Abstract
A mangiferin aglycon derivative J99745 has been identified as a potent xanthine oxidase (XOD) inhibitor by previous in vitro study. This study aimed to evaluate the hypouricemic effects of J99745 in experimental hyperuricemia mice, and explore the underlying mechanisms. Mice were orally administered 600 mg/kg xanthine once daily for 7 days and intraperitoneally injected 250 mg/kg oxonic acid on the 7th day to induce hyperuricemia. Meanwhile, J99745 (3, 10, and 30 mg/kg), allopurinol (20 mg/kg) or benzbromarone (20 mg/kg) were orally administered to mice for 7 days. On the 7th day, uric acid and creatinine in serum and urine, blood urea nitrogen (BUN), malondialdehyde (MDA) content and XOD activities in serum and liver were determined. Morphological changes in kidney were observed using hematoxylin and eosin (H&E) staining. Hepatic XOD, renal urate transporter 1 (URAT1), glucose transporter type 9 (GLUT9), organic anion transporter 1 (OAT1) and ATP-binding cassette transporter G2 (ABCG2) were detected by Western blot and real time polymerase chain reaction (PCR). The results showed that J99745 at doses of 10 and 30 mg/kg significantly reduced serum urate, and enhanced fractional excretion of uric acid (FEUA). H&E staining confirmed that J99745 provided greater nephroprotective effects than allopurinol and benzbromarone. Moreover, serum and hepatic XOD activities and renal URAT1 expression declined in J99745-treated hyperuricemia mice. In consistence with the ability to inhibit XOD, J99745 lowered serum MDA content in hyperuricemia mice. Our results suggest that J99745 exerts urate-lowering effect by inhibiting XOD activity and URAT1 expression, thus representing a promising candidate as an anti-hyperuricemia agent.
KEY WORDS: Antihyperuricemic effect, Mangiferin aglycon, Derivative, Xanthine oxidase, Urate transporter 1
Graphical abstract
Mangiferin aglycon derivative J99745 possessed potent xanthine oxidase (XOD) inhibition in vitro. In hyperuricemic mice, J99745 reduced serum urate and MDA content, enhanced fractional excretion of uric acid, and attenuated kidney damage. J99745 might represent a promising antihyperuricemia agent by inhibiting XOD activity and urate transporter 1 expression.
1. Introduction
Hyperuricemia characteristic with excessive uric acid in blood leads to gout, and increases the risk of cardiovascular diseases, metabolic disorder and chronic renal disease1, 2. It is widely regarded relevant to the progress and prognosis of gout, nephrosis and cardiovascular diseases. Adequate control of hyperuricemia contributes to prevention and treatment of these diseases3.
Uric acid is a product of the metabolic breakdown of purine nucleotides. The enzyme xanthine oxidase (XOD) catalyzes the oxidation of hypoxanthine or xanthine to uric acid. The renal excretion of uric acid is mainly dependent on the kidney urate transport system including glomerular filtration, as well as reabsorption into and secretion from proximal tubular cells. Urate transporter 1 (URAT1) located at the luminal membrane and glucose transporter type 9 (GLUT9) at the apical membranes of the renal proximal tubules constitute the main pathway of urate reabsorption in the kidney4, 5. However, 90% of urate reabsorption is obtained through renal URAT1. Moreover, renal organic anion transporter 1 (OAT1) localized in the basolateral membrane and ATP-binding cassette transporter G2 (ABCG2) located at the apical membrane have been identified as main secretory urate transporters6, 7.
Currently, there are a few urate-lowering drugs in clinical use while most of them have severe side effects or are insensitive to kidney function impairment8, 9. XOD inhibitors and uricosuric drugs are two major therapies to reduce blood urate levels. Allopurinol and febuxostat are two major XOD inhibitors used clinically. Both drugs must be administered at an initial low dose and are not suitable for patients with kidney function impairment. On the other hand, renal damage is a main side effect of uricosuric drugs including benzbromarone and probenecid10. A new uricosuric named lesinurad was approved by U. S. Food and Drug Administraion (FDA) in 2015. It is a specific URAT1 inhibitor which promotes urate excretion, but not recommended for patients with moderate to severe renal impairment either. Therefore, the discovery and development of a novel urate-lowering drug with better effect and safety profiles is still in urgent demand.
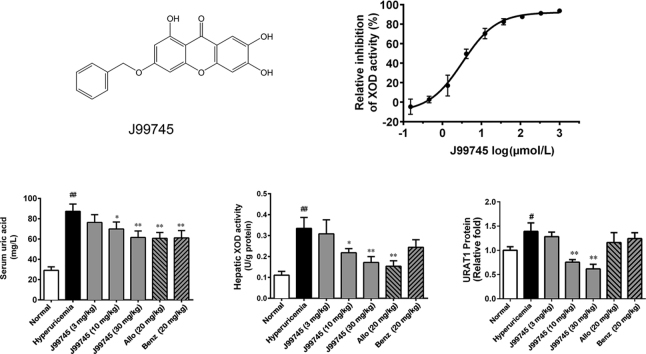
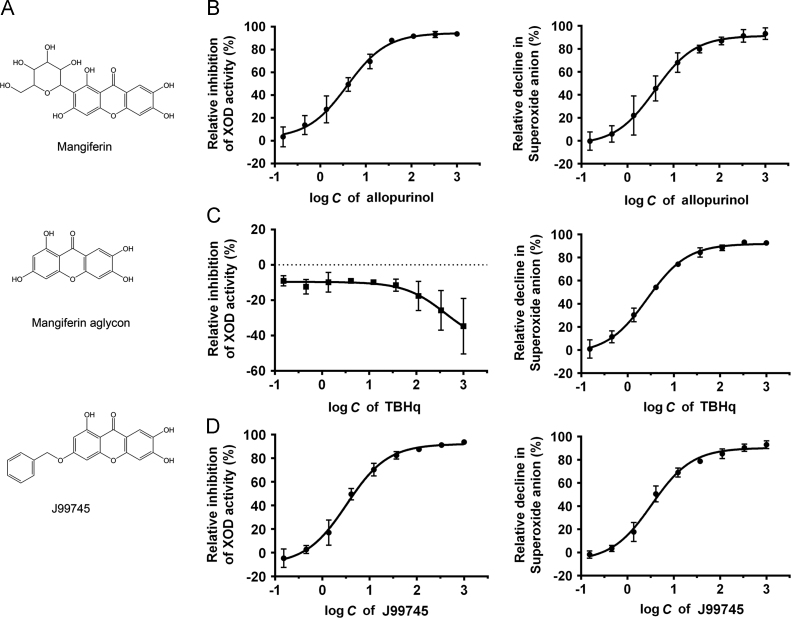
Mangiferin is a natural glucosyl xanthone enriched in the leaves of Mangifera indica L. and has been previously identified with a variety of potential pharmacological actions including antimicrobial and antioxidant activities, improving glycometabolism and lipid metabolism, and anti-diabetic effects11, 12, 13. Moreover, some studies showed that mangiferin exerted potent hypouricemic effects through modulation of uric acid production and excretion14, 15, 16. J99745 is a synthesized derivate from mangiferin aglycon (Fig. 1A) and has been identified as a potent XOD inhibitor by screening over 40,000 compounds using the method described previously17. The present study aimed to evaluate the urate-lowering effects of J99745 and explore the underlying mechanisms in experimental hyperuricemia mice.
