 2.
(A and B) Relationship between ethanol incorporated into a sawdust-based agar substrate at 0%, 0.1%, 0.5%, 1%, 2.5%, or 5% (vol/vol) and the presence of (A) larvae and (B) pupae of X. germanus, an ethanol-responsive ambrosia beetle. Data points represent mean values of larvae or pupae per tube as a function of ethanol percent using a standard weighting factor of 1/variance (n = 8 per ethanol concentration; see Table S1 for regression equations). (A) r2 = 0.90; F5 = 60.08; P = 0.002. (B) r2 = 0.96; F5 = 168.96; P = 0.0002). (C) White ambrosia garden of A. grosmanniae within the artificial rearing substrate.
Ethanol and Fungal Growth.
An agar plate bioassay examined the effect of ethanol on Ambrosiella grosmanniae, the fungal symbiont of the ethanol-responsive X. germanus. The dry weight of A. grosmanniae initially increased and then decreased with increasing amounts of ethanol in the medium from 0% to 5% (vol/vol) (Fig. 3 A and D and Table S1). The surface area of A. grosmanniae generally decreased (Fig. 3B) while biomass density increased (Fig. 3C) with increasing ethanol concentrations from 0% to 5%. The same procedure was applied to two other fungal symbionts associated with ethanol-responsive ambrosia beetles, namely, Ambrosiella roeperi associated with Xylosandrus crassiusculus and Raffaelea canadensis associated with Xyleborinus saxesenii. The dry weight of A. roeperi initially increased and then decreased in response to increasing ethanol concentrations (Fig. 4A and Table S1). The growth of R. canadensis also initially increased and then decreased in response to increasing amounts of ethanol (Fig. 4B and Table S1).
Fig. 3.
(A–C) Agar plate bioassays characterized the effects of ethanol incorporated into the medium at concentrations of 0%, 1%, 2.5%, or 5% (vol/vol) on the dry weight (A), surface area (B), and density (C) of A. grosmanniae, the fungal symbiont of the ethanol-responsive ambrosia beetle X. germanus. Data points represent mean growth values as a function of ethanol percentage using a standard weighting factor of 1/variance (n = 7‒10 per ethanol concentration; see Table S1 for regression equations). (A) r2 = 0.99; F4 = 22,685.60; P = 0.00004. (B) r2 = 0.99; F4 = 34,770.7; P = 0.00003. (C) r2 = 0.91; F4 = 31.68; P = 0.031. (D) Representative growth of A. grosmanniae on agar medium infused with ethanol.
Fig. 4.
(A and B) Agar plate bioassays characterized the effects of ethanol incorporated into the medium at concentrations of 0%, 1%, 2.5%, or 5% (vol/vol) on the growth of A. roeperi (A) and R. canadensis (B), fungal symbionts of the ethanol-responsive ambrosia beetles X. crassiusculus and X. saxesenii, respectively. (C–E) Growth of A. hylecoeti, the fungal symbiont of a ship-timber beetle (C) and fungal competitors of the symbionts including Penicillium sp. (D) and Aspergillus sp. (E). Data points represent mean growth values as a function of ethanol percentage using a standard weighting factor of 1/variance (n = 5 per ethanol concentration for A. roeperi and A. hylecoeti; n = 9‒10 for R. canadensis; n = 8‒10 for Aspergillus sp.; see Table S1 for regression equations). (A) r2 = 0.87; F4 = 21.81; P = 0.044. (B) r2 = 0.99; F4 = 2,773.97; P = 0.0004. (C) r2 = 0.72; F3 = 19.25; P = 0.0482. (D) r2 = 0.98; F4 = 173.71; P = 0.006. (E) r2 = 0.99; F4 = 12,658.3; P = 0.0001.
Growth was also measured for Ascoidea hylecoeti, the fungal symbiont of a ship-timber beetle (Elateroides dermestoides, Lymexylidae) that is also attracted to ethanol (38). The growth of the Ascoidea sp. steadily decreased with increasing amounts of ethanol from 0% to 5% (Fig. 4C and Table S1). A negative correlation occurred between ethanol concentration and dry weight of A. hylecoeti (Pearson correlation coefficient = −0.906; P < 0.0001).
