Significance
Contrasting with the dominance of conventional adaptive T cells in adult frogs, Xenopus tadpoles prominently rely on innate-like T (iT) cells expressing invariant TCRα rearrangements to combat pathogens. Here, we use three complementary loss-of-function approaches combining RNA interference with transgenesis or CRISPR/Cas9 genome editing to unveil an ancestral antimycobacterial immune surveillance system represented by MHC-like interacting innate-like T cells. Notably, this MHC-like (XNC4)/iVα45T cell system is critical for host defense against mycobacteria and distinctive from the key antiviral MHC-like XNC10-restricted iVα6T cells previously identified. These findings imply diversification and specialization of an ancestral innate-like T cell recognition system that is evolutionarily convergent to that of mammals.
Keywords: unconventional T cells, MHC evolution, innate-like T cells, ranavirus, reverse genetics
Abstract
The amphibian Xenopus laevis is to date the only species outside of mammals where a MHC class I-like (MHC-like) restricted innate-like (i) T cell subset (iVα6 T cells) reminiscent of CD1d-restricted iNKT cells has been identified and functionally characterized. This provides an attractive in vivo model to study the biological analogies and differences between mammalian iT cells and the evolutionarily antecedent Xenopus iT cell defense system. Here, we report the identification of a unique iT cell subset (Vα45-Jα1.14) requiring a distinct MHC-like molecule (mhc1b4.L or XNC4) for its development and function. We used two complementary reverse genetic approaches: RNA interference by transgenesis to impair expression of either XNC4 or the Vα45-Jα1.14 rearrangement, and CRISPR/Cas9-mediated disruption of the Jα1.14 gene segment. Both XNC4 deficiency that ablates iVα45T cell development and the direct disruption of the iVα45-Jα1.14 T cell receptor dramatically impairs tadpole resistance to Mycobacterium marinum (Mm) infection. The higher mortality of Mm-infected tadpoles deficient for iVα45T cells correlates with dysregulated expression responses of several immune genes. In contrast, iVα45-Jα1.14–deficient tadpoles remain fully competent against infection by the ranavirus FV3, which indicates a specialization of this unique iT cell subset toward mycobacterial rather than viral pathogens that involve iVα6 T cells. These data suggest that amphibians, which are evolutionarily separated from mammals by more than 350 My, have independently diversified a prominent and convergent immune surveillance system based on MHC-like interacting innate-like T cells.
MHC class I-like (MHC-like) restricted innate-like (i) T cells, including CD1d-restricted invariant natural killer T (iNKT) cells, MR1-restricted mucosal-associated invariant T (MAIT) cells and CD1a/b/c-restricted T cells such as germline-encoded mycolyl-reactive (GEM) T cells, constitute a substantial and immunologically relevant portion of the normal human T cell repertoire. Fundamental aspects of iT cells include their innate-like immune functional characteristics, the use of T cell receptor (TCR) with limited diversity, and restriction by distinct nonpolymorphic MHC-like genes (1). As iT cells lack the antigen specificity conferred by conventional αβ TCRs, it is generally considered that they are specialized to recognize and respond to more conserved ligands shared by groups of microbes. These ligands include small metabolites derived from the synthesis of vitamin B (a process used by a range of pathogenic bacteria) in the case of MR1 (2, 3), and structural glycolipids (4, 5) or endogenous molecules, such as alpha-glycosyl ceramides that are up-regulated in the case of stress for CD1 (6). These MHC-like/ligand complexes are then recognized by the semiinvariant TCRs expressed on iT cells. This recognition system is in many ways akin to that of germline-encoded, pattern recognition receptors, which suggests that iT cells represent a primordial T cell compartment with recognition/activation/regulation mechanisms distinct from conventional T cells.
To date, semiinvariant TCR-expressing iT cells and their respective restricting MHC-like molecules have been unequivocally described in humans and mice [MR1-restricted MAIT cells (7) and CD1d-restricted iNKT cells (8)] as well as in the amphibian Xenopus laevis [XNC10 restricted iVα6T cells (9)]. In addition, TCRα repertoire analysis by deep sequencing in rabbits, a species that has very few iNKT cells and no MAIT cells, recently identified a putative invariant T cell receptor alpha (TRAV41) and a MHC-like gene (MHX) consistent with the presence of a compensatory MHC-like/semiinvariant TCR pair in mammalian species that have lost their MR1 genes (10). This is intriguing, as it implies coevolution between MHC-like selecting elements and the TCRα genes used by their corresponding iT cells and suggests that additional, possibly species- and/or environment-adapted MHC-like/invariant TCR couples are present in other classes of vertebrates, providing a way to detect common microbial compounds. However, evolution of the MHC-like/iT cell immune components and the selective pressures exerted on these recognition systems remain enigmatic.
The amphibian X. laevis is an attractive model for iT cell biology and evolution, owing to the ease of genetic manipulation and visualization as well as its position as a “connecting” taxon linking mammals to vertebrates of more ancient origin (bony and cartilaginous fishes) that shared a common ancestor ∼350 Mya (11). Indeed, molecular and functional studies in X. laevis tadpoles have provided convincing evidence of an ancient origin of MHC-like restricted iT cells. Using a combination of MHC-like tetramers and RNA interference (RNAi) loss of function by transgenesis, we identified a population of XNC10-restricted iVα6T cells critical for antiviral immunity (9, 12). In addition, at least five other invariant Vα-Jα TCR rearrangements were identified in the CD8−/low T cell compartment in tadpoles, suggesting that multiple XNC/invariant TCR systems are present in Xenopus. These findings also indicated that despite the lack of orthology among MHC-like genes in different animal taxa, important functions of MHC-like molecules in relation to iT cells have been evolutionarily retained across vertebrates.
Intrathymic T cell development and peripheral T cell function are remarkably conserved between mammals and Xenopus. Unlike mammals, however, the Xenopus immune system and T cell differentiation in particular, are subject to an additional developmental program, including thymic remodeling and differential MHC class I regulation, during metamorphosis (11, 13). In contrast to adult frogs where cell surface MHC class I molecules are ubiquitously expressed, tadpoles have barely detectable MHC class I surface protein expression and lack significant expression of immunoproteasome subunit components in the thymus until the onset of metamorphosis (14, 15). Comparably, in the tadpole thymus, transcripts of numerous phylogenetically distinct MHC-like genes are readily detectable, suggesting that T cell selection is differentially regulated during the two life stages (i.e., tadpole versus adult) (16). Notably, in comparison with amniotes where the total number of T cells is sufficiently large to ensure the full diversification of the TCR repertoire, aquatic ectothermic vertebrates with external development, such as amphibians and fish, rely on a very limited T cell compartment. However, the need for a functional and efficient immune response in these animals is paramount, as they are exposed to pathogens of the aquatic environment from the first stage of development. Thus, we hypothesized that to overcome the limitations of their small conventional T cell compartment, Xenopus tadpoles generate a pool of functionally distinct iT cell lineages, each restricted by or interacting with a unique MHC-like element/ligand complex, capable of mounting rapid, albeit less specific, immune effector functions. Accordingly, we identified potential MHC-like gene products involved in iT cell development and used a combination of multiple reverse-genetic loss-of-function approaches (RNA interference and CRISPR/Cas9) to either knock down MHC-like transcripts or impair specific invariant TCRα rearrangement in combination with two ecologically relevant infectious agents, frog virus 3 (FV3) and Mm.
