Abstract
Increasing the leaf temperature of intact cotton (Gossypium hirsutum L.) and wheat (Triticum aestivum L.) plants caused a progressive decline in the light-saturated CO2-exchange rate (CER). CER was more sensitive to increased leaf temperature in wheat than in cotton, and both species demonstrated photosynthetic acclimation when leaf temperature was increased gradually. Inhibition of CER was not a consequence of stomatal closure, as indicated by a positive relationship between leaf temperature and transpiration. The activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which is regulated by Rubisco activase, was closely correlated with temperature-induced changes in CER. Nonphotochemical chlorophyll fluorescence quenching increased with leaf temperature in a manner consistent with inhibited CER and Rubisco activation. Both nonphotochemical fluorescence quenching and Rubisco activation were more sensitive to heat stress than the maximum quantum yield of photochemistry of photosystem II. Heat stress led to decreased 3-phosphoglyceric acid content and increased ribulose-1,5-bisphosphate content, which is indicative of inhibited metabolite flow through Rubisco. We conclude that heat stress inhibited CER primarily by decreasing the activation state of Rubisco via inhibition of Rubisco activase. Although Rubisco activation was more closely correlated with CER than the maximum quantum yield of photochemistry of photosystem II, both processes could be acclimated to heat stress by gradually increasing the leaf temperature.
Inhibition of photosynthetic CO2 fixation by high temperature has been documented in many plant species (for review, see Berry and Björkman, 1980). Several components of the photosynthetic apparatus and associated metabolic processes are heat labile. For example, PSII is thermally labile (Havaux, 1993; Havaux and Tardy, 1996) but can be acclimated to heat stress (Havaux, 1993). Recent evidence indicates that a chloroplast-localized heat-shock protein protects PSII from damage at high temperature (Heckathorn et al., 1998). Export of photoassimilate is another metabolic process that is sensitive to inhibition by high temperature. Jiao and Grodzinski (1996) reported that heat stress inhibited assimilate export to a greater degree than net photosynthesis. Inhibition was especially apparent at a high atmospheric CO2 concentration at which assimilate export, but not net photosynthesis, was inhibited by heat stress. However, in a similar study using a different plant species (Leonardos et al., 1996), heat stress under a high atmospheric CO2 concentration inhibited net photosynthesis but not assimilate export. Thus, the sensitivity of assimilate export to inhibition by heat stress may differ among plant species and/or associated environmental conditions.
Based on Chl fluorescence analysis, Bilger et al. (1987) reported that Calvin cycle activity was sensitive to rapid heat-stress treatments. Previous reports have documented that Rubisco activation is a primary site of inhibition (Weis, 1981a, 1981b; Kobza and Edwards, 1987; Feller et al., 1998). For example, Weis (1981a, 1981b) reported that rapid heat stress led to reversible inhibition of the light-dependent activation of Rubisco in spinach chloroplasts and leaves. Similar findings were reported for wheat (Triticum aestivum L.) leaves (Kobza and Edwards, 1987).
The close relationship between the activation state of Rubisco and photosynthesis in response to varying light intensity (Seemann et al., 1990) or altered activase content (Andrews et al., 1995; Eckardt et al., 1997) indicates the pivotal role of activase in the regulation of photosynthetic CO2 fixation. The activity of Rubisco assayed immediately after extraction from leaves exposed to light levels that are saturating for photosynthesis is directly related to the ability of Rubisco activase to activate Rubisco. At optimal temperatures in air, it has been shown that this ”initial activity” of Rubisco is similar to the activity after incubation of the enzyme with saturating levels of CO2 and Mg2+ (von Caemmerer and Edmondson, 1986; Seemann et al., 1988; Feller et al., 1998). Fully activated Rubisco activity, both in leaf extracts from heat-stressed leaves and using isolated Rubisco, has been shown to be very stable at high temperatures (Kobza and Edwards, 1987; Eckardt and Portis, 1997; Feller et al., 1998). Therefore, the altered initial activity of Rubisco from light-saturated, heat-stressed leaves is directly related to changes in the activity of Rubisco activase. The lack of any effect of heat stress on extractable Rubisco activity after incubation with CO2 and Mg2+ (Kobza and Edwards, 1987; Feller et al., 1998) precludes the possibility that decreases in initial Rubisco activity are associated with the formation of inhibitors of Rubisco.
Feller et al. (1998) proposed that heat stress rapidly and reversibly inhibited the light-dependent activation of Rubisco by inhibiting Rubisco activase activity. Evidence was presented that heat stress perturbed the structural properties of activase. In support of this hypothesis, the activity of isolated activase has been shown to be extremely sensitive to high temperature (Robinson and Portis, 1989; Holbrook et al., 1991; Crafts-Brandner et al., 1997; Eckardt and Portis, 1997). Crafts-Brandner et al. (1997) presented evidence that high temperature inhibited activase by disrupting subunit interactions.
In the present study, we have extended our previous work (Crafts-Brandner et al., 1997; Feller et al., 1998) to the level of the whole plant. We found the following: (a) Rubisco activation acclimates to heat stress in both cotton (Gossypium hirsutum L.) and wheat; (b) CER in wheat is more sensitive to inhibition by heat stress than CER in cotton under both acclimating and nonacclimating conditions; and (c) Rubisco activation and CER are remarkably well correlated during both rapid and gradual heat stress. For both plant species, Rubisco activation was more sensitive to heat stress than was Fv/Fm under both rapid and gradual heat stress.