Dry weight of a Penicillium sp., a microbial competitor of ambrosia beetle fungal cultivars isolated from galleries of X. saxesenii, rapidly decreased with increasing amounts of ethanol from 0% to 5% (Pearson correlation coefficient = −0.743; P = 0.0002) (Fig. 4D and Table S1). The growth of an Aspergillus sp. generalist pathogen, also isolated from X. saxesenii galleries, decreased and was negatively correlated (r = −0.965; P < 0.0001) with increasing amounts of ethanol from 0% to 5% (Fig. 4E and Table S1).
To further validate the observations, we measured the growth of liquid cultures in the presence or absence of ethanol and in the presence or absence of 2% glucose (Fig. S1). While increasing concentrations of ethanol generally led to prolonged lag phases, these were considerably shorter for A. grosmanniae and R. canadensis (maximum of about 20 h in the presence of 5% or 10% ethanol) than for a Penicillium competitor isolate (about 48 h) (Fig. S1). The growth rate was enhanced in most cases by the addition of 2% glucose, but this did not greatly affect the duration of the lag phase. While A. roeperi displayed extremely low overall growth rates, this symbiont grew only in the presence of ethanol in this bioassay.
Alcohol Dehydrogenase Activity.
Ethanol is generally toxic and in all organisms is detoxified to acetaldehyde by alcohol dehydrogenases (ADHs). Enzymatic ADH activity therefore determines ethanol tolerance, which was tested using a standard ADH activity assay before and after exposure to 2% ethanol on plates containing A. grosmanniae, A. roeperi, R. canadensis, an Aspergillus sp., and a Penicillium sp. (Fig. 5). Compared with the untreated control cultures, ADH activity was significantly (P < 0.05) higher in the ambrosia beetle cultivar A. grosmanniae after 6 and 93 h of exposure to ethanol (Fig. 5A) and in R. canadensis after 93 h of exposure (Fig. 5C). While Aspergillus sp. cultures showed almost no detectable ADH activity in the same conditions (Fig. 5D), activity was slightly induced in A. roeperi (Fig. 5B) and the Penicillium sp. (Fig. 5E).
Fig. 5.
(A–E) Enzymatic ADH activity of fungal symbionts (A. grosmanniae, A. roeperi, and R. canadensis) and symbiont competitors (Aspergillus sp., Penicillium sp.) immediately before (control) and after short-term (6 h) or long-term (93 h) exposure to 2% ethanol. First and third quartiles enclose the box plots with median and mean values represented by a bar and an “x” within the boxes, respectively; top and bottom whiskers represent maximum and minimum values, respectively. Different letters within a species denote significant differences between control vs. 6-h or 93-h mean values using one-way ANOVA and Dunnett’s test at P < 0.05 (n = 3−4 cultures per species and treatment). (A) F10 = 10.47; P = 0.006. (B) F9 = 1.61; P = 0.27. (C) F11 = 4.53; P = 0.044. (D) F10 = 1.45; P = 0.29. (E) F10 = 2.66; P = 0.13.
Discussion
The affinity of ambrosia beetles for host-derived ethanol has been viewed as a function of this compound acting as a chemical indicator of weakened, dying, or recently felled trees with compromised defenses that therefore are vulnerable to attack (27, 39). Based on our results, we propose that ambrosia beetles preferentially select host tissues containing ethanol because it provides a dual-functional benefit to their fungiculture: It promotes the growth of their nutritional fungal symbionts and reduces competition with fungal weeds that get suppressed. Our study shows an advantage for ambrosia beetles in selecting an ethanol-rich substrate to grow their coevolved fungal symbionts (i.e., A. grosmanniae, A. roeperi, and R. canadensis). Ethanol thereby benefits fungal crop production by ambrosia beetles and adds to other known behavioral or chemical means by which insects specifically promote their food fungi over other antagonists (40–43). Notably, attine ants apply fertilizers (44) and antibiotics (45, 46) to selectively facilitate the growth of food fungi over weeds and pathogens.
Promptly establishing fungal gardens within newly excavated host tunnels improves the chances of fungal symbionts outcompeting microbial competitors, including Aspergillus and Penicillium (24, 47), that can be passively introduced during tunnel excavation. We suggest that ethanol within host tree tissues facilitates this competitive advantage, as the growth of A. grosmanniae, A. roeperi, and R. canadensis benefitted from the presence of low concentrations of ethanol, while the Penicillium and Aspergillus competitors were inhibited. Ethanol could also provide a competitive advantage over entomopathogenic fungi since it inhibits the growth of Trichoderma harzianum (37).