Figure 1.
Chemical structure of J99745 (A) and its effect–concentration curve on XOD activity and superoxide anion scavenging in vitro (D). The effect–concentration curves of allopurinol (B) and TBHq (C) are also showed. Data are mean ± SD (n = 3). XOD, xanthine oxidase; TBHq, tert-butylhydroquinone.
2. Materials and methods
2.1. Chemicals and reagents
J99745 (purity > 95%, by HPLC) was synthesized by Department of New Drug Research & Development, Institute of Materia Medica (Beijing, China), and determined by electronic spray ion-mass spectrum (ESI-MS) and 1H NMR spectroscopy (Supplementary Information Fig. S1). m.p.: > 250 °C, ESI-MS (m/z): 351.31 [M+H]+, 1H NMR (400 MHz, DMSO-d6) δ: 13.03 (s, 1 H), 10.89 (s, 1 H), 9.83 (s, 1 H), 7.62 (t, J = 4 Hz, 1 H), 7.45 (m, 2H), 7.42 (s,1H), 7.31 (3, 1H), 7.27 (t, J = 4Hz, 1H), 6.34 (s, 1H), 6.17 (s, 1H), 5.23 (s, 2H).
XOD (SLBB1570V), xanthine, oxonic acid, allopurinol, tert-butylhydroquinone (TBHq, #112941) and benzbromarone were purchased from Sigma–Aldrich, St. Louis, USA. The XOD assay kit (#A002), uric acid (UA) assay kit (#C012), malondialdehyde (MDA) assay kit, blood urea nitrogen (BUN) assay kit (#C013-2), and creatinine assay kit (#C001-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Water-soluble tetrazolium (WST)-1 (2-[4-iodophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium monosodium salt, EQ. 756) was purchased from Dojindo Laboratory (Kumamoto, Japan).
2.2. Animals
All the animal experiments were carried out in accordance with the Institutional Guidelines for the Care of Laboratory Animals of the Chinese Academy of Medical Science, and were approved by the Institutional Ethics Committee for Research on Laboratory Animal Use. Healthy male Kunming strain mice of SPF grade, weighing 18–22 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (approval number SCXK (BJ) 2012-0001) and housed in air-conditioned room at 25 ± 2 °C on a 12 h light/dark cycle with free access to food and tap water.
2.3. Dual assay of xanthine oxidase activity and superoxide anion scavenging
The dual assay targeting XOD activity and superoxide anion scavenging ability was carried out according to the method described previously17. Briefly, WST-1 works as the probe for the superoxide anion. The superoxide anion (O2•−) accompanying with generation of uric acid reacts with WST-1 to form formazan, which has maximum absorption at the wavelength of 450 nm, indicating the level of O2•− in the assay system. Uric acid (which has maximum absorption at the wavelength of 295 nm) works as an indicator of xanthine oxidase activity. The 50 μL reaction system is composed of 4 U/L XOD, 250 μmol/L xanthine, 100 μmol/L WST-1, and the test compounds at various concentration in the reaction buffer (0.1 mol/L sodium paraphosphate, 0.3 mmol/L ethylene diamine tetra acetic acid (EDTA), pH 8.3). All reaction components were mixed in 384-well UV plate (Corning, USA). At last, XOD was added to initiate the reaction. The optical density at 295 nm (OD295) of the reaction system was monitored dynamically for 15 min at 37 °C with microplate reader SpectraMax M5, and the optical density at 450 nm (OD450) was just recorded once at the end of dynamic study. The initial velocity was calculated from the slope of the OD295–time curve which reflects the activity of XOD enzyme, and the slope of the OD450–time curve represents the level of O2•−. The effect-concentration curve was obtained and the IC50 value of each compound was calculated. XOD inhibitor allopurinol and antioxidant TBHq were used as references. By comparing the IC50 value, the compound could be determined to scavenge O2•− or not17. If the IC50 value of one compound on XOD inhibition is equal to the IC50 value on the level of O2•−, this compound could be regarded as just an XOD inhibitor. If the IC50 value of one compound on XOD inhibition is higher than the IC50 value on the level of O2•−, the compound could be thought to possess the ability to scavenge O2•− in addition to XOD inhibition.
2.4. Induction of hyperuricemia and drug administration
Hyperuricemia was induced in mice by xanthine and oxonic acid according to the previous studies with some modifications18, 19. Male mice were randomly allocated into 7 groups. The normal group was only treated orally with vehicle (0.5% carboxymethyl cellulose sodium, CMC-Na). In other groups, xanthine (600 mg/kg) was administered by gavage once daily for 7 days followed by single intraperitoneal injection of oxonic acid (250 mg/kg) to induce hyperuricemia. The hyperuricemia group received vehicle in addition to xanthine and oxonic acid treatment. J99745 (3, 10, and 30 mg/kg) group received hyperuricemia induction and oral administration of 3, 10, and 30 mg/kg J99745, respectively. Allo (20 mg/kg) group received hyperuricemia induction and oral administration of 20 mg/kg allopurinol. Benz (20 mg/kg) group received hyperuricemia induction and oral administration of 20 mg/kg benzbromarone. J99745, allopurinol and benzbromarone were dissolved in 0.5% CMC-Na and administered 30 min after xanthine administration each day during the experiment. The dosage of J99745 was set at doses of 3, 10, and 30 mg/kg according to the preliminary studies. On the 7th day, single intraperitoneal injection of oxonic acid (250 mg/kg) was administered immediately after the last dosage (Supplementary Information Fig. S2). Two hours later, animals were euthanized to collect blood and tissues.