MATERIALS AND METHODS
Plant Material
Cotton (Gossypium hirsutum L. cv Coker 100A-glandless) seeds and wheat (Triticum aestivum L. cv Arina) caryopses were planted in 15- × 15-cm pots containing a commercial potting mixture (Grow More, Gardena, CA1). Seeds were germinated in a greenhouse and transferred to a growth chamber 1 week before sampling. Cotton was grown under a photoperiod of 14 h at 28°C and a dark period of 10 h at 24°C. Wheat was grown under a photoperiod of 14 h at 25°C and a dark period of 10 h at 21°C. Light intensity was 800 μmol photons m−2 s−1 PAR, and RH was 50%. Cotton plants were fertilized twice a week with 750 mL of a solution containing 2 g L−1 Grow More 20–20-20 fertilizer. The nutrient solution was supplemented with 0.5 mL L−1 micronutrient solution containing 2 mm MnCl2, 10 mm H3BO3, 0.4 mm ZnSO4, 0.2 mm CuSO4, 0.4 mm Na2MoO4, and 0.1 mm NiCl2. Wheat plants were fertilized twice a week with 250 mL of the same nutrient solution. In addition, 750 mg of sodium ferric ethylenediamine,di-(o-hydroxyphenyl acetate) was incorporated into the upper 2 cm of the potting medium after the wheat plants had emerged. Fully expanded cotton leaves (fifth and sixth true leaves) or wheat leaves (second leaf) were used as experimental material. All experimental samples were from leaves that had been preilluminated with 1800 μmol photons m−2 s−1 PAR to maximize the light-dependent activation of Rubisco. Before the heat-stress experiments were conducted, the RH of the growth chamber was adjusted to >80% and maintained at this level throughout the experiment. Under these conditions, the leaf temperature, as measured with a thermocouple pressed to the bottom of a leaf, was increased in proportion to the air temperature of the growth chamber.
CER Measurements
CER was measured with a portable photosynthesis system (model 6400, Li-Cor, Lincoln, NE). CER was determined using a light intensity of 1800 μmol photons m−2 s−1 PAR. Partial pressure of CO2 in the sample chamber was maintained at a constant 350 μbar. The leaf chamber was set up inside the growth chamber to provide humidified air to the system. Leaf temperature was increased using the internal heater of the photosynthesis system in conjunction with the heated and humidified air supplied from the growth chamber. Under these conditions, the leaf temperature, as measured with a thermocouple pressed to the bottom of the leaf, could be increased to 45°C.
For rapid heat-stress treatments, steady-state CER was determined first at the control temperature, after which the leaf temperature was increased to the desired level at a rate of approximately 1°C min−1. One hour after the leaf temperature was increased, steady-state CER was again determined. A different leaf from a nonstressed plant was used for each measurement, and at least three measurements were made at each temperature. Two independent experiments were conducted. For gradual heat-stress experiments, CER was measured for an individual leaf over the entire range of temperatures. After steady-state CER was determined at the control temperature, the leaf temperature was increased in 2.5°C increments (at a rate of approximately 1°C min−1) and measurements were made after 1 h at each temperature. Three leaves, each from a different plant, were sampled in each of two independent experiments.
The effect of leaf temperature on dark respiration was determined as described above, except that the measurements were made in darkness. Temperature effects on photorespiration were determined by measuring CER as described above, except that CO2 was scrubbed from the sample-chamber inlet air. T50 values for the effect of temperature on CER were calculated using a nonlinear least-squares regression kinetics computer program (Brooks, 1992).
Determination of Light-Dependent Activation of Rubisco
All leaf samples were rapidly frozen between two pieces of metal cooled to the temperature of liquid N2. Control leaves were sampled after illumination at 1800 μmol photons m−2 s−1 for at least 20 min. For cotton, one-half of a leaf was sampled before heat treatment as a control and the other half of the same leaf was sampled after the heat-stress treatment. For wheat, separate leaves were sampled for all temperature treatments. Heat-stress treatments were initiated using leaves that were illuminated under high light at the control temperature for at least 20 min. For rapid heat-stress treatments, leaf temperature was increased at a rate of approximately 1°C min−1. After 1 h, the leaves were sampled. For gradual heat-stress experiments, leaf temperature was increased 2.5°C (at a rate of approximately 1°C min−1) and maintained at this temperature for 1 h, followed by another 2.5°C increase in leaf temperature for 1 h. This process was repeated until the desired leaf temperature was obtained. The leaf was sampled at the end of a 1-h period at the desired temperature. Three leaves were sampled for each temperature treatment in an experiment, and two independent experiments were conducted. Leaf tissue was either immediately assayed for initial Rubisco activity or stored at −80°C.
