Significance
Many populations and species have different strategies for maximizing reproductive success in changing environments. For example, some species of plants reproduce only once in their lifetime (annuals), whereas others can reproduce over multiple years (perennials). Such variation can also occur in fishes, and we examine how this variation in life history strategies is maintained in a Pacific salmonid species (Oncorhynchus mykiss). We find that fish that successfully migrate between the ocean and their freshwater spawning grounds multiple times (repeat spawners) have more than twice the lifetime reproductive success of fish that only spawn once (single spawners). We also find a striking pattern of negative frequency-dependent selection in which older, larger females have higher lifetime fitness when they are less abundant.
Keywords: Lotka-Volterra competition, evolutionary game theory, fitness, iteroparity, salmon
Abstract
The maintenance of diverse life history strategies within and among species remains a fundamental question in ecology and evolutionary biology. By using a near-complete 16-year pedigree of 12,579 winter-run steelhead (Oncorhynchus mykiss) from the Hood River, Oregon, we examined the continued maintenance of two life history traits: the number of lifetime spawning events (semelparous vs. iteroparous) and age at first spawning (2–5 years). We found that repeat-spawning fish had more than 2.5 times the lifetime reproductive success of single-spawning fish. However, first-time repeat-spawning fish had significantly lower reproductive success than single-spawning fish of the same age, suggesting that repeat-spawning fish forego early reproduction to devote additional energy to continued survival. For single-spawning fish, we also found evidence for a fitness trade-off for age at spawning: older, larger males had higher reproductive success than younger, smaller males. For females, in contrast, we found that 3-year-old fish had the highest mean lifetime reproductive success despite the observation that 4- and 5-year-old fish were both longer and heavier. This phenomenon was explained by negative frequency-dependent selection: as 4- and 5-year-old fish decreased in frequency on the spawning grounds, their lifetime reproductive success became greater than that of the 3-year-old fish. Using a combination of mathematical and individual-based models parameterized with our empirical estimates, we demonstrate that both fitness trade-offs and negative frequency-dependent selection observed in the empirical data can theoretically maintain the diverse life history strategies found in this population.
Natural scientists have long observed that there is considerable variation in the amount and diversity of life history strategies present within and among species (1, 2). The processes that generate and maintain this life history variation in some species, but not others, remains a fundamental question in ecology and evolutionary biology (3, 4). Here we identify two mechanisms that explain the maintenance of life history variation within a single population: fitness trade-offs, where a fitness benefit associated with one function (e.g., size at reproduction) is correlated with a fitness cost of another function (e.g., survivorship) (4–6), and negative frequency-dependent selection, where the relative fitness of a particular life history strategy declines as the life history strategy increases in frequency (7–10). These mechanisms create subtle, but testable, differences in patterns of reproductive success that can be examined in fully pedigreed populations.
Within a population, fitness trade-offs maintain life history variation because the cost and benefits associated with each “decision” can prevent any one strategy from reaching fixation. For example, there are well-documented trade-offs between growth and reproduction (11), where current reproduction comes at the cost of future growth such that two life history strategies may coexist: one that reproduces at an early age and small size at the expense of total reproductive output, and one that reproduces at a later age and larger size at the expense of increased cumulative risk of mortality (12, 13). Although trade-offs are not always sufficient for coexistence (10, 14), if a single strategy has consistently higher fitness with no trade-offs, then theory predicts that the single, optimal strategy should quickly reach fixation (15). Fitness trade-offs can be identified in fully pedigreed populations in which one can measure the sum of the costs and benefits of each strategy in terms of lifetime relative reproductive success (RRS) (16, 17).
Negative frequency-dependent selection, however, does not require a trade-off between any two characters to facilitate coexistence. Instead, the fitness of a phenotype (here a life history strategy) depends on its frequency relative to other phenotypes in a population, such that each phenotype has the highest fitness (relative to others) when it is the rarest. A classic example of negative frequency-dependent selection occurs in nectarless (nonreward-giving) orchids, where the fitness of each color polymorphism declines with increased frequency as a result of pollinators associating flower color with a lack of reward (18). To our knowledge, there are few empirical studies that have demonstrated the maintenance of behaviorally mediated life history strategies as a function of negative frequency-dependent selection, but the basic mechanism remains the same (19). As the frequency of a life history strategy increases, lifetime reproductive success decreases relative to the other life history strategies, thus preventing any one strategy from reaching fixation. This mechanism is testable in populations that have been pedigreed and phenotyped for multiple generations and in which the relationship between the frequency of a life history strategy and RRS can be measured over multiple years.
Steelhead trout (Oncorhynchus mykiss), a Pacific salmonid species, exhibits an extraordinary amount of life history variation both within and among populations. In fact, two alternative life history variants (the ocean-going, anadromous steelhead and the stream-residing resident rainbow trout) are so phenotypically divergent that they were once classified as separate species (20), although it is now well established that these life history variants routinely interbreed with one another (21, 22). There is also extensive variation within just the ocean-going, anadromous steelhead. Some steelhead are semelparous: they spawn once and die (23). Semelparous steelhead may return to spawn at different ages (2–5 y old), spending various amounts of time in freshwater or marine habitats (24). Other steelhead, known as repeat spawners, or kelts, spawn two and sometimes three times in their lifetime, returning to the ocean between each spawning event (23, 25). Furthermore, some steelhead mature in the ocean before returning to rivers to spawn (e.g., winter-run steelhead), whereas others return early and overwinter in freshwater before spawning (e.g., summer-run steelhead) (26, 27) and, in some populations, immature steelhead return to their natal rivers after spending only 4 mo in the ocean (“half pounders”) (28). Identifying how populations of this species maintain such an incredible diversity of often-interbreeding life history strategies can illuminate how phenotypic, life history, and species diversity are maintained across taxa.
