Abstract
Salicylic acid (SA) is known to induce alternative pathway respiration by activating expression of the alternative oxidase gene. In the present study we report a rapid mode of action by SA on plant mitochondrial functions. SA at concentrations as low as 20 μm induced inhibition of both ATP synthesis and respiratory O2 uptake within minutes of incubation in tobacco (Nicotiana tabacum) cell cultures. Biologically active SA analogs capable of inducing pathogenesis-related genes and enhanced resistance also caused rapid inhibition of ATP synthesis and respiratory O2 uptake, whereas biologically inactive analogs did not. Inhibition of ATP synthesis and respiratory O2 uptake by SA was insensitive to the protein synthesis inhibitor cycloheximide, but was substantially reduced by the antioxidant N-acetylcysteine, suggesting a possible role for reactive oxygen species in the inhibition of mitochondrial functions. With exogenous NADH as the respiratory substrate, mitochondria isolated from SA-treated tobacco cell cultures were found to have normal capacities for both ATP synthesis and respiratory O2 uptake; direct incubation of isolated mitochondria with SA had no significant effect on these mitochondrial functions. These results indicate that (a) the respiration capacities of isolated mitochondria do not correspond to the in vivo respiration activities in SA-treated cell cultures and (b) the SA-induced inhibition of respiration in tobacco cell cultures may involve other components that are not present in isolated mitochondria. Given the recently demonstrated roles of mitochondria in plant disease resistance and animal apoptosis, this rapid inhibition by SA of mitochondrial functions may play a role in SA-mediated biological processes, including plant defense responses.
Studies during the last several years have established that SA plays an essential role in plant disease resistance (Klessig and Malamy, 1994; Hunt et al., 1996; Yang et al., 1997). Application of exogenous SA has long been known to activate expression of PR genes and to induce resistance to plant diseases (White, 1979; Ward et al., 1991). In an increasing number of plant species, elevated levels of SA have been associated with resistance of the infected plant to the invading pathogen (Malamy et al., 1990; Metraux et al., 1990; Uknes et al., 1992). A large body of evidence documents the importance of this systemic increase in SA levels for the induction of systemic acquired resistance (Gaffney et al., 1993; Delaney et al., 1994). More recently, genetic and molecular studies have indicated that SA also participates in the development of the localized, hypersensitive disease resistance induced by pathogen infection (Levine et al., 1994; Weyman et al., 1995; Shirasu et al., 1997).
The signal transduction mechanism involved in the action of SA in plant defense responses is poorly understood. Biochemical studies have shown that SA and its analogs capable of inducing PR genes and disease resistance bind to and inhibit certain heme-containing catalases and peroxidases (Chen et al., 1993a, 1993b; Durner and Klessig, 1995). Further studies have suggested that SA may serve as one-electron-donating substrates for catalases/peroxidases and, in doing so, are converted into SA free radicals in the presence of H2O2 as electron acceptors (Durner and Klessig, 1996; Kvaratskhelia et al., 1997). More recently, Kawano et al. (1998) have demonstrated that in tobacco (Nicotiana tabacum) cell cultures SA induces extracellular superoxide generation by interacting with a SHAM-sensitive extracellular peroxidase. Based on their established roles in signal transduction, these SA-mediated ROS could be involved in certain aspects of action by SA in plant defense responses. Other studies on SA signaling pathways have focused on the regulation of the genes induced by SA. These studies have provided evidence for the possible involvement of protein phosphorylation in the transcriptional regulation of at least some of these SA-responsive genes (Jupin and Chua, 1996; Stange et al., 1997). A role for protein phosphorylation in SA signal transduction is supported by the observed suppression of SA action by protein kinases/phosphatase inhibitors and by the recent identification of an SA-activated MAP kinase (Conrath et al., 1997; Shirasu et al., 1997; Zhang and Klessig, 1997).
In addition to biochemical and molecular studies, genetic strategies have been applied to the dissection of SA signal transduction pathways by isolating and characterizing SA-insensitive mutants in Arabidopsis. These screenings have identified a number of mutants that fail to express PR genes and exhibit enhanced resistance to bacterial and fungal pathogens in response to treatment with SA or its functional analogs, 2,5-dichloroisonicotinic acid and benzothiodiazole (Cao et al., 1997; Ryals et al., 1997; Shah et al., 1997). It is interesting that all of these reported SA-insensitive mutants are allelic, caused by mutations in a gene encoding a 60-kD protein with ankyrin repeats and some homology to the animal IκB protein (Cao et al., 1997; Ryals et al., 1997). It is possible that other genes involved in this signal transduction pathway have not been identified by screening for SA-insensitive mutants because of their functional redundancy. In addition, some of these regulatory components may be essential for other important biological processes, so that mutations causing severe reduction in their biological activities may be deleterious or even lethal to the plants.
It has recently been demonstrated that SA-induced tobacco resistance to TMV is sensitive to SHAM, an inhibitor of the terminal oxidase of the mitochondrial alternative pathway (Chivasa et al., 1997). Moreover, the respiratory inhibitors antimycin A and cyanide induced alternative oxidase transcript accumulation and resistance to TMV (Chivasa and Carr, 1998). Furthermore, cyanide restores N gene-mediated resistance to TMV in transgenic tobacco that expresses the salicylate hydroxylase (nahG) gene (Chivasa and Carr, 1998). These results suggest that certain functions of plant mitochondria may play an important role not only in SA-induced thermogenesis, as previously demonstrated (Raskin et al., 1987), but also in SA-induced disease resistance.
