Abstract
Traditional Chinese herbs (TCH) are currently gaining attention in disease prevention and health care plans. However, their general bitter taste hinders their use. Despite the development of a variety of taste evaluation methods, it is still a major challenge to establish a quantitative detection technique that is objective, authentic and sensitive. Based on the two-bottle preference test (TBP), we proposed a novel quantitative strategy using a standardized animal test and a unified quantitative benchmark. To reduce the difference of results, the methodology of TBP was optimized. The relationship between the concentration of quinine and animal preference index (PI) was obtained. Then the PI of TCH was measured through TBP, and bitterness results were converted into a unified numerical system using the relationship of concentration and PI. To verify the authenticity and sensitivity of quantified results, human sensory testing and electronic tongue testing were applied. The quantified results showed a good discrimination ability. For example, the bitterness of Coptidis Rhizoma was equal to 0.0579 mg/mL quinine, and Nelumbinis Folium was equal to 0.0001 mg/mL. The validation results proved that the new assessment method for TCH was objective and reliable. In conclusion, this study provides an option for the quantification of bitterness and the evaluation of taste masking effects.
KEY WORDS: Bitter, Quantification, Two-bottle preference test, Quinine, Electronic tongue, Human sensory evaluation
Graphical abstract
A quantitative strategy for bitterness of traditional Chinese herbs was developed using a standardized animal test and a unified quantitative benchmark in this study. It provides an option for the quantification of bitterness and the evaluation of taste masking effects.
1. Introduction
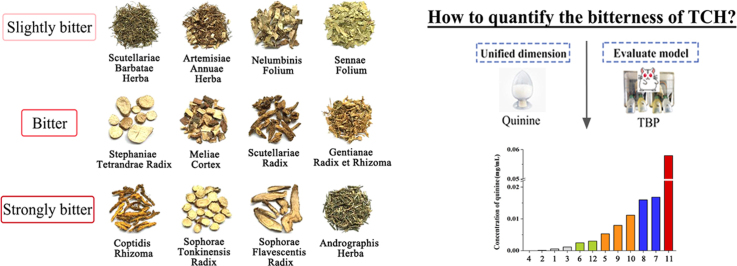
The popularity of herbal medicines is currently rising worldwide. However, the unpleasant taste of these substances is a common issue and is to some degree impeding the progress of ongoing efforts to give herbal medicines wider applications1. Among the offensive tastes, bitterness is one of the most prominent and can severely influence the compliance of patients, especially among infants and children2, 3. To develop effective taste masking strategies, it is essential to evaluate the bitterness objectively4. Our predecessors created a simple classification system for the bitter traditional Chinese herbs (TCH) that depended on sensory evaluation and included the following categories: strongly bitter, moderately bitter, slightly bitter, and not bitter. However, their evaluation methods are not exact, and they became increasingly unable to meet the demands of modern accurate quantification.
After decades of experimenting, several taste evaluation methods have been established5. The classic one is the human sensory test. It reflects genuine human perception, but is constrained by experience, identification ability, and individual differences. At the same time, the toxic or potentially hazardous substances in TCH make it difficult to conduct the experiments ethically. In general, this method is inconvenient, time consuming and has a low throughput6. To overcome these deficiencies, animal behavioral assays coupled with statistical methods, such as the animal preference test7 and the conditioned taste aversion tests8, were explored to evaluate the animals' degree of distaste. However, the assessment was comparative. It could not reflect the overall level of distaste, and animal individual differences could not be ignored. A third method is the chemical evaluation method, in which the dissolution profile of bitter compounds could directly represent the bitterness intensity9, 10, 11. However, it was unsuitable for TCH because of the complexity and uncertainty of the bitter ingredients12. The last method is a modern bionics evaluation represented by the electronic tongue (e-tongue)13. This method of detection is sensitive and fast, and even tiny differences can be resolved. However, it requires the sample to be controlled at a lower concentration, and the consistency of the evaluation results between the electronic tongue and real perception is still controversial. Through the analysis of these methods, it was found that they have become more precise over time; whereas there has been no investigations of improved precision in human bitterness detection. It seemed clear that further studies were needed to balance sensitive discrimination and genuine taste perception.
Animals tend to pursue their own interests and avoid risks. Accordingly, the two-bottle preference test (TBP) was designed to assess the unpleasant taste of food or beverage14, and the preference index (PI) was used as an evaluation indicator15. The experiment was performed by observing the preferences and dislikes in the animals' drinking habits. Rats are frequently chosen as test subjects, because rodents have bitter taste receptors that are highly homologous to those of humans and thus have a similar sense of taste16. Additionally, the individual differences existing among the rats can be decreased by observing them in large numbers and using a statistical approach. In addition, the bitterness of TCH needs to be fully and integrally characterized, as their components are complex. Therefore, a TBP at an integral level is suitable for TCH. However, there still are lots of challenges since the TBP has not been previously reported with TCH. Since TBP results are relative, and TCH are numerous, there needs to be a unified index and evaluation standard. To achieve the requirement of TCH, the standardization of an animal model, the determination of an evaluation index, the development of a quantification method, and the reliability of quantified results for realistic and sensitive discrimination in the TBP should be optimized.