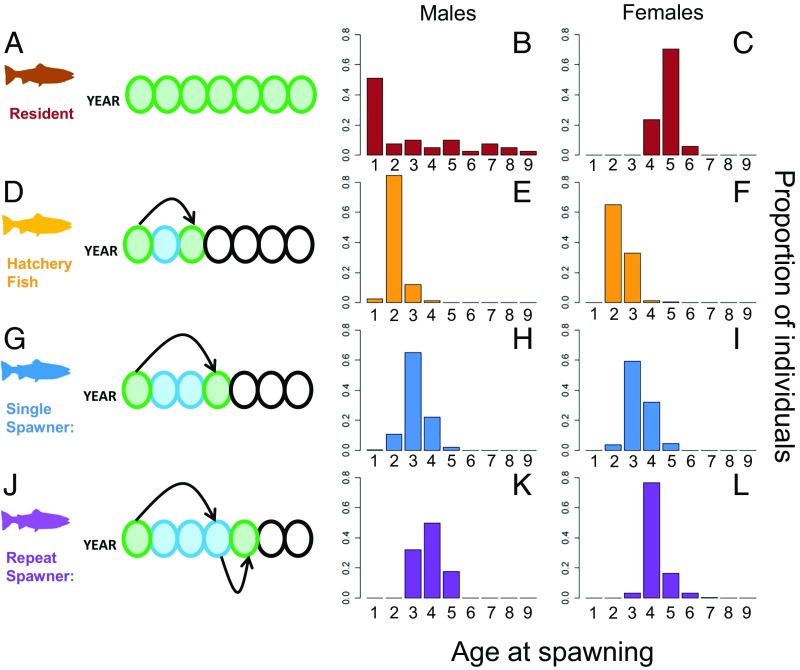
To examine the maintenance of this diversity, we focused on two life history strategies: number of spawning events and, for single-spawning fish, age of reproduction. For winter-run steelhead from the Hood River, Oregon, we examined 12,579 fish from 16 run-years and used a combination of multilocus genotypes, scale aging, and phenotypic measurements to identify repeat spawners, reconstruct pedigrees to estimate lifetime reproductive success, and determine size and age at spawning. We have near-complete samples of returning adult fish because all steelhead en route to their spawning grounds in the Hood River were physically transported over the Powerdale dam, which would otherwise be an impassable barrier to migrating adult fishes (SI Appendix, SI Text). Hood River winter-run steelhead have many interbreeding life-history variants including resident rainbow trout (21), semelparous steelhead that spawn between 2 and 5 y of age, and repeat spawners (Fig. 1). We identified repeat spawning fish as genetically identical adults that were present on the spawning grounds in the Hood River in at least two distinct run-years. The Hood River also has a hatchery supplementation program (29), and we could identify hatchery fish based on the presence or absence of year-specific fin clips (Fig. 1). We used parentage analysis to compare the lifetime reproductive success of repeat spawners with that of single spawners and to determine the lifetime reproductive success of single spawners that returned to spawn at different ages. We performed separate analyses for each year of spawning (run-year) and for males and females.
Fig. 1.
Ages at time of last spawning for the various life history strategies found within winter-run Hood River O. mykiss. (A–C) Resident fish data are from ref. 21 and are included here for a more complete description of the life history variation in this population. Residents are iteroparous, and most are wild-origin in the Hood River. Here, we could only infer the ages of spawning for resident fish born to hatchery parents. Male resident fish can begin successfully spawning as early as 1 y of age, whereas female residents do not successfully spawn until they are at least 4 y old. (D–I) Hatchery-origin fish have accelerated growth rates in fresh water in comparison with wild-origin fish, and so return to spawn a year earlier, on average. Furthermore, a greater proportion of hatchery-origin individuals occupy fewer spawning age classes in comparison with wild-origin fish, which return to spawn between ages 2 y and 5 y. (J–L) Repeat spawners appear on the spawning grounds with the same relative age distribution as single spawners, and most return to spawn the following year. A small proportion of repeat spawners skipped a year between spawning events, and although most repeat spawners only spawned twice, 6% of repeat spawning female adults successfully migrated between the river and ocean three times.
