The liver exerts important systemic functions at multiple levels. Even though the tissue looks macroscopically uniform, it is very heterogeneous at the cellular level. Beyond hepatocytes, stellate cells, endothelial cells, and Kupffer cells, many additional cell types contribute to its architecture. Beyond the cellular heterogeneity, there is a significant degree of functional heterogeneity present within the tissue, with profound differences apparent with respect to the degree that different hepatocytes dedicate themselves to specific biochemical pathways. Which set of anabolic or catabolic pathways a hepatocyte commits itself to is not a stochastic process, however. Rather, there is a discrete spatial organization put in place, referred to as “liver zonation,” that is critically involved in the separation of different metabolic pathways (1). In PNAS, Cheng et al. (2) report that they have established an additional signaling axis within the liver, “the glucagon axis,” by which liver zonation becomes initiated and maintained.
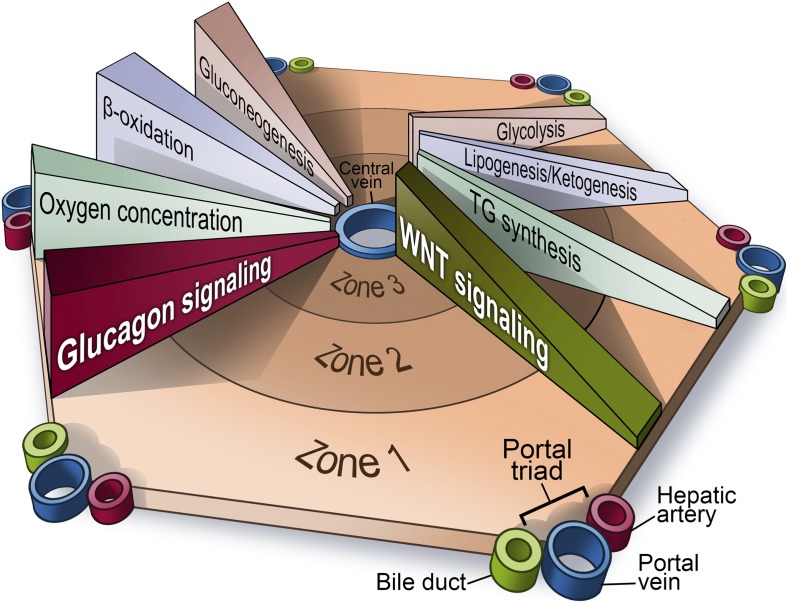
Hepatocytes within a functional liver unit, a hepatic lobule, assume distinct biochemical programs depending on their location. Blood flows from the portal vein and the hepatic artery through small blood vessels, referred to as sinusoids, to the central vein. While individual hepatocytes appear rather homogeneous along the axis, stretching from the periportal location (hepatic vein and hepatic artery) to the perivenous location (with the central vein), there are distinct differences within the microenvironment and biochemical properties of the cells themselves. The periportal region is exposed to nutrient- and oxygen-rich conditions. The portal vein supplies 75% of the blood supply to the liver and provides nutrients and hormones collected from the small intestine and much of the large intestine, the pancreas, and the spleen. The remaining 25% of the blood supply is contributed by the hepatic artery, which carries oxygen-rich blood (3). Together with the bile duct, these three vessels define the “portal triad.” With oxygen- and nutrient-rich conditions prevailing, there is a subset of oxygen-demanding pathways that are preferentially located in the periportal region. Mitochondrial β-oxidation (4), gluconeogenesis, and glycogen synthesis predominantly occur in the periportal region, while glycolysis, lipogenesis, and associated triglyceride synthesis are predominantly enriched in the pericentral region (5). While these pathways can be driven by substrate availability, it is widely accepted that there is a higher order imposed spatially by specific factors that help to establish a highly precise structure upon the lobules. Among these, the Wnt/β-catenin pathway is one of the most well-known mechanisms by which differential liver function is established (6). The Wnt pathway is most active in the pericentral region and favors the expression of pericentral genes (Fig. 1). The signaling heterogeneity for the Wnt pathways is maintained by the adenomatous polyposis coli (APC) tumor suppressor protein (7). APC is one of the key negative regulators of Wnt signaling, and, as such, governs the activity of the pathway across the different zones. Additional pathways tend to modulate gene expression along the axis as well. For instance, the Ras/MAPK/ERK signaling pathway is thought to be the periportal equivalent of the Wnt pathway, and, as such, directly induces components of pathways prevalent in the periportal region (8). In addition to this, HNF4α has been implicated as a driver for periportal gene expression, as well as suppressing pericentral genes by acting as a modulator of β-catenin (9). MicroRNAs (miRNAs) have also been implicated in the process, based on the observation that ablation of either β-catenin or the miRNA processing enzyme dicer leads to similar changes in the zonation in gene expression, suggesting that both components are involved in the proper regulation of pericentral gene expression (10).