Leaf tissue (20 mg fresh weight) was extracted using a glass homogenizer in 1.5 mL of CO2-free buffer containing 100 mm Tricine, pH 8.0, 5 mm MgCl2, 0.1 mm EDTA, 5 mm DTT, 1% (w/v) PVP, 1% (w/v) casein, 0.05% (v/v) Triton X-100, 1 mm PMSF, and 20 μm leupeptin. Within 30 s, an aliquot (25 μL) of extract was assayed at 30°C for 30 s to determine the Rubisco activation state (initial Rubisco activity). Rubisco assays were conducted as described by Salvucci and Anderson (1987), except that Triton X-100 and casein were not included in the assay medium. Activity was based on incorporation of 14CO2 into acid-stable products. T50 values for the effect of temperature on initial Rubisco activity were calculated as described for CER.
Determination of Chl Fluorescence
Modulated Chl fluorescence was measured using a fluorometer (PAM 2000, Heinz Walz, Effeltrich, Germany). Fluorescence induction and quenching of dark-adapted leaf tissue were measured as described by Schreiber et al. (1986) using a preprogrammed protocol (Standard Run 3). Initial Chl fluorescence was measured using a weak, modulated red light. Maximum Chl fluorescence was measured after a 0.8-s pulse of strong white light (>4000 μmol photons m−2 s−1 PAR). After a 2-s lag, a 5-min quenching analysis was initiated using continuous actinic light (125 μmol photons m−2 s−1 emitted at 665 nm) and saturating pulses of 0.8 s every 20 s. Experiments were designed such that leaves were illuminated for 40 min with 1800 μmol photons m−2 s−1 at the control temperature and then dark adapted for 20 min, using a leaf clip, before analysis. For rapid heat-stress experiments, a new leaf from a nonstressed plant was used for each temperature treatment. For gradual heat-stress experiments, the same leaf was used over the entire temperature range. After completion of the quenching analysis at a given temperature, the leaf was again illuminated with high light and leaf temperature was increased gradually as described for the Rubisco assays. Forty minutes after the temperature was increased, the leaves were again dark adapted for 20 min before analysis. Measurements were made on three separate leaves for each temperature treatment in each of two independent experiments. This analysis provided measurements of qN and Fv/Fm. In most cases, qN is reported as the steady-state value obtained at the end of the quenching analysis for a particular temperature treatment relative to the control. Likewise, the effect of high temperature on Fv/Fm is reported on a relative basis compared with controls. Because of the nature of the temperature response of Fv/Fm, in which abrupt rather than gradual perturbations occurred at the critical temperature, T50 values were estimated manually from the plotted data.
For selected heat-stress treatments, relaxation kinetics of qN were analyzed immediately after the fluorescence-quenching analysis. Relaxation kinetics were measured using a preprogrammed protocol (Standard Run 5) of the PAM 2000 fluorometer. After the quenching analysis, illumination with actinic light (125 μmol photons m−2 s−1 emitted at 665 nm) was continued for 1 min, after which the actinic light was turned off and 1.2-s pulses of strong white light (>4000 μmol photons m−2 s−1) were applied at exponentially increasing intervals for a 16-min period.
Determination of Metabolites
Metabolite levels were measured in leaves sampled as described for initial Rubisco activity. Freeze-clamped leaf samples were stored at −80°C before analysis. The leaf samples were rapidly weighed and then powdered with a prechilled mortar and pestle under liquid N2. Proteins were precipitated by homogenization in 500 μL of freshly prepared, ice-cold 5% (v/v) trifluoroacetic acid. Samples were transferred to microfuge tubes, kept on ice for 15 min, and then centrifuged for 5 min at 13,000g at 4°C. Supernatants were freeze dried and reconstituted in 200 μL of water. For pigment removal, a 1:5 (w/v) suspension of activated charcoal in water was prepared from which the fines had been twice removed. A constant amount of this suspension (100 μL g−1 fresh weight) was used for all of the samples. After the addition of charcoal, samples were vortexed, kept on ice for 30 min, and centrifuged as described above. The supernatants were assayed immediately or frozen in liquid N2 and kept at −80°C. Levels of PGA and RuBP were determined sequentially on the same sample using an enzyme-linked spectrophotometer assay as described by He et al. (1997), except that the sample volume was 150 μL, Tricine/NaOH was used as the buffer system, the assay medium contained 10% (v/v) glycerol, and the final volume of the assay was 1.15 mL. Recovery of the metabolites was tested using RuBP and PGA added to leaf extracts at levels up to 5-fold higher than those found in vivo. In all cases, recovery was in the range of 85% to 110%. Three leaves were sampled for each temperature treatment in each of two independent experiments.