Results
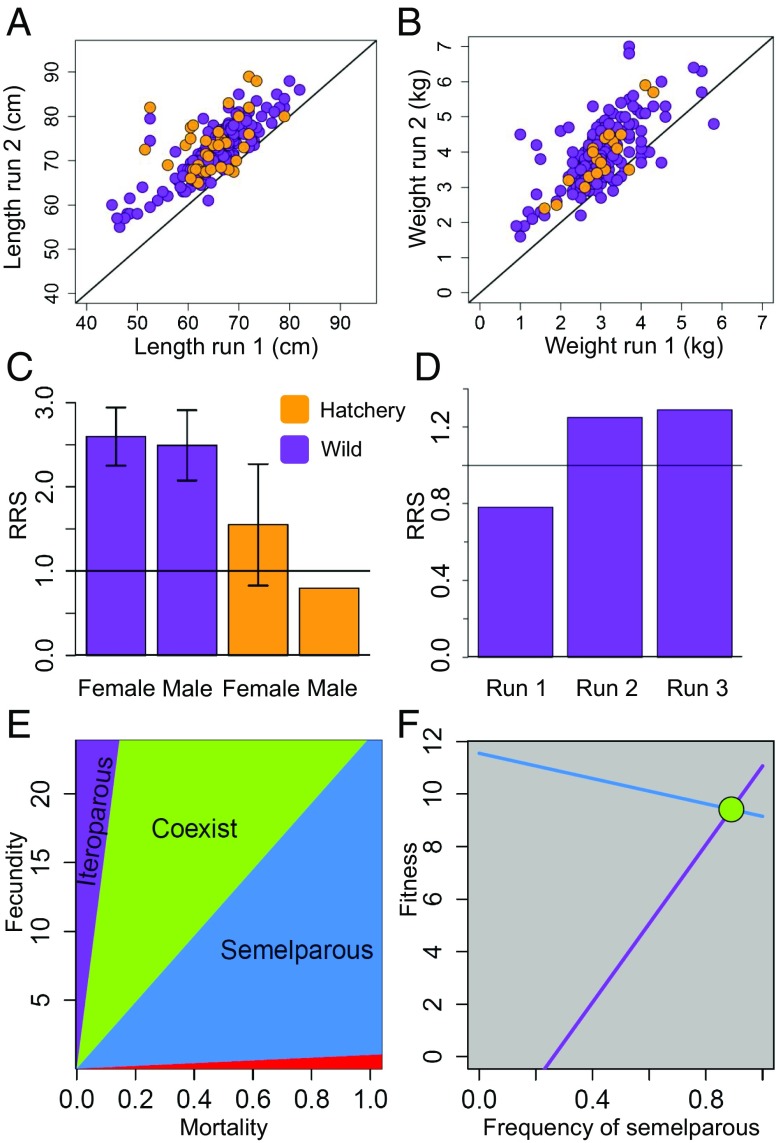
We identified a total of 299 repeat spawners, with 98% increasing in length and 95% increasing in weight between their first and second spawning attempts (Fig. 2 A and B). A ratio of 8:1 female:male repeat spawners was identified, which deviated substantially from equality (randomization test: P < 0.001; SI Appendix, Fig. S1) and is typical of steelhead populations, in which males are more likely to adopt the freshwater-resident life history strategy than females (30). There were more wild-origin repeat spawners than hatchery-origin repeat spawners, both as a proportion and in absolute numbers, despite hatchery and wild fish occurring in equal numbers on the spawning grounds (P < 0.01; SI Appendix, Fig. S1 and SI Text). This result suggests that something about the faster maturation (Fig. 1) or lower reproductive success of hatchery fish lowers their likelihood of becoming a successful repeat spawner. In comparison with wild-origin, single-spawning fish of the same sex, wild-origin repeat-spawning females had 2.59 times greater lifetime reproductive success (Fig. 2C; 95% CI, 2.25–2.94), and wild-origin repeat-spawning males had 2.48 times greater lifetime reproductive success (95% CI, 2.07–2.91). Hatchery-origin repeat-spawning females had 1.55 times the lifetime reproductive success of wild-origin, single-spawning females (95% CI, 0.83–2.27), whereas hatchery-origin repeat-spawning males had only 0.81 times the lifetime reproductive success of wild-origin, single-spawning males, although the sample size for hatchery-origin repeat spawning males was too small to construct informative confidence intervals (n = 5).
Fig. 2.
The vast majority of repeat spawners were longer and heavier the second time they spawned (A and B). There were no differences in length between hatchery repeat spawners (orange points) and wild repeat spawners (purple points). The average lifetime reproductive success for all wild repeat spawners relative to all single spawners of the same sex was 2.59 for females and 2.48 for males (C). Error bars illustrate 95% CIs. Hatchery repeat spawners had lower point estimates of RRS, but their smaller sample sizes resulted in much wider confidence intervals (not shown for hatchery males, which exceeded the y-axis scale). Females also had lower reproductive success relative to single spawners of the same age the first time they spawned (i.e., run 1), but higher RRS the second and third times they spawned (i.e., runs 2, 3) (D). Using a matrix game, we illustrate regions in fecundity-mortality space, where each strategy is the ESS (E). The semelparous strategy is shaded blue, and the iteroparous strategy is shaded purple. The region of overlap (shaded green) is the region of coexistence. The red shading demarcates where mortality exceeds fecundity, and below that line, all strategies go extinct. From the solutions in E, we can pick any point (mortality, fecundity) inside the region of coexistence and determine the fitness landscape (F). When coexistence is possible, we can plot the fitness landscape and the equilibrium frequency of each strategy (green circle).
We next examined whether there was a trade-off associated with repeat spawning, where first-time repeat spawners had lower RRS compared with single spawners of the same age, but higher RRS on their second attempts. For females, we made comparisons relative to age 3 single spawners both because the vast majority of single spawners were 3 y old when they first spawned and because 3-y-old single-spawning females had the highest fitness (Fig. 2D). For males, we made comparisons to both 3-y-old single spawners (the most numerically dominant age class) and 5-y-old single spawners (the age class with highest mean fitness; results were qualitatively similar; cf. SI Appendix, Figs. S2D and S3). Female repeat spawners were smaller in length and mass than single-spawning fish of all ages the first time they spawned (t tests: P < 0.0001 and P < 0.0001, respectively), and were larger in length and mass than single spawning fish the second time they spawned (t tests: P < 0.0001, P < 0.0001, respectively; SI Appendix, Fig. S2). However, although 4-y-old repeat-spawning females, the dominant age class (Fig. 1L), were not significantly shorter in length than 4-y-old single-spawning females (P < 0.128), they did weigh significantly less than 4-y-old single-spawning fish (3.1 vs. 3.9 kg; P < 0.001), suggesting a substantial energetic cost associated with migrating versus remaining at sea. Male repeat spawners were smaller in length and mass than single-spawning fish the first time they spawned (t tests: P < 0.0005 and P < 0.0001, respectively), but were not detectably larger in length or weight than single-spawning fish the second time they spawned (P < 0.7, P < 0.9, respectively; SI Appendix, Fig. S2). Both female and male repeat spawners had lower reproductive success than the single spawners the first time they spawned (randomization tests: P < 0.019 and P < 0.033, respectively), but higher reproductive success than single spawners the second time they spawned (randomization tests: P < 0.024 and P < 0.01, respectively; Fig. 2D and SI Appendix, Figs. S2 and S3). A small number (n = 17; 6%) of iteroparous females were present on the spawning grounds in three distinct run-years, and these individuals continued to have higher reproductive success than single spawners the third time they spawned (P < 0.1); no male fish were identified to have spawned more than twice.