To pursue these studies of the involvement of plant mitochondria in the response to SA, we have recently examined the effect of SA on the most important function of this organelle: oxidative phosphorylation (ATP synthesis). We have discovered that SA induces rapid inhibition of ATP synthesis when it is incubated with tobacco cell cultures. Based on the concomitant inhibition of respiratory O2 uptake, SA-induced inhibition of ATP synthesis appears to be caused by inhibited mitochondrial electron transport, rather than by enhanced alternative pathway respiration, which is known to be induced by SA (Kapulnik et al., 1992; Rhoads and McIntosh, 1992, 1993; Wagner, 1995; Lennon et al., 1997). Thus, in addition to the induction of alternative pathway respiration, which is dependent on the expression of the alternative oxidase gene, SA has a rapid mode of action on electron transport and oxidative phosphorylation of plant mitochondria. In the present paper, we discuss this rapid inhibition of plant mitochondrial functions by SA with respect to its possible mechanisms and biological roles in SA-induced biological processes, including plant defense responses.
MATERIALS AND METHODS
Chemicals and Plant Culture
We obtained [γ-32P]ATP and 32Pi from New England Nuclear and SA, SA analogs, and other common chemicals from Sigma. SA and its analogs were dissolved in water as 100 mm stock solutions and adjusted to pH 6.5 with KOH.
All experiments were carried out with tobacco (Nicotiana tabacum cv Xanthi-nc) cell cultures. The cell-suspension cultures were grown at room temperature on a rotary shaker at 110 rpm in the dark in Murashige and Skoog medium supplemented with 1 μg mL−1 α-NAA, 0.1 μg mL−1 2,4-D, and 0.1 μg mL−1 BA (Xie et al., 1998). Cells were maintained by a 10-fold dilution with fresh medium every 5 to 6 d. Two days after dilution the cells were used for all of the experiments.
Isolation of Mitochondria
We adopted the method of Rhoads and McIntosh (1991), which is a modification of the procedure of Schwitzguebel and Siegenthaler (1984), to prepare the mitochondria. We washed the tobacco suspension-culture cells (approximately 280 mL) twice with fresh medium and ground them with glass beads in a mortar and pestle in 20 mL of mitochondrial grinding buffer (0.35 m mannitol, 30 mm Mops, pH 7.5, 4 mm Cys, 1 mm EDTA, 0.2% BSA, and 0.6% PVP). The homogenate was centrifuged for 2 min at 5,000g. The supernatant was then centrifuged again for 10 min at 20,000g, and the pellet was resuspended directly in a reaction medium (250 mm Suc and 30 mm Mops, pH 6.8). The quality of the isolated mitochondria was determined by demonstrating the dependence of ATP synthesis and respiratory O2 uptake on the addition of an electron-donating substrate (e.g. NADH), which could be further enhanced by the addition of ADP and Pi.
Analysis of ATP Synthesis
ATP synthesis of tobacco cell cultures after chemical treatment was determined by direct labeling of [32P]Pi, followed by homogenization, extraction, and TLC separation. Cell cultures (1 mL) were incubated with SA at various concentrations for 10 to 30 min before the addition of 32Pi (4 μCi in 5 μL). After labeling for 10 min, the cells were washed with 1 mL of cold medium three times and resuspended in 200 μL of medium. After adding an equal volume of 6% perchloric acid, the cells were briefly sonicated. We then centrifuged the resulting homogenates for 10 min in a microcentrifuge to collect the supernatant. To every 300 μL of supernatant, we added 66 μL of 2 n KOH/0.5 m triethanolamine to neutralize the pH. After incubation on ice for 30 min followed by centrifugation in a microcentrifuge for 5 min, the supernatants (10 μL) with the same amount of radioactivity were loaded onto a TLC plate precoated with silica gel (Analtech, Newark, DE). Adenine nucleotides and 32Pi were separated using an elution medium containing dioxane:isopropanol:25% NH4OH:H2O (4:2:3:4, v/v) and 10 mm EDTA (Bronnikov and Zakharov, 1983). We identified the unmetabolized 32Pi and the synthesized [32P]ATP on the plates using [32P]ATP and 32Pi as the standards after autoradiography. ATP synthesis of isolated mitochondria was determined in the mitochondrial reaction buffer in the presence of 1 mm NADH.
Analysis of Total Cellular ATP Levels
We determined total cellular ATP levels using the luciferin-luciferase assay. After treatment we added 1 mL of cell cultures and 1 mL of 6% ice-cold perchloric acid. The cells were sonicated for 1 min and centrifuged for 10 min in a microcentrifuge. To adjust the pH from 7.5 to 8.0, we added 220 μL of 2 n KOH/0.5 m triethanolamine to 1 mL of supernatant. The tubes were placed on ice for 30 min to let the potassium perchloric acid precipitate. After centrifugation for 5 min, the supernatant was collected. To assay ATP levels, we added 100 μL of luciferine-luciferase buffer (50 mm Gly, pH 8.0, 7.5 mm DTT, 1 mm EDTA, 2 mm MgSO4, 15 μm luciferine, and 5 μg mL−1 luciferase) to 200 μL of 1:100 diluted supernatant. The signals were integrated for 10 s in a LUMAT luminometer (model LB9507, EG&G, Berthold, Germany). The actual ATP levels were calculated from an ATP standard curve constructed with commercially purchased ATP.
Respiration
Cells (5 mL, approximately 100 mg fresh weight) in the culture medium were placed in an O2 electrode unit (YSI, Yellow Springs, OH) at room temperature to measure respiratory O2 uptake. The respiratory O2 uptake of the isolated mitochondria was determined in the mitochondrial reaction buffer in the presence of 1 mm NADH. We assumed the O2 in the air-saturated medium to be 240 μm (Schwitzguebel and Siegenthaler, 1984). We repeated all of the assays for respiratory O2 uptake three times with either independently subcultured cells or prepared mitochondria.
