Significance
There has been an emerging interest in sleep and its association with β-amyloid burden as a risk factor for Alzheimer’s disease. Despite the evidence that acute sleep deprivation elevates β-amyloid levels in mouse interstitial fluid and in human cerebrospinal fluid, not much is known about the impact of sleep deprivation on β-amyloid burden in the human brain. Using positron emission tomography, here we show that acute sleep deprivation impacts β-amyloid burden in brain regions that have been implicated in Alzheimer’s disease. Our observations provide preliminary evidence for the negative effect of sleep deprivation on β-amyloid burden in the human brain.
Keywords: beta amyloid, sleep, hippocampus, Alzheimer’s disease, glymphatic system
Abstract
The effects of acute sleep deprivation on β-amyloid (Aβ) clearance in the human brain have not been documented. Here we used PET and 18F-florbetaben to measure brain Aβ burden (ABB) in 20 healthy controls tested after a night of rested sleep (baseline) and after a night of sleep deprivation. We show that one night of sleep deprivation, relative to baseline, resulted in a significant increase in Aβ burden in the right hippocampus and thalamus. These increases were associated with mood worsening following sleep deprivation, but were not related to the genetic risk (APOE genotype) for Alzheimer’s disease. Additionally, baseline ABB in a range of subcortical regions and the precuneus was inversely associated with reported night sleep hours. APOE genotyping was also linked to subcortical ABB, suggesting that different Alzheimer’s disease risk factors might independently affect ABB in nearby brain regions. In summary, our findings show adverse effects of one-night sleep deprivation on brain ABB and expand on prior findings of higher Aβ accumulation with chronic less sleep.
Beta-amyloid (Aβ) is present in the brain’s interstitial fluid (ISF) and is considered a metabolic “waste product” (1). Mechanisms by which Aβ is cleared from the brain are not completely understood (2), although there is evidence that sleep plays an important role in Aβ clearance (3). In rodents, chronic sleep restriction led to increases in ISF Aβ levels (4) and in a Drosophila model of Alzheimer’s disease (AD), chronic sleep deprivation (SD) resulted in higher Aβ accumulation (5). In healthy humans, imaging studies have revealed associations between self-reports of less sleep duration or poor sleep quality and higher Aβ burden (ABB) in the brain (6–8), which is a risk factor for AD. This association has been considered bidirectional because increased ABB could also lead to impairments in sleep (9, 10). Notably, increased ABB in the brain has been associated with impairment of brain function (11, 12). Thus, strategies that prevent Aβ accumulation in the brain could promote healthy brain aging and be useful in preventing AD. In this respect, there is increasing evidence that sleep disturbances might contribute to AD, in part by facilitating accumulation of Aβ in the brain (13).
To better characterize ABB dynamics, studies have focused on the effects of sleep patterns on ABB in the CNS. In rodents, it has been shown that Aβ clearance from the brain’s ISF predominately occurred during sleep (4), which was ascribed to the glymphatic pathway, operating most efficiently during sleep (3, 14, 15). Clinical studies have also shown that Aβ levels in the cerebrospinal fluid (CSF) are the highest before sleep and the lowest after wakening, while CSF Aβ clearance was counteracted by SD (16). However, there are some inconsistencies between animal models and findings in humans (17), and Aβ increases in human CSF could reflect factors other than ABB increases in the brain itself (18–21). Notably, the effects of acute SD on Aβ clearance in the human brain have not been documented. This observation will be important for understanding the contribution of sleep to Aβ clearance from the brain and the regional specificity of such effects.
Here we evaluated the effects of one-night SD on ABB in healthy controls to investigate whether sleep affects clearance of Aβ from the human brain. For this purpose, we used positron emission tomography (PET) with which it is now possible to measure ABB in the living human brain. There are several validated PET radiotracers for this purpose, including 18F-florbetaben (FBB) (22, 23). It is believed that such radiotracers predominantly bind to insoluble Aβ42 plaques (24–27), but there is recent evidence that they also bind to soluble Aβ42 forms (28). Thus, we reasoned that PET and FBB could be used to detect increases in ABB because of acute SD, directly in the human brain (3). First, we aimed to assess the effect of one-night SD on brain ABB with PET-FBB in healthy controls (n = 20, 22–72 y old, 10 females) (Table S1), and compared the measures to baseline brain ABB captured at the same time of the day but following a night of rested sleep [referred to as rested-wakefulness (RW)]. Second, we aimed to replicate in our sample the previously reported association between sleep history and brain ABB (when measured after RW) (6–8). For our first aim, we hypothesized that one night of SD would increase ABB in the hippocampus, which shows some of the earliest structural and functional changes in AD (29, 30). For our second aim, we hypothesized that history of poor sleep would be associated with higher ABB in the hippocampus, precuneus, and medial prefrontal cortex (6, 8, 31).