Results
XNC4 Loss-of-Function Impairs iVα45-Jα1.14 Expression and Increases Larval Susceptibility to Viral and Mycobacterial Infections.
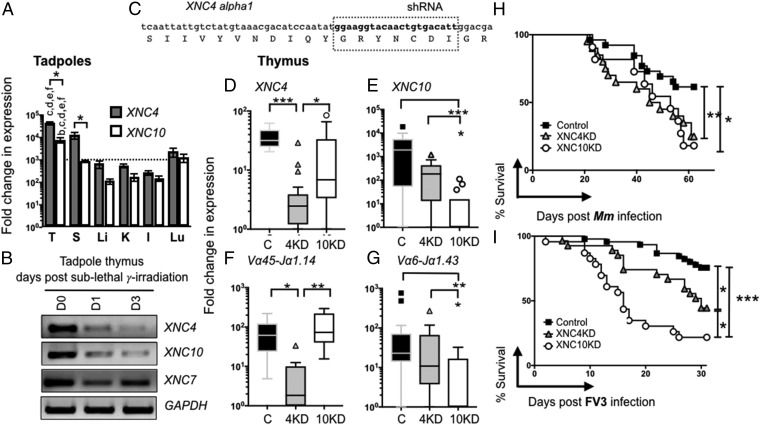
To select candidate MHC-like genes putatively involved in iT cell biology in Xenopus, we assessed and compared expression patterns, phylogenetic relationships, and interspecies conservation of the 23 XNC genes identified to date (17–19). Among these, XNC4 (mhc1b4.L) caught our attention because it represents a unique monogenic lineage phylogenetically distinct from classical X. laevis MHC class I (mhc1a.L) and other XNC genes. XNC4 is highly conserved between two divergent Xenopus species (>80% nt identity to the Xenopus tropicalis gene SNC4, mhc1b4.L) with an especially high interspecies sequence conservation in its putative antigen binding domain (19). In addition, the XNC4 gene is predominantly expressed in thymus and spleen (Fig. 1A). To obtain further indication of the possible role of XNC4 in iT cell development, we determined whether XNC4 was preferentially expressed by immature thymocytes by subjecting tadpoles to sublethal γ-irradiation, which preferentially depletes radiosensitive cortical thymocytes. Compared with untreated controls, thymic XNC4 gene expression was markedly reduced at 1 and 3 d postirradiation (Fig. 1B). This suggests that XNC4, similarly to XNC10 (mhc1b10.L) the MHC-like gene that we have previously shown to be required for the development and function of iVα6T cells, is mainly expressed by thymocytes (20). In contrast, XNC7 (mhc1b7.L), similar to classical MHC class I, is expressed by the more resistant thymic stoma cells (Fig. 1B).
Fig. 1.
Identification of a unique MHC class I-like interacting iT cell population (iVα45) using reverse genetics. (A) Outbred premetamorphic (stage 54, n = 12) tadpoles were assessed for XNC10 (white) and XNC4 (gray) gene expression. *P < 0.05, **P < 0.005, ***P < 0.0005 statistical difference between indicated groups. Tissues examined are as follows: I, intestine; K, kidney; L, liver; Lu, lung; S, spleen; and T, thymus. Letters at the Top of the bars indicate tissues exhibiting significantly different (P < 0.05) gene expression levels in the thymus for either XNC4 or XNC10 compared with b = S; c = L, d = K, e = I, and f = Lu. (B) Representative RT-PCR analysis of total thymocytes from premetamorphic tadpoles untreated and 1 and 3 d postsublethal gamma-irradiation (10 Gy). (C) Schematic of the shRNA targeting the XNC4 alpha 1 domain. (D–G) Relative gene expression by qPCR of XNC4 (D), XNC10 (E), iVα45-Jα1.14 (F), and iVα6-Jα1.43 (G) in F0 tadpole at developmental stage 52/53 transgenic (Tg) with knockdown (KD) for XNC4 Tg (gray), XNC10 Tg (white), or age-matched dejellied controls (black). Results were normalized to two endodgenous controls (GAPDH and L13) and presented as fold change in expression compared with the lowest level of expression for each individual gene. All results were pools from two independent experiments and presented as individual tadpoles with the mean ± SEM (n = 10–12) with outliers. *P < 0.05, **P < 0.005, ***P < 0.0005 (one-way ANOVA followed by post hoc analysis using Tukey’s multiple comparisons test). (H and I) F0 transgenic or control tadpoles (stage 52/53) were i.p. infected with 1 × 104 pfu FV3 (H) or 300,000 cfu Mm (I), and survival was monitored daily over a 30- and 60-d period, respectively. Each experiment was repeated three times independently (n = 15 in each cohort) and the results were pooled. Survival was determined using Kaplan–Meier, *P < 0.05, **P < 0.005, ***P < 0.0005. Dejellied controls (black squares), XNC4shRNA Tg (gray triangles), and XNC10shRNA Tg (white circles).
We next determined the requirement of XNC4 for iT cell development using a reverse-genetic loss-of-function approach that combines I-SecI meganuclease-mediated transgenesis with a shRNA targeting the alpha 1 domain of XNC4 (Fig. 1C). As a comparison, XNC10 knockdown (KD) was used as previously described (9). The protective jelly membranes of experimental and control embryos were removed postfertilization by incubation with l-cysteine (dejellying) to facilitate microinjections. Successful XNC4 and XNC10 KD and any resulting changes in the expression of the six previously identified predominant iTCR alpha rearrangements were determined at stage 53 (25 d postfertilization) tadpoles using quantitative (q)PCR with primers specific to each unique CDR3 region (Fig. 1 D–F and SI Appendix, Fig. S1). Each shRNA was assessed and revealed to be specific for its intended target, XNC4 and XNC10, respectively (Fig. 1 D and E). Notably, XNC4 KD in transgenic animals resulted in a drastic decreased expression of the invariant iVα45-Jα1.14 rearrangement compared with both dejellied controls and, more importantly, XNC10-deficient tadpoles (Fig. 1F). In contrast, XNC4 silencing did not significantly alter the expression of the other five iTCRα rearrangements (Fig. 1G and SI Appendix, Fig. S1). Consistent with our previously published observations, XNC10 silencing specifically ablated expression of iVα6-Jα1.43 (9), but did not alter iVα45-Jα1.14 expression (Fig. 1 E–G). These results indicate that successful differentiation of the iVα45 T cell lineage is XNC4 dependent.