RESULTS
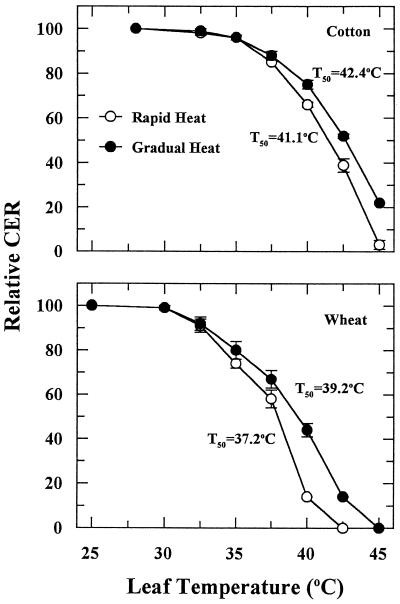
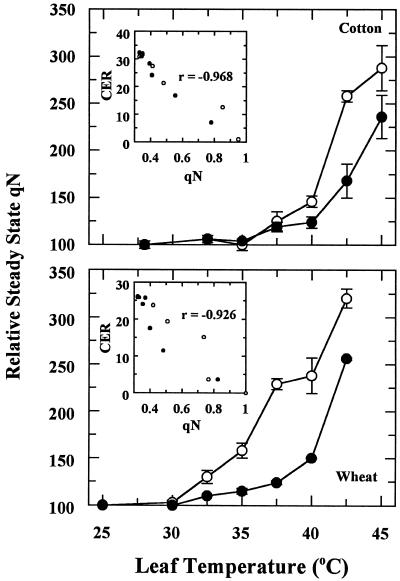
CER was inhibited at leaf temperatures greater than 35°C and 30°C for cotton and wheat, respectively (Fig. 1). Complete inhibition occurred when the leaf temperature was increased at a rate of 1°C min−1 to more than 42.5°C for cotton or 40°C for wheat. Acclimation to heat stress occurred for both plant species if the leaf temperature was increased gradually by 2.5°C every hour (Fig. 1). Acclimation was most pronounced at the higher leaf temperatures. For example, wheat leaf CER at 40°C was 43% or 14% of the 25°C controls when temperature was increased gradually or rapidly, respectively. When cotton leaf temperature was gradually increased to 45°C, the leaves maintained a CER that was 20% of the controls.
Figure 1.
The effect of rapid and gradual increases in leaf temperature on CER of cotton and wheat leaves. Values are reported relative to the CER of the control, which was set at 100%. Each point is the mean ± se of two independent experiments in which three measurements were made for each temperature treatment. CER for the controls averaged 32.3 ± 1.8 and 26.2 ± 1.9 μmol CO2 m−2 s−1 for cotton and wheat, respectively.
Dark respiration rates of both species were approximately 2.5 ± 1.0 μmol m−2 s−1 at the control leaf temperature. Increasing the leaf temperature to 37.5°C and 40°C for wheat and cotton, respectively, caused a nearly 2-fold increase in the dark respiration rate (data not shown). At higher leaf temperatures, the dark respiration rate declined to the level of the control. The rate of photorespiration, as estimated by CO2 evolution into CO2-free air in the light, was approximately 3.0 ± 1.0 μmol m−2 s−1 at the control temperature for both species. Photorespiration decreased approximately 3-fold as leaf temperature was increased gradually to 42.5°C and 45°C for wheat and cotton, respectively (data not shown). Thus, although dark respiration and photorespiration were significantly altered by heat stress, the magnitude of the effect was small relative to the large changes in CER (Fig. 1).
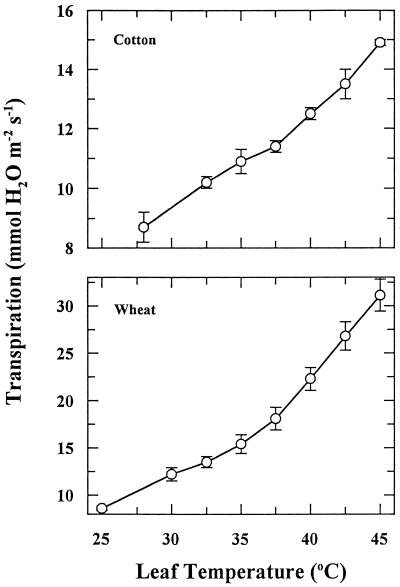
For well-watered plants, high leaf temperatures could be attained only under conditions of high (>75%) RH. Under these conditions, there was no evidence that stomatal closure had any influence on the heat-stress-induced inhibition of CER. Leaf transpiration increased progressively as leaf temperature was increased (Fig. 2). In addition, both leaf conductance to CO2 and internal CO2 concentration were increased as leaf temperature was increased (data not shown). Leonardos et al. (1996) reported a similar relationship between leaf temperature and transpiration for leaves of Alstroemeria.
Figure 2.
The effect of gradual increases in leaf temperature on the transpiration rate of cotton and wheat leaves. Each point is the mean ± se of two independent experiments in which three measurements were made for each temperature treatment.
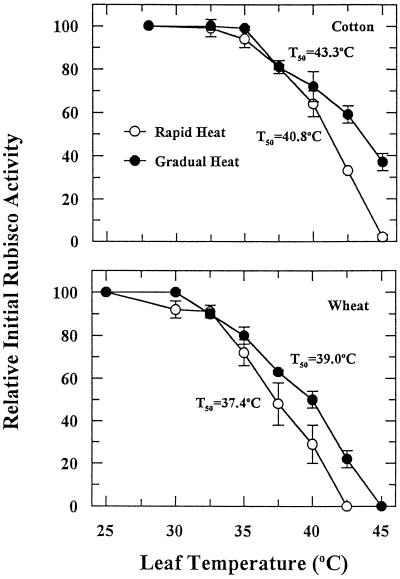
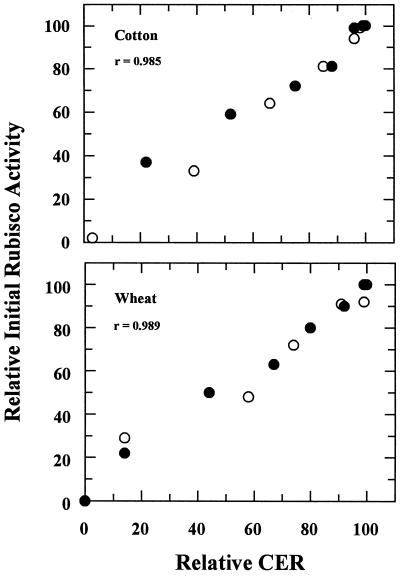
At leaf temperatures greater than 35°C and 30°C for cotton and wheat, respectively, the initial activity of Rubisco (Fig. 3) was inhibited to an extent that was very similar to the inhibition of CER (Fig. 1). Acclimation to high temperature was apparent based on the differences between the rapid and the gradual heat-stress treatments at the higher leaf temperatures. The T50 values for initial Rubisco activity (Fig. 3) were nearly identical to those calculated for CER (Fig. 1). Analysis of the entire data set, including both rapid and gradual heat-stress treatments, indicated a close correlation between CER and initial Rubisco activity, with correlation coefficients > 0.98 for both cotton and wheat (Fig. 4).
