Biofilms are loosely defined as aggregates of bacteria encased in a self-produced matrix (1–3). Many bacterial species are known to produce biofilms when they attach to surfaces. They are commonly found in the natural environment, industrial settings, and the clinic where they can be either beneficial or problematic depending upon the context (4–6). The last two decades have seen a rapid rise in the study of biofilms. Two key questions that have occupied researchers in the field are (i) how do bacteria sense a surface? and (ii) what are the important developmental steps involved in building a biofilm community? In PNAS, Lee et al. (7) describe the contribution of a surface-sensing mechanism in Pseudomonas aeruginosa to its behavior during early stages of biofilm development. This study provides novel insight regarding how these two questions are linked.
In the laboratory, biofilm formation by flagellated, rod-shaped bacterial species has been shown to involve multiple steps in a flowing, aqueous environment (1, 2). Newly adherent cells are loosely associated with a surface, readily able to detach. This is called the reversible attachment stage and is often characterized by polarly attached cells (8, 9). Given time, some individual cells then enter the irreversible attachment stage where the cells lay flat against the surface and resist attempts to physically dislodge them (8, 9). Following irreversible attachment, cells multiply and start producing biofilm matrix components, forming small aggregates of bacteria called microcolonies. Eventually they develop into large cellular aggregates encased by a matrix. For many Gram-negative species, a key intracellular signaling molecule involved in this process is called cyclic di-GMP (c-di-GMP). In the biofilm state, cells tend to exhibit high c-di-GMP, which promotes production of biofilm matrix and represses flagellar-mediated swimming motility (10). Thus, the bacterium sensing and responding to surfaces after initial attachment is a vital step in biofilm formation.
Surface sensing is an important feature of the biology of many bacterial species. Some of the very best early work on surface sensing was conducted in the marine bacterial species, Vibrio parahaemolyticus. V. parahaemolyticus has long been known to undergo a dramatic phenotypic transformation when interacting with a surface. In free swimming, or planktonic culture, this rod-shaped bacterium has a single polar flagellum. However, upon surface attachment, V. parahaemolyticus sprouts several lateral flagella, which facilitates a type of surface motility called swarming (11, 12). McCarter and Silverman demonstrated that the surface “signal” being perceived by the bacterium was impeded rotation of the polar flagellum (a consequence of being surface associated). A critical experiment to support their model was to show that application of the drug phenamil, which disrupts ion transport within the basal body and stops flagellar rotation, was able to induce lateral flagellar gene expression in the absence of a surface (11).
In a model laboratory organism for studying biofilms, Pseudomonas aeruginosa, two distinct surface-sensing mechanisms have been identified. The first involves the Wsp system that was described by Hickman et al. (13). This system is analogous to a chemotaxis signal transduction network, with a methyl-accepting chemotaxis protein (MCP), methylase and methyl esterase, and the other associated proteins. Unlike chemotaxis systems, the output of the Wsp system is a c-di-GMP cyclase or synthase. Thus, in response to a surface, the Wsp system stimulates production of c-di-GMP. The exact nature of the signal perceived by the Wsp system is a mystery. One hypothesis is that surface contact exerts stress on the membrane, which then activates the Wsp system through the MCP-like protein, WspA. Supporting this hypothesis is that exogenous addition of the membrane stressor ethanol activates this system (14).
The second surface-sensing mechanism, and a focus of the study by Lee et al., involves the Pil-Chp system. This surface-sensing mechanism is more complex and involves a cascade of intracellular signaling molecules (15–18). Type IV pili are an important surface appendage that is central to this mechanism. Upon surface association, the MCP-like protein PilJ transduces a signal to the protein CyaB, stimulating its activity. CyaB is an adenylate cyclase, so ∼2 h after attachment, cellular levels of cAMP rise (19). Another activity regulated by the Pil-Chp system in response to a surface is type IV pili production and twitching motility. This is controlled through the regulators, Vfr and FimS, which are stimulated by high cAMP levels. Finally, production of the PilY1 protein is also induced in response to cAMP. PilY1 is associated with the type IV pilus and is thought to perceive a second surface-related signal. PilY1 harbors a von Willebrand motif, which is involved in mechanosensing in eukaryotic systems. Thus, Persat et al. (16) hypothesized that this protein may be involved in the mechanosensing of surfaces. The output of this second signal is through the c-di-GMP cyclase, SadC. Therefore, the second signal results in an increase in cellular c-di-GMP levels. One potentially important aspect of the Pil-Chp surface-sensing system is that its impact on cellular physiology in response to a surface is not particularly fast (on the order of hours). Additionally, like the Wsp system, the exact nature of the signal being sensed is unclear.