2.5. Urine, blood, and tissues collection
On the 6th day, the mice were transferred to metabolic cages to collect 24 h urine samples for each mouse. Urine samples were then centrifuged at 2000×g for 10 min to obtain the supernatant for uric acid and creatinine analysis. Whole blood samples were collected after final administration on the 7th day and centrifuged at 10,000×g for 5 min to obtain the serum. Simultaneously, liver tissues were excised and stored at –80 °C until XOD analysis. Kidney tissues were quickly and carefully dissected on an ice plate and some parts were immediately fixed for H&E staining. Other parts were stored at –80 °C for PCR and Western blot analysis.
2.6. Determination of levels of uric acid, creatinine, MDA, and XOD activities
Uric acid levels in serum (Sur) and urine (Uur), creatinine levels in serum (Scr) and urine (Ucr), as well as BUN were determined using commercially available kits according to the manufacturers׳ instructions20, 21. Fractional excretion of uric acid (FEUA) was calculated as follows: FEUA (%) = (Uur/Sur)/(Ucr/Scr) × 10022, 23. Serum MDA content was determined using commercially available colorimetric kits according to the manufacturer׳s instruction24, 25. Hepatic and serum XOD activities were determined using commercially available colorimetric kits according to the manufacturer׳s instruction26.
2.7. Histopathological examination
Renal tissues were rapidly fixed with 4% paraformaldehyde, and then embedded in paraffin and sectioned at 5 μm for H&E staining. The prepared sections were visualized under light microscopy.
2.8. RNA isolation and real-time PCR analysis
Liver and kidney were extracted to prepare total RNA using Trizol reagent, followed by the synthesis of cDNA. 10 μg of total RNA isolated were subjected to reverse transcription-PCR using Superscript III First Strand (Invitrogen, USA). Then 20–50 ng of cDNA was amplified by real-time PCR. The used primers were listed as below: Xod, 5ʹ-TCAGAAGCCAAGAAGGTG-3ʹ and 5ʹ-ATGTTCTGGGGTGTCAGC-3ʹ; Urat1, 5ʹ-AGCTCTTGGACCCCAATGC-3ʹ and 5ʹ-CTTCAGAGCGTGAGAGTCACACA-3ʹ; Gapdh, 5ʹ-GCTGAGTATGTGGAGT-3ʹ and 5ʹ-GTTCACACCCATCACAAAC-3ʹ; Abcg2, 5ʹ-TGCCAGATAAGAGGGGTTAGGT-3ʹ and 5ʹ-TGCTTGCAGTGGAGTTGAGA-3ʹ; Glut9, 5ʹ-TCTCAGTTGCTTGGGAGCAG-3ʹ and 5ʹ-AGCTAAAGCAAGCTCCCTGG-3ʹ; Oat1, 5ʹ-GCTGGTACTCCTCCTCTGGA-3ʹ and 5ʹ-TCCATGACCAGCCCGTAGTA-3ʹ.
2.9. Western blot analysis
Liver or renal tissues were homogenized in ice-cold RIPA buffer supplemented with 1 mmol/L phenylmethylsulfonyl fluoride and then centrifugated at 12,000×g for 20 min. The protein concentration of supernatant was determined by bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Nanjing, China). Protein were loaded by a SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Then, the PVDF membranes were blocked with 5% skim milk and incubated with the primary antibodies for XOD (ab109235), URAT1 (ab198791), GLUT9 (ab82910), OAT1 (ab131087), ABCG2 (ab108312) or GAPDH (ab9984) at 4 °C overnight. The membranes were incubated with secondary antibody at room temperature. The bands were visualized by a gel imaging system using enhanced chemiluminescence detection kit (Beijing Kangwei Century Biological Technology, Beijing, China) and densitometric analysis were performed by Image J software (National Institutes of Health, USA).
2.10. Statistical analysis
All data were presented as the mean ± standard deviation (SD). The significant differences were statistically assessed by one-way analysis of variance (ANOVA) followed by Dunnett׳s test for post hoc analysis using the GraphPad Prism 6.0 software, and the difference was considered statistically significant when P < 0.05.
3. Results
3.1. J99745 exhibited potent XOD inhibition in vitro
In the dual assay, allopurinol inhibited the activities of XOD enzyme in a dose-dependent manner with an IC50 of 3.902 μmol/L while the IC50 on the level of O2•− was 3.966 μmol/L (Fig. 1B). In contrast, TBHq slightly increased the XOD activity, this may be due to the decline in the level to the product O2•− shifted the reaction equilibrium to the right side (Fig. 1C). The IC50 of TBHq on the level of O2•− was 2.575 μmol/L. J99745 presented a valid XOD inhibition (IC50 = 3.297 μmol/L). J99745 also exhibited an inhibition on the level of O2•− with an IC50 of 3.353 μmol/L (Fig. 1D). Like allopurinol, the IC50 on XOD inhibition is almost equal to the IC50 on the level of O2•−, indicating that J99745 did not show the ability to scavenge O2•− in addition to XOD inhibition. The results demonstrated that J99745 is a potent XOD inhibitor.