Results
Acute SD Effects.
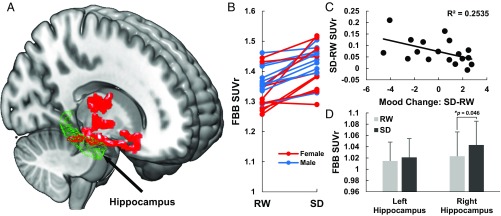
To compare the differences in FBB binding [quantified as relative standard uptake value (SUVr) and used as a marker of ABB] (Methods) after acute SD versus that obtained after RW, we used a voxelwise paired t test in statistical parametric mapping (SPM) (Methods). This analysis showed that images obtained after SD compared with those obtained after RW had significantly higher FBB binding (ABB increases) in a right lateralized cluster (Fig. 1A) that comprised hippocampal, parahippocampal, and thalamic regions (Table S2). Of note, the increases in FBB SUVr in this cluster were robust and observed in 19 of 20 participants (Fig. 1B) from RW (mean = 1.35, standard deviation = 0.06) to SD (mean = 1.42, standard deviation = 0.07; a 5% increase, P < 0.0001). To further confirm this finding, we quantified FBB SUVr in an a priori hippocampal region of interest (ROI) (Methods and Fig. 1D) and compared the measures after SD to those after RW. This ROI analysis also showed a significant increase in FBB SUVr in the right hippocampus ROI from RW to SD (P = 0.046, two-tailed, Cohen’s d = 0.48) but not in the left hippocampus (P = 0.4). The magnitude of the Aβ changes in the hippocampal cluster varied significantly between subjects (Fig. 1B) (−0.58% to +16.1%). We found that this variability was not associated with gender, age, or apolipoprotein E (APOE)-based odds ratio for AD (ORAD) (Methods) (P > 0.3). Notably, changes in FBB SUVr in the subcortical cluster were significant in both males (P = 0.0008) and females (P = 0.003). In addition, reported sleep hours (SH) and total score (TS) for sleep quality (Pittsburgh Sleep Questionnaire Inventory, PSQI) (Methods) were not associated with these SD-related increases. Thus, the mechanisms accounting for the observed between-subject variability are still unclear. Subjective behavioral assessment revealed that SD negatively impacted mood compared with RW (Methods and Fig. S1). We assessed if the effects of SD on mood were correlated with increases in ABB in the right hippocampal cluster. This analysis showed that mood worsening was negatively associated with changes in FBB SUVr [r (16) = −0.50, P = 0.03] (Fig. 1C) such that participants with larger increases in ABB in the hippocampal cluster (Fig. 1A) had more mood worsening after SD. Because the quantification of ABB using FBB SUVr can be sensitive to blood perfusion effects, we quantified FBB accumulation using measures of binding potential (BPnd), which are less sensitive to blood perfusion effects than SUVr measures. BPnd in the cluster where we observed the SD effect (Methods and Fig. 1A) was also significantly higher in SD relative to RW [t(19) = 3.57, P = 0.002] (Fig. S2A). Moreover, SD-related changes in BPnd were significantly correlated with those observed with FBB SUVr [r(18) = 0.53, P = 0.016] (Fig. S2B), further supporting that SD-related increases in FBB SUVr are not primarily driven by perfusion effects.
Fig. 1.
Effects of one-night SD on ABB. (A) Voxelwise paired t test between RW and SD conditions highlighting the hippocampus as well as other subcortical structures (PFWE < 0.05, cluster-size corrected) (Table S1). (B) Subject-level changes in FBB SUVr (in the red cluster identified in A) from RW to SD. There was no significant effect of gender, or gender × sleep interaction (P > 0.15). (C) Association between changes in mood from RW to SD and changes in the FBB SUVr for the cluster identified in A. Mood change was quantified using the principal component of the changes in self-report measures from RW to SD, which accounted for 35.5% of the variance. Self-report measures of alert, friendly, happy, social, and energetic significantly decreased, and measures of tired and difficulty staying awake significantly increased from RW to SD (P < 0.001, two-tailed) (see also Fig. S1). (D) Average FBB SUVr in a priori hippocampus ROIs across subjects. Error bars show standard deviation (Methods).
Sleep, APOE, and ABB.