To test whether the discrete strategies of semelparity and iteroparity would be theoretically expected to coexist, we developed a two by two matrix game (SI Appendix, Table S1) (31), parameterized with our empirical estimates of RRS for repeat spawners (i.e., Fig. 2C). The matrix game assumes only that population growth changes according to births minus deaths, and that single-strategy and mixed-strategy populations differ in fecundity and mortality according to a scaling factor. Importantly, the model can be solved without specific knowledge of these scaling factors or of mortality rates. The model results demonstrate that coexistence of the two life history strategies is theoretically possible for a substantial portion of the parameter space (Fig. 2E). These analyses also further demonstrate two important points: repeat spawners are unlikely to become the single dominant life history strategy, despite having more than twice the reproductive success of single spawners (the model suggests that this is because of the nontrivial rates of ocean mortality), and fitness trade-offs between fecundity and mortality can lead to the coexistence of both life-histories because there is a fitness equalizing effect in which both strategies are able to attain similar fitness (32), and that this point is a stable attractor that promotes coexistence (green circle, Fig. 2F).
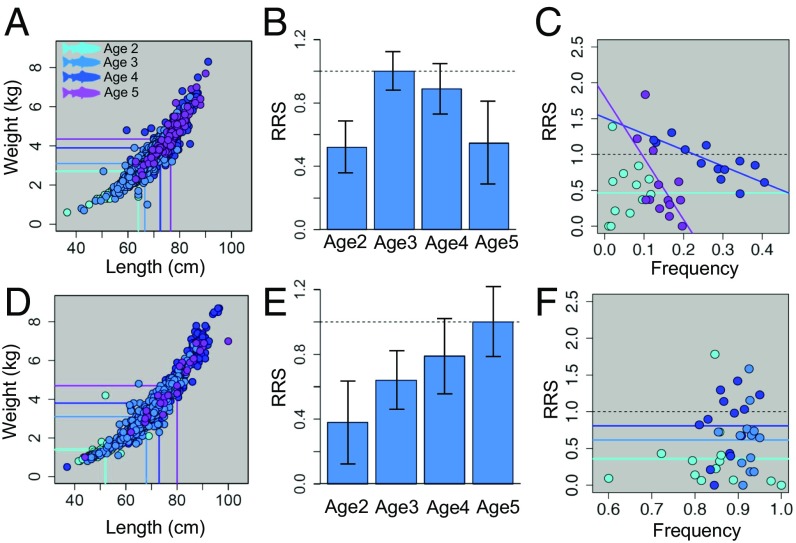
We next examined which mechanism for the maintenance of life history variation could explain why semelparous fish spend different amounts of time in the ocean, a life history trait that has been shown to have high heritability in Chinook salmon (33). For both females and males, we found that each subsequent age class is both longer and heavier than the next (Fig. 3 A and D; ANOVA, Tukey’s honest significant difference, all P < 0.001). For females, there is a clear payoff for spending an extra year at sea and spawning at age 3 y rather than at age 2 y (Fig. 3B; P < 0.001). However, we were surprised to find that 3-y-old fish had the highest reproductive success of any age class (Fig. 3B) because 4- and 5-y-old fish are both longer and heavier and have been exposed to considerably increased risk for mortality by staying out at sea for a longer time (34). These differences in RRS can be explained by negative frequency-dependent selection, in which age 4 females have reduced lifetime reproductive success relative to 3-y-old females, as 4-y-old fish increase in frequency on the spawning grounds [linear model (lm): slope = −2.24; P < 0.0009; R2 = 0.61; Fig. 3C]. Conversely, age 4 females have higher lifetime reproductive success relative to 3-y-old females when 4-y-old fish are at low frequency within the spawning population. We found the same general pattern for 5-y-old females relative to 3-y-old females (lm: slope = −8.56; P < 0.022; R2 = 0.33; Fig. 3C). We found no evidence of negative frequency-dependent selection in males, but strong support for the well-established pattern that older fish are both larger and fitter than younger fish when they spawn (Fig. 3 D–F; age 2 vs. age 5: P < 0.001; age 3 vs. age 5: P < 0.001; age 4 vs. age 5: P < 0.0172). Given that the probability of survival at sea is low each year (35–37), we assume that male Hood River steelhead are exhibiting the expected trade-off between age (size) at reproduction and probability of survival to that age (5).
Fig. 3.
For single-spawning females (A) and males (D), older fish that spawned later in life were larger than younger fish. Of all of the ages at which steelhead return to spawn, 3-y-old females had the highest RRS (B). This observation is explained by a combination of fitness trade-offs for 2- vs. 3-y-olds and negative frequency-dependent selection for 4- and 5-y-olds vs. 3-y-olds (C). Each point represents a single run-year, the frequency of that particular age group at spawning, and its lifetime reproductive success relative to 3-y-old females. For males, in contrast, older, larger individuals had greater lifetime reproductive success across all age classes found on the spawning grounds (E), and there was no relationship between frequency of age class and its fitness (F).