To investigate the relevance of XNC4-dependent iVα45 T cells during immune responses, we used two different ecologically relevant Xenopus pathogens: the ranavirus FV3, a large double-stranded DNA virus implicated in amphibian decline (21), and Mycobacterium marinum (Mm), an acid-fast pathogen infecting humans and many aquatic vertebrates, including Xenopus. Tadpole immune defenses against FV3, which primarily occur in the kidney, were previously shown to critically depend on a functional XNC10/iVα6 T cell immune surveillance system (9). Comparably, Mm primarily accumulates in the liver with little to no bacteria detected in the kidney. Accordingly, we intraperitoneally (i.p.) infected F0 XNC4 and XNC10 KD transgenic tadpoles (stage 52–53, 25 d postfertilization) with either Mm (Fig. 1H) or FV3 (Fig. 1I). Following Mm infections, both XNC4 and XNC10 deficiency resulted in a markedly increased susceptibility (P = 0.0009 and P = 0.0294, respectively, compared with dejellied controls). However, neither the respective cumulative mortality nor the median survival of XNC4 and XNC10 KD tadpoles was significantly different. With regard to FV3 infection, XNC4 and XNC10 deficiency also resulted in a statistically significant increase in susceptibility compared with dejellied controls (P = 0.0112 and P < 0.0001, respectively), but the defect was significantly more pronounced for XNC10 than XNC4 KD transgenic tadpole siblings (Fig. 1I). The median survival time following infection of XNC10-deficient tadpoles was significantly shorter than XNC4-deficient tadpoles (16 versus 27.5 d, P = 0.0098). Interestingly, postmortem FV3 genome copy numbers determined by absolute qPCR to verify successful infection indicated that, while XNC10-deficient tadpoles succumbed to infection markedly faster than XNC4-deficient and dejellied controls, viral loads were not significantly different among the groups (SI Appendix, Fig. S2A). Since iT cells are generally more critical during early stages of infection, we hypothesized that XNC10 rather than XNC4 deficiency resulted in impaired early antiviral responses in tadpoles. Indeed, peritoneal leukocytes (site of infection) isolated from XNC10-deficient tadpoles had higher viral loads compared with controls at 2 d postinfection (dpi), whereas no significant difference was observed between controls and XNC4-deficient tadpoles (SI Appendix, Fig. S2B). Moreover, only XNC10 KD, and not XNC4 KD, impaired early FV3-induced increase in gene expression of the antiviral cytokine type I IFN as well as the two proinflammatory cytokines IL-1β and TNFα in peritoneal leukocytes compared with dejellied controls (SI Appendix, Fig. S3).
Next, to obtain additional evidence regarding the roles of iVα45 T and iVα6 T cells in tadpole immunity, we i.p. infected wild-type (WT) X. laevis tadpoles with either FV3 or Mm and quantified transcript levels of the invariant Vα45-Jα1.14 and Vα6-Jα1.43 rearrangements in immunologically relevant tissues using qPCR. Consistent with the iVα6 T cell anti-FV3 function, iVα6-Jα1.43 transcripts were reduced in the spleen and concomitantly elevated in the peritoneal cavity (site of infection) and kidneys (main site of viral replication) at 2 dpi, then decreased near baseline at 6 dpi, indicating a rapid and transitory influx of iVα6 T to virally infected tissues (SI Appendix, Fig. S4). In stark contrast, no significant changes in the expression of iVα45-Jα1.14 were observed following FV3 infection in any of the tissues examined (SI Appendix, Fig. S4). Conversely, following i.p. infection with dsRED-labeled Mm, Vα45-Jα1.14 expression but not Vα6-Jα1.43 was elevated in the liver (main site of bacterial accumulation) at 3 dpi, became statistically significant at 6 dpi, and then returned to low but detectable levels from 12 to 21 dpi. Furthermore, iVα45-Jα1.14 expression was again elevated at 36 dpi when mortality due to infection occurs (Fig. 1H), whereas no significant changes in the expression of iVα6-Jα1.43 were observed (SI Appendix, Fig. S5). Collectively, these data suggest that iVα45 and iVα6 represent two functionally distinct iT cell populations interacting with different MHC-like molecules, which likely exhibit different ligand specificities.
Targeted Disruption of iVα45 T Cells Using Two Different Reverse-Genetic Approaches Results in Increased Larval Susceptibility to Mycobacterial Infection but Not to Viral Challenge.
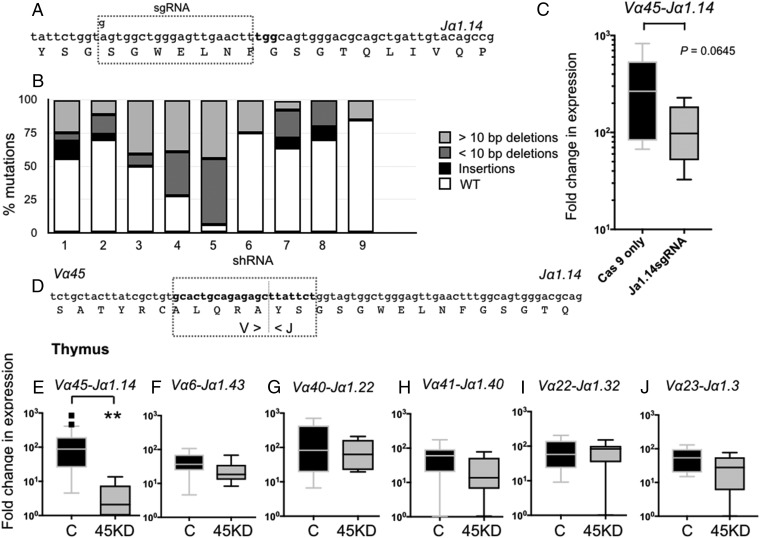
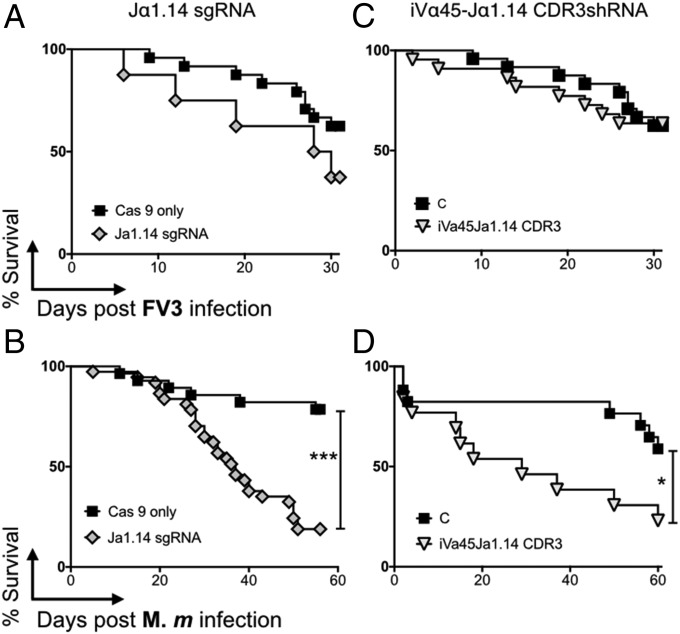
The silencing of MHC-like genes is likely to result in biological effects other than those inferred via the reciprocal loss of a unique iT cell population. Therefore, to elucidate more specifically the roles of iVα45 T cells in immune responses, we used two different reverse-genetic approaches to directly target and ablate iVα45 T cells in vivo (Fig. 2): (i) the disruption of the Jα1.14 gene segment by the CRISPR/Cas9 genome editing system to prevent the iVα45-Jα1.14 TCR rearrangement; and (ii) I-SecI meganuclease-mediated transgenesis targeting the CDR3 region to silence the Vα45-Jα1.14 rearrangement. We have shown that the CRISPR/Cas9 system is a robust method for targeting with high specificity a single member of multigene families in X. laevis (22). Accordingly, we designed a single guide RNA (sgRNA) against the Jα1.14 gene (Fig. 2A). Due to the limited number of target sites conforming to the criteria defined for designing optimal sgRNAs within a single Jα segment, the 5′-end “A” nucleotide of this anti-Jα1.14 sgRNA sequence was changed to a “G” in order to provide the “GG” sequence required for efficient T7 promoter transcription. Genomic PCR and sequencing data from nine randomly selected F0 tadpoles indicate that the sgRNA effectively targeted the Jα1.14 gene with mutations ranging from 25% to 98% (Fig. 2B), and an overall 49% mutagenesis efficiency (SI Appendix, Fig. S6). The majority of mutations (40.8%) were deletions, ranging from 3 to 47 bp, with 6.2% of the sequences carrying nucleotide insertions and/or substitutions (SI Appendix, Fig. S6). The relatively high degree of mosaicism within individual F0 tadpoles has been previously reported and is likely due to a rapid embryogenesis and continuous CRISPR/Cas9-mediated genome modifications after the one-cell embryo