RESULTS
Rapid Inhibition of ATP by SA
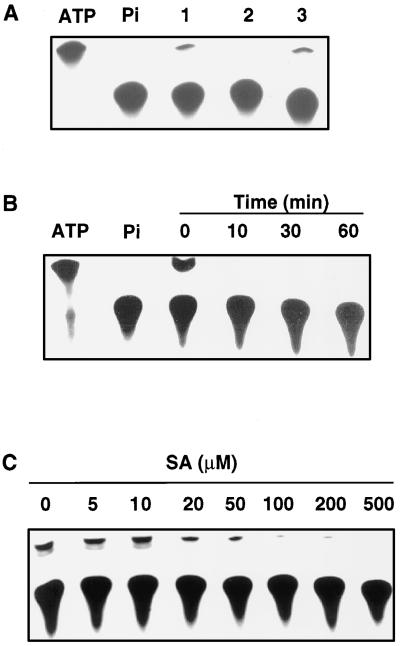
We made our initial observation on SA-induced rapid inhibition of ATP synthesis from experiments designed to test for a possible change in protein phosphorylation in tobacco cell cultures after treatment with SA. In these experiments, tobacco cell cultures were treated with SA for 30 min and labeled with 32Pi. The treated/labeled cells were homogenized and subjected to SDS-PAGE to detect proteins with altered phosphorylation as a result of SA treatment. These experiments revealed drastically decreased intensities of a low-Mr species in SA-treated samples that migrated behind 32Pi molecules, but ahead of all detectable protein molecules. Based on the migration rate and the high abundance in untreated cell cultures, we suspect that this band corresponded to the labeled ATP molecules, which appeared to be drastically decreased in the SA-treated samples. To verify this possibility, we used more reliable TLC procedures with [γ-32P]ATP and 32Pi as the standards to establish the identity of the labeled band. As shown in Figure 1A (lane 1), brief labeling of untreated cells with 32Pi produced a band on the TLC plates that comigrated with the [γ-32P]ATP standard, in addition to the much more abundant band that corresponded to the unmetabolized 32Pi molecules. The identity of the minor band as 32P-labeled ATP was further established by its susceptibility to treatment with ATP-hydrolyzing apyrase (Fig. 1A, lane 2). Apyrase catalyzes the hydrolysis of phosphoanhydride bonds of nucleoside tri- and diphosphates (e.g. ATP and ADP), but not the hydrolysis of phosphomonoester bonds of nucleoside monophosphate (e.g. AMP) or sugar phosphates (Cori et al., 1965).
Figure 1.
Inhibition of ATP synthesis of tobacco cell cultures by SA. A, Separation and identification of ATP and 32Pi on TLC plates. Separations were for supernatants from untreated cell cultures (lane 1) or the same supernatants that had been incubated at 30°C for 30 min in the presence (lane 2) or absence (lane 3) of 10 units of the ATP-hydrolyzing apyrase. B, Time course of inhibition of ATP synthesis by 1 mm SA. C, Concentration curve for inhibition of ATP synthesis after incubation for 10 min with 20 to 500 μm SA. The first two lanes in A and B are commercially purchased [γ-32P]ATP and 32Pi used as the standards.
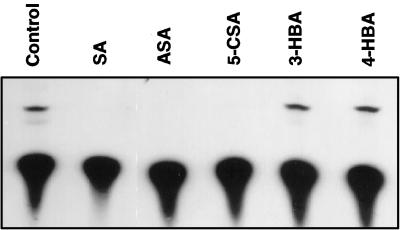
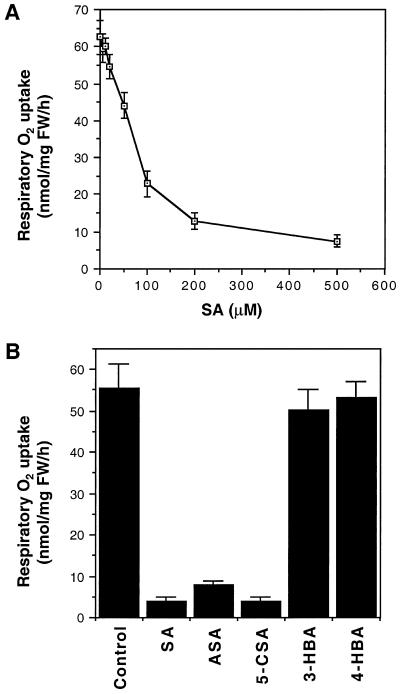
Treatment with 1 mm SA for as short as 10 min effectively inhibited the synthesis of cellular ATP in the tobacco cell cultures (Fig. 1B). In fact, treatment of the cell cultures for 10 min with SA at concentrations as low as 20 μm inhibited ATP synthesis, although a high level of inhibition was observed only at SA concentrations higher than 50 μm (Fig. 1C). To determine the specificity, we tested SA analogs for their ability to inhibit ATP synthesis in tobacco cell cultures. Among these analogs, 5-chlorosalicylic acid and acetylsalicylic acid (aspirin) are biologically active analogs that induce both PR gene expression and disease resistance in tobacco, whereas 3-hydroxybenzoic acid and 4-hydroxybenzoic acid are not biologically active in inducing these defense responses (Conrath et al., 1995). As shown in Figure 2, although biologically active SA, acetylsalicylic acid, and 5-chlorosalicylic acid effectively inhibited ATP synthesis at a similar potency, biologically inactive 3hydroxybenzoic acid and 4-hydroxybenzoic acid failed to have any significant effect on ATP synthesis. Two additional biologically inactive benzoic acid derivatives (2,3-dihydroxybenzoic acid, and 2,4-dihydroxybenzoic acid) have also been tested and found to be ineffective in inhibiting ATP synthesis of tobacco cell cultures (data not shown).