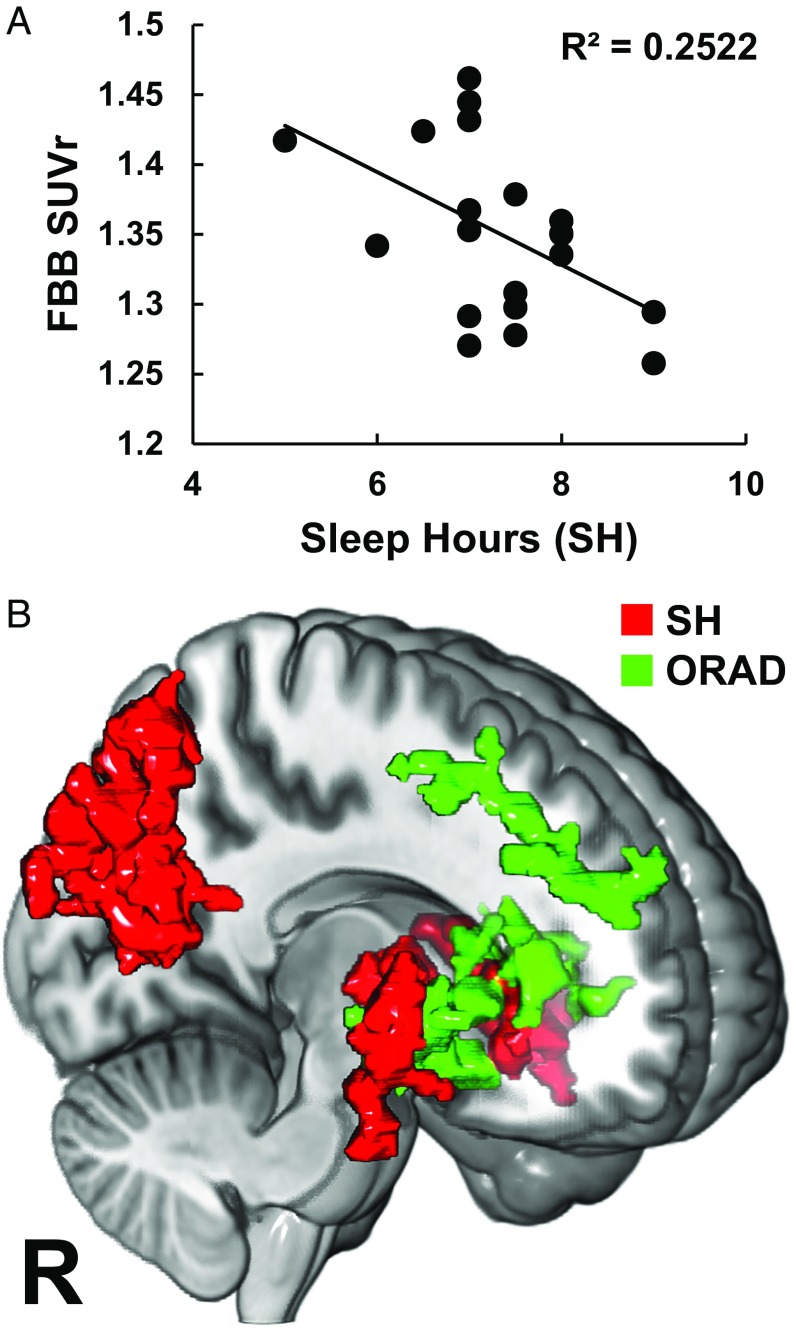
Prior studies had reported an association between reported SH and sleep quality and (cortical) ABB in healthy middle-aged and older individuals (6–8). We tested whether we would corroborate those observations in our sample using the measures obtained during RW. We found that reported average SH inversely correlated with FBB SUVr [r(18) = −0.5, P = 0.024] and with BPnd [r(18) = −0.57, p = 0.009] at RW in the subcortical cluster that showed increases in ABB with SD (Fig. 2A), thus supporting long-term susceptibility of these regions to increased ABB with less SH. Voxelwise regression analysis of FBB SUVr on SH showed that less SH was associated with higher FBB SUVr in the bilateral putamen, parahippocampus, and right precuneus (Fig. 2B and Table S3). Interestingly, the SH-related brain areas were more extensive than the areas associated with (acute) SD-induced ABB increases. For regional ABB, SH-related subcortical regions (Table S3) had minimal overlap with subcortical areas related to ORAD (Fig. 2B), which included the bilateral lentiform nucleus and pallidum (Table S4). These observations suggested that different brain regions could be independently affected by different AD-risk factors (i.e., sleep vs. APOE). This observation is consistent with prior findings reporting that the APOE genotype did not moderate the relationship between sleep measures and ABB in the brain (7).
Fig. 2.