Alcohol-detoxifying enzymes were strongly induced in A. grosmanniae and R. canadensis after exposure to ethanol, while ADH activity was low to absent in Penicillium sp. and Aspergillus sp. Genetic mutations allowing the comparatively fast metabolism of ethanol might permit the fungal symbionts to consume or at least rapidly detoxify ethanol present in the host tissues and thereby achieve improved growth. Bacteria (Acinetobacter spp., Pseudomonas aeruginosa) (48, 49), many yeasts (50, 51), and the fungal tree pathogen Armillaria mellea (52) have also been proposed to use ethanol as a carbon source. In addition to detoxifying ethanol, ambrosia beetle fungal symbionts are known to produce ethanol, among other alcohols (53 and 54), which may maintain an alcohol-rich gallery environment even after its production by plant cells has ceased (i.e., because of tree death). Such consumption, production, and environmental accumulation of ethanol has been reported so far from only three lineages of yeasts, in which it has been shown to be a potent tactic for securing their food resource against other microbial and possibly also arthropod competitors (50, 51, 55). Therefore, the presence of ethanol may explain why ethanol-resistant yeasts are the dominant symbionts, next to ambrosia fungi, in ambrosia beetle galleries (56, 57).
The role of screening by hosts to generate and maintain a beneficial community of symbionts in animal–microbe mutualisms is generally understudied. Currently, the clearest case of biological screening appears in Euprymna scolopes squids, which create a demanding environment in which bacterial bioluminescence protects the symbionts against the host’s lethal reactive oxygen species, thus allowing only bioluminescent Vibrio fisheri bacteria to colonize the light organ (3, 58). Fungus-farming insects select and prepare substrates for their nutritional fungi to grow on, so they should be predisposed for screening. They may choose selective substrates varying in bioactive compounds (e.g., ethanol in this study), or they (or their symbionts) may actively incorporate such compounds into the medium (4, 45, 59). This would add to mechanisms known to maintain associations with beneficial fungal cultivars by farming insects, such as partner choice through signaling (fungus-farming termites) and vertical symbiont transmission in the fungus spore-carrying organs of nest-founding individuals [ambrosia beetles and attine ants (5)].
Since our current study and previous ones (26, 28, 34) demonstrate that X. germanus does not colonize healthy trees, attacks on living but weakened trees emitting stress-induced ethanol are arguably a function of maximizing successful fungus farming. Following their introduction to new habitats, the establishment and proliferation of ambrosia beetles could benefit from the combination of a broad host range and the ability to utilize host tissues containing ethanol. Host tissue chemistry could also reduce interspecific competition by excluding non–ethanol-responsive ambrosia beetles (e.g., Xyleborus glabratus and Raffaelea lauricola) vs. opportunistic species with broad host ranges that have an affinity for ethanol and living but weakened trees (X. germanus and A. grosmanniae). Presumably this effect is not only restricted to other ambrosia beetles but is also found in other competitors (e.g., wood-boring beetles) that are not resistant to ethanol. Likewise, the tolerance toward alcohol produced by certain yeasts has been shown to determine the relative success of particular Drosophila spp. (60, 61).
The high-level food production of ambrosia beetles suggests the evolution of horticultural practices used by other insect and human farmers, including crop fertilization and chemical control of competitors and pathogens (5). Still, additional facets of ambrosia beetle fungiculture remain to be elucidated. For instance, the production of defensive compounds such as antibiotics by symbionts of farming ants and termites to help defend against weedy competitors (45, 62) has also been described for bacterial symbionts of a bark beetle (41) and a mold-like fungus of an ambrosia beetle (63). In microbes, such defense reactions are usually induced under stress (64), so it is likely that alcohol-rich environments not only screen in alcohol-producing microbes but also induce antibiotic production. Interestingly, many of the ophiostomatoid fungal mutualists of ambrosia beetles, including Ambrosiella and Raffaelea, are resistant to the fungicide cycloheximide (65, 66), which may indicate the presence of microbes that produce this antibiotic. Again, this would screen in antibiotic-producing symbionts and screen out nonresistant species.
Taken together, the experimental findings presented here reveal that the affinity of X. germanus for ethanol benefits their fungicultural lifestyle. The failure of ambrosia beetle foundresses to inoculate a host and establish a fungal garden probably represents a key weak point in the evolution of this obligate mutualism, because beetles will die or abandon the freshly excavated tunnels if inoculation is unsuccessful (26). Understanding the role of host chemistry in promoting and inhibiting the establishment of ambrosia beetle fungal symbionts could lead to novel management strategies for these devastating pests of trees in horticultural, ornamental, and forested settings.