Figure 2.
Inhibition of ATP synthesis by SA analogs. Tobacco cell cultures were incubated with 0.5 mm SA, acetylsalicylic acid (ASA), 5-chlorosalicylic acid (5-CSA), 3-hydroxybenzoic acid (3-HBA), or 4-hydroxybenzoic acid (4-HBA) for 30 min before assay for ATP synthesis.
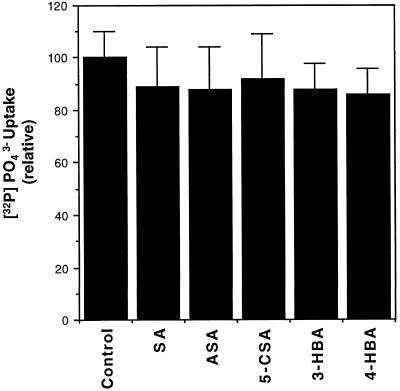
The decreased levels of 32P-labeled ATP in SA-treated cells could result from a decreased uptake of 32Pi, rather than from inhibited ATP synthesis. To test this possibility we directly measured the uptake of 32Pi by tobacco cell cultures. After treatment with SA or its analogs for 30 min, these tobacco cells were labeled with 32Pi for 10 min, washed three times in cold medium, and counted for retained radioactivities. These experiments established that treatment with SA or its analogs had no significant effect on the uptake of 32Pi by tobacco cell cultures (Fig. 3).
Figure 3.
Uptake of 32Pi by tobacco cells after pretreatment with SA and analogs. Tobacco cell cultures were pretreated with 0.5 mm SA, acetylsalicylic acid (ASA), 5-chlorosalicylic acid (5-CSA), 3-hydroxybenzoic acid (3-HBA), or 4-hydroxybenzoic acid (4-HBA) for 30 min followed by addition of 32Pi. After labeling for 10 min, the cells were washed three times with cold medium, and the radioactivity retained by cells was determined by scintillation counting and reported (+se calculated from three independent experiments).
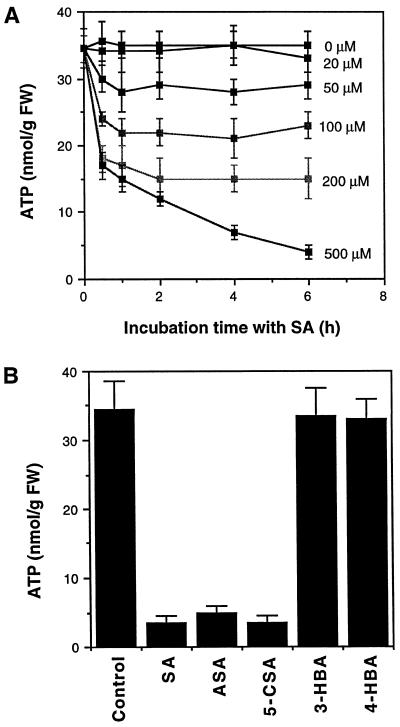
To further confirm the inhibition of ATP synthesis by SA, we directly measured the total cellular ATP levels in the tobacco cell cultures after SA treatment. As shown in Figure 4A, treatment with SA at concentrations from 50 to 200 μm significantly decreased total ATP levels. Treatment with 500 μm SA decreased ATP levels by 50% within the first 30 min of incubation, after which the ATP level continued to decrease to as low as 15% of the control levels at the end of assays (Fig. 4A). Furthermore, those SA analogs (acetylsalicylic acid and 5-chlorosalicylic acid) capable of inhibiting ATP synthesis were also able to deplete total cellular ATP levels, whereas the SA analogs (3-hydroxybenzoic acid and 4-hydroxybenzoic acid) incapable of inhibiting ATP synthesis did not significantly affect the cellular ATP levels of tobacco cell cultures (Fig. 4B).
Figure 4.
Depletion of total cellular ATP in tobacco cell cultures after treatment with SA or its analogs. A, Tobacco cell cultures were incubated with various concentrations of SA. At indicated time points, cell samples were taken for determining total cellular ATP levels. B, Tobacco cell cultures were incubated with 0.5 mm SA, acetylsalicylic acid (ASA), 5-chlorosalicylic acid (5-CSA), 3-hydroxybenzoic acid (3-HBA), or 4-hydroxybenzoic acid (4-HBA). ATP levels were determined after 6 h of incubation and reported (+se calculated from three independent assays). FW, Fresh weight.
Rapid Inhibition of Respiratory O2 Uptake by SA
Inhibition of ATP synthesis in SA-treated tobacco cell cultures could be caused by (a) blockage of mitochondrial electron transport, (b) conversion of electron transport from the cytochrome oxidase pathway to the alternative oxidase pathway, which differ in their capacity for ATP synthesis, and/or (c) uncoupling of electron transport from oxidative phosphorylation as a result, for example, of depolarization of the mitochondria inner membrane potential. For the first mechanism, inhibited ATP synthesis is associated with decreased respiratory O2 uptake. For the second or third mechanism, however, inhibition of ATP synthesis may not necessarily lead to a decrease in respiratory O2 uptake. To distinguish between these possibilities, we directly measured the respiratory O2 uptake of tobacco cell cultures after SA treatment. As shown in Figure 5A, treatment of the cell cultures for 10 min with SA decreased respiratory O2 uptake with a dose response similar to that observed for inhibition of ATP synthesis (Fig. 1C). Furthermore, those SA analogs that inhibited ATP synthesis also inhibited respiratory O2 uptake, whereas those analogs incapable of inhibiting ATP synthesis failed to inhibit respiratory O2 uptake (Fig. 5B). Thus, inhibition of ATP synthesis by SA or its analogs was associated with inhibited respiratory O2 uptake. In an earlier report, Kapulnik et al. (1992) showed that 200 μm SA inhibited respiratory O2 uptake of tobacco cell cultures by approximately 50%. This relatively low sensitivity of respiratory O2 uptake to SA may have been due to the old age (7 d) of the cells used in the earlier study.