Relationship between SH, APOE genotype, and ABB. (A) Regression of FBB SUVr (indexing ABB) for the red cluster shown in Fig. 1A at RW against SH. (B) Three-dimensional rendering of the areas showing an association between higher FBB SUVr at RW and lower SH (red clusters, PFWE < 0.05, cluster-size corrected) (Table S3), as well as areas showing an association between higher FBB SUVr and higher APOE-based genetic risk for AD (quantified as log of ORAD) (green clusters, PFWE < 0.05, cluster-size corrected, except the right-sided subcortical cluster, which was cluster size-corrected at qFDR < 0.05) (Table S4). The subcortical clusters related to SH and ORAD had minimal spatial overlap (18 voxels, <3%) (Tables S3 and S4). Notably, FBB SUVr within the SH-related subcortical clusters (red, B) was not associated with ORAD (P = 0.25) and FBB SUVr within the ORAD-related subcortical clusters (green, B) was not associated with SH (P = 0.2).
Discussion
Our findings provide preliminary evidence for the role of SD on Aβ accumulation in the human brain. The increases in ABB after SD in the hippocampus, which is considered among the most-sensitive brain regions to AD neuropathology (29), is consistent with epidemiological data identifying impaired sleep as a risk factor for AD (9, 10) and with recent evidence showing that disruption of deep sleep increases Aβ in human CSF (32). We also showed Aβ increases in the thalamus after SD, which is a brain region that shows increases in Aβ in the early stages of AD (33). The regional increases in ABB that we observed from RW to SD might reflect decreased clearance of Aβ, presumably from lack of sleep, thus supporting the role of glymphatic system in clearing Aβ from the brain during sleep. While this effect was observed in rodents (3), no study as of now has been able to directly measure Aβ clearance reflecting glymphatic function in the human brain. Other mechanisms have been shown to stimulate Aβ clearance during sleep, such as γ-oscillations during the rapid eye-movement cycle (34). Alternatively, Aβ increases in the hippocampus after SD could reflect increases in Aβ synthesis associated with endogenous neuronal activity during SD (35), or sleep-related changes in hippocampal neurogenesis (36, 37). While we are interpreting our findings of increases in ABB after SD to reflect Aβ accumulation due to lack of glymphatic clearance (or other unknown mechanisms), we cannot rule out the possibility that they reflect increases in the synthesis of Aβ (38) from lack of sleep. Preclinical studies that monitor Aβ clearance during sleep using PET radiotracers alongside optical imaging of ISF are needed to corroborate whether Aβ ligands and PET have potential as markers of glymphatic function in the human brain. Future work should also study the extent that elevated ABB in the brain is related to increases in Aβ levels in the peripheral tissue following SD (18, 20).
Detection of Aβ plaques with PET is clinically relevant for the diagnosis of AD, while elevated brain ABB in mild cognitive impairment (MCI) has been suggested as a risk factor for progression to AD (22, 39). ABB increases as a function of aging and AD severity, with an estimated 17% increase from younger to older adults (40). Relative to healthy elderly, estimated increases of 21% and 43% have been reported in individuals with MCI and AD, respectively (40). In our study, the increases in ABB due to one-night SD were smaller (5%) (Fig. 1 A and B) and were observed in a subcortical cluster that included the hippocampus. At this stage, we are uncertain whether such SD-related increases in ABB may subside following rested sleep. In addition, the magnitude of the observed effects should be interpreted with caution considering the methodological limitations and potential confounds, such as the effects of blood-flow changes and the limited sensitivity of current PET radiotracer to soluble Aβ (28). While, prior studies have reported associations with chronic poor sleep and higher average ABB across large a priori-selected cortical areas (6–8), our results highlight the relevance of studying subcortical regions for the associations between ABB and sleep.
It has been shown in rodents that Aβ plaques can form very rapidly (41), while Aβ plaques in their earliest stages show the highest levels of neuritic dystrophy (42), thus suggesting that increases in ABB due to one-night SD could adversely impact the brain. Chronic poor sleep could then result in higher baseline ABB levels, helping to explain the association with higher ABB at RW (in the cluster showing SD effects) (Fig. 1A) and less reported SH (Fig. 2A). In addition, SH negatively correlated with ABB in the precuneus, putamen, and parahippocampus (Fig. 2B and Table S3). These findings are consistent with studies showing that chronic sleep restriction lead to elevated cortical Aβ oligomers levels (that are neurotoxic) in the mouse cortex (43), which might in part reflect up-regulation of β-secretase 1 (44). However, a confounder is that increased ABB could in turn exacerbate sleep problems (10).