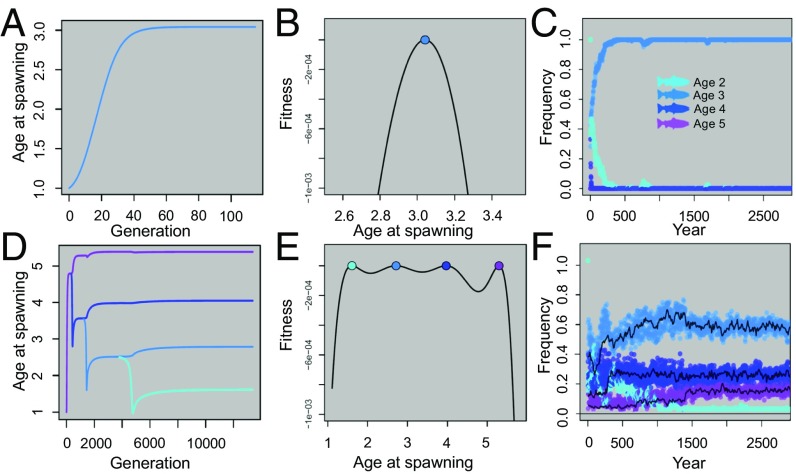
To test whether the observed variation in the continuous strategy of age at reproduction could be theoretically maintained by negative frequency dependence (NFD), we examined two complimentary models: first, we adapted a well-known, preexisting evolutionary game theoretic model that incorporates continuous ecological and evolutionary dynamics (38–40) and has been extensively analyzed previously, and second, we developed an individual-based model in which the fitness of each individual depends on its age at spawning and its relative frequency within the population in accordance with the fitness values and slopes illustrating NFD (i.e., Fig. 3 B and C). When using our empirical estimates of female fitness (i.e., Fig. 3B), but assuming no NFD, both models independently illustrate that the age 3 spawners represent the evolutionarily stable strategy (ESS) and quickly reach fixation (Fig. 4 A–C). However, when NFD is introduced, four age-at-spawning life histories evolve in the mathematical model (Fig. 4D), are found to coexist in both the mathematical and individual-based modeling approaches (Fig. 4 E and F), and were also found to occur at similar relative frequencies as observed in the Hood River population (Fig. 4F). These combined empirical and model-based results demonstrate that NFD is a reasonable explanation for the continued maintenance of life history variation in this population.
Fig. 4.
Using a continuous game theory model and our empirical estimates of female RRS, we demonstrate that only a single life history variant (age 3 spawners) can evolve (A), and that only a single ESS exists (age 3 spawners) when the resource niche is narrower than the competitive niche (B). (The mathematical meaning of resource and competitive niche width are defined in the SI Appendix and shown graphically in SI Appendix, Fig. S9.) An individual-based model independently illustrates that only age 3 spawners can exist if there is no negative frequency dependent selection, even if all four life history strategies are initially present within the population (C). When we widen the resource niche, four life history variants evolve in the game theory model (D), resulting in four ESSs coexisting because NFD, as illustrated on a fitness landscape (E). The individual-based model also independently illustrates that coexistence of the four different age-at-spawning strategies occurs when parameterized with our estimates of NFD (F).
Discussion
We found evidence that both fitness trade-offs and negative frequency-dependent selection maintain life history variation within a single population. Repeat spawners have more than twice the lifetime reproductive success as single-spawning fish, indicating a substantial benefit associated with this life history strategy. However, this life history strategy has not become fixed in the population as a result of expected trade-offs between mortality and reproduction. Our matrix game, which was parameterized with our empirical estimates of RRS, clearly illustrated that low rates of mortality are theoretically required for a fully iteroparous population to evolve (Fig. 2E). However, additional migrations likely result in high rates of mortality, given that only 2.4% (299/12,579) of fish were identified as repeat spawners. Although we do not know the exact percentage of adult steelhead that attempt to become repeat spawners in the Hood River, anecdotal evidence from the capture of out-migrating fish suggests the proportion is high; an observation that is consistent with data on steelhead from neighboring populations (25) and suggests that rates of migration-associated mortality are nontrivial. The high proportion of out-migrating adults also suggests that although there may be low variation in the proportion of fish that attempt to repeat spawn (perhaps allowing for a fitness boon during years of good ocean conditions), there is likely high variation in the investment toward their first spawning effort, and we suspect that this is the trait under selection (41). To decrease rates of migration-associated mortality, it is clear that the repeat spawners are employing a different strategy in their first spawning run to increase their chances of survival during an additional, perilous journey back out to sea (Fig. 2D). Why first-time repeat spawners have lower reproductive success remains unknown, but they may be slightly smaller on average (certainly the case for males; SI Appendix, Fig. S2), or they may hold back during spawning in response to environmental cues (e.g., ocean conditions, adult spawner density). It is of considerable interest that the six additional, and more recently derived, Pacific salmon species are all semelparous (i.e., there are no repeat spawners). How this life history variation was lost is unknown, but one possibility suggested by our model is increased rates of migration-associated mortality.
We also found clear evidence of trade-offs with respect to the ages at which semelparous fish return to spawn. For males, older and larger individuals have greater lifetime reproductive success across all age classes. This pattern is suggestive of sexual selection by male–male competition, which has been well documented in other salmonids (42, 43). We also find a similar trade-off between 2- and 3-y-old females, in which 2-y-old fish have lower lifetime reproductive success than 3-y-old fish, but higher survivorship. The larger, older 3-y-old fish can produce greater numbers of bigger eggs than 2-y-old fish (44), and may be more attractive to males, both of which likely increases their fitness. Surprisingly, this pattern did not continue with 4- and 5-y-old females. Instead, 4-y-old females had lower lifetime reproductive success, on average, compared with 3-y-old females, and 5-y-old females had lower lifetime reproductive success, on average, than both 3- and 4-y-old females. Although this was the average pattern across years, there were several years in which both 4- and 5-y-old fish had higher reproductive success than 3-y-old fish (Fig. 3C). Furthermore, there was strong negative frequency-dependent selection; the years in which the 4 and 5-y-old fish had the highest relative lifetime reproductive success were also the years in which they were less common than the other age classes.