stage, which results in independent double-strand breaks with different mutations upon imprecise DNA repair mechanism (22). To evaluate the potential off-target effects on Jα segments proximal to, or sharing sgRNA motifs with Jα1.14, we sequenced three Jα gene segments in the three tadpoles carrying the highest number of Jα1.14 mutations and did not find any off-target mutations (SI Appendix, Fig. S7). We also performed a genome-wide search for any significant sequence matching the Jα1.14 sgRNA that could represent a potential off-target site. In total, three locations were identified: (i) an intronic region within the second TCRα locus located between Jα2.1 and the Cα2 region on scaffold 131 (position 94528–94509); (ii) an intergenic region on chromosome 4S (position 98485229–98485210); and (iii) an intergenic region on chromosome 5S (position 20771451–20771432) containing three, five, and two mismatches to the Jα1.14 sgRNA, respectively. No mutations were observed in the chromosome 4S and scaffold 131 locations (SI Appendix, Fig. S7). However, in two of the tadpoles sequenced, 50% and 22% of the chromosome 5S sequences, respectively, were mutated, indicating that while the Jα1.14 sgRNA has no detectable off-target effects on Jα segments, one cannot definitely exclude potential genome-wide off-target effects. Although transcript levels of the iVα45-Jα1.14 rearrangement were decreased in whole Jα1.14 CRISPRized tadpoles compared to age-matched controls injected only with Cas9 protein, it did not reach statistical significance (Fig. 2C, P = 0.0645), presumably because of the high mutation variability between individuals. Nevertheless, we proceeded to determine whether the disruption of the Jα1.14 gene had functional phenotypes. We i.p infected F0 CRISPRized tadpoles with either FV3 or Mm. Notably, despite the variability in Jα1.14 mutation efficiency and the iVα45-Jα1.14 defect, Jα1.14 CRISPRized tadpoles were drastically more susceptible to Mm than Cas9-only injected controls of the same parents (Fig. 3B, P > 0.0001), whereas they remained as resistant to FV3 infection as Cas9-only injected controls (Fig. 3A).
Fig. 2.
iVα45 T cell loss of function using two different reverse-genetic approaches. (A) Schematic representation showing the modified sgRNA targeting the Jα1.14 gene segment. (B) Bar graph showing the relative contributions of different types of mutations divided into insertions (black), >10-bp deletions (light gray), and <10-bp deletions (dark gray) observed among 10 randomly selected sgRNA/Cas9-treated tadpoles. Note: Multiple alignments of all mutations are shown in SI Appendix, Fig. S6. (C) Expression of the invariant Vα45-Jα1.14 transcript by qPCR in age-matched Cas9-only injected WT controls (black) and CRISPR/Cas9 Jα1.14-injected tadpoles (gray). P = 0.0645 (Student’s t test). (D) Schematic of the shRNA targeting the iVα45-Jα1.14 CDR3 region, dotted line indicates the Vα/Jα junction. Note: No n-nucleotide diversity was observed in any of the iTCR rearrangments. (E–J) Relative gene expression by qPCR in the thymus of stage 52/53 tadpoles transgenic with iVα45-Jα1.14 CDR3 KD (gray) and age-matched dejellied controls (black) for iVα45-Jα1.14 (E), iVα6-Jα1.43 (F), iVα40-Jα1.22 (G), iVα41-Jα1.40 (H), iVα22-Jα1.32 (I), and iVα23-Jα1.3 (J). Results were normalized to two endogenous controls (GAPDH and L13) and presented as fold change in expression compared with the lowest level of expression for each individual gene. All results are pools from two separate experiments and presented as individual tadpoles with the mean ± SE (n = 10–12). **P < 0.005 (one-way ANOVA test followed by a post hoc analysis using Tukey’s multiple comparisons test).
Fig. 3.
iVα45 T cells are required for host resistance to Mm but not FV3 infection. F0 transgenic iVα45-Jα1.14 CDR3 KD and control tadpoles (stage 52/53) were sham infected (controls) or either i.p. infected with 1 × 104 pfu FV3 (A and C) or 300,000 cfu Mm (B and D), and survival was monitored daily over a 30- and 60-d period, respectively. Each experiment was repeated three times independently (n = 15 in each cohort) and the results were pooled. Survival was determined using Kaplan–Meier, *P < 0.05, ***P < 0.0005. Shown are dejellied controls (black squares), iVα45-Jα1.14 CDR3shRNA Tg (inverted gray triangles), and Jα1.14sgRNA/CRISPR Tg (gray diamonds).
Since the Jα1.14 sgRNA may have some unclear off-target effects and since disrupting this single Jα segment may also affect other T cell clones utilizing this specific Jα in its TCR, we developed a second complementary method to specifically silence the Vα45-Jα1.14 rearrangement. This was accomplished using a shRNA targeting the CDR3 region generated by joining Vα45 and Jα1.14 (Fig. 2D). The specificity and efficiency of Vα45-Jα1.14 KD were assessed by comparing the relative expression of the six different iTCRα rearrangements in the thymus of transgenic and age-matched dejellied controls using qPCR (Fig. 2 E–J). Targeting the Vα45-Jα1.14 CDR3 region severely abrogated the expression of the iVα45-Jα1.14 rearrangement compared with dejellied controls (Fig. 2E). Comparably, no significant differences were observed in the expression of any of the other five iTCR rearrangements (Fig. 2 F–J). Importantly, iVα45-Jα1.14 loss of function dramatically increased tadpole susceptible to Mm (Fig. 3D, P = 0.0343) but not FV3 (Fig. 3C). It is also noteworthy that the major drop in survival resulting from this highly targeted and effective iVα45-Jα1.14 silencing occurred at early stage of infection (50% death within 18 dpi), which is consistent with the preponderant role of iT cells early during immune response.
Collectively these data strongly support the hypothesis that iVα45 T cells through interaction with XNC4 are critical in antimycobacterial immunity, while being dispensable during a FV3 challenge. In contrast, the impairment of both antiviral and antimycobacterial immune response obtained when loss of function is applied to XNC4 implies that this class I-like molecule is not only required for iVα45T cell development and function, but is also involved in other, as yet undefined, tadpole immune defenses.
iVα45 T Cell Deficiency Impairs Th1 Promoting Cytokine Gene Expression Profiles in Liver of Mm-Infected Tadpoles.