It is noteworthy that ABB increases in the hippocampus are less pronounced in MCI (45) or early-stage AD than in the precuneus (22). Instead, changes in hippocampal volume, glucose metabolism (30), and the blood–brain barrier (46) were most evident in patients with early-stage AD. Thus, it is possible that the increases in ABB in the hippocampus with sleep disruptions might trigger local neurotoxicity without necessarily resulting in marked plaque accumulation. The precuneus was not significantly affected by SD; however, we found an association between reported SH and ABB in this region (Fig. 2B and Table S3), again suggesting that distinct processes might mediate the effects of acute vs. chronic SD on regional ABB. Future work should investigate the extent to which the effects of acute SD and less SH might reflect distinct mechanisms (i.e., neuroinflammation triggered by chronic poor sleep) (47, 48) or sensitivity of FBB to different forms of Aβ (soluble oligomers vs. plaques) (28). One limitation of this work includes the inability of PET-FBB to distinguish soluble from insoluble Aβ (27, 28, 49). We suggested that the interruption of glymphatic clearance by SD would increase ABB in the brain, yet our findings do not demonstrate the mechanisms that account for the Aβ accumulation with SD. In our study, estimates of ABB were obtained using FBB SUVr, which is sensitive to physiological factors such as blood flow, which could have been impacted by SD (50, 51). However, recent evidence suggests that blood-flow effects are small on FBB SUVr estimated with later time points (52) (Methods). We also corroborated the FBB SUVr findings with BPnd measures that are less sensitive to blood-flow effects (Fig. S2).
In our study, we did not predict the laterality effect for the SD-related increases in ABB, and while this could reflect the sensitivity of the glymphatic system to orientation of the head during sleep (14), we did not record head position. Consistent with the recognized role of the hippocampus and thalamus in mood disorders (53), the association between SD-related increases in ABB and mood worsening (Fig. 1C) supported the functional significance of elevated ABB. This association could reflect the previously reported contribution of the hippocampus in modulating mood changes that follow SD (54). The effects of SD on the hippocampus have also been implicated in the memory impairment associated with SD, although we did not measure effects of SD on memory in our study. Even though our sample was small (n = 20), we were able to identify a significant effect of SD on brain ABB with no significant interaction with gender. Because of the small sample size of our study, future studies are needed to assess the generalizability to a larger and more diverse population and to more reliably characterize potential gender effects.
In summary, this study documents an effect of one-night SD on ABB in the hippocampus, thus providing preliminary evidence that sleep, among other factors, could influence Aβ clearance in the human brain. Our results highlight the relevance of good sleep hygiene for proper brain function and as a potential target for prevention of AD (31, 55).
Methods
Participants.
Twenty-two healthy individuals were recruited at the National Institutes of Health, of which 20 (10 females, age: 39.8 y ± 10.4, range: 22–72 y old) completed two PET scan sessions to measure ABB. All participants provided informed consent to participate in the study that was approved by the Institutional Review Board at the NIH (Combined Neurosciences White Panel). Exclusion criteria were: (i) urine positive for psychotropic drugs; (ii) history of alcohol or drug use disorders; (iii) present or past history of neurological or psychiatric disorder, including evidence of cognitive impairment; (iv) use of psychoactive medications in the past month (i.e., opiate analgesics, stimulants, sedatives); (v) currently taking prescription medications (i.e., antihistamines, antihypertensive, antibiotics); (vi) medical conditions that may alter cerebral function; (vii) cardiovascular and metabolic diseases; and (viii) history of head trauma with loss of consciousness longer than 30 min. Table S1 summarizes physiological and neuropsychological assessment to ensure participants were healthy and were not cognitively impaired.
Structural MRI.
Participants also underwent MRI in a 3.0 T Magnetom Prisma scanner (Siemens Medical Solutions) using a 32-channel head coil to collect T1-weighted 3D MPRAGE (TR/TE = 2,400/2.24 ms, 0.8-mm isotropic resolution) and T2-weighted spin-echo multislice (TR/TE = 3,200/564 ms, 0.8-mm in-plane resolution). MRI was processed using the minimal preprocessing pipeline of the Human Connectome Project (56). Specifically, FreeSurfer v5.3 (Martinos Center for Biomedical Imaging; https://surfer.nmr.mgh.harvard.edu/) was used for anatomical data segmentation. In addition, each MRI image underwent gradient distortion correction, field map processing, spatial normalization to the stereotactic space of the Montreal Neurological Institute (MNI) with 2-mm isotropic resolution, and brain masking using routines from University of Oxford’s Center for Functional Magnetic Resonance Imaging of the Brain Software Library release 5.0 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). FreeSurfer segmentation (‘wmparc.nii’) in the MNI space was used for generating a subject-specific mask of the hippocampus (label numbers: 17 and 53 for left and right hippocampus, respectively).
PET Data Acquisition.