Figure 5.
Inhibition of respiratory O2 uptake by SA and its analogs. A, Tobacco cell cultures were incubated with various concentrations of SA for 10 min before the measurement for respiratory O2 uptake. B, Tobacco cell cultures were incubated with 0.5 mm SA, acetylsalicylic acid (ASA), 5-chlorosalicylic acid (5-CSA), 3-hydroxybenzoic acid (3-HBA), or 4-hydroxybenzoic acid (4-HBA) for 30 min before the measurement for respiratory O2 uptake. FW, Fresh weight.
Effect of the Protein Synthesis Inhibitor Cycloheximide
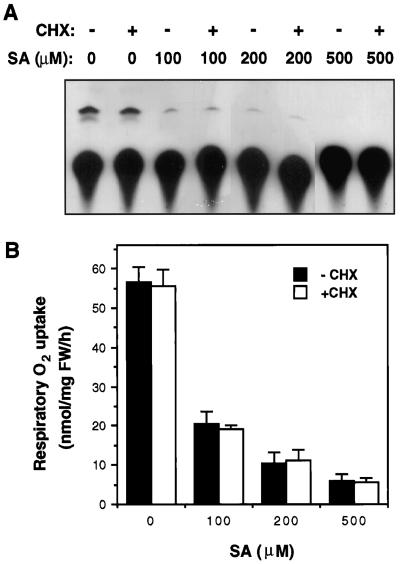
It has been shown previously that SA induced alternative pathway respiration in tobacco cell cultures by activating expression of the alternative oxidase gene (Kapulnik et al., 1992; Rhoads and McIntosh, 1993). Thus SA-induced alternative pathway respiration was sensitive to inhibitors of transcription and protein synthesis (Rhoads and McIntosh, 1993). To test whether the rapid inhibition of ATP synthesis and respiratory O2 uptake by SA is also dependent upon protein synthesis, we tested the sensitivity of SA inhibition effects to the protein synthesis inhibitor cycloheximide. Tobacco cell cultures were pretreated with 100 μg mL−1 cycloheximide for 30 min before treatment with SA for 30 min; both the ATP synthesis and the respiratory O2 uptake were then analyzed and compared with cell cultures without the pretreatment. As shown in Figure 6, pretreatment with cycloheximide did not block SA-induced inhibition of ATP synthesis or respiratory O2 uptake. Rhoads and McIntosh (1993) showed that cycloheximide at this concentration effectively blocked SA-induced expression of the alternative oxidase gene and alternative pathway respiration in tobacco cell cultures.
Figure 6.
Effects of cycloheximide (CHX) on SA-induced inhibition of ATP synthesis (A) and respiratory O2 uptake (B). Tobacco cell cultures were preincubated with 100 μg mL−1 cycloheximide for 30 min before treatment with 100 to 500 μm SA. After SA treatment for 30 min, ATP synthesis and respiration of treated cells were determined. FW, Fresh weight; lanes −, no CHX; lanes +, with CHX.
Effect of the Antioxidant NAC
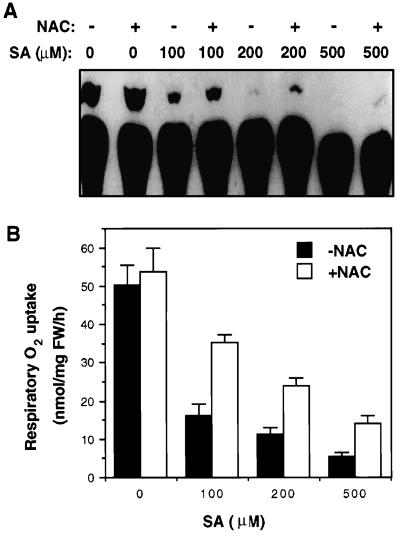
Several recent studies have shown that SA and its biologically active analogs inhibit catalases/peroxidases and induce lipid peroxidation and generation of ROS in tobacco cell cultures (Chen et al., 1993b; Conrath et al., 1995; Durner and Klessig, 1995, 1996; Kvaratskhelia et al., 1997; Kawano et al., 1998). To test for the possible involvement of ROS in SA-induced inhibition of ATP synthesis and respiratory O2 uptake, we tested the effects of a commonly used antioxidant, NAC, which was previously shown to block SA- and BTH-induced PR-1 protein synthesis in tobacco (Wendehenne et al., 1998). Tobacco cell cultures were pretreated with 20 mm NAC for 1 h before treatment with SA for 30 min; both the ATP synthesis and the respiratory O2 uptake were then analyzed and compared with cell cultures without antioxidant pretreatment. As shown in Figure 7, pretreatment with NAC without subsequent treatment with SA had no significant effect on either ATP synthesis or respiratory O2 uptake in control cell cultures. However, for cell cultures that were subsequently treated with SA, NAC pretreatment decreased SA-induced inhibition of ATP synthesis and respiratory O2 uptake by approximately 50%. This result demonstrates that ROS production probably plays a significant role in the SA-induced rapid inhibition of ATP synthesis and respiratory O2 uptake.