Methods
Ethanol and Host Tree Colonization Success.
The influence of ethanol was examined by comparing attacks, presence of fungal gardens, and X. germanus offspring production in trees that were either baited or irrigated with aqueous solutions of ethanol. In particular, the experiment sought to compare colonization success in stem tissues that were in the vicinity of but lacking in vivo ethanol (i.e., ethanol-baited trees) vs. trees with tissues containing ethanol (ethanol-irrigated). Two deciduous species commonly attacked by X. germanus were selected (28): C. florida was tested during the first field experiment followed by C. canadensis. C. florida trees of stems measuring 2.54 cm in diameter and C. canadensis trees of 3.81-cm caliper were grown in 26.5-L pots containing a pine bark and peat moss mix amended with lime and micronutrients.
Ethanol-baited trees were prepared by attaching three lures (95% ethanol; 65 mg/d at 30 °C; AgBio, Inc.) using nylon cable ties to a metal rod in parallel with the main stem. Lures were attached to the metal rod rather than to the actual stems to avoid potential adsorption of ethanol by the stem tissue. Lures were positioned about 25.4 cm apart at the base, middle, and upper portions of C. florida and C. canadensis stems.
Ethanol-irrigated trees were prepared by irrigating the soil of each potted C. florida and C. canadensis tree with an aqueous solution containing 0%, 1% (i.e., 7.9 mg/mL), 2.5% (i.e., 19.7 mg/mL), or 5% (39.5 mg/mL) ethanol (vol/vol) (99.5%; Acros Organics). Each pot received an average of 2.8 L of solution every 2‒3 d throughout the duration of the two experiments. Ethanol-baited, nonbaited, and ethanol-irrigated C. florida trees were arranged in five randomized complete blocks within a deciduous woodland in Wayne County, Ohio (40°45′41.54″N; 81°51′15.92″W) and were deployed under field conditions from 24 April 2017 to 19 May 2017. C. canadensis trees were arranged in six randomized complete blocks within a deciduous woodland in Wayne County., Ohio (40°47′4.11″N; 81°50′4.82″W) and were deployed from 15 May 2017 to 12 June 2017. Trees within a block were 2 m apart, and adjacent blocks were 10 m apart. New ambrosia beetle attacks were recorded every 2‒3 d. Trees were cut on the last day of field deployment and were transferred to a refrigerator held at 5 °C. Stems were dissected using pruning shears and were examined under a stereomicroscope. Specimens of X. germanus foundresses and offspring (i.e., eggs and larvae) within their respective galleries were collected and preserved in 70% ethanol.
Ethanol and Ambrosia Beetle Offspring Production.
A rearing substrate was prepared to examine the effects of ethanol on offspring production by X. germanus. A minimal medium substrate was prepared based on refs. 67 and 68 by dry mixing 75 g of C. canadensis sawdust and 20 g of malt extract agar followed by 325 mL of distilled water. About 20 mL of substrate was dispensed into 50-mL polystyrene tubes. Substrate tubes were autoclaved for 30 min at 120 °C and were allowed to cool slightly in a laminar flow hood; then ethanol (99.5%; Acros Organics) was pipetted into the substrate to achieve ethanol concentrations of 0%, 0.1%, 0.5%, 1%, 2.5%, or 5% (vol:wt) per substrate tube. After pipetting, the substrate within each tube was immediately stirred using a flame-sterilized spatula for 1.5 min. Tubes were loosely screw-capped and held in a laminar flow hood under UV light for 4 d.
Live adult female X. germanus were collected (26), transferred to Petri dishes lined with filter paper, and held overnight at 7 °C. Active beetles were selected after the Petri dishes were held at room temperature for 3‒4 h, and four beetles were transferred to each substrate tube. Previous studies recommend surface-sterilizing beetles with ethanol to minimize contamination by secondary microbes passively carried on the cuticle (67, 69), but we intentionally omitted this sterilization procedure to avoid biasing the competition of A. grosmanniae with microbial contaminants. Substrate tubes were incubated at 25 °C for 22 d, after which specimens within each tube were quantified.
Ethanol and Fungal Growth.