The PET scans were performed using a high-resolution research tomography Siemens scanner on two separate scan days with FBB. FBB was injected through an intravenous catheter in about 1 min using a Harvard pump. Dynamic scanning started immediately after FBB injection, with 1.23-mm isotropic resolution using list-mode acquisition. During the PET imaging procedures, the participants rested quietly under dim illumination. To ensure that subjects did not fall asleep, they were monitored throughout the procedure and asked to keep their eyes open (no fixation cross). Information about head movement was collected using a cap with small light reflectors and a Polaris Vicra (Northern Digital) head-tracking system to minimize motion-related image blurring. Before FBB injection, a transmission scan was obtained using cesium-137 to correct for attenuation.
FBB-PET Scans.
Each participant underwent two FBB-PET scans to measure ABB, one scan on a day following RW and another scan on a day following a night of SD. For this purpose, participants stayed overnight at the Clinical Center at the NIH before their scheduled SD or RW scans. For the SD condition, participants were instructed to wake up at 8:00 AM on the morning before the SD night. Upon arrival, participants were continuously accompanied by a nurse to ensure that they stayed awake for the SD condition during their stay. For the RW conditions, nurses observed whether patients were asleep every hour from 10:00 PM to 7:00 AM. On the day of RW, the participants were woken up at 7:00 AM. On both days, participants remained under supervision of the nurse and were brought to the PET imaging center before the scan, which started around 1:30 PM. Thus, participants remained awake for a total of about 31 h (including the scan length) during the SD condition. No food was given after midnight and caffeinated beverages were discontinued 24 h before the study. Patients had a light breakfast and lunch on the scan day. The order of RW and SD scans was counterbalanced across subjects and were, on average, 15 d apart (standard deviation = 19 d). In preparation for the scans, a catheter was placed for radiotracer injection. Dynamic scanning started right after intravenous injection of about 9.5 mCi (or less) of FBB, which lasted for 120 min. During FBB scans, participants were encouraged to listen to music to stay awake.
PET Data Analysis.
FBB uptake in the brain was quantified using the SUVr using the whole cerebellum as reference region for images obtained from later time points (90–110 min) (57, 58). We chose the SUVr method given the recent evidence (for a radiotracer from the same family as FBB) that the SUVr approach of estimating tracer accumulation, relative to other noninvasive kinetic modeling approaches, had one of the highest correlations, with arterial input compartment modeling results (R2 = 0.95), and had comparable accuracy, while maintaining much simpler modeling requirements and not suffering from voxel-level noise on model fitting (59). Despite the concern that SUVr estimates of radiotracer accumulation are affected by confounds such as blood-flow changes, recent work has suggested a limited influence of blood-flow changes on FBB SUVr measures obtained from the later time points, similar to the range used in our study (i.e., 90–110 min) (52). SUVr images for FBB were coregistered with individual subjects’ T1-weighted images and resampled into 2-mm isotropic resolution before being transformed into the MNI space using the same normalization parameters that were generated for T1-weighted (and T2-weighted) images. For statistical parametric mapping, images were smoothed with a 4-mm kernel and masked at 0.5 SUVr to remove voxels outside the brain. We also performed a follow-up BPnd analysis to address the concern that FBB SUVr might have been affected by confounds, such as changes in blood flow and tracer clearance properties (52, 60). Specifically, regional BPnd was calculated using a noninvasive simplified reference tissue model (SRTM) (61, 62) that was fitted to the time activity curve (0–120 min) derived from the subcortical cluster showing a significant effect of SD on FBB SUVr (Fig. 1A). We also corroborated SRTM-based BPnd findings using the reference Logan approach (Fig. S2) (59, 63). Kinetic modeling of dynamic PET data was performed in the PMOD Kinetic Modeling Tool v3.605 (PMOD Technologies). For consistency with the SUVr approach, the whole cerebellum was used as the reference region for the BPnd analyses.
Pittsburgh Sleep Quality Index.
The PSQI questionnaire (64) was administered to all participants (n = 20). For the analyses, we used the number of SH and the TS quality score from the PSQI, because prior studies have linked these sleep measures to brain ABB (6, 65, 66). While SH is a self-report of participants’ SH at night (excluding times spent awake in bed), TS is a composite of scores of sleep quality, latency, hours, efficiency, medication, disturbances because of one’s health or sleep partners, and daytime life quality (lower TS indicates better sleep quality).
APOE Genotyping.
SNPs of the APOE gene (rs7412 and rs429358) have been shown to influence brain glucose metabolism and ABB (67, 68). Accordingly, in all participants we genotyped rs7412 and rs429358 SNPs of APOE and computed a log of ORAD for each participant, following the methods of a previous study (69), normalized relative to the population risk for AD. We used ORAD to evaluate whether APOE influenced the association between sleep and ABB.