Figure 3.
The effect of rapid and gradual increases in leaf temperature on initial Rubisco activity. Values are reported relative to the initial Rubisco activity of the control, which was set at 100%. Each point is the mean ± se of two independent experiments in which three measurements were made for each temperature treatment. Initial Rubisco activity of the controls averaged 0.452 ± 0.016 and 0.433 ± 0.018 μmol CO2 g−1 fresh weight s−1 for cotton and wheat, respectively.
Figure 4.
Correlation between initial Rubisco activity and CER of cotton and wheat leaves. Data points are from Figures 1 and 3. ○, Rapid heat stress; •, gradual heat stress.
Differences in Chl fluorescence quenching were apparent when leaf temperature was gradually increased compared with rapidly increased. Steady-state qN increased at leaf temperatures greater than 35°C and 30°C for cotton and wheat, respectively (Fig. 5). Compared with rapid heat stress, gradual heat stress markedly decreased the magnitude of the increase in qN, especially for wheat. However, for both types of heat-stress treatments, this trait reflected inhibition of CER (Fig. 5, insets).
Figure 5.
The effect of rapid and gradual increases in leaf temperature on steady-state qN of cotton and wheat leaves. Values are reported relative to the qN of the control, which was set at 100%. Each point is the mean ± se of two independent experiments in which three measurements were made for each temperature treatment. Steady-state qN values for control cotton and wheat leaves averaged 0.330 ± 0.028 and 0.322 ± 0.030, respectively. The insets represent the correlation between CER (from data in Fig. 1) and steady-state qN. ○, Rapid heat stress; •, gradual heat stress.
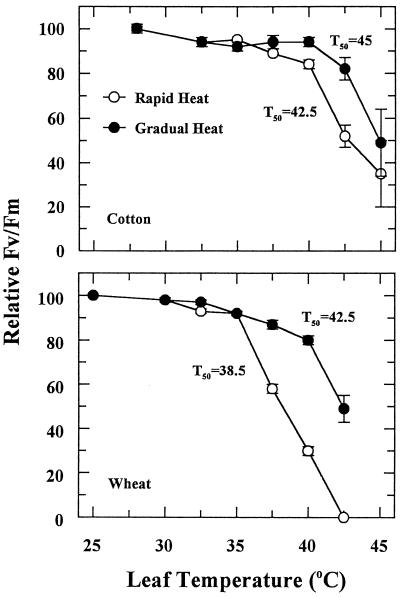
High-temperature stress also inhibited Fv/Fm (Fig. 6), but the inhibition occurred at higher temperatures than the corresponding perturbations in qN (Fig. 5). The T50 values for Fv/Fm were higher than those for CER and initial Rubisco activity (Fig. 6). For cotton, Fv/Fm was relatively stable until leaf temperature exceeded 40°C. The small decrease in Fv/Fm between 35°C and 40°C for the rapid heat-stress treatment was caused by a gradual increase in the initial Chl fluorescence (data not shown). As leaf temperature exceeded 35°C for wheat, there was an abrupt decrease in Fv/Fm for the rapid heat-stress treatment. For the gradual heat-stress treatment, however, Fv/Fm at 40°C was 80% of the control Fv/Fm. As in cotton, the decrease in Fv/Fm between 32.5°C and 40°C for the gradual heat-stress treatment was associated with a gradual increase in the initial Chl fluorescence (data not shown).
Figure 6.
The effect of rapid and gradual increases in leaf temperature on Fv/Fm of cotton and wheat leaves. Values are reported relative to the Fv/Fm of the control, which was set at 100%. Each point is the mean ± se of two independent experiments in which three measurements were made for each temperature treatment. Fv/Fm for control cotton and wheat leaves averaged 0.769 ± 0.073 and 0.766 ± 0.063, respectively.