Figure 7.
Effects of NAC on SA-induced inhibition of ATP synthesis (A) and respiratory O2 uptake (B). Tobacco cell cultures were preincubated with 20 mm NAC for 1 h before treatment with 100 to 500 μm SA. After SA treatment for 30 min, ATP synthesis and respiration of treated cells was determined. FW, Fresh weight; lanes −, no NAC; lanes +, with NAC.
Isolated Mitochondria
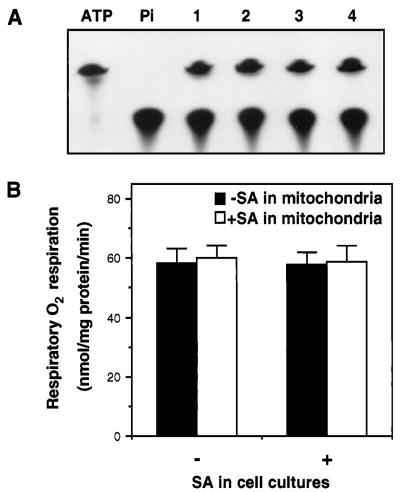
The rapid inhibition of ATP synthesis and respiration by SA in the tobacco cell cultures observed in this study was not consistent with an earlier report that treatment of tobacco cell cultures with 1 mm SA resulted in increased capacities of alternative pathway respiration without significant change in cytochrome pathway respiration (Rhoads and McIntosh, 1993). However, unlike the current study, which measured ATP synthesis and respiratory O2 uptake directly in cell cultures, the earlier report measured respiration in mitochondria isolated from SA-treated cell cultures. Thus, the mitochondria isolated from SA-treated tobacco cell cultures may be capable of respiratory O2 uptake. To confirm this, we isolated mitochondria from SA-treated cell cultures and determined their capacities for both ATP synthesis and respiratory O2 uptake with exogenously added NADH as the respiratory substrate. As shown in Figure 8, SA-treated tobacco cell cultures showed inhibition of both ATP synthesis and respiratory O2 uptake, whereas the mitochondria isolated from these respiration-suppressed cell cultures exhibited normal capacities in both ATP synthesis and respiratory O2 uptake. Thus, the high capacities in respiration found in mitochondria isolated from SA-treated cells did not reflect the in vivo rates of respiration.
Figure 8.
Capacities of ATP synthesis and respiratory O2 uptake of isolated mitochondria. A, Capacity of ATP synthesis for mitochondria isolated from cell cultures treated with 0 mm (lanes 1 and 2) or 0.5 mm (lanes 3 and 4) SA for 30 min. Before ATP synthesis assays, the isolated mitochondria were again treated with 0 mm (lanes 1 and 3) or 0.5 mm (lanes 2 and 4) SA for 30 min. B, Respiratory O2 uptake for isolated mitochondria. −, No SA; +, with SA.
Inhibition of respiration in SA-treated cell cultures could be caused by the direct effects of SA on the components of mitochondria that are important for electron transport and oxidative phosphorylation. Alternatively, SA may induce generation of certain molecules such as ROS, which subsequently inhibit respiration in SA-treated cells. To distinguish between these two possibilities, we tested the effects of the direct incubation of isolated mitochondria with SA on their capacities of ATP synthesis and respiratory O2 uptake. As shown in Figure 8, no significant inhibition of respiration was observed in SA-treated mitochondria when assayed with exogenous NADH as the reducing substrates.
DISCUSSION
In the present study we show that 20 to 500 μm SA inhibited ATP synthesis in tobacco cell cultures. Two lines of evidence indicated that this effect did not appear to depend on the induction of alternative pathway respiration by SA that had previously been documented (Kapulnik et al., 1992; Rhoads and McIntosh, 1992, 1993; Wagner, 1995). First, inhibition of ATP synthesis by SA required only minutes of SA incubation and was insensitive to the protein synthesis inhibitor cycloheximide. In contrast, SA-induced alternative respiration is associated with de novo synthesis of the alternative oxidase protein and requires hours to reach maximum levels (Kapulnik et al., 1992; Rhoads and McIntosh, 1993). Second, the rapid inhibition of ATP synthesis by SA is associated with decreased respiratory O2 uptake, suggesting the blockage of electron transport for both the cytochrome oxidase and alternative oxidase pathways.
How SA incubation leads to such a rapid inhibition of ATP synthesis and respiratory O2 uptake in tobacco cell cultures is unclear. Because inhibition of respiratory O2 uptake was nearly complete at relatively high SA concentrations, SA appears to inhibit both the cytochrome oxidase pathway and the alternative oxidase pathway. Therefore, the potential SA target(s) must be important for both respiratory pathways. One possible candidate is complex I of the mitochondrial electron transport chain, which provides electrons for both electron transport pathways. Alternatively, SA may target components in the TCA cycle that provide the reducing substrates for electron transport. Aconitase, an enzyme in the TCA cycle, was previously shown to bind SA at relatively high affinity (Rüffer et al., 1995). Either of these two mechanisms should predict normal respiratory O2 uptake in mitochondria isolated from SA-treated cells, as long as exogenously added NADH is available to provide electrons directly to ubiquinone pools through the external NADH dehydrogenase. This route of electron transport bypasses the TCA cycle and/or complex I of the electron transport chain. This mechanism may also explain why respiratory O2 uptake in tobacco leaves did not change after SA treatment (Lennon et al., 1997): cytosolic NADPH or NADH generated from photosynthesis would feed into the mitochondrial electron chain without passing through inhibited SA target(s).