3.2. J99745 reduced serum urate levels and enhanced excretion of urate in hyperuricemia mice
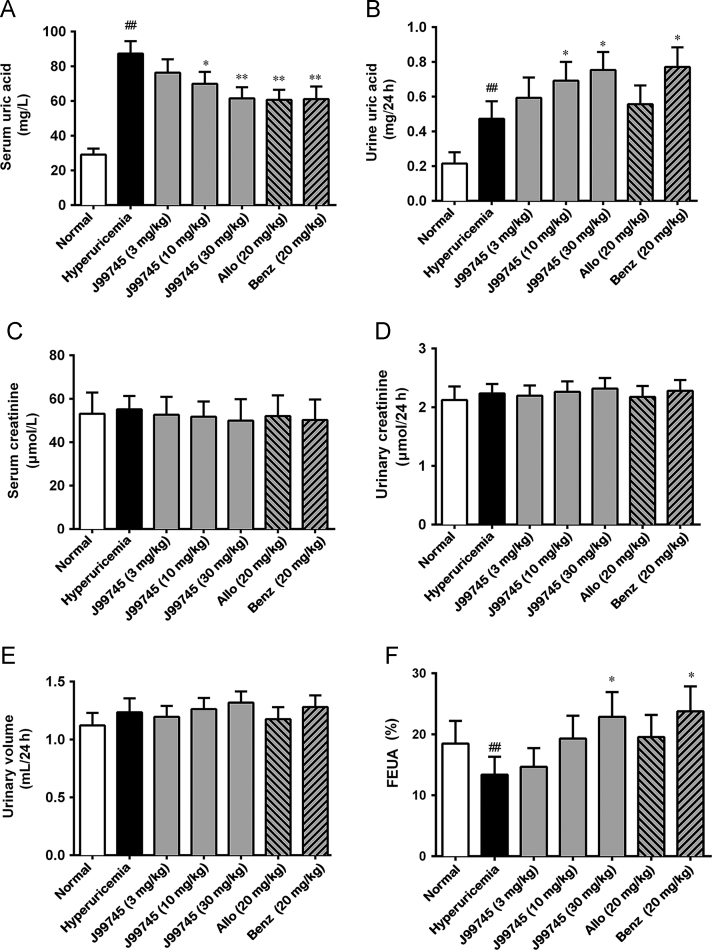
Oral administration of xanthine for 7 days and single oxonic acid injection significantly increased serum urate in the hyperuricemia mice compared to those in the normal mice (Fig. 2A). J99745 at 3 mg/kg failed to alter serum urate levels, while J99745 at 10 and 30 mg/kg effectively reduced serum urate in hyperuricemic mice after 7 days of gavage administration. Moreover, animals receiving allopurinol and benzbromarone exhibited significant decline in serum urate levels. Nevertheless, the effect of benzbromarone seemed to be weaker than that of J99745 at 30 mg/kg. Under hyperuricemia induction, the hyperuricemia group presented a significant increase in urine urate level, which was aggravated by J99745 at 10 and 30 mg/kg or benzbromarone at 20 mg/kg. In addition, all test compounds showed no significant impact on the serum and urinary creatinine as well as urine volume over 24 h in mice (Fig. 2C–E). On the other hand, a remarkable reduction in FEUA was observed in the hyperuricemia mice, which seemed to be restored in 10 and 30 mg/kg J99745-treated mice or benzbromarone-treated mice (Fig. 2F).
Figure 2.
Effects of J99745 on the levels of serum uric acid (A), urine uric acid in 24 h (B), serum cratinine (C), urinary cratinine in 24 h (D) and urine volume (E) in hyperuricemic mice. J99745 (3, 10 and 30 mg/kg), allopurinol (Allo, 20 mg/kg) or benzbromarone (Benz, 20 mg/kg) were respectively administrated to mice in addition to xanthine and oxonic acid treatment. The hyperuricemia group was adminstrated with vehicle. FEUA (F) was calculated and expressed as the percentage. Data are displayed as the mean ± SD (n =6). #P < 0.05, ##P < 0.01 compared with the normal group; *P < 0.05, **P < 0.01 compared with the hyperuricemia group.
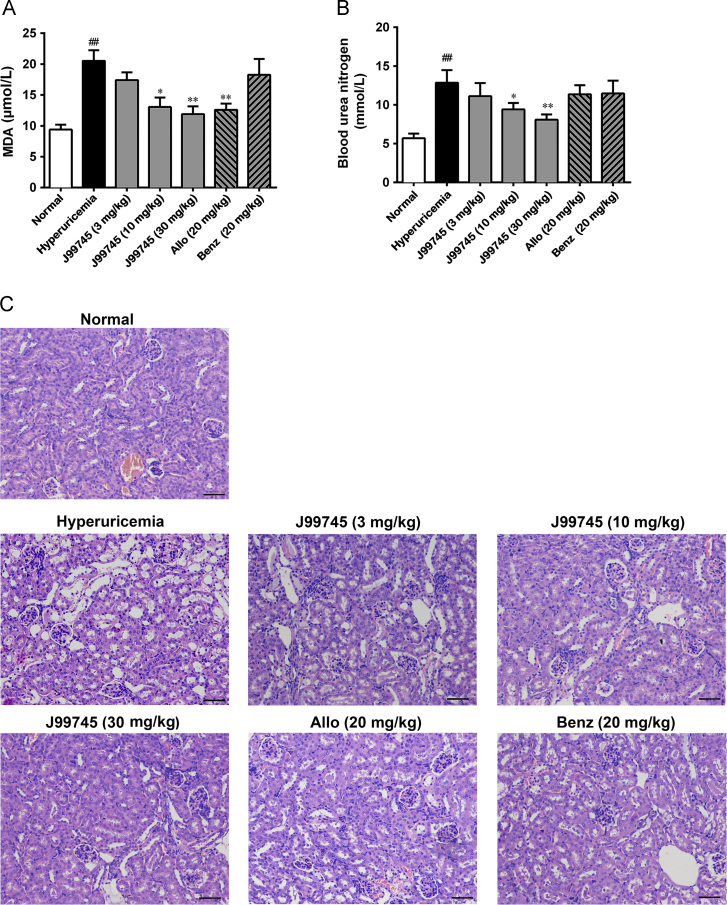
3.3. J99745 improved renal function and histopathological changes
Compared to the normal mice, the hyperuricemia mice showed increased BUN level, but no significant change in serum creatinine, indicating mild renal function damage. J99745 at doses of 10 and 30 mg/kg reduced BUN compared to the hyperuricemia group, while allopurinol and benzbromarone treatment failed to reduce BUN (Fig. 3B). H&E staining on kidney sections from the hyperuricemia group showed obvious vacuolization in renal tissue, irregular cell arrangement, and renal tubular dilatation, which could be significantly alleviated following the treatment of J99745 at 10 and 30 mg/kg (Fig. 3C). Dispersed vacuolization and slightly dilated renal tubule were observed in the 3 mg/kg J99745-treated group as well as the benzbromarone group and the allopurinol group.