Cultures of A. grosmanniae were grown and maintained on malt extract agar (MEA) [3% malt extract and 0.5% soya peptone (wt/vol)]. The effects of ethanol on the dry weight, surface area, and density of A. grosmanniae were determined using an agar plate method. Ethanol (99.5%; Pharmco-AAPER) was added to the MEA to achieve concentrations of 1%, 2.5%, or 5% (vol/vol). Sterile distilled water was used for the control. The solidified agar was overlaid with a sterile cellophane membrane (Research Products International Corp.), and a mycelial plug (3 mm in diameter) of A. grosmanniae was transferred to the center of each plate. Inoculated plates were photographed every 2 d, and the surface area was measured using ImageJ v. 1.47 software (NIH). At 6 d after inoculation, the mycelial mat was scraped, and the fresh weight was determined. The mycelia were then allowed to dry at 37 °C until the weight was constant. The density of the mycelia was calculated by dividing the dry weight by the surface area. Using the same approach, the effects of ethanol were examined on A. roeperi, R. canadensis, A. hylecoeti, Penicillium sp., and Aspergillus sp. Cultures were grown under the following conditions: A. roeperi for 4 d at 28 °C; R. canadensis for 10 d at 23 °C; A. hylecoeti for 6 d at 23 °C; Penicillium sp. for 4 d at 28 °C; and Aspergillus sp. for 4 d at 28 °C. Dry weight was measured as described above.
The effect of ethanol was further assessed using liquid cultures of A. grosmanniae, A. roeperi, R. canadensis, Penicillium sp., and Aspergillus sp. grown in the presence of 0%, 5%, or 10% ethanol. The optical density of the liquid cultures was measured using a microplate reader to quantify growth rate (Fig. S1). (See SI Methods for specific methods.)
ADH Activity.
The enzymatic activity of ADH and the protein concentration were measured using A. grosmanniae, A. roeperi, R. canadensis, A. hylecoeti, Penicillium sp., and Aspergillus sp. cultures grown on yeast extract/mannitol/agar (YEMA) plates flooded with 0% or 2% ethanol. Proteinaceous supernatant prepared from each fungal culture was analyzed for ADH activity using a standard kit (catalog no. MAK053; Sigma-Aldrich). Total protein concentrations were determined using the Bradford assay (Roti-Quant; Roth). (See SI Methods for specific methods.)
Ethanol Quantification.
Ethanol was quantified within flood-stressed C. florida trees, ethanol-irrigated C. florida trees, and the sawdust-based substrate to confirm that biologically relevant levels of ethanol were tested as part of our study (Fig. S2). Solid-phase microextraction-gas chromatography-mass spectrometry (SPME-GC-MS) was used to analyze ethanol concentrations in the various tissues/substrates (26). (See SI Methods for specific methods.)
Statistics.
Weighted regression analyses were conducted on fungal growth parameters (i.e., surface area, dry weight, and density) and offspring production (larvae and pupae) as a function of the percent of ethanol incorporated into the growth medium. A weighted regression analysis was used on mean parameter values (e.g., dry weight) as a function of the percent of ethanol using a standard weighting factor of 1/variance. TableCurve 2D v. 5.01 (Systat Software Inc., 2002) was used for regression equations, and SYSTAT v.11 was used to obtain graphs. The Pearson correlation coefficient was used to assess the correlation between ethanol percentage and fungal growth parameters. One-way ANOVA and Tukey’s honest significant difference (HSD) or Dunnett’s multiple comparisons test were used as indicated to separate means.
Supplementary Material
Acknowledgments
We thank Emma Trapp (Ohio State University), Betsy Anderson [US Department of Agriculture–Agricultural Research Service (USDA-ARS)], Pamela Baumann and Hammad Achmed (Max Planck Institute for Chemical Ecology), Franziska Ruf (Julius Maximilian University Würzburg), and Nicole Ganske (Technical University of Munich) for technical assistance; Rabiu Olatinwo (USDA Forest Service) for sharing the A. roeperi isolate; the anonymous reviewers for useful comments; and Douglas Yu (University of East Anglia) for explaining the concept of biological screening and discussions about our findings. This research was supported by the USDA Floriculture and Nursery Research Initiative, the Horticultural Research Institute, and USDA-ARS Grant NP304 3607-22000-012-00D. P.H.W.B. is funded by German Research Foundation Emmy Noether Grant BI 1956/1-1 and Marie Curie Intra-European Fellowship Project 626279. Mention of proprietary products or companies does not imply any endorsement or preferential treatment by the USDA-ARS.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716852115/-/DCSupplemental.