In this study, the methodology of the animal model was optimized first. Quinine, which is recognized as a standard substance in the study of bitterness perception17, was selected as the quantified standard. Then, according to the classifications in the Chinese Pharmacopoeia18, the PI of 12 types of TCH, without other tastes, from different bitterness levels was obtained by the optimized method. After that, the bitterness of TCH was quantified based on the standard concentration–PI equation of quinine and the PI of TCH was expressed as the amount of quinine. Finally, the quantified result was verified by human sensory test to get a real sense in vivo and e-tongue for sensitive discrimination in vitro. At the end of the study, the bitterness of TCH was converted to a united standard based on its biological assessment. This standard could provide a method for an exemplification of the sensory evaluation of natural medicines, and could be a potential tool for taste masking study of bitter TCH preparations.
2. Materials and methods
2.1. Ethics statement
This study was conducted in strict accordance with the recommendations of the Guidelines for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China. The protocol and experimental designs were approved by the Ethical Committee of Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Approval ID: 2014KL-016). All animals remained healthy throughout the experiment, and all possible steps were taken to avoid the animals' suffering at any stage of the experiment. Volunteers were given written informed consent regarding the purpose of the study and their right to keep information confidential. Informed written consent was obtained from all participants.
2.2. Chemicals and animals
TCH were purchased from Rongle Pharmacy (Chengdu, China) and identified by Professor Runchun Xu of Chengdu University of Traditional Chinese Medicine (Chengdu, China). Their bitterness was of different level (Table 1). Quinine sulfate (No. YM0317BA14, purity>98.0%) was purchased from Yuanye Biological Technology Co., Ltd. (Shanghai, China), and was dissolved in distilled water before the experiment to a serial of concentrations (0, 0.004, 0.02, 0.1, 0.5, and 1 mg/mL), respectively. Water was purified using a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals used were of analytical grade and available locally.
Table 1.
Bitterness of the studied TCH.
| Number | TCH | Chinese name | Bitter description |
|---|---|---|---|
| 1 | Artemisiae Annuae Herba | Qinghao | Slightly |
| 2 | Sennae Folium | Fanxieye | Slightly |
| 3 | Scutellariae Barbatae Herba | Banzhilian | Slightly |
| 4 | Nelumbinis Folium | Heye | Slightly |
| 5 | Meliae Cortex | Kulianpi | Bitter |
| 6 | Scutellariae Radix | Huangqin | Bitter |
| 7 | Gentianae Radix et Rhizoma | Longdan | Bitter |
| 8 | Stephaniae Tetrandrae Radix | Fangji | Bitter |
| 9 | Sophorae Flavescentis Radix | Kushen | Strongly |
| 10 | Sophorae Tonkinensis Radix et Rhizoma | Shandougen | Strongly |
| 11 | Coptidis Rhizoma | Huanglian | Strongly |
| 12 | Andrographis Herba | Chuanxinlian | Strongly |
Sprague–Dawley (SD) rats were obtained from the Institute of Laboratory Animals of Sichuan Provincial People's Hospital (Permit No. SCXK (chuan) 2013-15, Chengdu, China). The animals were housed in plastic cages under standard conditions (23.5 ± 1.5 °C, under a 12-h light–dark cycle with lights on at 8:00 am, food and water provided ad libitum). Rats were adapted to laboratory conditions for at least 1 week prior to data collection. All experiments were performed during the light phase of the light–dark cycle. All possible steps were taken to avoid animal suffering at any stage of the experiments.