Figure 3.
Effects of J99745 on serum MDA content (A), BUN (B) and renal morphological changes in mice (C). Serum MDA content and BUN was expressed as mean ± SD (n = 6). Kidney sections for HE staining (n = 4) were presented at a magnification of 200×. Scale bar, 20 μm. J99745 (3, 10 and 30 mg/kg), allopurinol (Allo, 20 mg/kg) or benzbromarone (Benz, 20 mg/kg) were respectively administrated to mice in addition to xanthine and oxonic acid treatment. The hyperuricemia group was adminstrated with vehicle. #P < 0.05, ##P < 0.01 vs. the normal group; *P < 0.05, **P < 0.01 vs. the hyperuricemia group.
3.4. J99745 lowered serum MDA content
To investigate the effects of J99745 on oxidative stress injury, serum MDA content was checked, which is an important readout of lipid and protein peroxidation. Serum MDA increased significantly in the hyperuricemia group. J99745 at 10 and 30 mg/kg and allopurinol dramatically decreased serum MDA content, indicating that oxidative stress was relieved under J99745 treatment (Fig. 3A).
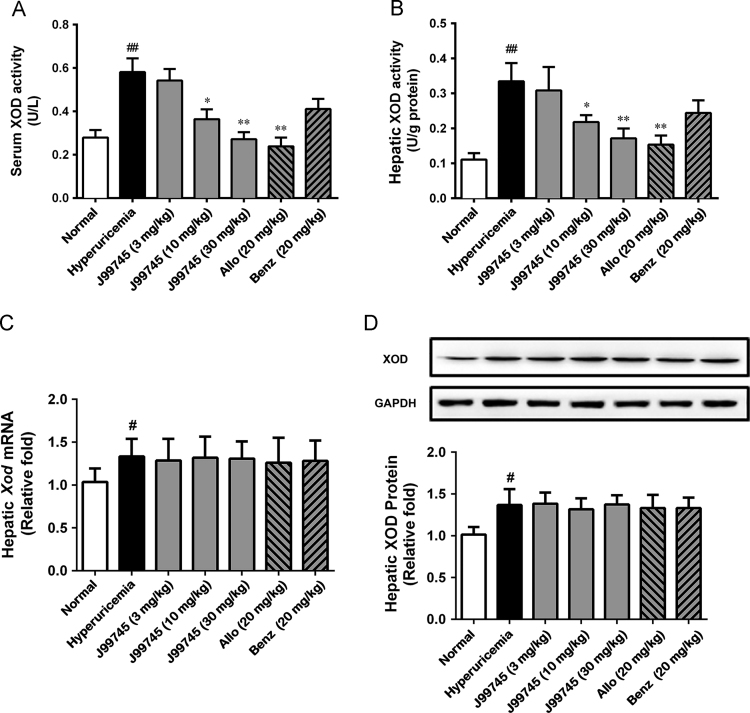
3.5. J99745 decreased XOD activity with no impact on XOD expression
J99745 exhibited potent XOD inhibition in vitro. Thus, its effect on XOD activity was evaluated in the hyperuricemia mice. Serum and hepatic XOD activities were significantly increased in the hyperuricemic mice (Fig. 4A and B). In contrast, J99745 with two high doses and allopurinol at 20 mg/kg significantly lowered the XOD activities in serum and liver. However, J99745 and allopurinol exerted no impact on the mRNA and protein levels of XOD (Fig. 4B). Treatment with 20 mg/kg benzbromarone showed no influence on serum and hepatic XOD activities as well as mRNA and protein levels. These data indicated that J99745 inhibited XOD activity to reduce uric acid production.
Figure 4.
Effects of J99745 on serum and hepatic XOD activities, mRNA and protein levels in mice. XOD activities were expressed as mean ± SD (n = 6). Xod mRNA and protein data were normalized to Gapdh and expressed as relative fold (n = 4). J99745 (3, 10 and 30 mg/kg), allopurinol (Allo, 20 mg/kg) or benzbromarone (Benz, 20 mg/kg) were respectively administrated to mice in addition to xanthine and oxonic acid treatment. The hyperuricemia group was adminstrated with vehicle. #P < 0.05, ##P < 0.01 vs. the control group; *P < 0.05, **P < 0.01 vs. the hyperuricemia vehicle group.
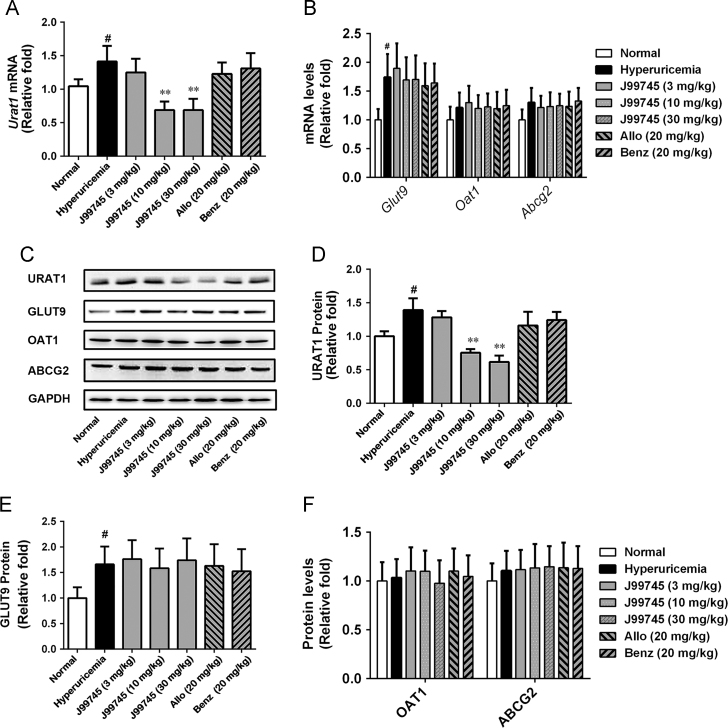
3.6. J99745 downregulated URAT1 expression
Renal mRNA and protein levels of URAT1 and GLUT9 increased remarkably in the hyperuricemic mice (Fig. 5A–C). J99745 at doses of 10 and 30 mg/kg downregulated the mRNA and protein levels of URAT1, but failed to alter GLUT9 expression (Fig. 5D and E). Compared to the normal group, renal mRNA as well as protein levels of OAT1 and ABCG2 in hyperuricemic mice did not alter significantly. None of the treatments of J99745, allopurinol or benzbromarone exerted impact on OAT1 and ABCG2 expression (Fig. 5B, C, and F). These results indicated that J99745 downregulated renal URAT1 expression to promote the excretion of uric acid.