Because observational data are challenging to collect on the Hood River spawning grounds, we can only speculate as to how this negative frequency-dependent selection operates. One possibility is that larger, older fish target, and potentially compete for, limited higher-quality spawning habitat to build their redds (43). For example, larger fish are better able to ascend high gradients and to access habitat closer to the headwaters than smaller fish. If there are few large, old females, then most of them can mate in the high-quality habitat and have high average reproductive success. However, if there are many large, old females competing for limited spawning habitat, then many of them may have to spawn in habitat that is more suitable for 3-y-old fish, but less suitable for older fish [e.g., perhaps they dig redds that are too shallow or too deep (45)], driving down the mean reproductive success of the entire age class. Another possibility is that predators, such as river otters, fixate on certain sizes (and thus age classes) as those sizes increase in frequency (43, 46, 47). We did not include predation in our models, although game theoretic models of NFD via predation also exist (48). Regardless of the mechanism, this pattern suggests that negative frequency-dependent selection can maintain diverse life-history strategies in this population, and our models support this interpretation.
Negative frequency-dependent selection is a form of balancing selection that can easily maintain genetic variation within populations, perhaps partially explaining how this species is able to rapidly adapt to novel environments (49, 50). Negative frequency-dependent processes can maintain phenotypic diversity, life history strategies, and species diversity, and our models show how it could theoretically drive our observed data. From a pragmatic viewpoint, understanding how life history variation is maintained both empirically and theoretically can lead to more effective conservation measures. For example, restricting the harvest of older, larger females in years that they are rare may facilitate negative frequency-dependent processes, and allowing these mechanisms to naturally maintain genetic and phenotypic diversity may help reverse declining populations. Here we show that fitness trade-offs and negative frequency-dependent selection can maintain life history variation; how these processes interact across genomes, sexes, and life-history variants remains an exciting avenue for continued exploration in ecology and evolution.
Methods
Identification of Repeat Spawners.
To identify repeat-spawning fish, we first examined the scale reading data collected by Oregon Department of Fish and Wildlife (ODFW) on all fish. When steelhead spawn in freshwater, a distinct mark known as a “spawning check” is laid down in a scale (51), such that repeat spawners can be identified if growth continues past this mark. For example, if a steelhead spawned after spending 2 y in the ocean, and then returned 1 y later to spawn again, it would be designated as a “2S.3,” where the S indicates the spawning check. Because all fish were also genotyped at a minimum of eight highly polymorphic microsatellite loci (29, 52), we used these joint data to confirm the identification of repeat spawners. A permutation procedure illustrated that two randomly selected individuals were unlikely to share both alleles at more than 3 loci (SI Appendix, Fig. S4). Details on this study system, laboratory methods, and the identification of repeat spawners are found in SI Appendix, SI Text.
RRS.
We calculated the lifetime reproductive success (adult to adult) for all repeat spawning fish and all single-spawning fish. We performed all analyses by run-year, and for each run-year, we included all wild adults as parents. We further added any hatchery fish identified as repeat spawners to the set of candidate parents. We next identified all candidate offspring as wild individuals assigned to appropriate brood-year. The hatchery-born adults spawned in the wild, such that all their offspring were also wild-born individuals. We performed parentage analysis using a Bayesian approach (53–55) (see SI Appendix, SI Text for details).
We next calculated the reproductive success of repeat-spawning fish relative to single-spawning fish for males and females and hatchery and wild repeat spawners separately. Because the repeat spawners were present in a minimum of two run-years, and because average fitness can vary substantially among run-years, it is important to consider the appropriate reference for repeat-spawning fish. We accounted for this variation in two ways. First, we simply compared the average fitness of repeat spawners to the fitness of single spawning fish in the first year in which repeat spawners reproduced (Fig. 2). Second, we calculated a weighted, average fitness for single-spawning fish, where we weighted the mean fitness values of single-spawning fish by the proportion of repeat spawners present in each year (SI Appendix, Fig. S5). Regardless of the approach used to calculate the average fitness of the single spawners, RRS was calculated by dividing the mean reproductive success of the repeat-spawning fish by the mean reproductive success of the single-spawning fish. We constructed 95% confidence intervals using the SE across all 13 estimates. We also calculated RRS for different ages at spawning among wild, semelparous fish (no hatchery fish or repeat spawners were included in these analyses). Within each run-year, we compared the mean lifetime reproductive success of one age class with the age class that had the highest mean fitness over all run-years (females, age 3 y; males, age 5 y).
Trade-Offs, Negative Frequency Dependence, and Theoretical Models.
We tested for significant differences in RRS in three categories: first-time repeat spawners vs. single spawners of the same age (Fig. 2D), second-time (and third-time) repeat spawners vs. single spawners in that same year (Fig. 2D), and different age classes for single spawners (Fig. 3) using randomization tests in which we pulled randomly without replacement from the assignments offspring to parents, by the total number of parents found in each of the two groups, and calculated the number of times (out of 1,000 iterations) that the randomized estimate of RRS was as, or more, extreme than the observed value (56). Outcomes for repeat spawners are presented in Results. For females, we found that 2- and 5-y-olds have lower RRS than 3-y-olds (P < 0.001 and P < 0.001, respectively), but the pattern was not significant for 3- vs. 4-y-olds (P < 0.22). To test for NFD, we calculated the frequency of each age class in each run-year and plotted it against the RRS of that age class relative to the age class with highest fitness. We fit a linear model in R, using the lm function. Log transformations had a minimal effect on the model results, and are thus not reported. Possible environmental correlates (e.g., ocean conditions, frequency of males) could not explain the observed patterns of NFD (SI Appendix, Fig. S6), nor could decreases in the relative abundance of age classes through time (SI Appendix, Fig. S7) or the total number of spawning individuals (SI Appendix, Fig. S8). Extensive details regarding the three theoretical models can be found in SI Appendix, SI Text.