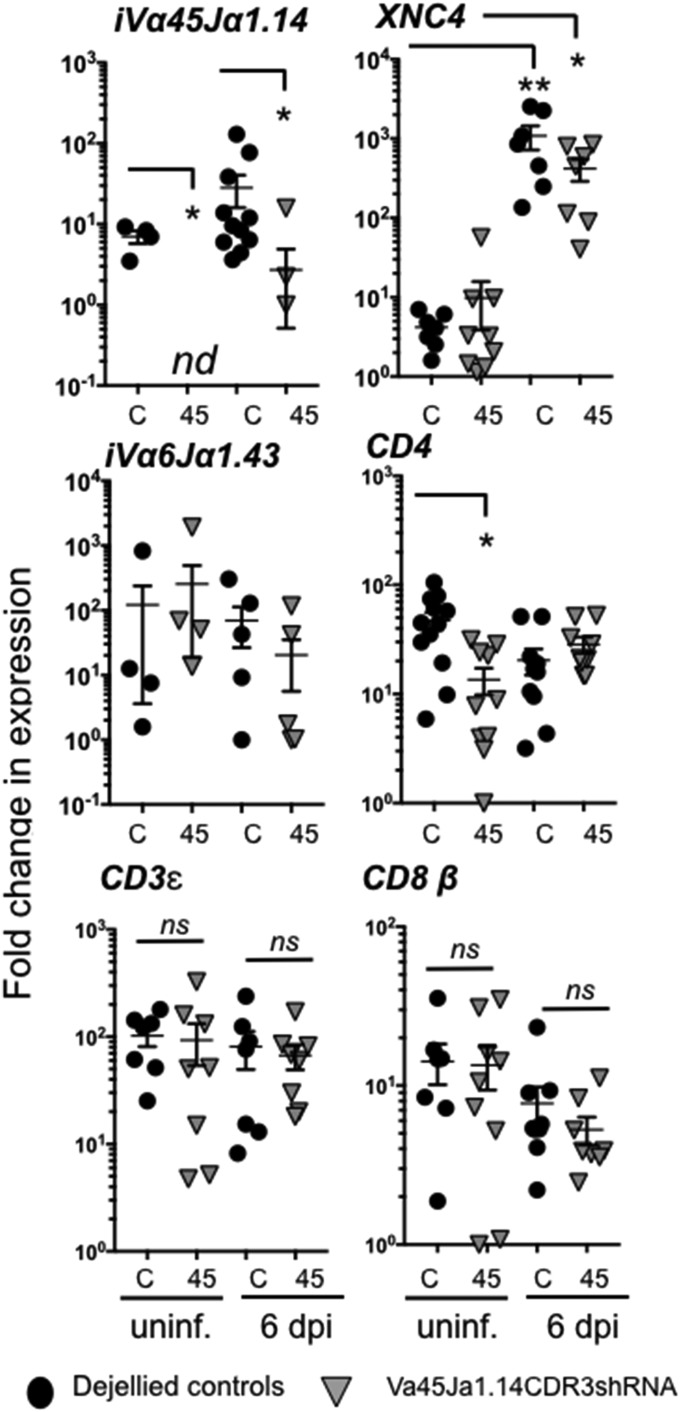
Since more specific and efficient F0 iVα45 T cell loss of function was obtained using Vα45-Jα1.14CDR3 silencing, we further examined host immune responses to Mm infection in iVα45 T cell-deficient tadpoles. Based on kinetic studies indicating an initial and significant increase of iVα45 gene expression in the tadpole liver at 6 dpi, we focused our investigation on this early time point (SI Appendix, Fig. S5). Compared with controls, iVα45-Jα1.14 transcript levels were undetectable in the liver before Mm infection and significantly reduced at 6 dpi in transgenic KD tadpoles (Fig. 4). Also, uninfected iVα45 T cell-deficient tadpoles had significantly less CD4 gene expression than controls. In contrast, during the time span of our study, no differences in iVα6-Jα1.43, CD3ε, or CD8β transcript levels or in the frequency of CD8+ T cells were observed in the liver of transgenic and control groups (Fig. 4). Following Mm infection, XNC4 expression was significantly increased in both groups. Collectively, these data reinforce the specificity of our CDR3-shRNA loss-of-function approach in strictly ablating iVα45 T cell function.
Fig. 4.
iVα45-Jα1.14 CDR3shRNA KD specifically ablates iVα45 T cells. Quantitative gene expression analysis of iVα45-Jα1.14, iVα6-Jα143, CD3ε, CD4, CD8β, and XNC4 in liver isolated from either uninfected (unif.) or Mm (300,000 cfu) infected F0 transgenic tadpoles KD for Vα45Jα1.14 CDR3 (gray triangles, n = 10) or age-matched dejellied control (black circles, n = 10) at 0 and 6 dpi. Gene expression was determined relative to the endogenous control GAPDH and normalized against respective uninfected gene expression. Results are representative of two independent experiments and presented with the mean ± SEM (n = 10–12). *P < 0.05, **P < 0.005, (one-way ANOVA test followed by a post hoc analysis using Tukey’s multiple comparisons test). ns, nonsignificant.
To investigate whether increased susceptibility to Mm of iVα45 T cell-deficient tadpoles resulted from uncontrolled bacterial growth and/or dissemination, we determined Mm loads in different tissues following i.p. infection by absolute qPCR. By 6 dpi, with the exception of the spleen, (the main lymphoid organ) where Mm loads were significantly higher in transgenic KD tadpoles, no difference in Mm loads was observed between the two groups, including peritoneal leukocytes (site of infection), thymus, liver (main site of Mm accumulation during late-stage infection), kidney, and intestine tissues (SI Appendix, Fig. S8). The increased susceptibility of iVα45-KD transgenic tadpoles to Mm infection was, therefore, unlikely due to uncontrolled bacterial growth and dissemination. This raises the possibility of an Mm-mediated immune dysregulation negatively affecting immune responses, although more definitive studies using direct cellular assays will be required to fully answer this question.