Fig. 1.
“Zonation” of different biochemical pathways in the liver. Zone 1 is defined as the region closest to the “portal triad,” consisting of the portal vein, the hepatic artery, and the bile duct. The innermost zone is located near the central vein, and is referred to as the pericentral region. Different anabolic and catabolic pathways are differentially active in different zones. A key “zonation modulator,” the Wnt/β-catenin pathway is active in the pericentral region near the central vein. The glucagon pathway, in contrast, displays its highest activity near the periportal region. TG, triglycerides. Image created by Richard Howdy (Visually Medical).
Cheng et al. (2) now provide evidence that the absence of glucagon action in the liver also results in a loss of proper liver zonation, as judged by gene expression analyses. This is based on the observation that glucagon-null mice display an altered gene expression pattern, particularly in the periportal region. The expression of almost 300 genes is altered in mice lacking glucagon. Glutamine synthase is a classical marker for the pericentral region, and its expression changes from being in the innermost three cell layers in wild-type mice to extending out almost twice as far in glucagon-null mice. Supplying exogenous glucagon restores the original expression pattern. Of interest in this context is the observation that Wnt/β-catenin target genes are not zonated or regulated by glucagon. As such, it would appear that glucagon action is reminiscent of the effects of HNF4α, which shows similar opposing transcriptional effects. Remarkably, the regional differences of glucagon action cannot be explained on the basis of differential receptor expression, since the glucagon receptor is uniformly expressed throughout the liver. However, glucagon shows, of course, a concentration gradient, as the pancreatic α cells dispense their secretory products through the portal vein, giving rise to higher periportal glucagon levels. Based on their reported observations, Cheng et al. (2) firmly establish the glucagon axis as an additional factor prompting differential gene expression across the different zones in the liver (Fig. 1).
There have been various hints in the literature that point to a potential involvement of glucagon in this process. As early as 1986, Kinugasa and Thurman (11) examined the effects of glucagon on gluconeogenesis in periportal and pericentral hepatocytes. They noticed that glucagon had much more pronounced effects in the periportal region under gluconeogenic conditions in the fasted state. Constantin et al. (12) further confirmed this, and observed higher effects of glucagon in the periportal regions. Aggarwal et al. (13) examined the differential response to glucagon with respect to protein phosphorylation in periportal versus pericentral regions, and identified that major differences are indeed present, although overall phosphorylation was not restricted to just one region. Cheng et al. (2) are, however, the first group to systematically address the issue of the effects of glucagon on establishing a zonation pattern.
While the zonation of gene expression and biochemical pathways have been reported and firmly established for more than 50 y of research, the physiological significance is only slowly emerging. It makes physiological sense to physically separate anabolic and catabolic pathways in the liver. Fatty acid oxidation and synthesis occur in different cells. This may prevent futile cycling and also avoid competition for substrates. Furthermore, this may also at least partially explain the selective insulin resistance observed for gluconeogenesis and de novo lipogenesis. The increased uptake of free fatty acids (FFAs) in the periportal region may prompt a “spillover” effect, as frequently observed in cells where the FFA concentrations exceed the demand and are consequently shunted into the ceramide synthesis pathway. Ceramides, in turn, have been directly implicated in insulin resistance through actions on protein kinase C ζ and protein phosphatase 2A (14). The pericentral region is exposed to lower FFA concentrations and, as a result, may be less prone to a reduction of insulin action, and therefore at liberty to follow its full lipogenic potential. Cheng et al. now provide evidence that the absence of glucagon action in the liver also results in a loss of proper liver zonation, as judged by gene expression analyses. Given the at
Cheng et al. now provide evidence that the absence of glucagon action in the liver also results in a loss of proper liver zonation, as judged by gene expression analyses.
least partial redundance of the regulatory pathways governing the zonation, it is not surprising that the disruption of these pathways does not necessarily lead to severe metabolic dysregulation, or even a lethal phenotype. Glucagon receptor-null mice survive quite well and actually even display resistance to type 1 and type 2 diabetes (15). Mice lacking β-catenin in the liver simply display a more “periportal” gene expression pattern in the pericentral region; however, they also survive (16). In contrast, APC-null livers become “pericentral-like,” leading to a lethal phenotype (7). Many of these factors, including HNF4α, may exert many additional unrelated functions, but one could speculate that a liver with an exclusive pericentral expression pattern is incompatible with normal metabolic function and life, whereas a liver with a ubiquitous periportal gene expression pattern seems to fare better.