Supplementary Material
Acknowledgments
We thank W. Ardren, B. Cooper, V. Amarasinghe, M. Marine, B. Van Orman, and the Oregon State Center for Genome Research and Biotechnology for laboratory protocols and genotyping efforts. We also thank M. Ford, C. Searle, and M. Sparks for insightful discussions and the editor and anonymous reviewers for thought-provoking comments that greatly improved the manuscript. We acknowledge all ODFW staff members who collected field data, performed scale aging, and acquired tissue samples for this data set. This research was funded by support to M.R.C. from the Purdue Biological Sciences and Forestry and Natural Resources Departments and by a grant to M.S.B. from the Bonneville Power Administration.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801779115/-/DCSupplemental.
References
- 1.Stearns SC. The Evolution of Life Histories. Oxford Univ Press; Oxford: 1992. [Google Scholar]
- 2.Darwin C. The Origin of Species. John Murray; London: 1859. [Google Scholar]
- 3.Ricklefs RE, Relyea R. Ecology: The Economy of Nature. WH Freeman; New York: 2014. [Google Scholar]
- 4.Futuyma D, Kirkpatrick M. Evolution. 4th Ed Sinauer; Sunderland, MA: 2017. [Google Scholar]
- 5.Stearns SC. Trade-offs in life-history evolution. Funct Ecol. 1989;3:259–268. [Google Scholar]
- 6.Metcalf JC, Rose KE, Rees M. Evolutionary demography of monocarpic perennials. Trends Ecol Evol. 2003;18:471–480. [Google Scholar]
- 7.Koskella B, Lively CM. Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution. 2009;63:2213–2221. doi: 10.1111/j.1558-5646.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 8.Carius HJ, Little TJ, Ebert D. Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 9.Adler PB, Hillerislambers J, Levine JM. A niche for neutrality. Ecol Lett. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 10.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 11.Roff D. Trade-offs between growth and reproduction: An analysis of the quantitative genetic evidence. J Evol Biol. 2000;13:434–445. [Google Scholar]
- 12.Fleming IA. Reproductive strategies of Atlantic salmon: Ecology and evolution. Rev Fish Biol Fish. 1996;6:379–416. [Google Scholar]
- 13.Gross MR. Sunfish, salmon, and the evolution of alternative reproductive strategies and tactics in fishes. In: Potts G, Wootton R, editors. Fish Reproduction: Strategies and Tactics. Academic Press; London: 1984. pp. 55–75. [Google Scholar]
- 14.Hatchwell BJ, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. [DOI] [PubMed] [Google Scholar]
- 15.Charnov EL, Schaffer WM. Life-history consequences of natural selection: Cole’s result revisited. Am Nat. 1973;107:791–793. [Google Scholar]
- 16.Grafen A. On the uses of data on lifetime reproductive success. In: Clutton-Brock TH, editor. Studies of Individual Variation in Contrasting Breeding Systems. The University of Chicago Press; Chicago: 1988. pp. 454–471. [Google Scholar]
- 17.Christie MR, Ford MJ, Blouin MS. On the reproductive success of early-generation hatchery fish in the wild. Evol Appl. 2014;7:883–896. doi: 10.1111/eva.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gigord LD, Macnair MR, Smithson A. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proc Natl Acad Sci USA. 2001;98:6253–6255. doi: 10.1073/pnas.111162598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross MR. Evolution of alternative reproductive strategies: Frequency-dependent sexual selection in male bluegill sunfish. Philos Trans R Soc Lond B Biol Sci. 1991;332:59–66. [Google Scholar]
- 20.Jordan DS, Evermann BW. American Food and Game Fishes: A Popular Account of All Species Found in America North of the Equator, with Keys for Ready Identification, Life Histories and Methods of Capture. Doubleday, Page & Company; New York: 1923. [Google Scholar]
- 21.Christie MR, Marine ML, Blouin MS. Who are the missing parents? Grandparentage analysis identifies multiple sources of gene flow into a wild population. Mol Ecol. 2011;20:1263–1276. doi: 10.1111/j.1365-294X.2010.04994.x. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman CE, Reeves GH. Identification of steelhead and resident rainbow trout progeny in the Deschutes River, Oregon, revealed with otolith microchemistry. Trans Am Fish Soc. 2002;131:986–993. [Google Scholar]
- 23.Seamons TR, Quinn TP. Sex-specific patterns of lifetime reproductive success in single and repeat breeding steelhead trout (Oncorhynchus mykiss) Behav Ecol Sociobiol. 2010;64:505–513. [Google Scholar]
- 24.Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. American Fisheries Society; Bethesda, MD: 2005. [Google Scholar]
- 25.Keefer ML, Wertheimer RH, Evans AF, Boggs CT, Peery CA. Iteroparity in Columbia river summer-run steelhead (Oncorhynchus mykiss): Implications for conservation. Can J Fish Aquat Sci. 2008;65:2592–2605. [Google Scholar]
- 26.Withler I. Variability in life history characteristics of steelhead trout (Salmo gairdneri) along the Pacific coast of North America. J Fish Res Board Can. 1966;23:365–393. [Google Scholar]
- 27.Quinn TP, McGinnity P, Reed TE. The paradox of “premature migration” by adult anadromous salmonid fishes: Patterns and hypotheses. Can J Fish Aquat Sci. 2015;73:1015–1030. [Google Scholar]
- 28.Kesner WD, Barnhart RA. Characteristics of the fall-run steelhead trout (Salmo gairdneri gairdneri) of the Klamath River system with emphasis on the half-pounder. Calif Fish Game. 1972;58:204–220. [Google Scholar]