The observed antagonism of the SA-induced inhibition of ATP synthesis and respiratory O2 uptake by the antioxidant NAC suggests that SA-induced inhibition of mitochondrial functions may involve ROS. Very recently, Kawano et al. (1998) have shown that SA induces rapid generation of extracellular superoxides in tobacco cell cultures. This rapid generation of ROS was found to be caused by the interaction of SA with a SHAM-sensitive extracellular guaiacol-utilizing peroxidase in tobacco cells. Because SA can serve as one-electron-donating substrate for heme-containing peroxidases/catalases (Durner and Klessig, 1996; Kvaratskhelia et al., 1997), this interaction of SA with extracellular peroxidase should lead to the oxidation of SA into its free radical form and, consequently, other ROS (Kawano et al., 1998). Because intracellular heme-containing catalases and peroxidases are known to interact with SA (Chen et al., 1993b; Conrath et al., 1995; Durner and Klessig, 1995), intracellular generation of superoxides is also possible through a similar mechanism. These SA-mediated ROS could lead to the inhibition of key enzymes involved in the TCA cycles and/or the mitochondrial electron transport. For example, Hausladen and Fridovich (1994) found that several dehydratases bearing Fe-S prosthetic groups were inactivated by superoxide. Aconitase in the TCA cycle and several components in the electron transport chain of mitochondria contained Fe-S prosthetic groups and, therefore, could serve as potential targets for ROS.
SA is cytotoxic to plant cells at high concentrations (Allan and Fluhr, 1997; Anderson et al., 1998; Kawano et al., 1998). The rapid depletion of ATP in cell cultures incubated with high levels of SA (>200 μm) could be one of the mechanisms responsible for the cytotoxicity of SA. At relatively low levels of SA (20–100 μm, close to the physiological levels found in pathogen-infected plants), perturbation of mitochondrial functions may not be sufficient to cause cell death directly but could still play a role in SA-mediated defense responses. The observation that only SA and its analogs capable of inducing PR genes and disease resistance can also inhibit these mitochondrial functions is consistent with a possible role for the rapid inhibition of ATP synthesis and respiratory O2 uptake in SA-mediated plant defense responses.
In TMV-infected resistant tobacco plants, SA levels rose to approximately 50 to 100 μm in the cells immediately surrounding the lesions (Enyedi et al., 1992). These levels of SA should lead to significant inhibition of mitochondrial functions. In upper uninfected leaves, however, SA levels increased by only 1.2- to 4-fold to approximately 0.06 to 0.2 μg g−1 fresh weight or 0.5 to 1.5 μm (Enyedi et al., 1992; Vernooij et al., 1994). At these low levels, inhibition of mitochondrial functions would be insignificant based on the dose-response curve established in this study (Figs. 1 and 4). However, a comparison of exogenously applied SA concentrations with endogenously produced SA levels as a means for determining physiological relevance is misleading, because it does not take into consideration a number of important factors that affect SA uptake, metabolism, and compartmentalization by plant cells. In fact, although TMV-induced, systemic-acquired resistance in resistant tobacco plants is associated with only a small increase of endogenous SA levels in upper uninfected leaves, much higher levels of exogenous SA (typically in the range of 1–5 mm) are often required to induce a similar level of resistance and defense gene expression. For determining the physiological relevance of a possible biochemical mechanism induced by exogenous SA, it may be more appropriate to compare it with the concentrations of exogenous SA required for inducing disease resistance and defense gene expression.
The most direct evidence for a role of mitochondrial functions in SA signal transduction comes from the studies on the effects of oxidative respiration inhibitors on SA-induced resistance to viral pathogens (Chivasa et al., 1997; Chivasa and Carr, 1998). These studies suggest that SA-induced resistance to virus may be mediated by an induced SHAM-sensitive signaling pathway, which probably involves alternative oxidases. Because inhibitors of electron transport and certain enzymes (e.g. aconitase) in the TCA cycle induced alternative oxidase gene expression (Vanlerberghe and McIntosh, 1994, 1996), SA may induce alternative oxidase gene expression through its rapid inhibitory effect on mitochondrial electron transport. Furthermore, inhibition of aconitase by SA or SA-induced ROS may lead to the increased levels of citrate that serve as a signal for inducing alternative oxidase gene expression and, consequently, the capacity of alternative pathway respiration (Vanlerberghe and McIntosh, 1996).
SA-induced rapid inhibition of ATP synthesis and respiratory O2 uptake may also play a role in the involvement of SA in pathogen-induced hypersensitive response. Recent studies have shown that pathogen-induced hypersensitive cell death in plants is a form of programmed cell death and shares many mechanistic features with animal apoptosis (Greenberg, 1996; Mittler et al., 1997). A variety of key events in animal apoptosis are associated with mitochondria, including the release of caspase activators (such as cytochrome c), changes in electron transport, disruption of oxidative phosphorylation, loss of mitochondrial transmembrane potential, and altered cellular reduction-oxidation (redox) potential (Green and Reed, 1998). Based on the present studies of the rapid alteration of mitochondrial functions and the previously demonstrated induction by SA of ROS (Chen et al., 1993b; Rao et al., 1997; Kawano et al., 1998), the involvement of SA in pathogen-induced hypersensitive cell death could be mediated in part by these interrelated events associated with the functions of plant mitochondria.
ACKNOWLEDGMENTS
We would like to thank Dr. Allan Caplan for critically reading the manuscript and Dr. Rolf Ingermann for providing access to the luminometer for ATP assays.