2.3. Optimization of methodology of TBP
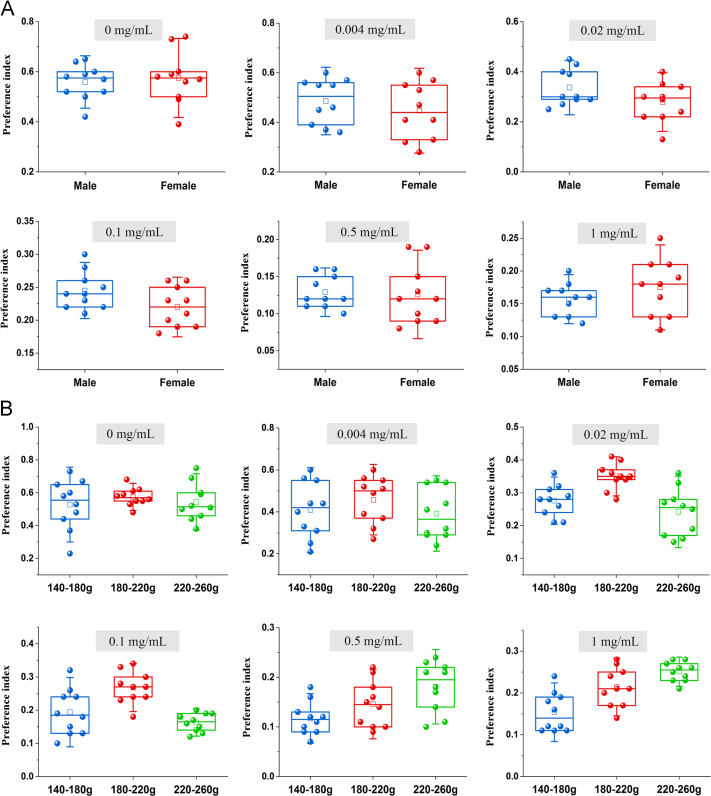
A previous study showed that animals with different genders19 and weights20 had different sensitivity levels to taste. Moreover, to reduce the number of animals used and to achieve the 3 R principles of animal ethics, the rats were recycled. Therefore, the recycling times, the gender and the weight were all investigated to obtain the optimal parameters.
In each test series, the rats first received two drinking tubes21 containing distilled water for 48 h; and then were given a choice between distilled water and ascending concentrations of quinine solutions, with each test lasting 48 h. The positions of the two drinking tubes were switched every 24 h. Intakes amounts from each tube were obtained by recording the level of fluid (on a volumetric scale to the nearest 0.1 mL) at approximately 8:00 am for each 48-h test period. The PI was used as the index by comparing the ratio of sample consumption to the total amount of water and sample over 48 h. When the PI was greater than 50%, it indicated that the rats liked the sample. A PI that was lower than 50% suggested that the rats disliked the sample.
2.3.1. Screening of gender
To avoid sensitivity to the concentration, the above mentioned six concentrations of quinine were provided. Twenty rats weighing 180–220 g were divided equally into male and female groups. The gender parameter was optimized using the standard deviation (SD) of the PI.
2.3.2. Screening of weight
Based on the survey of gender, thirty rats were divided equally into 140–180 g, 180–220 g and 220–260 g groups. The weight parameter was optimized using the SD of PI.
2.3.3. Relationship between concentrations of quinine and PI
Twenty rats were used to obtain the relationship between the concentration of quinine and the PI based on the optimized gender and weight parameters. And the above mentioned six concentrations of quinine were provided.
2.4. Quantification of the bitterness of TCH
The TCH were respectively extracted by reflux extraction with distilled water to a concentration of 1 mg/mL (quantified by the crude drug amount). The PI was obtained through the above optimized method of TBP. The bitterness intensities of the TCH were calculated by combining the standard coordinate–concentration relationship, and expressed by concentrations of quinine.
2.5. Verification of results
2.5.1. Electronic tongue measurement
To study the applicability of the quantified method, e-tongue was used to verify the results as it has a good resolution.
2.5.1.1. Preparation of samples
Quinine was dissolved in purified water and diluted to 1, 0.7, 0.5, 0.3, 0.1, 0.02, and 0.004 mg/mL. The preparation of TCH was the same to that in TBP. Each solution was then filtered through 0.45 μm nylon membrane filters (Tianjin Jinteng Co., Ltd., Tianjin, China).
2.5.1.2. Analysis method
The samples were measured by a sensor-based system: ASTREE II e-tongue system (Alpha M.O.S., Toulouse, France) equipped with seven liquid cross-selective sensors (ZZ, AB, GA, BB, CA, DA, and JE). The response intensity of each sensor was measured with an Ag/AgCl reference electrode. The potentiometric differences between each coated sensor and the reference electrode contributed to the intensity value of the measured samples. The acquisition time was fixed at 120 s22. Sensors were rinsed with distilled water between each measurement. Measured data were recorded and analyzed by AlphaSoft Software (Alpha MOS, Toulouse, France). Each sample was replicated 10 times, and the last three measurements were used for subsequent data analysis because it was observed that the last three measurements had lower variation in the sensors' potentiometric response23, 24, 25.
At present, e-tongue commonly uses Euclidean distance (Ed) in the principal component analysis (PCA) map to quantify the taste26. Ed is the true distance between two points in the dimension space. It is commonly used by e-tongue to predict the taste masking efficiency of different formulations. It can be calculated by the center coordinates of each sample on a PCA map.
| (1) |
where k represents the number of variables of each sample. xi represents the value of variable i of the first sample. yi represents the value of variable i of the second sample. The shorter is the Ed, the similar the bitter intensity. In this study, Ed represents the distance between each TCH and quinine at 0.5 mg/mL. A smaller Ed indicates a more bitter formulation.