References
- 1.Biedermann PH, Rohlfs M. Evolutionary feedbacks between insect sociality and microbial management. Curr Opin Insect Sci. 2017;22:92–100. doi: 10.1016/j.cois.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archetti M, et al. Economic game theory for mutualism and cooperation. Ecol Lett. 2011;14:1300–1312. doi: 10.1111/j.1461-0248.2011.01697.x. [DOI] [PubMed] [Google Scholar]
- 4.Scheuring I, Yu DW. How to assemble a beneficial microbiome in three easy steps. Ecol Lett. 2012;15:1300–1307. doi: 10.1111/j.1461-0248.2012.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller UG, Gerardo N. Fungus-farming insects: Multiple origins and diverse evolutionary histories. Proc Natl Acad Sci USA. 2002;99:15247–15249. doi: 10.1073/pnas.242594799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Syst. 2005;36:563–595. [Google Scholar]
- 7.Aanen DK, et al. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordal BH, Cognato AI. Molecular phylogeny of bark and ambrosia beetles reveals multiple origins of fungus farming during periods of global warming. BMC Evol Biol. 2012;12:133. doi: 10.1186/1471-2148-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulcr J, Atkinson TH, Cognato AI, Jordal BH, McKenna DD. Morphology, taxonomy, and phylogenetics of bark beetles. In: Vega FE, Hofstetter RW, editors. Bark Beetles: Biology and Ecology of Native and Invasive Species. Academic; New York: 2015. pp. 41–84. [Google Scholar]
- 10.French JR, Roeper RA. Interactions of the ambrosia beetle, Xyleborus dispar (Coleoptera: Scolytidae), with its symbiotic fungus Ambrosiella hartigii (Fungi: Imperfecti) Can Entomol. 1972;104:1635–1641. [Google Scholar]
- 11.Weber BC, McPherson JE. Life history of the ambrosia beetle Xylosandrus germanus (Coleoptera: Scolytidae) Ann Entomol Soc Am. 1983;76:455–462. [Google Scholar]
- 12.Kajimura H, Hijii N. Dynamics of the fungal symbionts in the gallery system and the mycangia of the ambrosia beetle, Xylosandrus mutilatus (Blandford) (Coleoptera, Scolytidae) Ecol Res. 1992;7:107–117. [Google Scholar]
- 13.Norris DM. Chemical interdependencies among Xyleborus spp. ambrosia beetles and their symbiotic microbes. Mater Org. 1975;3:479–788. [Google Scholar]
- 14.Francke-Grosmann H. Hautdrüsen als träger der pilzsymbiose bei ambrosiakäfern. Z Morphol Oekol Tiere. 1956;45:275–308. [Google Scholar]
- 15.Batra LR. Ambrosia beetles and their associated fungi: Research trends and techniques. Proc Plant Sci. 1985;94:137–148. [Google Scholar]
- 16.Mayers CG, et al. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol. 2015;119:1075–1092. doi: 10.1016/j.funbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Wingfield MJ, et al. Novel associations between ophiostomatoid fungi, insects and tree hosts: Current status—Future prospects. Biol Invasions. 2017;19:3215–3228. [Google Scholar]
- 18.Harrington TC, McNew D, Mayers C, Fraedrich SW, Reed SE. Ambrosiella roeperi sp. nov. is the mycangial symbiont of the granulate ambrosia beetle, Xylosandrus crassiusculus. Mycologia. 2014;106:835–845. doi: 10.3852/13-354. [DOI] [PubMed] [Google Scholar]
- 19.Kostovcik M, et al. The ambrosia symbiosis is specific in some species and promiscuous in others: Evidence from community pyrosequencing. ISME J. 2015;9:126–138. doi: 10.1038/ismej.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkendall LR, Biedermann PHW, Jordal BH. Evolution and diversity of bark and ambrosia beetles. In: Vega FE, Hofstetter RW, editors. Bark Beetles: Biology and Ecology of Native and Invasive Species. Academic; New York: 2015. pp. 85–156. [Google Scholar]
- 21.Haanstad JO, Norris DM. Microbial symbiotes of the ambrosia beetle Xyloterinus politus. Microb Ecol. 1985;11:267–276. doi: 10.1007/BF02010605. [DOI] [PubMed] [Google Scholar]