- 29.Araki H, Ardren WR, Olsen E, Cooper B, Blouin MS. Reproductive success of captive-bred steelhead trout in the wild: evaluation of three hatchery programs in the Hood river. Conserv Biol. 2007;21:181–190. doi: 10.1111/j.1523-1739.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 30.McMillan JR, Dunham JB, Reeves GH, Mills JS, Jordan CE. Individual condition and stream temperature influence early maturation of rainbow and steelhead trout, Oncorhynchus mykiss. Environ Biol Fishes. 2012;93:343–355. [Google Scholar]
- 31.Vincent TL, Brown JS. Evolutionary Game Theory, Natural Selection, and Darwinian Dynamics. Cambridge Univ Press; Cambridge: 2005. [Google Scholar]
- 32.Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- 33.Hankin DG, Nicholas JW, Downey TW. Evidence for inheritance of age of maturity in Chinook salmon (Oncorhynchus tshawytscha) Can J Fish Aquat Sci. 1993;50:347–358. [Google Scholar]
- 34.Ward B, Slaney P, Facchin A, Land R. Size-biased survival in steelhead trout (Oncorhynchus mykiss): Back-calculated lengths from adults’ scales compared to migrating smolts at the Keogh River, British Columbia. Can J Fish Aquat Sci. 1989;46:1853–1858. [Google Scholar]
- 35.Parker RR. Estimations of ocean mortality rates for Pacific salmon (Oncorhynchus) J Fish Res Board Can. 1962;19:561–589. [Google Scholar]
- 36.Ricker W. Review of the rate of growth and mortality of Pacific salmon in salt water, and noncatch mortality caused by fishing. J Fish Res Board Can. 1976;33:1483–1524. [Google Scholar]
- 37.Bradford MJ. Comparative review of Pacific salmon survival rates. Can J Fish Aquat Sci. 1995;52:1327–1338. [Google Scholar]
- 38.Roughgarden J. Evolution of niche width. Am Nat. 1972;106:683–718. [Google Scholar]
- 39.Cressman R, et al. Unlimited niche packing in a Lotka-Volterra competition game. Theor Popul Biol. 2017;116:1–17. doi: 10.1016/j.tpb.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Leimar O, Sasaki A, Doebeli M, Dieckmann U. Limiting similarity, species packing, and the shape of competition kernels. J Theor Biol. 2013;339:3–13. doi: 10.1016/j.jtbi.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Hendry AP, Morbey YE, Berg OK, Wenburg JK. Adaptive variation in senescence: reproductive lifespan in a wild salmon population. Proc Biol Sci. 2004;271:259–266. doi: 10.1098/rspb.2003.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleming IA, Gross MR. Breeding competition in a Pacific salmon (coho: Oncorhynchus kisutch): Measures of natural and sexual selection. Evolution. 1994;48:637–657. doi: 10.1111/j.1558-5646.1994.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 43.Quinn TP, Foote CJ. The effects of body size and sexual dimorphism on the reproductive behaviour of sockeye salmon, Oncorhynchus nerka. Anim Behav. 1994;48:751–761. [Google Scholar]
- 44.Quinn TP, Seamons TR, Vøllestad LA, Duffy E. Effects of growth and reproductive history on the egg size–Fecundity trade-off in steelhead. Trans Am Fish Soc. 2011;140:45–51. [Google Scholar]
- 45.Holtby LB, Healey M. Selection for adult size in female coho salmon (Oncorhynchus kisutch) Can J Fish Aquat Sci. 1986;43:1946–1959. [Google Scholar]
- 46.Cunningham CJ, Ruggerone GT, Quinn TP. Size selectivity of predation by brown bears depends on the density of their sockeye salmon prey. Am Nat. 2013;181:663–673. doi: 10.1086/670026. [DOI] [PubMed] [Google Scholar]
- 47.Carlson SM, Quinn TP, Hendry AP. Eco-evolutionary dynamics in Pacific salmon. Heredity (Edinb) 2011;106:438–447. doi: 10.1038/hdy.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ripa J, Storlind L, Lundberg P, Brown JS. Niche co-evolution in consumer–Resource communities. Evol Ecol Res. 2009;11:305–323. [Google Scholar]
- 49.Christie MR, Marine ML, French RA, Blouin MS. Genetic adaptation to captivity can occur in a single generation. Proc Natl Acad Sci USA. 2012;109:238–242. doi: 10.1073/pnas.1111073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christie MR, Marine ML, Fox SE, French RA, Blouin MS. A single generation of domestication heritably alters the expression of hundreds of genes. Nat Commun. 2016;7:10676. doi: 10.1038/ncomms10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seamons TR, Dauer MB, Sneva J, Quinn TP. Use of parentage assignment and DNA genotyping to validate scale analysis for estimating steelhead age and spawning history. N Am J Fish Manage. 2009;29:396–403. [Google Scholar]
- 52.Christie MR, Marine ML, French RA, Waples RS, Blouin MS. Effective size of a wild salmonid population is greatly reduced by hatchery supplementation. Heredity (Edinb) 2012;109:254–260. doi: 10.1038/hdy.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christie MR. Parentage in natural populations: novel methods to detect parent-offspring pairs in large data sets. Mol Ecol Resour. 2010;10:115–128. doi: 10.1111/j.1755-0998.2009.02687.x. [DOI] [PubMed] [Google Scholar]
- 54.Christie MR, Tennessen JA, Blouin MS. Bayesian parentage analysis with systematic accountability of genotyping error, missing data and false matching. Bioinformatics. 2013;29:725–732. doi: 10.1093/bioinformatics/btt039. [DOI] [PubMed] [Google Scholar]
- 55.Anderson EC, Ng TC. Comment on ‘Bayesian parentage analysis with systematic accountability of genotyping error, missing data and false matching’. Bioinformatics. 2014;30:743–745. doi: 10.1093/bioinformatics/btt588. [DOI] [PubMed] [Google Scholar]
- 56.Manly BF. Randomization, Bootstrap and Monte Carlo Methods in Biology. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.