It is clear that the zonation pattern is not firmly cast in stone. Different metabolic conditions (feeding versus fasting) affect the process. Coupled to that are profound circadian effects on the process as well. Sympathetic innervation and parasympathetic innervation are clearly present near the hepatic artery and the portal vein, although the degree of innervation further inward toward the pericentral region seems to differ between species.
Liver zonation is clearly a vastly underestimated phenomenon that deserves attention every time we manipulate hepatic metabolism. Grinding up liver tissue and performing gene expression and metabolomics on the entire hepatic extract will, at best, reduce the extent of differences observed in the microenvironment and, at worst, completely obscure existing differences. Based on the recent results reported by Cheng et al. (2), we need to bear that in mind when studying glucagon action in the liver. Furthermore, pathophysiological changes imposed by glucagon on the liver in the context of type 1 and type 2 diabetes, both states of insulinopenia and impaired insulin action, result in unopposed glucagon action. Determining the effects of glucagon on the differential metabolic zones under these diabetic conditions will be a challenge for the future.
Acknowledgments
We are supported by US NIH Grants R01-DK55758, P01-DK088761, R01-DK099110, and P01-AG051459 Project 3 (to. P.E.S.) and JDRF Grant 2-SRA-2016-149-Q-R (to C.M.K. and P.E.S.).
Footnotes
The authors declare no conflict of interest.
See companion article on page E4111.
References
- 1.Jungermann K. Metabolic zonation of liver parenchyma: Significance for the regulation of glycogen metabolism, gluconeogenesis, and glycolysis. Diabetes Metab Rev. 1987;3:269–293. doi: 10.1002/dmr.5610030112. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X, et al. Glucagon contributes to liver zonation. Proc Natl Acad Sci USA. 2018;115:E4111–E4119. doi: 10.1073/pnas.1721403115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre C, Perret C, Colnot S. Molecular determinants of liver zonation. Prog Mol Biol Transl Sci. 2010;97:127–150. doi: 10.1016/B978-0-12-385233-5.00005-2. [DOI] [PubMed] [Google Scholar]
- 4.Guzmán M, Castro J. Zonation of fatty acid metabolism in rat liver. Biochem J. 1989;264:107–113. doi: 10.1042/bj2640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: Mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt R, Hovhannisyan A. Organ patterning in the adult stage: The role of Wnt/beta-catenin signaling in liver zonation and beyond. Dev Dyn. 2010;239:45–55. doi: 10.1002/dvdy.22041. [DOI] [PubMed] [Google Scholar]
- 7.Benhamouche S, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Braeuning A, Ittrich C, Köhle C, Buchmann A, Schwarz M. Zonal gene expression in mouse liver resembles expression patterns of Ha-ras and beta-catenin mutated hepatomas. Drug Metab Dispos. 2007;35:503–507. doi: 10.1124/dmd.106.013656. [DOI] [PubMed] [Google Scholar]
- 9.Stanulović VS, et al. Hepatic HNF4alpha deficiency induces periportal expression of glutamine synthetase and other pericentral enzymes. Hepatology. 2007;45:433–444. doi: 10.1002/hep.21456. [DOI] [PubMed] [Google Scholar]
- 10.Sekine S, Ogawa R, Mcmanus MT, Kanai Y, Hebrok M. Dicer is required for proper liver zonation. J Pathol. 2009;219:365–372. doi: 10.1002/path.2606. [DOI] [PubMed] [Google Scholar]
- 11.Kinugasa A, Thurman RG. Differential effect of glucagon on gluconeogenesis in periportal and pericentral regions of the liver lobule. Biochem J. 1986;236:425–430. doi: 10.1042/bj2360425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantin J, Ishii-Iwamoto E, Suzuki-Kemmelmeier F, Bracht A. Zonation of the action of glucagon on gluconeogenesis studied in the bivascularly perfused rat liver. FEBS Lett. 1994;352:24–26. doi: 10.1016/0014-5793(94)00915-5. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal SR, Lindros KO, Palmer TN. Glucagon stimulates phosphorylation of different peptides in isolated periportal and perivenous hepatocytes. FEBS Lett. 1995;377:439–443. doi: 10.1016/0014-5793(95)01387-3. [DOI] [PubMed] [Google Scholar]
- 14.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci USA. 2012;109:14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed KR, et al. B-catenin deficiency, but not Myc deletion, suppresses the immediate phenotypes of APC loss in the liver. Proc Natl Acad Sci USA. 2008;105:18919–18923. doi: 10.1073/pnas.0805778105. [DOI] [PMC free article] [PubMed] [Google Scholar]