2.5.2. Human sensory test
To study the authenticity of the quantified method, a human sensory test using the visual analog scale (VAS)27 was proposed to verify the results. VAS was a measurement instrument for subjective characteristics or attitudes that cannot be directly measured. It was widely applied in pain score, and was borrowed to bitter evaluation.
In the test, 24 well-trained and healthy volunteers (11 male and 13 female, aged 20–26) participated in the sensory evaluation. Volunteers were selected from graduate students at Chengdu University of Traditional Chinese Medicine. During the training sessions, volunteers were trained with different concentrations of quinine solutions (1, 0.7, 0.5, 0.3, 0.1, 0.02, and 0.004 mg/mL) so they were accustomed to the evaluation scales and bitterness intensities. After that, the samples were evaluated. A drop of approximately 10 mL of each solution was applied to the upper surface of the tongue for 15 s. Then, the test solution was expectorated. Volunteers were asked to score the “bitterness” using the 100 mm VAS by placing a mark along a 100 mm line23. Between each test interval, the mouth was rinsed well with distilled water so that no bitter taste remained. Volunteers were given a break between each session.
2.6. Statistical analysis
Statistical analyses were performed using SPSS 22.0 package (SPSS Inc., Chicago, IL, USA). Data were reported as mean ± standard deviation (SD) and as individual values in the figures. Differences were considered statistically significant at P value <0.05. Data obtained by e-tongue were firstly normalized by SPSS 22.0 package. SIMCA-P 11.0 version (Umetrics AB, Umea, Sweden) was used to carry on PCA. Oringin 9.0 (OriginLab, Hampton, Massachusetts, USA) was used to fit the concentration and PI of quinine.
3. Results
3.1. Optimization of methodology of TBP
The results of gender and weight studies were shown in Fig. 1. Comparison of the overall SD of PI showed that the data from male rats were more stable than those of female rats, and the data of 180–220 g rats were more stable than those of 140–180 g and 220–260 g rats. Therefore, male rats weighing 180–220 g were selected for the follow-up studies.
Figure 1.
Influence of sex (A) and weight (B) on preference index.
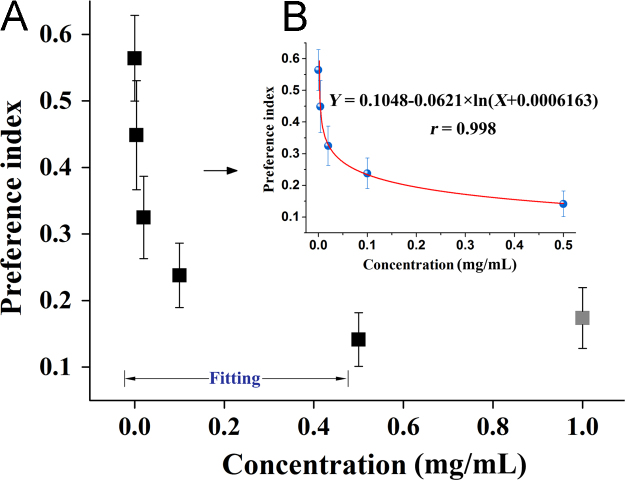
The result of PI based on the optimized parameters was shown in Fig. 2A. When the concentration of quinine was higher than 1 mg/mL, the PI did not decrease with the increasing concentration. This may have been due to the tolerance level of the rats. Thus, rats was not used for more than five times. Therefore, the relationship between the concentration of quinine and PI was established according to the first five concentrations. Finally, the logarithmic model was selected to fit the curve (Table 2 and Fig. 2B).
Figure 2.
Concentration–preference index (PI) relation of quinine.
Table 2.
Fitting equation between concentrations of quinine and preference index (PI).
| Model | Fitting equation | R |
|---|---|---|
| Linear | Y= 0.3937−0.5347X | 0.728 |
| Polynomial | Y= 0.4766−2.9922X+4.6449X2 | 0.876 |
| Allometric | Y= 0.1401X−0.2195 | 0.941 |
| Hill1 | Y= 0.5692–0.8678X0.3196/(0.8370+X0.3196) | 0.982 |
| Log3P1 | Y= 0.1048−0.0621×ln(X+0.0006163) | 0.998 |
3.2. Quantification of bitterness of TCH
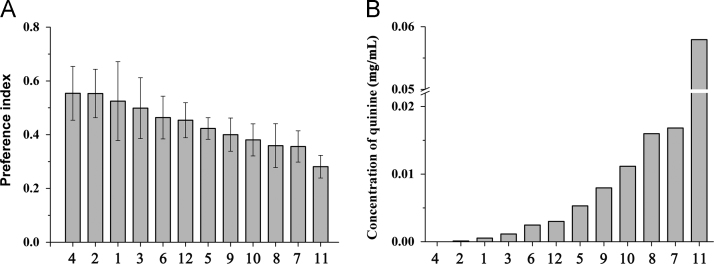
Fig. 3A showed the PIs of TCH. Through the standard coordinate–concentration fitting equation, the quantified bitterness of TCH expressed by concentrations of quinine was displayed in Fig. 3B. Based on the quantified bitterness, these TCM could be classified to 5 levels. Bitterness was strongest at quinine concentrations over 0.02 mg/mL. The taste was remarkably bitter at concentrations between 0.01 to 0.02 mg/mL. The taste was bitter when concentration was between 0.005 to 0.01 mg/mL. The bitterness was slight at concentration between 0.001 to 0.005 mg/mL. The taste was not bitter when concentrations were lower than 0.001 mg/mL (Table 2).