Figure 5.
Effects of J99745 on mRNA and protein levels of renal URAT1, GLUT9, OAT1 and ABCG2 in mice. Data were normalized to GAPDH and expressed as relative fold (n = 4). J99745 (3, 10 and 30 mg/kg), allopurinol (Allo, 20 mg/kg) or benzbromarone (Benz, 20 mg/kg) were respectively administrated to mice in addition to xanthine and oxonic acid treatment. The hyperuricemia group was adminstrated with vehicle. #P < 0.05, ##P < 0.01 vs. the normal group; *P < 0.05, **P < 0.01 vs. the hyperuricemia group.
4. Discussion
This study is the first to demonstrate that xanthone flavone J99745 analogous to mangiferin aglycon exerts urate-lowering and nephroprotective effects in hyperuricemia mice. Furthermore, the underlying mechanism of J99745 might be through inhibiting hepatic XOD activity and renal URAT1 expression.
In the present study, hyperuricemia was reproduced in mice using xanthine gavage for 7 days and single injection of potassium oxonic acid at the last day, in accordance with some reports18, 27, 28, 29. In contrast, consecutive administration of potassium oxonic acid for 7 days was adopted in some other reports to stimulate the hyperuricemia30, 31. The two protocols shared similar outcome of hyperuricemia. Our protocol induced milder renal function impairment indicated by decreased BUN without the alteration in serum creatinine. H&E staining in kidney sections confirmed the mild impairment. Interestingly, some kidney injuries were also observed following allopurinol or benzbromarone treatment. The renal damage observed may be partially attributable to the fact that benzbromarone, as a uricosuric drug, promotes renal urate excretion and elevates urinary urate concentration; thereby increasing the excretory burden of kidney and induces damage32.
XOD as a typical oxidant-producing enzyme and a major source of reactive oxygen species (ROS) is upregulated in experimental hyperuricemia33, 34, 35. The uric acid conversion from hypoxanthine and xanthine by XOD is accompanied by the generation of O2•−36, 37. In patients with gout, the overproduction of ROS causes oxidative stress and increases the risks of diseases secondary to hyperuricemia including cardiovascular events35, 36. Moreover, the evidence that uric acid within the cell may function as a pro-oxidant instead of an antioxidant (primarily in plasma) and play a contributory role in the pathogenesis of many diseases has been established1, 38, 39. Therefore, antioxidant could be of therapeutic value in retarding these pathogenic processes. It was demonstrated that XOD inhibitors could play a role as antioxidant to reduce the ROS level and subsequent oxidative stress injury, and protected against ischemia–reperfusion injury, endothelial injury, and heart failure40, 41, 42, 43. In the study, the dual in vitro assay demonstrated that J99745 potently inhibited the activity of XOD enzyme, although it did not exhibit the ability to scavenge O2•−. In hyperuricemic mice, J99745 also exerted effective inhibition on serum and hepatic XOD activities as well as relieved the oxidative stress injury indicated by decline in serum MDA content. These observations might explain why J99745 performed with better profile than benzbromarone, a uricosuric drug without antioxidant action, in hyperuricemic mice.
Under physiological conditions, a fine balance exists between urate production and excretion in humans. Hence, any pathological process stimulating urate overproduction due to increased hepatic XOD activity or decreased renal excretion could result in hyperuricemia. Recent studies also exhibited that insufficient renal excretion of uric acid contributed to the majority of hyperuricemia44. In the present study, the hyperuricemia group demonstrated insufficient excretion of uric acid as compared to it overproduction although the urine urate level was elevated. J99745 treatment at doses of 10 and 30 mg/kg further increased urine uric acid excretion and FEUA as well. The beneficial effects of J99745 may result from efficient reduction in serum urate levels through not only blocking overproduction but also reversing underexcretion of uric acid. Some previous studies have suggested that the urate handling was mainly dependent on the renal urate transport system, and OAT1, URAT1, GLUT9, and ABCG2 were regarded as the potential therapeutic targets for the treatment of hyperuricemia4, 5, 6, 7. Our results demonstrated that xanthine- and oxonic acid-treatment remarkably increased renal URAT1 and GLUT9 protein levels but not OAT1 and ABCG2 protein levels in mice. J99745 downregulated renal URAT1 expression in hyperuricemic mice, but failed to alter the expression of GLUT9, OAT1 and ABCG2. Actually, the effect of J99745 on uric acid transfer across cell membrane has been checked on manipulating HEK293 cell line stably overexpressing human URAT1 combined HPLC method for supernatant uric acid detection, and found that J99745 had no significant influence on human URAT1 function (data not shown). J99745 treatment showed no such injury in renal function and morphology compared to the benzbromarone treated mice. Maybe the phenomenon that J99745 exerted merely inhibition on URAT1 promoted uric acid excretion so moderately as not to induce the subsequent kidney damage. The mechanisms by which J99745 showed specificity on URAT1 expression need to be elucidated in future study.