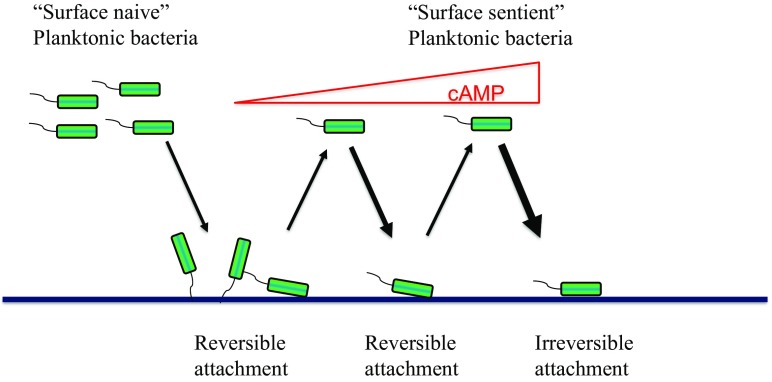
Current dogma in the field suggests that biofilm formation progresses linearly through a number of distinct steps. One important contribution by Lee et al. is that through successive surface interactions and detachments, cells become surface adapted. They demonstrate that, in P. aeruginosa, this results from a progressive increase in cellular cAMP levels and a gradual corresponding increase in type IV pili. Their experimental setup to show this involved flow cell chambers. These chambers are amenable to microscopy and present abiotic surfaces for bacteria to attach. Therefore, the authors were able to use time-lapse microscopy to evaluate the surface behavior of bacteria. The bacteria used in these experiments contained a fluorescent reporter that allowed for the monitoring of intracellular cAMP levels. Shortly after inoculation, P. aeruginosa was found to exhibit rapid attachment/detachment kinetics. These cells were termed “surface naive” and corresponded to low type IV pili/cAMP levels. However, these transient surface associations ultimately led to a buildup in cAMP levels resulting in a population the authors called “surface sentient.” These cells had a greater tendency to attach, remain surface associated, and progress to the irreversible attachment stage (Fig. 1). Sentience was found to diminish over time (i.e., cAMP levels gradually fall after cells return to the planktonic phase), suggesting that this system may represent an aspect of “memory” in the population. These points highlight one of the key findings of this study—progression to the irreversible attachment stage may involve multiple, transient attachment/detachment events for an individual cell. They go on to verify that this transition to the surface-sentient state is mediated by the Pil-Chp surface-sensing system.
Fig. 1.
A diagram depicting early events in biofilm formation. Planktonic cells that have not previously encountered cells and are exhibiting low cAMP levels are called surface naive. These cells initially exhibit very brief, transient interactions with a surface called reversible attachment. After each successive attachment/detachment event, the levels of cAMP gradually build due to the activity of the Pil-Chp surface-sensing system. Planktonic cells that have recently associated with surfaces and exhibit high cAMP levels are called surface sentient. These increases in cAMP eventually result in bacteria remaining associated with the surface for longer periods of time and ultimately contribute to progression to the irreversible attachment stage, where cells remain surface adhered and ultimately develop into biofilms.