- 22.Aylward FO, et al. Convergent bacterial microbiotas in the fungal agricultural systems of insects. MBio. 2014;5:e02077. doi: 10.1128/mBio.02077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie CR, Mueller UG, Malloch D. The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA. 1999;96:7998–8002. doi: 10.1073/pnas.96.14.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biedermann PHW, Taborsky M. Larval helpers and age polyethism in ambrosia beetles. Proc Natl Acad Sci USA. 2011;108:17064–17069. doi: 10.1073/pnas.1107758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Fine Licht HH, Biedermann PHW. Patterns of functional enzyme activity in fungus farming ambrosia beetles. Front Zool. 2012;9:13. doi: 10.1186/1742-9994-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranger CM, Schultz PB, Frank SD, Chong JH, Reding ME. Non-native ambrosia beetles as opportunistic exploiters of living but weakened trees. PLoS One. 2015;10:e0131496. doi: 10.1371/journal.pone.0131496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kühnholz S, Borden JH, Uzunovic A. Secondary ambrosia beetles in apparently healthy trees: Adaptations, potential causes and suggested research. Integr Pest Manage Rev. 2001;6:209–219. [Google Scholar]
- 28.Ranger CM, Tobin PC, Reding ME. Ubiquitous volatile compound facilitates efficient host location by a non-native ambrosia beetle. Biol Invasions. 2015;17:675–686. [Google Scholar]
- 29.Hulcr J, Dunn RR. The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc Biol Sci. 2011;278:2866–2873. doi: 10.1098/rspb.2011.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Spina S, De Canniére C, Dekri A, Grégoire J-C. Frost increases beech susceptibility to scolytine ambrosia beetles. Agric For Entomol. 2013;15:157–167. [Google Scholar]
- 31.Graham K. Anaerobic induction of primary chemical attractancy for ambrosia beetles. Can J Zool. 1968;46:905–908. [Google Scholar]
- 32.Kimmerer TW, Kozlowski TT. Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol. 1982;69:840–847. doi: 10.1104/pp.69.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald RC, Kimmerer TW. Ethanol in the stems of trees. Physiol Plant. 1991;82:582–588. [Google Scholar]
- 34.Ranger CM, Reding ME, Schultz P, Oliver J. Influence of flood-stress on ambrosia beetle (Coleoptera: Curculionidae, Scolytinae) host-selection and implications for their management in a changing climate. Agric For Entomol. 2013;15:56–64. [Google Scholar]
- 35.Kelsey RG, Beh MM, Shaw DC, Manter DK. Ethanol attracts scolytid beetles to Phytophthora ramorum cankers on coast live oak. J Chem Ecol. 2013;39:494–506. doi: 10.1007/s10886-013-0271-6. [DOI] [PubMed] [Google Scholar]
- 36.McGovern PE, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dao T, Dantigny P. Control of food spoilage fungi by ethanol. Food Control. 2011;22:360–368. [Google Scholar]
- 38.Klimetzek D, Kohler J, Vite JP, Kohnle U. Dosage response to ethanol mediates host selection by ‘secondary’ bark beetles. Naturwissenschaften. 1986;73:270–272. [Google Scholar]
- 39.Wood DL. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol. 1982;27:411–446. [Google Scholar]
- 40.Cardoza YJ, Klepzig KD, Raffa KF. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol Entomol. 2006;31:636–645. [Google Scholar]
- 41.Scott JJ, et al. Bacterial protection of beetle-fungus mutualism. Science. 2008;322:63. doi: 10.1126/science.1160423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Six DL. The bark beetle holobiont: Why microbes matter. J Chem Ecol. 2013;39:989–1002. doi: 10.1007/s10886-013-0318-8. [DOI] [PubMed] [Google Scholar]
- 43.Flórez LV, Biedermann PH, Engl T, Kaltenpoth M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep. 2015;32:904–936. doi: 10.1039/c5np00010f. [DOI] [PubMed] [Google Scholar]
- 44.Martin MM. The biochemical basis of the fungus-attine ant symbiosis. Science. 1970;169:16–20. doi: 10.1126/science.169.3940.16. [DOI] [PubMed] [Google Scholar]
- 45.Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. [Google Scholar]
- 46.Sen R, et al. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci USA. 2009;106:17805–17810. doi: 10.1073/pnas.0904827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biedermann PHW, Klepzig KD, Taborsky M, Six DL. Abundance and dynamics of filamentous fungi in the complex ambrosia gardens of the primitively eusocial beetle Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae) FEMS Microbiol Ecol. 2013;83:711–723. doi: 10.1111/1574-6941.12026. [DOI] [PubMed] [Google Scholar]