5. Conclusions
In summary, this study showed that J99745 significantly decreased the serum urate level and protected against hyperuricemia-associated renal damage in xanthine- and oxonic acid-induced hyperuricemia mice. The mechanism by which J99745 lowered urate might be inhibiting XOD activity and URAT1 expression. Therefore, J99745 may represent a promising candidate as an anti-hyperuricemia drug candidate.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81573645, 81202538 and 81673422), CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-3-007) and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2013ZX09508104 and 2013ZX09402203).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2017.05.004.
Appendix A. Supplementary material
J99745 was determined by ESI-MS and 1H NMR. m.p.: > 250 °C, ESI-MS (m/z): 351.31 [M+H]+, 1H NMR (400 MHz, DMSO-d6) δ: 13.03 (s, 1 H), 10.89 (s, 1 H), 9.83 (s, 1 H), 7.62 (t, J = 4 Hz, 1 H), 7.45 (m, 2 H), 7.42 (s,1 H), 7.31 (3, 1 H), 7.27 (t, J = 4 Hz, 1 H), 6.34 (s, 1 H), 6.17 (s, 1 H), 5.23 (s, 2 H).
.
A schematic diagram on induction of hyperuricemia and drug administration in mice. The test compound means J99745, allopurinol or benzbromarone dissolved in 0.5% CMC-Na, respectively.
.
References
- 1.Kanbay M., Jensen T., Solak Y., Le M., Roncal-Jimenez C., Rivard C. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 2016;29:3–8. doi: 10.1016/j.ejim.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Quintana E., Tugores A., Rodriguez-Gonzalez F. Serum uric acid levels and cardiovascular disease: the gordian knot. J Thorac Dis. 2016;8:E1462–E1466. doi: 10.21037/jtd.2016.11.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson J., Quinn T., Walters M. Uric acid reduction: a new paradigm in the management of cardiovascular risk? Curr Med Chem. 2007;14:1879–1886. doi: 10.2174/092986707781058797. [DOI] [PubMed] [Google Scholar]
- 4.Dinour D., Gray N.K., Campbell S., Shu X., Sawyer L., Richardson W. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21:64–72. doi: 10.1681/ASN.2009040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichida K., Hosoyamada M., Hisatome I., Enomoto A., Hikita M., Endou H. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15:164–173. doi: 10.1097/01.asn.0000105320.04395.d0. [DOI] [PubMed] [Google Scholar]
- 6.Ding X.W., Wu J.H., Jiang C.P. ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010;86:631–637. doi: 10.1016/j.lfs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Y., Zhang R., Wu J., Liu M., Peng W., Yu X. Organic anion transporter 1 (OAT1) involved in renal cell transport of aristolochic acid I. Hum Exp Toxicol. 2012;31:759–770. doi: 10.1177/0960327111424302. [DOI] [PubMed] [Google Scholar]
- 8.Grassi D., Pontremoli R., Bocale R., Ferri C., Desideri G. Therapeutic approaches to chronic hyperuricemia and gout. High Blood Press Cardiovasc Prev. 2014;21:243–250. doi: 10.1007/s40292-014-0051-6. [DOI] [PubMed] [Google Scholar]
- 9.Khanna D., Fitzgerald J.D., Khanna P.P., Bae S., Singh M.K., Neogi T. 2012 American college of rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azevedo V.F., Buiar P.G., Giovanella L.H., Severo C.R., Carvalho M. Allopurinol, benzbromarone, or a combination in treating patients with gout: analysis of a series of outpatients. Int J Rheumatol. 2014;2014:263720. doi: 10.1155/2014/263720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apontes P., Liu Z., Su K., Benard O., Youn D.Y., Li X. Mangiferin stimulates carbohydrate oxidation and protects against metabolic disorders induced by high-fat diets. Diabetes. 2014;63:3626–3636. doi: 10.2337/db14-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J., Liu Z., Apontes P., Feng D., Pessin J.E., Sauve A.A. Dual mode action of mangiferin in mouse liver under high fat diet. PLoS One. 2014;9:e90137. doi: 10.1371/journal.pone.0090137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H.L., Li C.Y., Zhang B., Liu Y.D., Lu B.M., Shi Z. Mangiferin facilitates islet regeneration and β-cell proliferation through upregulation of cell cycle and β-cell regeneration regulators. Int J Mol Sci. 2014;15:9016–9035. doi: 10.3390/ijms15059016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Q.H., Zhang X., Wang Y., Kong L.D. Mangiferin promotes uric acid excretion and kidney function improvement and modulates related renal transporters in hyperuricemic mice. Acta Pharm Sin. 2010;45:1239–1246. [PubMed] [Google Scholar]
- 15.Niu Y., Lu W., Gao L., Lin H., Liu X., Li L. Reducing effect of mangiferin on serum uric acid levels in mice. Pharm Biol. 2012;50:1177–1182. doi: 10.3109/13880209.2012.663763. [DOI] [PubMed] [Google Scholar]
- 16.Yang H., Gao L., Niu Y., Zhou Y., Lin H., Jiang J. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol Pharm Bull. 2015;38:1591–1598. doi: 10.1248/bpb.b15-00402. [DOI] [PubMed] [Google Scholar]
- 17.Xie T., Qin Z.Z., Zhou R., Zhao Y., Du G.H. Establishment of double targets of high throughput screening model for xanthine oxidase inhibitors and superoxide anion scavengers. Acta Pharm Sin. 2015;50:447–452. [PubMed] [Google Scholar]