Abbreviations:
- apyrase
ATP-diphosphohydrolase
- NAC
N-acetylcysteine
- PR
pathogenesis-related
- ROS
reactive oxygen species
- SA
salicylic acid
- SHAM
salicylhydroxamic acid
- TMV
tobacco mosaic virus
Footnotes
This work was supported in part by Idaho Agricultural Experimental Station and U.S. Department of Agriculture grant no. 96-36301-3316 to Z.C. Z.X. was supported by a Plant Biotechnology Graduate Assistantship from the University of Idaho Institute for Molecular and Agricultural Genetic Engineering.
LITERATURE CITED
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MD, Chen Z, Klessig DF. Possible involvement of lipid peroxidation in salicylic acid-mediated induction of PR-1 gene expression. Phytochemistry. 1998;47:555–566. [Google Scholar]
- Bronnikov GE, Zakharov SD. Microquantitative determination of Pi-ATP and ADP-ATP exchange kinetics using thin-layer chromatography on silica gel. Anal Biochem. 1983;131:69–74. doi: 10.1016/0003-2697(83)90136-7. [DOI] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ricigliano JW, Klessig DF. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proc Natl Acad Sci U S A. 1993a;90:9533–9537. doi: 10.1073/pnas.90.20.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993b;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Chivasa S, Carr JP. Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell. 1998;10:1489–1498. doi: 10.1105/tpc.10.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Naylor M, Carr JP. Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell. 1997;9:547–557. doi: 10.1105/tpc.9.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JW, Klessig DF. Two inducers of plant defense responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Silva H, Klessig DF. Protein dephosphorylation mediates salicylic acid-induced expression of PR-1 genes in tobacco. Plant J. 1997;11:747–757. [Google Scholar]
- Cori O, Traverso-Cori A, Tetas M, Chaimovich H. Substrate specificity and inhibition studies on potato apyrase. Biochem Z. 1965;342:345–358. [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E and others. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci USA. 1995;92:11312–11315. doi: 10.1073/pnas.92.24.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem. 1996;271:28492–28501. doi: 10.1074/jbc.271.45.28492. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Yalpani N, Silverman P, Raskin I. Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA. 1992;89:2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A, Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- Hunt MD, Neuenschwander UH, Delaney TP, Weymann KB, Friedrich LB, Lawton KA, Steiner HY, Ryals JA. Recent advances in systemic acquired resistance research: a review. Gene. 1996;179:89–95. doi: 10.1016/s0378-1119(96)00429-5. [DOI] [PubMed] [Google Scholar]
- Jupin I, Chua NH. Activation of the CaMV as-1 cis-element by salicylic acid: differential DNA-binding of a factor related to TGA1a. EMBO J. 1996;15:5679–5689. [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Yalpani N, Raskin I. Salicylic acid induces cyanide-resistant respiration in tobacco cell-suspension cultures. Plant Physiol. 1992;100:1921–1926. doi: 10.1104/pp.100.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Kvaratskhelia M, George SJ, Thorneley RN. Salicylic acid is a reducing substrate and not an effective inhibitor of ascorbate peroxidase. J Biol Chem. 1997;272:20998–21001. doi: 10.1074/jbc.272.34.20998. [DOI] [PubMed] [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Metraux J-P, Signer H, Ryals JA, Ward E, Wyss-Benz M, Gaudin J, Raschorf K, Schmid E, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Mittler R, Del Pozo O, Meisel L, Lam E. Pathogen-induced programmed cell death in plants, a possible defense mechanism. Dev Genet. 1997;21:279–289. doi: 10.1002/(SICI)1520-6408(1997)21:4<279::AID-DVG5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production in Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Isolation and characterization of a cDNA clone encoding an alternative oxidase protein of Sauromatum guttatum (Schott) Proc Natl Acad Sci USA. 1991;88:2122–2126. doi: 10.1073/pnas.88.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell. 1992;4:1131–1139. doi: 10.1105/tpc.4.9.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Cytochrome and alternative pathway respiration in tobacco. Plant Physiol. 1993;103:877–883. doi: 10.1104/pp.103.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüffer M, Steipe B, Zenk MH. Evidence against specific binding of salicylic acid to plant catalase. FEBS Lett. 1995;377:175–180. doi: 10.1016/0014-5793(95)01334-2. [DOI] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P and others. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzguebel JP, Siegenthaler PA. Purification of peroxisomes and mitochondria from spinach leaf by Percoll gradient centrifugation. Plant Physiol. 1984;75:670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF. Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant-Microbe Interact. 1997;10:69–78. doi: 10.1094/MPMI.1997.10.1.69. [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange C, Ramirez I, Gomez I, Jordana X, Holuigue L. Phosphorylation of nuclear proteins directs binding to salicylic acid-responsive elements. Plant J. 1997;11:1315–1324. doi: 10.1046/j.1365-313x.1997.11061315.x. [DOI] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis plant. Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell. 1994;6:959–969. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM. A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in petunia hybrida cells. FEBS Lett. 1995;368:339–342. doi: 10.1016/0014-5793(95)00688-6. [DOI] [PubMed] [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Metraux J-P, Ryals JA. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Chen Z, Klessig DF. Benzothiadiazole, an inducer of plant defense inhibits catalase and ascorbate peroxidase. Phytochemistry. 1998;47:651–657. [Google Scholar]
- Weyman KHM, Uknes S, Neuenschwander U, Lawton K, Steiner H, Ryals J. Suppression and restoration of lesion formation in Arabidopsis Isd mutants. Plant Cell. 1995;7:2013–2022. doi: 10.1105/tpc.7.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Xie Z, Fan B, Chen Z. Induction of PR-1 proteins and potentiation of pathogen signals by salicylic acid exhibit the same dose response and structural specificity in plant cell cultures. Mol Plant-Microbe Interact. 1998;11:568–571. [Google Scholar]
- Yang Y, Shah J, Klessig DF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]