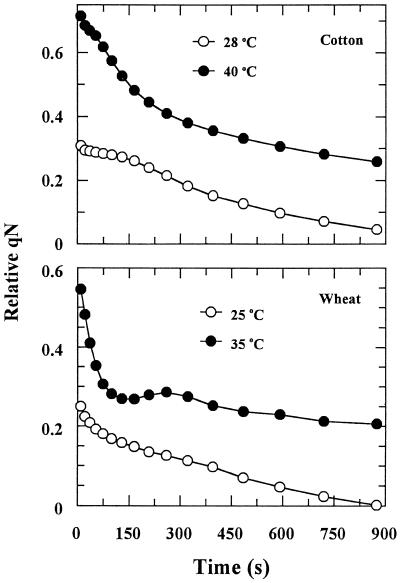
Figure 7 shows the effect of heat stress on the time course of the relaxation kinetics of steady-state qN developed during 5 min in the light. For controls of both species, qN relaxed to levels comparable to those of dark-adapted leaves during the 15-min time course. When leaf temperature was rapidly increased to 40°C and 35°C for cotton and wheat, respectively, relaxation of qN occurred but not nearly to the extent seen in the controls.
Figure 7.
The effect of rapid increases in leaf temperature on the relaxation kinetics of qN of a cotton and a wheat leaf. Relaxation kinetics were analyzed immediately after quenching analysis, during which steady-state qN had developed in the light. Experiments were conducted first at the control temperatures of 28°C and 25°C for cotton and wheat, respectively, and then again after the leaf temperature was rapidly increased to 40°C and 35°C for cotton and wheat, respectively. The data reported were obtained from one representative leaf.
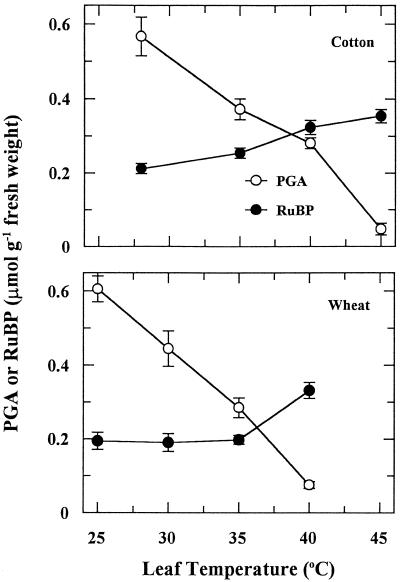
Heat stress altered the pools of PGA and RuBP in a manner consistent with a decrease in the activation state of Rubisco (Fig. 8). The level of PGA was very sensitive to increases in leaf temperature for both cotton and wheat, with significant decreases occurring before heat-stress-induced inhibition of CER (Fig. 1). At leaf temperatures of 45°C and 40°C for cotton and wheat, respectively, the level of PGA was barely detectable. The content of RuBP was relatively stable until leaf temperature exceeded 35°C for both plant species. Significant increases in RuBP content were observed at leaf temperatures greater than 35°C.
Figure 8.
The effect of rapid increases in leaf temperature on the levels of PGA and RuBP in leaves of cotton and wheat. Each point is the mean ± se of two independent experiments in which three leaves were analyzed for each experiment.
DISCUSSION
Isolated activase is extremely heat labile (Robinson and Portis, 1989; Holbrook et al., 1991; Crafts-Brandner et al., 1997; Eckardt and Portis, 1997), and the different polypeptide forms of activase differ in their sensitivity to inactivation by high temperature (Crafts-Brandner et al., 1997). The basis of thermal sensitivity appears to be disruption of subunit interactions that are necessary for activity (Crafts-Brandner et al., 1997). Feller et al. (1998) confirmed and extended the earlier reports of Weis (1981a, 1981b, 1982) and Kobza and Edwards (1987) by showing that Rubisco activation in intact leaf tissue was sensitive to rapid increases in leaf temperature. This inhibition was attributed to activase, which was shown to be denatured at temperatures greater than 40°C (Feller et al., 1998). Here we demonstrate that the activation state of Rubisco can acclimate to high-temperature stress in intact cotton and wheat plants and that the degree of acclimation is directly related to photosynthetic CO2 fixation. CER and Rubisco activation were closely correlated for both plant species analyzed in all of the temperature treatments (Fig. 4). Based on previous data (Crafts-Brandner et al., 1997; Feller et al., 1998), we attribute heat-stress-induced inhibition and acclimation of CER and Rubisco activation state to inhibition of activase and specifically to disrupted activase subunit interactions. Direct measurement of activase activity in leaf extracts of heat-stressed leaves is not feasible because the in situ stromal environment that promotes or disrupts subunit interactions would not be preserved upon tissue homogenization.
Under both acclimating and nonacclimating heat-stress conditions, wheat was more sensitive to heat stress than cotton. For example, rapidly increasing the leaf temperature to 40°C caused a much more severe inhibition of CER for wheat than for cotton (Fig. 1). Both species, however, were able to acclimate to heat stress if the leaf temperature was increased gradually. Under acclimating conditions, the T50 values of both CER and Rubisco activation were increased approximately 1.5°C to 2.0°C (Figs. 1 and 3). This degree of acclimation to heat stress was similar to that observed for kudzu when photosynthesis was determined in the presence of isoprene added to the air supplied to the leaves (Singsaas et al., 1997). It would be interesting to determine if isoprene influences Rubisco activation during heat stress of isoprene-emitting species.