- 18.Zhu J.X., Wang Y., Kong L.D., Yang C., Zhang X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol. 2004;93:133–140. doi: 10.1016/j.jep.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Tung Y.T., Lin L.C., Liu Y.L., Ho S.T., Lin C.Y., Chuang H.L. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Complement Altern Med. 2015;15:423. doi: 10.1186/s12906-015-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M.X., Liu Y.L., Yang Y., Zhang D.M., Kong L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur J Pharmacol. 2015;747:59–70. doi: 10.1016/j.ejphar.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Zhang D.M., Liu J.H., Hu L.S., Xue Q.C., Ding X.Q. Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose-induced hyperuricemic mice. J Ethnopharmacol. 2015;169:49–59. doi: 10.1016/j.jep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Ruiz F., Calabozo M., Erauskin G.G., Ruibal A., Herrero-Beites A.M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheumatol. 2002;47:610–613. doi: 10.1002/art.10792. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y.C., Lin K.S., Jhai Y.F., Lee B.H., Han Y., Cui Z. Miracle fruit (Synsepalum dulcificum) exhibits as a novel anti-hyperuricaemia agent. Molecules. 2016;21:140. doi: 10.3390/molecules21020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu G., Zhang Y.F., Wei W., Li L., Zhang Y., Yang J. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol Med Rep. 2016;13:2320–2326. doi: 10.3892/mmr.2016.4797. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J., Xu S., Song F., Nian L., Zhou X., Wang S. 2,3,5,4ʹ-tetrahydroxystilbene-2-O-β-D-glucoside protects human umbilical vein endothelial cells against lysophosphatidylcholine-induced apoptosis by upregulating superoxide dismutase and glutathione peroxidase. IUBMB Life. 2014;66:711–722. doi: 10.1002/iub.1321. [DOI] [PubMed] [Google Scholar]
- 26.Chen G.L., Wei W., Xu S.Y. Effect and mechanism of total saponin of Dioscorea on animal experimental hyperuricemia. Am J Chin Med. 2006;34:77–85. doi: 10.1142/S0192415X06003655. [DOI] [PubMed] [Google Scholar]
- 27.Hall I.H., Scoville J.P., Reynolds D.J., Simlot R., Duncan P. Substituted cyclic imides as potential anti-gout agents. Life Sci. 1990;46:1923–1927. doi: 10.1016/0024-3205(90)90507-n. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z., Fong W.P., Cheng C.H. The dual actions of morin (3,5,7,2ʹ,4ʹ-pentahydroxyflavone) as a hypouricemic agent: uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther. 2006;316:169–175. doi: 10.1124/jpet.105.092684. [DOI] [PubMed] [Google Scholar]
- 29.Tung Y.T., Hsu C.A., Chen C.S., Yang S.C., Huang C.C., Chang S.T. Phytochemicals from Acacia confusa heartwood extracts reduce serum uric acid levels in oxonate-induced mice: their potential use as xanthine oxidase inhibitors. J Agric Food Chem. 2010;58:9936–9941. doi: 10.1021/jf102689k. [DOI] [PubMed] [Google Scholar]
- 30.Chen G., Tan M.L., Li K.K., Leung P.C., Ko C.H. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J Ethnopharmacol. 2015;175:14–20. doi: 10.1016/j.jep.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Wang R., Ma C.H., Zhou F., Kong L.D. Siwu decoction attenuates oxonate-induced hyperuricemia and kidney inflammation in mice. Chin J Nat Med. 2016;14:499–507. doi: 10.1016/S1875-5364(16)30059-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q., Su J., Zhou T., Tian J., Chen X., Zhu J. A study comparing the safety and efficacy of febuxostat, allopurinol, and benzbromarone in Chinese gout patients: a retrospective cohort study. Int J Clin Pharmacol Ther. 2017;55:163–168. doi: 10.5414/CP202629. [DOI] [PubMed] [Google Scholar]
- 33.Huo L.N., Wang W., Zhang C.Y., Shi H.B., Liu Y., Liu X.H. Bioassay-guided isolation and identification of xanthine oxidase inhibitory constituents from the leaves of Perilla frutescens. Molecules. 2015;20:17848–17859. doi: 10.3390/molecules201017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou P.Y., Mi C., He Y., Zhang J., Wang S.Q., Yu F. Pallidifloside D from Smilax riparia enhanced allopurinol effects in hyperuricemia mice. Fitoterapia. 2015;105:43–48. doi: 10.1016/j.fitote.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Cristobal-Garcia M., Garcia-Arroyo F.E., Tapia E., Osorio H., Arellano-Buendia A.S., Madero M. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxid Med Cell Longev. 2015;2015:535686. doi: 10.1155/2015/535686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayyan M., Hashim M.A., AlNashef I.M. Superoxide ion: generation and chemical implications. Chem Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 37.Enroth C., Eger B.T., Okamoto K., Nishino T., Nishino T., Pai E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA. 2000;97:10723–10728. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viazzi F., Garneri D., Leoncini G., Gonnella A., Muiesan M.L., Ambrosioni E. Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis. 2014;24:921–927. doi: 10.1016/j.numecd.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Soltani Z., Rasheed K., Kapusta D.R., Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15:175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borghi C., Desideri G. Urate-lowering drugs and prevention of cardiovascular disease: the emerging role of xanthine oxidase inhibition. Hypertension. 2016;67:496–498. doi: 10.1161/HYPERTENSIONAHA.115.06531. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Dierckx R., Cleland J.G. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Ther. 2014;32:57–58. doi: 10.1111/1755-5922.12059. [DOI] [PubMed] [Google Scholar]
- 42.Peglow S., Toledo A.H., Anaya-Prado R., Lopez-Neblina F., Toledo-Pereyra L.H. Allopurinol and xanthine oxidase inhibition in liver ischemia reperfusion. J Hepatobiliary Pancreat Sci. 2011;18:137–146. doi: 10.1007/s00534-010-0328-7. [DOI] [PubMed] [Google Scholar]
- 43.Sun K., Fan J., Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anzai N., Jutabha P., Amonpatumrat-Takahashi S., Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol. 2012;16:89–95. doi: 10.1007/s10157-011-0532-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
J99745 was determined by ESI-MS and 1H NMR. m.p.: > 250 °C, ESI-MS (m/z): 351.31 [M+H]+, 1H NMR (400 MHz, DMSO-d6) δ: 13.03 (s, 1 H), 10.89 (s, 1 H), 9.83 (s, 1 H), 7.62 (t, J = 4 Hz, 1 H), 7.45 (m, 2 H), 7.42 (s,1 H), 7.31 (3, 1 H), 7.27 (t, J = 4 Hz, 1 H), 6.34 (s, 1 H), 6.17 (s, 1 H), 5.23 (s, 2 H).
A schematic diagram on induction of hyperuricemia and drug administration in mice. The test compound means J99745, allopurinol or benzbromarone dissolved in 0.5% CMC-Na, respectively.