Mood Questionnaires.
Mood questionnaires were collected periodically (five times) throughout the RW and SD scans in 18 of the 20 participants. Questionnaires were acquired at least an hour apart, prior (three measures), during (one measure), and after (one measure) the PET scans. On a scale of 0–10, subjects rated whether they felt alert, tired, hungry, friendly, happy, sad, anxious, irritable, social, confused, bored, comfortable, energetic, caffeine craving, and difficulty staying awake. The scores across the five time-points were averaged for each mood measure for each scan day. The first principle component of the standardized SD–RW difference scores for the 15 self-report mood measures was computed to summarize the change in mood from RW to SD into one component. This component accounted for 35.5% of the variance of the mood change measures (SD–RW) and was positively correlated with changes in ratings of feeling alert, friendly, happy, social, and energetic (P < 0.01, two-tailed), and negatively correlated with changes in ratings of feeling tired, anxious, and irritable from RW to SD (P < 0.01, two-tailed). Higher scores along this component reflected positive changes in mood from RW to SD.
Statistical Parametric Mapping.
SPM8 (Wellcome Trust Centre for Neuroimaging) (70) was used for performing a voxelwise paired t test between SD and RW in FBB SUVr and voxelwise correlation between behavioral or genotyping measures and PET data. All effects were thresholded at P ≤ 0.015 in SPM8 and a minimum cluster size of 300 voxels (2-mm isotropic). We chose this threshold based on the low sensitivity of FBB to soluble Aβ (28), but effects were corrected for multiple comparisons for cluster size using the random field theory (71) (family-wise error, FWE), or unless indicated, with false-discovery rate (FDR) in SPM8.
Data Availability.
Subject-level measures used in the manuscript are available in Dataset S1. Please contact corresponding authors for additional information.
Supplementary Material
Acknowledgments
We thank Joanna Fowler for the insightful comments; Christopher Wong, Lori Talagala, and Minoo McFarland for their assistance with behavioral and imaging data collection; David Goldman, Hui Sun, and Melanie Schwandt for assistance with APOE genotyping; Jeih-San Liow and Santi Bullich for insights about PET data analysis; and Kimberly Herman, Tom Lionetti, and Rosa Clark for assistance with monitoring participants. This work was supported by NIH/National Institute on Alcohol Abuse and Alcoholism intramural research program (Grant Y1AA3009).
Footnotes
Conflict of interest statement: S.D.S. was an employee of Piramal Pharma Inc., which partly supported the radiotracer for this study.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721694115/-/DCSupplemental.
References
- 1.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017;131:2257–2274. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J-E, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabuchi M, et al. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr Biol. 2015;25:702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spira AP, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown BM, et al. AIBL Research Group The relationship between sleep quality and brain amyloid burden. Sleep (Basel) 2016;39:1063–1068. doi: 10.5665/sleep.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprecher KE, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju Y-ES, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagust W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. doi: 10.1093/brain/awv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spira AP, Gottesman RF. Sleep disturbance: An emerging opportunity for Alzheimer’s disease prevention? Int Psychogeriatr. 2017;29:529–531. doi: 10.1017/S1041610216002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, et al. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ooms S, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 17.LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006320. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu XL, et al. Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry. October 31, 2017 doi: 10.1038/mp.2017.204. [DOI] [PubMed] [Google Scholar]
- 19.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 20.Wei M, et al. Sleep deprivation induced plasma amyloid-β transport disturbance in healthy young adults. J Alzheimers Dis. 2017;57:899–906. doi: 10.3233/JAD-161213. [DOI] [PubMed] [Google Scholar]
- 21.Lucey BP, et al. Effect of sleep on overnight CSF amyloid-β kinetics. Ann Neurol. December 8, 2017 doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 23.Rowe CC, et al. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: Proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 24.Fodero-Tavoletti MT, et al. In vitro characterization of [18F]-florbetaben, an Aβ imaging radiotracer. Nucl Med Biol. 2012;39:1042–1048. doi: 10.1016/j.nucmedbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Villemagne VL, et al. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011;52:1210–1217. doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- 26.Sabri O, Seibyl J, Rowe C, Barthel H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26. doi: 10.1007/s40336-015-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni R, Gillberg P-G, Bergfors A, Marutle A, Nordberg A. Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain. 2013;136:2217–2227. doi: 10.1093/brain/awt142. [DOI] [PubMed] [Google Scholar]