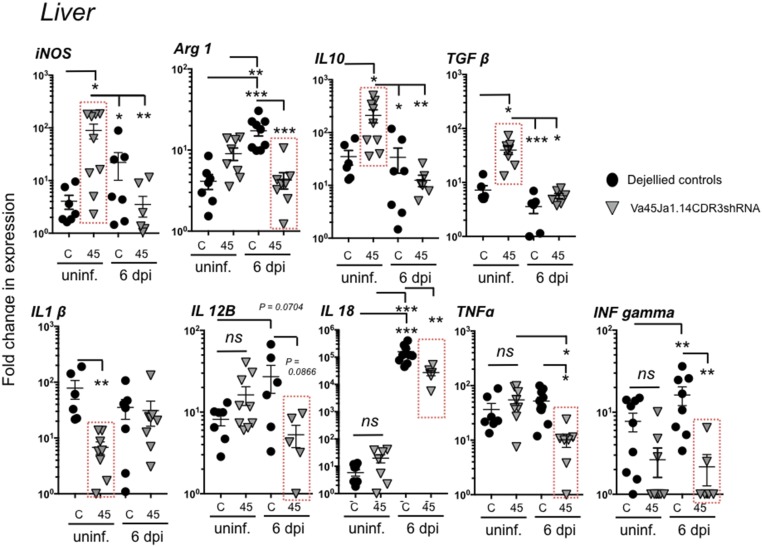
In humans, the outcome of Mycobacterium tuberculosis (Mtb) infection can vary greatly ranging from asymptomatic clearance to clinical disease. One key element generally considered to mediate resistance to mycobacterial infections is the activity of T helper type I (Th1) cells and their associated cytokines, such as TNFα, IFNγ, IL-1β, IL-12, and IL-18 that are involved in promoting cell-mediated immunity and that can activate antimicrobial effector functions in infected macrophages to enhance mycobacterial killing (23). In contrast, development of Th2 and/or Treg responses typically antagonize the protective cellular immunity and correlates with disease susceptibility and TB pathology in humans (24, 25). While to date the Th1/Th2 dichotomy has not been studied in Xenopus, both gene expression analysis and functional assays are consistent with the presence of CD4+ T-helper subsets in this species (26). Thus, as an indirect way to reveal a putative role of XNC4 and iVα45 T cells as early protagonists shaping the cytokine milieu and promoting tadpole antimycobacterial immunity, we compared the cytokine expression profiles in the liver of Vα45-deficient and control tadpoles (Fig. 4). Notably, even at steady state before Mm infection, iVα45-KD transgenic tadpoles exhibited elevated gene expression levels of the antiinflammatory cytokines IL-10 and TGFβ as well as decreased expression of the proinflammatory cytokine IL1β (Fig. 4). These data indicate that iVα45T cell deficiency has an impact on immune cytokine homeostasis in unchallenged tadpole liver, suggesting that iVα45T cells may exert regulatory functions and actively participate in immune homeostasis. Consistent with a role of iVα45 T cells in promoting cell-mediated antimicrobial T cell responses, Mm-infected iVα45 T-KD tadpoles had significantly impaired gene expression response of TNFα and IL-18 as well as a markedly, albeit not significantly, reduced IL-12β expression compared with controls (Fig. 4). In addition, compared with the slight but consistent increased expression of IFN-γ (IFN-γ) induced by Mm infection in control tadpoles, INF-γ gene expression was significantly impaired in iVα45 T cell-deficient tadpoles (Fig. 4). Type I and type III INF gene expression response was not altered in iVα45-KD tadpoles at 6 dpi (SI Appendix, Fig. 10).
Importantly, both Mtb and Mm invade and persist inside host phagocytes (27, 28). This ability to survive inside phagocytic cells such as macrophages, provides Mycobacterium species with an opportunity to multiply and alter immune response in favor of bacterial persistence. Thus, we sought to determine whether part of the impaired anti-Mm immune response in iVα45 T cell-deficient tadpoles could reflect altered effector functions in cells of the myeloid lineage, including macrophages and polymorphonuclear phagocytes. Accordingly, we first established that Mm infection in X. laevis, similar to fish (e.g., zebrafish and goldfish) (29, 30) resulted in accumulation of bacteria inside macrophages (SI Appendix, Fig. S9). As the main bacterial accumulation occurs in the liver, we again focused our investigation on this tissue using gene expression as proxy. At 6 dpi CCR2 (monocytes) and GCSFR (polymorphonuclear granulocytes) but not MCSFR (mononuclear phagocytes) gene expression levels were increased in the liver in both groups (SI Appendix, Fig. S10). These results are consistent with an early infiltration of monocytes and polymorphonuclear granulocytes following Mm infection. Notably, while the expression profiles of the X. laevis macrophage-associated cytokines CSF-1 and IL-34 genes were not significantly different, both inducible NO synthase (iNOS) and Arginase 1 gene expression was dramatically altered in iVα45 T cell-KD tadpoles, and this alteration was detected at steady state as well as following Mm infection (Fig. 5). Compared with controls, expression of iNOS gene was exacerbated before and severely dampened following Mm infection in transgenic tadpoles (Fig. 5). With regard to Arginase 1 expression, the baseline levels were not significantly different between transgenic and control tadpoles; however, transgenic tadpoles were severely hampered in their induction of Arginase 1 following Mm infection, suggesting that loss of iVα45 T cells, either directly or indirectly, impact macrophage-associated functions.
Fig. 5.
iVα45 T cell deficiency impacts cytokine expression profile in liver of Mm-infected tadpoles. Relative gene expression analysis by qPCR for inducible nitric oxide (iNOS), Arginase 1 (Arg1), interleukin 10 (IL-10), transforming growth factor β (TGFβ), interleukin 1β (IL1β), interleukin 12 (IL12-B), interleukin 18 (IL-18), tumor necrosis factor α (TNFα), and interferon gamma (IFN-γ) in liver isolated from either uninfected or Mm (300,000 cfu)-infected F0 transgenic tadpoles with KD of Vα45Jα1.14 CDR3 (gray triangles, n = 10) or age-matched dejellied control (black circles, n = 10) at 0 and 6 dpi. Gene expression was determined relative to the endogenous control GAPDH and normalized against respective uninfected gene expression. Results are representative of two independent experiments and presented with the mean ± SEM (n = 10–12). *P < 0.05, **P < 0.005, ***P < 0.0005 (one-way ANOVA test followed by a post hoc analysis using Tukey’s multiple comparisons test). ns, nonsignificant.
Discussion
Our identification of a distinct antimycobacteria MHC-like/iT cell recognition system, represented by XNC4/i iVα45 T cells, in an ectothermic tetrapod, X. laevis, emphasizes the ancestral relevance of iT cell immune surveillance across jawed vertebrates. Importantly, either ablating the MHC-like gene XNC4 or strictly impairing the iVα45 TCR function has enabled us to show the critical role of iVα45 T cells in X. laevis tadpole immune defense against mycobacteria, which are resilient pathogens plaguing a wide spectrum of vertebrate species, including humans. Considering the lack of orthology of both MHC-like and TCRα genes between mammals and amphibians, we bring to light a remarkable case of evolutionary convergence possibly driven by continual or repeated pressure from pathogenic mycobacterial species. While we previously identified a XNC10/iVα6 T cell axis critical for tadpole antiviral immunity (9), in this report, we provide evidence showing the importance of a distinct XNC4/iVα45 T cell system important for antimycobacterial immunity. The specialization of different iT cell subsets in Xenopus is manifested by the fact that the loss of iVα45 T cell function markedly impaired host immune resistance to Mm but not ranaviral (FV3) infection. Consistent with this specialized antimycobacterial effector function, transcript levels of iVα45-Jα1.14 were significantly elevated in the liver—the main site of bacterial accumulation—following Mm infection, but were unaffected during FV3 infections.
While our data unequivocally show the requirement of XNC4 and a productive iVα45-Jα1.14 rearrangement for the function of the XNC4/iVα45 T cell system, the contribution of the TCRβ chain is currently unknown. Based on mammalian studies (4, 5) and XNC10/iVα6 T cell in Xenopus (12), one can envision a lesser constraint, the TCRβ chain resulting in a more limited but not strictly invariant repertoire. More specific reagents such as XNC4 tetramers and TCR-specific antibodies will be required to address the role of TCRβ chains in iVα45 T cell function, since our reverse-genetic approach will not be suitable.