Figure 3.
Preference index (PI) of traditional Chinese herbs (A) and quantified bitterness expressed by concentration of quinine (B). See herb numbers in Table 1.
3.3. Verification of results
3.3.1. E-tongue measurement
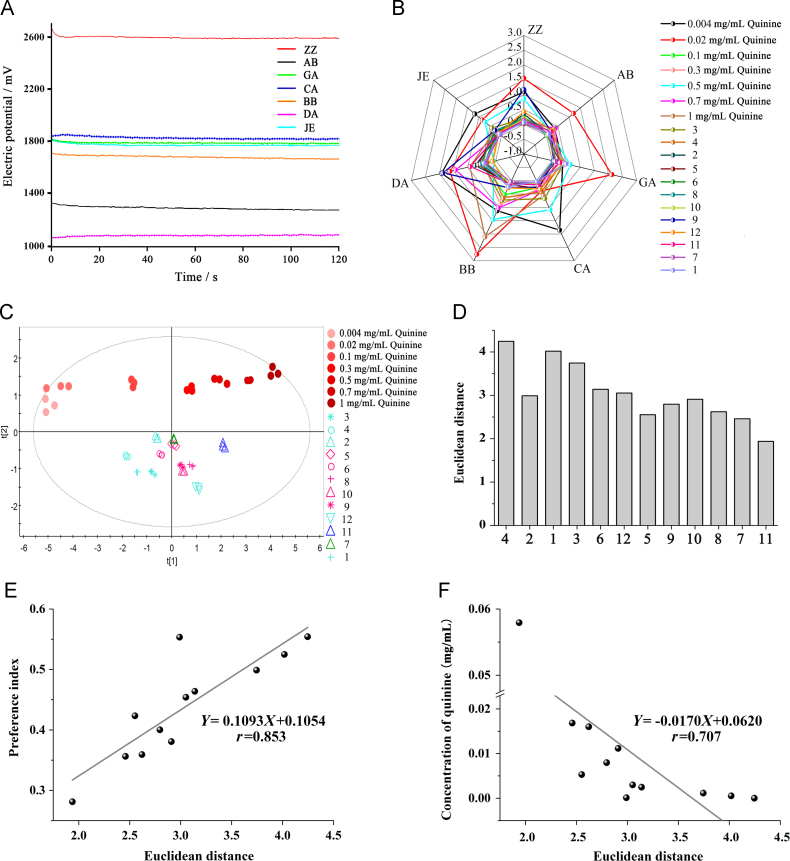
In order to determine the resolution of the quantified method, e-tongue was utilized to verify the results in vitro. As shown in Fig. 4B, the R.S.D. values of all samples were less than 3%. This suggested that the assay variation of the sensors was minimal and that reproducible results could be generated. The PCA map was shown in Fig. 4C. The cluster of each sample was small, indicating the analysis was reproducible. Furthermore, a clear discrimination between different samples was observed. As shown in Fig. 4D, the Ed between the quinine at 0.5 mg/mL and the TCH could compare the bitterness levels of TCH. For example, the distance between Coptidis Rhizoma and quinine was the smallest, so Coptidis Rhizoma was the most bitter of the TCH. This was consistent with the results of TBP.
Figure 4.
Validation effects of electronic tongue. (A) Sensory potential. (B) Radar map of RSD% of sensors. (C) PCA map of TCH. (D) Euclidean distance of TCH and 0.5 mg/mL quinine. (E) Correlation analysis between Euclidean distance and preference index (PI) of TCH. (F) Correlation analysis between Euclidean distance and corresponding concentration of quinine of TCH. See herb numbers in Table 1.
Correlations were determined with Pearson's coefficient. The correlation coefficient (r) of fitted equation (Fig. 5E) between PI and Ed of TCH was 0.853, and the r of fitted equation (Fig. 4F) between the predicted bitterness and Ed was 0.707. Thus, the established method has good resolution.