- 48.Smith MG, Des Etages SG, Snyder M. Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol. 2004;24:3874–3884. doi: 10.1128/MCB.24.9.3874-3884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen AI, et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10:e1004480. doi: 10.1371/journal.ppat.1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson JM, et al. Resurrecting ancestral alcohol dehydrogenases from yeast. Nat Genet. 2005;37:630–635. doi: 10.1038/ng1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dashko S, Zhou N, Compagno C, Piškur J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014;14:826–832. doi: 10.1111/1567-1364.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinhold AR, Garraway MO. Nitrogen and carbon nutrition of Armillaria mellea in relation to growth-promoting effects of ethanol. Phytopathology. 1966;56:108–112. [Google Scholar]
- 53.Kuhns EH, et al. Volatiles from the symbiotic fungus Raffaelea lauricola are synergistic with manuka lures for increased capture of the redbay ambrosia beetle Xyleborus glabratus. Agric For Entomol. 2014;16:87–94. [Google Scholar]
- 54.Kandasamy D, Gershenzon J, Hammerbacher A. Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J Chem Ecol. 2016;42:952–969. doi: 10.1007/s10886-016-0768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou N, et al. Coevolution with bacteria drives the evolution of aerobic fermentation in Lachancea kluyveri. PLoS One. 2017;12:e0173318. doi: 10.1371/journal.pone.0173318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paine TD, Raffa KF, Harrington TC. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol. 1997;42:179–206. doi: 10.1146/annurev.ento.42.1.179. [DOI] [PubMed] [Google Scholar]
- 57.Davis TS. The ecology of yeasts in the bark beetle holobiont: A century of research revisited. Microb Ecol. 2015;69:723–732. doi: 10.1007/s00248-014-0479-1. [DOI] [PubMed] [Google Scholar]
- 58.Ruby EG, McFall-Ngai MJ. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 1999;7:414–420. doi: 10.1016/s0966-842x(99)01588-7. [DOI] [PubMed] [Google Scholar]
- 59.Biedermann PH, Kaltenpoth M. New synthesis: The chemistry of partner choice in insect-microbe mutualisms. J Chem Ecol. 2014;40:99. doi: 10.1007/s10886-014-0382-8. [DOI] [PubMed] [Google Scholar]
- 60.McKenzie JA, Parsons PA. Alcohol tolerance: An ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- 61.Becher PG, et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol. 2012;26:822–828. [Google Scholar]
- 62.Matsui T, Tanaka J, Namihira T, Shinzato N. Antibiotics production by an actinomycete isolated from the termite gut. J Basic Microbiol. 2012;52:731–735. doi: 10.1002/jobm.201100500. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima T, Iizawa T, Ogura K, Maeda M, Tanaka T. Isolation of some microorganisms associated with five species of ambrosia beetles and two kinds of antibiotics produced by Xv-3 strain in these isolates. J Fac Agric Hokkaido Univ. 1982;61:60–72. [Google Scholar]
- 64.van der Meij A, Worsley SF, Hutchings MI, van Wezel GP. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol Rev. 2017;41:392–416. doi: 10.1093/femsre/fux005. [DOI] [PubMed] [Google Scholar]
- 65.Harrington TC. Cycloheximide sensitivity as a taxonomic character in Ceratocystis. Mycologia. 1981;73:1123–1129. [Google Scholar]
- 66.Human ZR, Slippers B, de Beer ZW, Wingfield MJ, Venter SN. Antifungal actinomycetes associated with the pine bark beetle, Orthotomicus erosus, in South Africa. S Afr J Sci. 2017;113:34–40. [Google Scholar]
- 67.Castrillo LA, Griggs MH, Vandenberg JD. Brood production by Xylosandrus germanus (Coleoptera: Curculionidae) and growth of its fungal symbiont on artificial diet based on sawdust of different tree species. Environ Entomol. 2012;41:822–827. [Google Scholar]
- 68.Peer K, Taborsky M. Female ambrosia beetles adjust their offspring sex ratio according to outbreeding opportunities for their sons. J Evol Biol. 2004;17:257–264. doi: 10.1111/j.1420-9101.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 69.Biedermann PH, Klepzig KD, Taborsky M. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three xyleborine species. Environ Entomol. 2009;38:1096–1105. doi: 10.1603/022.038.0417. [DOI] [PubMed] [Google Scholar]
- 70.Corseuil HX, Moreno FN. Phytoremediation potential of willow trees for aquifers contaminated with ethanol-blended gasoline. Water Res. 2001;35:3013–3017. doi: 10.1016/s0043-1354(00)00588-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.