Although the correlation between CER and initial Rubisco activity was very strong (Fig. 4), close inspection of the data indicated that there was a tendency for CER to be inhibited more than initial Rubisco activity at the higher leaf temperatures. Based on results using antisense activase plants, He et al. (1997) proposed that activase promotes the catalytic turnover of carbamylated Rubisco in addition to facilitating Rubisco carbamylation. It is possible that increasing leaf temperature has a differential effect on activase-mediated carbamylation, compared with activase-mediated catalytic turnover, of Rubisco. In addition, the specificity of Rubisco for O2 increases with temperature (Jordan and Ogren, 1984), which would influence the relationship between CER and Rubisco activation at higher leaf temperatures.
It is known that the sensitivity of the photosynthetic apparatus to heat stress is altered by light intensity. Based on Chl fluorescence analysis, Schreiber and Berry (1977) reported that light protects PSII from heat damage, whereas Weis (1982) found little effect of light on PSII activity. Weis (1982) also reported that photosynthesis and Rubisco activation were more sensitive if rapid heat treatments were imposed under dark versus light conditions. In our experiments, measurements of Chl fluorescence were made using leaves that were heat stressed under conditions of saturating light and subsequently dark adapted for a minimal amount of time. This protocol was used to best approximate the conditions used for CER and Rubisco activation experiments and to best approximate the environmental conditions in which heat stress would likely occur. Chl fluorescence analysis (Figs. 5 and 6) proved to be a sensitive indicator of heat-stress-induced inhibition of CER (Fig. 1) and initial Rubisco activity (Fig. 3). Heat stress was associated with increased qN, an indicator of Calvin cycle activity, and decreased Fv/Fm. Thus, heat stress inhibited both Calvin cycle and electron-transport processes. Under both rapid and gradual heat-stress treatments, however, significant perturbations in qN were detected at lower leaf temperatures than were required to alter Fv/Fm. Furthermore, relaxation of steady-state qN was delayed at temperatures that did not significantly alter Fv/Fm (Fig. 7), suggesting that the dissipation of the transthylakoid energy gradient was inhibited by heat stress. Heat-stress-related effects on xanthophyll metabolism could also be associated with the decreased relaxation of qN (Havaux and Tardy, 1996). Overall, Chl fluorescence analysis corroborated Rubisco activation assays and indicated that Calvin cycle activity was more sensitive to high temperature than Fv/Fm for both plant species under acclimating and nonacclimating conditions.
Using Chl fluorescence techniques, Havaux (1993) demonstrated that PSII activity in potato leaves could be acclimated to heat stress. Our Chl fluorescence experiments for cotton and wheat confirmed that PSII activity, as well as Calvin cycle activity, could acclimate to heat stress. Furthermore, comparison of the two species indicated that heat tolerance of PSII was greater in cotton than in wheat (Fig. 6). Because Calvin cycle activity (based on qN measurements; Fig. 5) and, more specifically, activase-dependent activation of Rubisco (Fig. 3) acclimated to heat stress in a species-specific manner similar to that seen in PSII, it appeared that sensitivity/tolerance to heat stress was manifested throughout the photosynthetic apparatus.
Perturbations in the levels of the substrate and product of Rubisco (Fig. 8) provided support for our conclusion that heat stress inhibited the flow of carbon through Rubisco. For both plant species, PGA content declined markedly in response to rapid increases in leaf temperature, such that PGA was barely detectable at the highest temperature. PGA levels were decreased (Fig. 8) before any detectable change was seen in CER or initial Rubisco activity (Figs. 1 and 3). PGA is a substrate in numerous metabolic reactions, and its content and allocation to the Calvin cycle could be influenced according to the temperature dependence of several enzymes. On the other hand, RuBP content is directly indicative of Rubisco activity, and inhibition of any other Calvin cycle enzyme would lead to depletion of RuBP. There was no evidence of heat-related depletion of RuBP for either species. At temperatures greater than 35°C, RuBP levels were increased significantly, which is indicative of inhibited carbon flow through Rubisco. Gradual increases in leaf temperature had a similar effect on PGA and RuBP levels, as observed for rapid temperature increases (data not shown). Kobza and Edwards (1987) reported similar effects of rapid heat stress on PGA and RuBP levels in wheat. Additionally, PGA content was more sensitive than RuBP content when the flow of carbon through Rubisco was restricted in antisense Rubisco plants (Quick et al., 1991) or antisense activase plants grown under ambient CO2 (He et al., 1997).
We conclude that the light-dependent activation of Rubisco, which is mediated by Rubisco activase, is one of the most thermally labile reactions associated with the photosynthetic apparatus. Inhibition of this reaction is directly related to inhibition of CER and, as such, could have a significant effect on plant growth and development. Our results indicate that activase sensitivity to high temperature varies among plant species and that activase activity can acclimate during a relatively short period when the leaf temperature is increased in gradual increments. It will be important to determine the mechanism associated with activase acclimation and why cotton activase is more heat tolerant than wheat activase. Differences in heat tolerance between the two forms of activase from spinach (Crafts-Brandner et al., 1997) indicate that the inherent thermal properties of the subunits of the enzyme may differ both within and among species. Heat-stress-induced changes in the pools of ATP and ADP, which are substrates known to stabilize activase (Robinson and Portis, 1989; Wang et al., 1993), could influence the acclimation of activase as leaf temperature is gradually increased. Acclimation to high temperature may be associated with altered biosynthesis of the molecular forms of activase.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance provided by Donald L. Brummett. We also thank M.E. Salvucci for many insightful discussions and H.C. Huppe for helpful suggestions concerning the metabolite analysis.