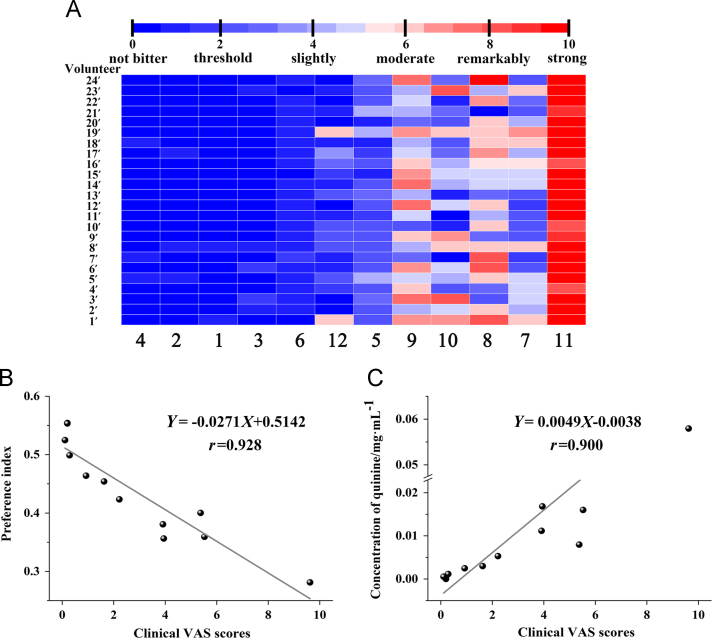
Figure 5.
Validation effects of human sensory test. (A) Clinical VAS scores of TCH. (B) Correlation analysis between VAS scores and preference index (PI) of TCH. (C) Correlation analysis between VAS scores and corresponding concentration of quinine of TCH. See herb numbers in Table 1.
3.3.2. Human sensory test
The result of human sensory test was shown in Fig. 5A. It reflected a human's true sense of taste. The correlation coefficient (r) of fitted equation (Fig. 5C) between PI and VAS scores of TCH was 0.928, and r of fitted equation (Fig. 5C) between the predicted bitterness and VAS scores was 0.900. It showed that the established method was realistic.
The validation results of e-tongue and human sensory test both exhibited a high correlation (r>0.7) with quantified bitterness. Thus, this established method has a strong level of sensitivity and discrimination and can reflect a human's real sense of taste. Through further analysis, it was found that the correlation between the PI, quantified bitterness and the VAS scores was higher than that of the Ed. This finding demonstrated that the quantification method used in this paper was more capable of reflecting real taste than discriminating different samples. The correlation coefficient of Ed and VAS scores was 0.723. This indicated that e-tongue could not detect the true bitterness like TBP, as the bitter taste of TCH was different and more complex. Using animal models would allow a comparison of their behaviors with the psychophysical results obtained by human subjects, providing a potential bridge between the animal neurobiological data and human taste perception.
4. Discussion
Bitterness exists in many natural products, natural medicines and the preparations of those products. For instance, andrographolide (a natural antibiotic)28 and tetrandrine (an analgesic)29 both taste strongly bitter. We counted the number of TCH in the 2015 edition of the Chinese Pharmacopoeia18. It was found that 618 TCH were recorded, and 22% of these were classified as bitter, including Coptidis Rhizoma (a well-known antibacterial herb)30 and Gentianae Radix et Rhizoma (an anti-inflammatory herb)31. Moreover, bitterness is also common in traditional patent drugs, such as Ginaton (containing Ginkgo biloba leaf extract, for anti-cerebral ischemia)32 and Kushen Tablets (containing Sophorae Flavescentis Radix, a well-known antineoplastic medication)33. Therefore, bitterness is ubiquitous in TCH and their preparations. In some cases, the degree of bitterness may influence doctors' decisions to prescribe these medications. Thus, quantifying the bitterness is important. Moreover, it is also essential for evaluation of the taste masking effectiveness.
TBP utilized the idea that rats would seek advantages and avoid disadvantages as an external stimulus to assess taste. This was thought to reflect the overall true taste in vivo. To reduce the impact of the individual differences among rats on results, the influence of gender and weight was investigated. Fig. 2 demonstrated that rats could be recycled in this experiment. It could keep the rat number to a minimum to achieve the 3R (reducing, reusing, and recycling) principles of animal ethics. To solve the problem that the evaluation standards were disunity and the indicators were difficult to quantify, quinine was used as a standardized bitter substance, and the bitterness of TCH was quantified by corresponding concentrations of quinine. Eventually, TBP could be able to determine the overall quantitative characterization of the intensity level of the bitterness for TCH.The taste assessment of pediatric drugs is difficult. Sensory evaluation in children is challenging, particularly with sick children, and is likely to be limited or even prohibited by ethics boards34. One could argue that adults could be used to test the medications' tastes. Thus far, very little data exist to indicate that using adults to assess the palatability of children's medication would be of any value35. Thus, we are attempting to use young rats for TBP to alternate children for the evaluation of pediatric drugs in further research.