- 28.Yamin G, Teplow DB. Pittsburgh compound-B (PiB) binds amyloid β-protein protofibrils. J Neurochem. 2017;140:210–215. doi: 10.1111/jnc.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuff N, et al. Alzheimer’s Disease Neuroimaging Initiative MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Santi S, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 31.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39:552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ju YS, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thal DR, Rüb U, Orantes M, Braak H. Phases of A β-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 34.Aron L, Yankner BA. Neurodegenerative disorders: Neural synchronization in Alzheimer’s disease. Nature. 2016;540:207–208. doi: 10.1038/540207a. [DOI] [PubMed] [Google Scholar]
- 35.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng O, et al. Short-term sleep deprivation stimulates hippocampal neurogenesis in rats following global cerebral ischemia/reperfusion. PLoS One. 2015;10:e0125877. doi: 10.1371/journal.pone.0125877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes C, et al. Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front Cell Neurosci. 2015;9:140. doi: 10.3389/fncel.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Jr, et al. Alzheimer’s Disease Neuroimaging Initiative Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenberghe R, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 41.Meyer-Luehmann M, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condello C, Schain A, Grutzendler J. Multicolor time-stamp reveals the dynamics and toxicity of amyloid deposition. Sci Rep. 2011;1:19. doi: 10.1038/srep00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao HY, et al. Chronic sleep restriction induces cognitive deficits and cortical beta-amyloid deposition in mice via BACE1-antisense activation. CNS Neurosci Ther. 2017;23:233–240. doi: 10.1111/cns.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camus V, et al. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging. 2012;39:621–631. doi: 10.1007/s00259-011-2021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagne A, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai Z, Hussain MD, Yan L-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci. 2014;124:307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 48.Zhu B, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–355. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sehlin D, et al. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat Commun. 2016;7:10759. doi: 10.1038/ncomms10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu JC, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 51.Ma N, Dinges DF, Basner M, Rao H. How acute total sleep loss affects the attending brain: A meta-analysis of neuroimaging studies. Sleep (Basel) 2015;38:233–240. doi: 10.5665/sleep.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bullich S, et al. Validation of non-invasive tracer kinetic analysis of 18F-florbetaben PET using a dual time-window acquisition protocol. J Nucl Med. 2017:jnumed.117.200964. doi: 10.2967/jnumed.117.200964. [DOI] [PubMed] [Google Scholar]
- 53.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller AD, Meerlo P, McGinty D, Mistlberger RE. Sleep and adult neurogenesis: Implications for cognition and mood. In: Meerlo P, Benca RM, Abel T, editors. Sleep, Neuronal Plasticity and Brain Function. Springer; Heidelberg, Germany: 2013. pp. 151–181. [DOI] [PubMed] [Google Scholar]
- 55.Dissel S, et al. Enhanced sleep reverses memory deficits and underlying pathology in Drosophila models of Alzheimer’s disease. Neurobiol Sleep Circadian Rhythms. 2017;2:15–26. doi: 10.1016/j.nbscr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glasser MF, et al. WU-Minn HCP Consortium The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catafau AM, et al. Cerebellar amyloid-β plaques: How frequent are they, and do they influence 18F-florbetaben SUV ratios? J Nucl Med. 2016;57:1740–1745. doi: 10.2967/jnumed.115.171652. [DOI] [PubMed] [Google Scholar]
- 58.Bullich S, et al. Optimal reference region to measure longitudinal amyloid-beta change with 18F-florbetaben PET. J Nucl Med. 2017:jnumed.116.187351. doi: 10.2967/jnumed.116.187351. [DOI] [PubMed] [Google Scholar]
- 59.Heurling K, Buckley C, Van Laere K, Vandenberghe R, Lubberink M. Parametric imaging and quantitative analysis of the PET amyloid ligand [(18)F]flutemetamol. Neuroimage. 2015;121:184–192. doi: 10.1016/j.neuroimage.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 60.Ossenkoppele R, Prins ND, van Berckel BN. Amyloid imaging in clinical trials. Alzheimers Res Ther. 2013;5:36. doi: 10.1186/alzrt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 62.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 63.Logan J, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 65.Choe YM, et al. Sleep quality in young and middle age-period is associated with cerebral amyloid burden in cognitively normal elderly people. Alzheimers Dement. 2016;12(Suppl):P171–P172. [Google Scholar]
- 66.Westwood AJ, et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology. 2017;88:1172–1179. doi: 10.1212/WNL.0000000000003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118, and erratum (2013), 10.1038/nmeurol.2013.32. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiman EM, et al. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corneveaux JJ, et al. Association of CR1, CLU and PICALM with Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. Hum Mol Genet. 2010;19:3295–3301. doi: 10.1093/hmg/ddq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friston KJ, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 71.Poline J-B, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Subject-level measures used in the manuscript are available in Dataset S1. Please contact corresponding authors for additional information.