Although the effector functions of iVα45 T cells remain to be determined by more direct cellular assays, our data suggest that these cells help to establish a more robust antimycobacterial state by modulating the function of other immune cells. The regulatory potential of iVα45 T cells is already revealed at steady state in the liver of unchallenged KD transgenic tadpoles. In comparison with controls, iVα45 T cell-deficient tadpoles had significantly dysregulated expression of iNOS, IL-10, TGFβ, and IL-1β genes. Furthermore, the inefficient increase of IFN-γ and TNFα transcript levels in the liver during Mm infection likely contributed to the higher tadpole mortality. Notably, both IFN-γ and TNFα have been recognized as crucial host-protective factors in Mtb. In fact, IFN-γ is one of the main mediators of macrophage activation and promotion of Th1 immune responses associated with protection against TB in humans (31). Similarly, TNFα has been shown to induce macrophage apoptosis, which eliminates the protective niche and exposes the pathogen to the immune system (32). Whether iVα45 T cells interact directly or indirectly with Mm-infected macrophages remains to be determined. However, it is noteworthy that loss of iVα45T cells negatively impacted the expression of the macrophage polarization factors iNOS and Arginase 1. Indeed, iVα45 T cell-deficient transgenic animals exhibited elevated iNOS transcript levels before Mm infection compared with controls, and iNOS gene expression was almost completely ablated during Mm infection. A similar trend was observed with Arginase 1 gene expression. Whether the absence of iVα45 T cell influences the activation and/or effector function of macrophages needs further investigation. Nevertheless, these findings illustrate the importance of iVα45 T cells in maintaining a controlled inflammatory state to counteract Mm pathogenesis.
An ongoing question about iT cell biology, especially in Xenopus, is their interactions with MHC class I-like elements. Here we find that in contrast to direct iVα45 T cell deficiency, the silencing of XNC4 resulted in more intricate outcomes. While XNC4 KD clearly abrogated iVα45 T cell development in vivo, it impaired host resistance not only to Mm but also to viral (FV3) infections. The XNC4 gene is located within the MHC class I-like locus in the telomeric region of X. laevis’ long chromosome 8 and is phylogenetically distinct from XNC10, the MHC-like gene restricting iVα6 T cells. Although the nature of the XNC4 ligand is currently under investigation, it is noteworthy that XNC4 exhibits a unique five amino acid stretch (IPFSS) in its alpha 1 domain that is absent in both classical MHC and XNC10 sequences. This argues for a distinctive putative antigen-presenting groove and biochemical features for XNC4. Furthermore, while XNC4 and XNC10 genes are highly expressed in immature and mature CD8 thymocytes, both tissue and developmental gene expression patterns of XNC4 are distinct from XNC10, which is likely affecting the nature of antigenic compounds presented. This has the important implication that the two MHC-like/iT cell surveillance systems identified in X. laevis recognize and respond to different immunological stimuli and likely have nonoverlapping effector functions.
Our findings adhere to a unique paradigm based on a MHC class I-like/iT system more conserved and widespread across jawed vertebrates than previously thought. Such a system may represent a highly adaptable species-specific immune recognition system subject to strong coevolutionary pressures among the iTCR, MHC-like, and pathogenic ligands. It is tempting to speculate that this type of recognition system is adapted to specific commensals and/or pathogens, providing an early recognition system working in conjunction with conventional T/MHC recognition. Indeed, while the high polymorphism of MHC genes reflects their ability for an arms race with pathogens that can rapidly harbor mutations, oligomorphic MHC-like genes are more adapted to present antigens that are part of conserved pathways that cannot readily undergo change without losing essential functions, as exemplified by MR1 binding to antigens generated from the vitamin B biosynthesis, a pathway that is highly prevalent and essential to a majority of prokaryotes (33, 34).
In addition to providing in vivo evidence of the critical roles of multiple MHC-like–interacting iT cells in amphibian immunity, this study places X. laevis tadpoles as an attractive alternative animal model to explore the role of iT cells in establishment and persistence of Mtb, the causative agent of TB in humans. Despite years of research, Mtb remains a leading cause of infectious mortality worldwide and although TB is generally curable, current treatment strategies are complex and of long duration with the emergence of multidrug and extensive drug-resistant TB (35, 36). Although it is well established that cellular immunity is critical in controlling mycobacterial growth, there are still major gaps in our understanding of how the early initiation and regulation of the immune response occurs (37). Recent studies have highlighted the potential of early responding CD1d-restricted iNKT and NKT cells as key modulators of Mtb growth and pathogenesis (38–40). However, studies aimed at deciphering the specific roles of iT cells in host defense against Mtb have been difficult to ascertain in large part due to compensatory effects exerted by conventional T cells (41, 42). Comparably, tadpoles rely predominantly on iT cells to mediate protective immunity, making the X. laevis–Mm model attractive to determine the requirement and function of iT cells during mycobacterial infections at the whole organism level.
In comparison with the Xenopus larval stage, wherein MHC class I surface protein expression is barely detectable, adult frogs ubiquitously express MHC class I molecules and exhibit a more fully developed and prominent conventional T cell compartment. Nevertheless, we have previously shown that XNC10-iVα6 T cell deficiency in adults significantly delayed and weakened antiviral immune response to FV3 (12). Although the iVα6 T cell defect did not increase mortality in adult frogs as it did in tadpoles, the extensive tissue damage in adult kidneys that resulted from increased viral loads would likely reduce adult fitness. Given that XNC4 has a gene expression pattern as similar in tadpoles as in adults, and that iVα45 TCR transcripts are readily detectable in adult thymus and spleen, it is tempting to speculate that this immune surveillance also operates in adults with a fully competent conventional T cell compartment. While the roles of the XNC4-iVα45 T cell axis in adult antimycobacterial immunity remains to be determined, it stands to reason that their presence at adult stage should have functional relevance in antimycobacterial immunity. It is plausible for example that iVα45 T cells in adults, similar to tadpoles, would promote Th1 responses, thereby enhancing the cell-mediated immunity-enhancing mycobacterial killing, although they may not be as critical as in tadpoles.
In summary, using different complementary reverse-genetic approaches, we provide evidence of a distinct MHC-like/iT cell system in Xenopus tadpoles implicated in homeostasis and in immune response against mycobacteria. In connection to our previous study, we unveil the coexistence in X. laevis tadpoles of at least two MHC-like/iT cell immune surveillance systems that recognize and respond to distinct immunological stimuli and have different effector functions. Importantly this system would allow Xenopus tadpoles to generate a pool of iT cells guided by or interacting with MHC-like molecules that are capable of rapidly, albeit less specifically, mounting immune effector functions against various pathogens, providing an essential immune adaptation to the larval developmental stage.
These data are of fundamental significance as the biological roles and evolutionary ancestry of the MHC-like/iT cell immune surveillance system is, despite marked progress in the understanding of mammalian iT cells, still far from fully appreciated and is likely to be highly multifaceted.
Materials and Methods
Animals.
Tadpoles were from the Xenopus laevis Research Resource for Immunology at the University of Rochester (https://www.urmc.rochester.edu/microbiology-immunology/xenopus-laevis.aspx). Transgenesis was performed as described in SI Appendix, SI Materials and Methods. All animals were handled under strict laboratory and University Committee on Animal Resources regulations (100577/2003–151), and discomfort was minimized at all times.
FV3 Infections.
FV3 was produced at high titer as previously described (43). Tadpoles were i.p. infected with 1 × 104 pfu. Viral loads were determined by absolute qPCR on isolated genomic DNA as previously described (44).
M. marinum Infections.
M. marinum strains used for infection were either a clinical isolate (stock no. PM2690) generously provided by D. Hardy, University of Rochester, Rochester, NY, or strain PM3495, which is strain PM2690 transformed with plasmid pMV261.DsRed (kindly provided by W. R. Jacobs, Albert Einstein College of Medicine, Bronx, NY). Mm were cultured as described in SI Appendix, SI Materials and Methods. Tadpoles were i.p. infected with thawed and diluted cell suspensions at 300,000 cfu per tadpole.