The quantified method could be developed for equipment aiming at taste assessment. The samples in bottles were weighed through the electrical potential instead of volume by pouring samples into graduated cylinders. Then, the electrical potential was connected to computer, and the taste level could be determined by real-time computing.
5. Conclusions
This study is the first to apply TBP to the field of TCH and a new method suitable for the quantitative evaluation of bitterness of TCH was first established. Quantitative results from this study can be used to supply the taste description of TCH. This method contributed to the assessment of taste masking effect of TCH preparations. Moreover, it may also be feasible for quality control evaluation of herbs containing complex and numerous components by comparing the taste intensity. Further, it may provide a new perspective for the quantitative evaluation of other taste. Overall, this study may be significant in promoting the modernization of the character estimation of TCH.
Acknowledgements
We thank Prof. Wenquan Zou for the help guiding animal procedures and the reviewers for their critical comments in the manuscript. This work was financially supported by the National Natural Science Foundation of China (Grant No. 81403115).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Dingkun Zhang, Email: zdklester@163.com.
Junzhi Lin, Email: linjunzhi2007@126.com.
References
- 1.Zheng J.Y., Keeney M.P. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int J Pharm. 2006;310:118–124. doi: 10.1016/j.ijpharm.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Exp Opin Drug Deliv. 2011;8:1521–1529. doi: 10.1517/17425247.2011.628311. [DOI] [PubMed] [Google Scholar]
- 3.Negri R., Di Feola M., Di Domenico S., Scala M.G., Artesi G., Valente S. Taste perception and food choices. J Pediatr Gastroenterol Nutr. 2012;54:624–629. doi: 10.1097/MPG.0b013e3182473308. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z., Wu F., Singh V., Guo T., Ren X., Yin X. Host-guest kinetic interactions between HP-β-cyclodextrin and drugs for prediction of bitter taste masking. J Pharm Biomed Anal. 2017;140:232–238. doi: 10.1016/j.jpba.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Anand V., Kataria M., Kukkar V., Saharan V., Choudhury P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov Today. 2007;12:257–265. doi: 10.1016/j.drudis.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Phat C., Moon B., Lee C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 2016;192:1068–1077. doi: 10.1016/j.foodchem.2015.07.113. [DOI] [PubMed] [Google Scholar]
- 7.Tordoff M.G., Ellis H.T., Aleman T.R., Downing A., Marambaud P., Foskett J.K. Salty taste deficits in CALHM1 knockout mice. Chem Senses. 2014;39:515–528. doi: 10.1093/chemse/bju020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore-Langton J.K., Flax S.M., Pomfrey R.L., Wetzell B.B., Riley A.L. Measures of the aversive effects of drugs: a comparison of conditioned taste and place aversions. Pharmacol Biochem Behav. 2015;134:99–105. doi: 10.1016/j.pbb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Bovet L.L., Zhao K. Taste masking microspheres for orally disintegrating tablets. Int J Pharm. 2008;359:63–69. doi: 10.1016/j.ijpharm.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Guhmann M., Preis M., Gerber F., Pollinger N., Breitkreutz J., Weitschies W. Design, development and in-vitro evaluation of diclofenac taste-masked orodispersible tablet formulations. Drug Dev Ind Pharm. 2015;41:540–551. doi: 10.3109/03639045.2014.884122. [DOI] [PubMed] [Google Scholar]
- 11.Draskovic M., Medarevic D., Aleksic I., Parojcic J. In vitro and in vivo investigation of taste-masking effectiveness of Eudragit E PO as drug particle coating agent in orally disintegrating tablets. Drug Dev Ind Pharm. 2017;43:723–731. doi: 10.1080/03639045.2016.1220572. [DOI] [PubMed] [Google Scholar]
- 12.Yajima T., Fukushima Y., Itai S., Kawashima Y. Method of evaluation of the bitterness of clarithromycin dry syrup. Chem Pharm Bull. 2002;50:147–152. doi: 10.1248/cpb.50.147. [DOI] [PubMed] [Google Scholar]
- 13.Yi E.J., Kim J.Y., Rhee Y.S., Kim S.H., Lee H.J., Park C.W. Preparation of sildenafil citrate microcapsules and in vitro/in vivo evaluation of taste masking efficiency. Int J Pharm. 2014;466:286–295. doi: 10.1016/j.ijpharm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Yoneda T., Saitou K., Mizushige T., Matsumura S., Manabe Y., Tsuzuki S. The palatability of corn oil and linoleic acid to mice as measured by short-term two-bottle choice and licking tests. Physiol Behav. 2007;91:304–309. doi: 10.1016/j.physbeh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Loney G.C., Torregrossa A.M., Smith J.C., Sclafani A., Eckel L.A. Rats display a robust bimodal preference profile for sucralose. Chem Senses. 2011;36:733–745. doi: 10.1093/chemse/bjr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noorjahan A., Amrita B., Kavita S. In vivo evaluation of taste masking for developed chewable and orodispersible tablets in humans and rats. Pharm Dev Technol. 2014;19:290–295. doi: 10.3109/10837450.2013.778870. [DOI] [PubMed] [Google Scholar]