Another interesting finding related to this study relates to the multigenerational analysis of these communities over time. During the course of biofilm formation, bacteria stop moving and ultimately multiply and form aggregates. One conventional mechanistic explanation for this would be that there is a gradual buildup of c-di-GMP in the surface-associated cells. The authors show that an additional/alternative mechanism might contribute to this behavior. The timing of the cAMP signal increase and its impact on
In PNAS, Lee et al. describe the contribution of a surface-sensing mechanism in Pseudomonas aeruginosa to its behavior during early stages of biofilm development.
physiology through type IV pili is offset by about 5 h. This is quite lengthy from the standpoint of the bacterium (i.e., the doubling time for P. aeruginosa in most laboratory growth media is on the order of 40–60 min). Therefore, the effects of cAMP signaling events would be potentially spread out vertically in a surface-associated lineage. The authors suggest that this would lead to coupled oscillations of peak cAMP levels and type IV pili activity that is multigenerational. These coupled oscillations would produce a state where cAMP signal levels are high and type IV pili activity is low. This was both predicted by a model and demonstrated experimentally. This is a rather nonintuitive observation that was only made possible by the authors’ unique experimental/modeling approach.
This study involved a collaboration between the O’Toole and Wong research groups. The O’Toole laboratory has long specialized in using molecular microbiology to study biofilms, while the Wong group excels in the application of physics-inspired methods such as large-scale cell tracking to characterize complex community behavior of surface-associated bacteria at single-cell resolution. The inherent multidisciplinary nature of this work reflects the perspectives of the two groups and led to new insight into some long-studied questions. The power of time-lapse microscopy in addressing the questions being posed was very impressive, and the amount of information that can be extracted from these datasets is enormous. Tracking algorithms allowed the authors not only to attribute surface behavior to individual bacterial cells but also to evaluate this behavior within “family trees.” This type of analysis could be extremely valuable in examining the division of labor or activities between different subpopulations that contribute to different biofilm-related processes.
There are many obvious questions moving forward. From the standpoint of mechanism, it is not clear how c-di-GMP signaling is coordinated with the study’s findings. Do c-di-GMP signals also propagate through multiple generations in a given lineage of bacteria? If so, then second messengers may be a channel of communication from one generation to the next that allows “long-term planning” in the context of biofilm formation. Is c-di-GMP signaling carefully orchestrated with the cAMP signaling events/oscillations? Does peak c-di-GMP signaling exhibit similar coupled oscillations with its outputs (production of biofilm matrix components)? It would also be interesting to know how or whether the Wsp surface-sensing system impacts the observed surface behaviors. There are also questions surrounding the ecological significance of the findings. The concept of surface sentience might be relevant to making lifestyle decisions in accordance with the environment. In a surface-rich environment, you might expect surface sentience to help accelerate surface colonization. Are there clinical or environmental systems that exhibit different levels of surface availability that vary over time or circumstances, and if so do the authors’ observations impact biofilm formation in these systems? Ultimately, the authors raise many new questions through their novel observations.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4471.
References
- 1.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 2.Tolker-Nielsen T. Biofilm development. Microbiol Spectr. 2015;3:MB-0001-2014. doi: 10.1128/microbiolspec.MB-0001-2014. [DOI] [PubMed] [Google Scholar]
- 3.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 4.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 5.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol Med Microbiol. 2012;65:127–145. doi: 10.1111/j.1574-695X.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, et al. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc Natl Acad Sci USA. 2018;115:4471–4476. doi: 10.1073/pnas.1720071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caiazza NC, O’Toole GA. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J Bacteriol. 2004;186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinsa SM, Espinosa-Urgel M, Ramos JL, O’Toole GA. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol Microbiol. 2003;49:905–918. doi: 10.1046/j.1365-2958.2003.03615.x. [DOI] [PubMed] [Google Scholar]
- 10.Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 12.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 13.Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen AI, et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog. 2014;10:e1004480. doi: 10.1371/journal.ppat.1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Toole GA, Wong GC. Sensational biofilms: Surface sensing in bacteria. Curr Opin Microbiol. 2016;30:139–146. doi: 10.1016/j.mib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inclan YF, et al. A scaffold protein connects type IV pili with the Chp chemosensory system to mediate activation of virulence signaling in Pseudomonas aeruginosa. Mol Microbiol. 2016;101:590–605. doi: 10.1111/mmi.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, et al. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. MBio. 2015;6:e02456-14. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs EL, et al. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J Bacteriol. 2010;192:2779–2790. doi: 10.1128/JB.00168-10. [DOI] [PMC free article] [PubMed] [Google Scholar]