Abbreviations:
- CER
CO2-exchange rate
- Chl
chlorophyll
- Fv/Fm
maximum quantum yield of photochemistry of PSII
- PGA
3-phosphoglyceric acid
- qN
nonphotochemical Chl fluorescence quenching
- RuBP
ribulose-1,5-bisphosphate
- T50
temperature that causes 50% inhibition
Footnotes
Mention of a trade name does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval over other products that may also be suitable.
LITERATURE CITED
- Andrews TJ, Hudson GS, Mate CJ, von Caemmerer S, Evans JR, Avridsson YBC. Rubisco, consequences of altering its expression and activation in transgenic plants. J Exp Bot. 1995;46:1293–1300. [Google Scholar]
- Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bilger W, Schreiber U, Lange OL. Chlorophyll fluorescence as an indicator of heat induced limitation of photosynthesis in Arbutus unedo L. In: Tenhunen JD, Catarino FM, Lange OL, editors. Plant Responses to Stress. Berlin: Springer; 1987. pp. 391–399. [Google Scholar]
- Brooks SPG. A simple computer program with statistical tests for the analysis of enzyme kinetics. BioTechniques. 1992;17:1154–1161. [PubMed] [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME. The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol. 1997;114:439–444. doi: 10.1104/pp.114.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt NA, Portis AR., Jr Heat denaturation profiles of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase and the inability of Rubisco activase to restore activity of heat-denatured Rubisco. Plant Physiol. 1997;113:243–248. doi: 10.1104/pp.113.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt NA, Snyder GW, Portis AR, Jr, Ogren WL. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose-1,5-bisphosphate carboxylase/oxygenase activase content. Plant Physiol. 1997;113:575–586. doi: 10.1104/pp.113.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998;116:539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993;16:461–467. [Google Scholar]
- Havaux M, Tardy F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta. 1996;198:324–333. [Google Scholar]
- He Z, von Caemmerer S, Hudson GS, Price GD, Badger MR, Andrews TJ. Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development. Plant Physiol. 1997;115:1569–1580. doi: 10.1104/pp.115.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook GP, Galasinski SC, Salvucci ME. Regulation of 2-carboxyarabinitol 1-phosphatase. Plant Physiol. 1991;97:894–899. doi: 10.1104/pp.97.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Grodzinski B. The effect of leaf temperature and photorespiratory conditions on export of sugars during steady-state photosynthesis in Salvia splendens. Plant Physiol. 1996;111:169–178. doi: 10.1104/pp.111.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. The CO2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase: dependence on ribulose-bisphosphate concentration, pH and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987;83:69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardos ED, Tsujita MJ, Grodzinski B. The effect of source or sink temperature on photosynthesis and 14C partitioning in and export from a source leaf of Alstroemeria. Physiol Plant. 1996;97:563–575. [Google Scholar]
- Quick WP, Schurr U, Scheibe R, Schulze E-D, Rodermel SR, Bogorad L, Stitt M. Decreased ribulose-1,5-bisphosphate carboxylase/oxygenase in transgenic tobacco transformed with “antisense” rbcS. I. Impact on photosynthesis in ambient growth conditions. Planta. 1991;183:542–554. doi: 10.1007/BF00194276. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Portis AR., Jr Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch Biochem Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Anderson JC. Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol. 1987;85:66–71. doi: 10.1104/pp.85.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Berry JA. Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus. Planta. 1977;136:233–238. doi: 10.1007/BF00385990. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Seemann J, Kobza J, Moore B. Metabolism of 2-carboxyarabinitol-phosphate and regulation of ribulose-1,5-bisphosphate carboxylase activity. Photosynth Res. 1990;23:119–130. doi: 10.1007/BF00035005. [DOI] [PubMed] [Google Scholar]
- Seemann JR, Kirschbaum MUF, Sharkey TD, Pearcy RW. Regulation of ribulose-1,5-bisphosphate carboxylase activity in Alocasia macrorrhiza in response to step changes in irradiance. Plant Physiol. 1988;88:148–152. doi: 10.1104/pp.88.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Edmondson DL. Relationship between steady-state gas exchange, in vivo ribulose bisphosphate carboxylase activity and some carbon reduction cycle intermediates in Raphanus sativus. Aust J Plant Physiol. 1986;13:669–688. [Google Scholar]
- Wang ZY, Ramage RT, Portis AR., Jr Mg2+ and ATP or adenosine 5′-[γ-thio]-triphosphate (ATPγS) enhances intrinsic fluorescence and induces aggregation which increases the activity of spinach Rubisco activase. Biochim Biophys Acta. 1993;1202:47–55. doi: 10.1016/0167-4838(93)90061-u. [DOI] [PubMed] [Google Scholar]
- Weis E. The temperature sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett. 1981a;129:197–200. [Google Scholar]
- Weis E. Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta. 1981b;151:33–39. doi: 10.1007/BF00384234. [DOI] [PubMed] [Google Scholar]
- Weis E. Influence of light on the heat sensitivity of the photosynthetic apparatus in isolated spinach chloroplasts. Plant Physiol. 1982;70:1530–1534. doi: 10.1104/pp.70.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]