- 17.Takeshi A., Naoko T., Tomoko N., Matzno S., Shinozuka K., Uchida T. Effects of quinine on the intracellular calcium level and membrane potential of PC 12 cultures. J Pharm Pharmacol. 2007;59:1521–1526. doi: 10.1211/jpp.59.11.0009. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2015. Pharmacopoeia of the People's Republic of China (Part 1) [Google Scholar]
- 19.Nesil T., Kanit L., Pogun S. Bitter taste and nicotine preference: evidence for sex differences in rats. Am J Drug Alcohol Abus. 2015;41:57–67. doi: 10.3109/00952990.2014.990091. [DOI] [PubMed] [Google Scholar]
- 20.Miura H., Ooki M., Kanemaru N., Harada S. Decline of umami preference in aged rats. Neurosci Lett. 2014;577:56–60. doi: 10.1016/j.neulet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Tordoff M.G., Bachmanov A.A. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–324. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz J.K., Reo J.P., Hendl O., Worthington J.H., Petrossian V.D. Evaluation of a taste sensor instrument (electronic tongue) for use in formulation development. Int J Pharm. 2009;367:65–72. doi: 10.1016/j.ijpharm.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H., Uchida S., Sugiura T., Namiki N. The prediction of the palatability of orally disintegrating tablets by an electronic gustatory system. Int J Pharm. 2015;493:305–312. doi: 10.1016/j.ijpharm.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Xu M., Yang S.L., Peng W., Liu Y.J., Xie D.S., Li X.Y. A novel method for the discrimination of Semen Arecae and its processed products by using computer vision, electronic nose, and electronic tongue. Evid Based Complement Altern Med. 2015;2015:753942. doi: 10.1155/2015/753942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell G.A., Charles J.A., Roberts-Skilton K., Oh C.K., Weinecke A., Wagner R. Evaluating the taste masking effectiveness of various flavors in a stable formulated pediatric suspension and solution using the Astree™ electronic tongue. Powder Technol. 2012;224:109–123. [Google Scholar]
- 26.Woertz K., Tissen C., Kleinebudde P., Breitkreutz J. Taste sensing systems (electronic tongues) for pharmaceutical applications. Int J Pharm. 2011;417:256–271. doi: 10.1016/j.ijpharm.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Mennella J.A., Spector A.C., Reed D.R., Coldwell S.E. The bad taste of medicines: overview of basic research on bitter taste. Clin Ther. 2013;35:1225–1246. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aromdee C., Sriubolmas N., Wiyakrutta S., Suebsasna S., Khunkitti W. Effect of the derivatives of andrographolide on the morphology of Bacillus subtilis. Arch Pharm Res. 2011;34:71–77. doi: 10.1007/s12272-011-0108-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H., Luo F., Li H., Zhang L., Yi Y., Wan J. Antinociceptive effect of tetrandrine on LPS-induced hyperalgesia via the inhibition of IKKβ phosphorylation and the COX-2/PGE2 pathway in mice. PLos One. 2014;9:e94586. doi: 10.1371/journal.pone.0094586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang C.H., Yu B., Su C.H., Chen D.S., Hou Y.C., Chen Y.S. Coptidis Rhizome and Si Jun Zi Tang can prevent Salmonella enterica serovar typhimurium infection in mice. PLoS One. 2014;9:e105362. doi: 10.1371/journal.pone.0105362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y.M., Zhu S., Ge Y.W., Kazuma K., Zou K., Cai S.Q. The anti-inflammatory secoiridoid glycosides from Gentianae Scabrae Radix: the root and rhizome of Gentiana scabra. J Nat Med. 2015;69:303–312. doi: 10.1007/s11418-015-0894-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y.Y., Li P.F., Li D. Effect of Ginkgo biloba leaf extract on electroencephalography of rat with cerebral ischemia and reperfusion. Acta Pharmacol Sin. 2003;24:157–162. [PubMed] [Google Scholar]
- 33.Xiao Z.M., Wang A.M., Wang X.Y., Shen S.R. Effects of ethanol extract of Radix Sophorae Flavescentis on activity of colon cancer HT29 cells. Afr J Tradit Complement Altern Med. 2013;10:353–355. doi: 10.4314/ajtcam.v10i5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coupland J.N., Hayes J.E. Physical approaches to masking bitter taste: lessons from food and pharmaceuticals. Pharm Res. 2014;31:2921–2939. doi: 10.1007/s11095-014-1480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies E.H., Tuleu C. Medicines for children: a matter of taste. J Pediatr. 2008;153:539–540. doi: 10.1016/j.jpeds.2008.06.030. [DOI] [PubMed] [Google Scholar]