RNA and gDNA Isolation, RT-PCR, and qPCR.
These procedures were performed using standard protocols as described in SI Appendix, SI Materials and Methods. Sequences of all primers used are listed in SI Appendix, Table S1.
Statistical Analysis.
Statistical significance of survival data was determined by a log-rank (Mantel–Cox) test using the GraphPad Prism 7 software (GraphPad Software, Inc.). All quantitative expression data were analyzed by a one-way test of variance (one-way ANOVA) followed by a post hoc test using the GraphPad Prism 7 software. P value >0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Ms. Tina Martin for expert animal husbandry and Ms. Fayth Kim and Mr. Ryan Hecht for their significant technical contribution. This research was supported by R24-AI-059830 from the NIH National Institute of Allergy and Infectious Diseases and IOS-1456213 from the National Science Foundation. M.B. was supported by Ruth L. Kirschstein Predoctoral Fellowship F31 (F31CA192664) from the NIH National Cancer Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722129115/-/DCSupplemental.
References
- 1.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 2.Kjer-Nielsen L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 3.Corbett AJ, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 5.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15:643–654. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kain L, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity. 2014;41:543–554, and erratum (2014) 41:867. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilloy F, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269:185–186. doi: 10.1126/science.7542402. [DOI] [PubMed] [Google Scholar]
- 9.Edholm ES, et al. Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Natl Acad Sci USA. 2013;110:14342–14347. doi: 10.1073/pnas.1309840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudinot P, et al. Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals. Proc Natl Acad Sci USA. 2016;113:E2983–E2992. doi: 10.1073/pnas.1600674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238:1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edholm ES, Grayfer L, De Jesús Andino F, Robert J. Nonclassical MHC-restricted invariant Vα6 T cells are critical for efficient early innate antiviral immunity in the amphibian Xenopus laevis. J Immunol. 2015;195:576–586. doi: 10.4049/jimmunol.1500458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Pasquier L, Weiss N. The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur J Immunol. 1973;3:773–777. doi: 10.1002/eji.1830031207. [DOI] [PubMed] [Google Scholar]
- 14.Flajnik MF, et al. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137:3891–3899. [PubMed] [Google Scholar]
- 15.Salter-Cid L, Nonaka M, Flajnik MF. Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol. 1998;160:2853–2861. [PubMed] [Google Scholar]
- 16.Edholm ES, Grayfer L, Robert J. Evolution of nonclassical MHC-dependent invariant T cells. Cell Mol Life Sci. 2014;71:4763–4780. doi: 10.1007/s00018-014-1701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flajnik MF, et al. A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J. 1993;12:4385–4396. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyos A, Sowa J, Ohta Y, Robert J. Remarkable conservation of distinct nonclassical MHC class I lineages in divergent amphibian species. J Immunol. 2011;186:372–381. doi: 10.4049/jimmunol.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edholm ES, et al. Unusual evolutionary conservation and further species-specific adaptations of a large family of nonclassical MHC class Ib genes across different degrees of genome ploidy in the amphibian subfamily Xenopodinae. Immunogenetics. 2014;66:411–426. doi: 10.1007/s00251-014-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyos A, Ohta Y, Guselnikov S, Robert J. Novel nonclassical MHC class Ib genes associated with CD8 T cell development and thymic tumors. Mol Immunol. 2009;46:1775–1786. doi: 10.1016/j.molimm.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinchar VG. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Arch Virol. 2002;147:447–470. doi: 10.1007/s007050200000. [DOI] [PubMed] [Google Scholar]
- 22.Banach M, Edholm ES, Robert J. Exploring the functions of nonclassical MHC class Ib genes in Xenopus laevis by the CRISPR/Cas9 system. Dev Biol. 2017;426:261–269. doi: 10.1016/j.ydbio.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng G, Zhang G, Chen X. Th1 cytokines, true functional signatures for protective immunity against TB? Cell Mol Immunol. 2018;15:206–215. doi: 10.1038/cmi.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: What is beneficial for the host and for the bacterium? Front Microbiol. 2011;2:2. doi: 10.3389/fmicb.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hossain MM, Norazmi MN. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection—the double-edged sword? BioMed Res Int. 2013;2013:179174. doi: 10.1155/2013/179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chida AS, Goyos A, Robert J. Phylogenetic and developmental study of CD4, CD8 α and β T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis. Dev Comp Immunol. 2011;35:366–377. doi: 10.1016/j.dci.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan C. Macrophages’ choice: Take it in or keep it out. Immunity. 2016;45:710–711. doi: 10.1016/j.immuni.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Awuh JA, Flo TH. Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci. 2017;74:1625–1648. doi: 10.1007/s00018-016-2422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grayfer L, Hodgkinson JW, Belosevic M. Analysis of the antimicrobial responses of primary phagocytes of the goldfish (Carassius auratus L.) against Mycobacterium marinum. Dev Comp Immunol. 2011;35:1146–1158. doi: 10.1016/j.dci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Cronan MR, Tobin DM. Fit for consumption: zebrafish as a model for tuberculosis. Dis Model Mech. 2014;7:777–784. doi: 10.1242/dmm.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin KL, Anis FZ, Sarmiento ME, Norazmi MN, Acosta A. Role of interferons in the development of diagnostics, vaccines, and therapy for tuberculosis. J Immunol Res. 2017;2017:5212910. doi: 10.1155/2017/5212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50:1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of thiamin biosynthesis in procaryotes. New genes and regulatory mechanisms. J Biol Chem. 2002;277:48949–48959. doi: 10.1074/jbc.M208965200. [DOI] [PubMed] [Google Scholar]
- 34.Mondot S, Boudinot P, Lantz O. MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules? Immunogenetics. 2016;68:537–548. doi: 10.1007/s00251-016-0927-9. [DOI] [PubMed] [Google Scholar]
- 35.Sizemore CF, Schleif AC, Bernstein JB, Heilman CA. The role of biomedical research in global tuberculosis control: gaps and challenges: A perspective from the US National Institute of Allergy and Infectious Diseases, National Institutes of Health. Emerg Microbes Infect. 2012;1:e9. doi: 10.1038/emi.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadosch D, Abel Zur Wiesch P, Kouyos R, Bonhoeffer S. The role of adherence and retreatment in de novo emergence of MDR-TB. PLOS Comput Biol. 2016;12:e1004749. doi: 10.1371/journal.pcbi.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkataswamy MM, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora P, Foster EL, Porcelli SA. CD1d and natural killer T cells in immunity to Mycobacterium tuberculosis. Adv Exp Med Biol. 2013;783:199–223. doi: 10.1007/978-1-4614-6111-1_11. [DOI] [PubMed] [Google Scholar]
- 40.Rothchild AC, Jayaraman P, Nunes-Alves C, Behar SM. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1003805. doi: 10.1371/journal.ppat.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa AO, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci USA. 2000;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawakami K, et al. Minimal contribution of Valpha14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol Immunol. 2002;46:207–210. doi: 10.1111/j.1348-0421.2002.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 43.Morales HD, et al. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J Virol. 2010;84:4912–4922. doi: 10.1128/JVI.02486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sifkarovski J, Grayfer L, De Jesús Andino F, Lawrence BP, Robert J. Negative effects of low dose atrazine exposure on the development of effective immunity to FV3 in Xenopus laevis. Dev Comp Immunol. 2014;47:52–58. doi: 10.1016/j.dci.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.