Abstract
Photodynamic therapy (PDT), based on the photoactivation of photosensitizers (PSs), has become a well-studied therapy for cancer. Photofrin®, belonging to the first generation of PS, is still widely used for the treatment of different kinds of cancers; however, it has several drawbacks that significantly limit its general clinical use. Consequently, there has been extensive research on the design of PS molecules with optimized pharmaceutical properties, with aiming of overcoming the disadvantages of traditional PS, such as poor chemical purity, long half-life, excessive accumulation into the skin, and low attenuation coefficients. The rational design of novel PS with desirable properties has attracted considerable research in the pharmaceutical field. This review presents an overview on the classical photosensitizers and the most significant recent advances in the development of PS with regard to their potential application in oncology.
KEY WORDS: Anti-cancer, Photosensitizers, Photodynamic therapy, Photochemical reactions, Oncology, BODIPY
Graphical abstract
Photodynamic therapy, based on the photoactivation of photosensitizers (PS), has become a well-studied therapy for cancer. This review presents an overview on the classical photosensitizers and the most significant recent advances in the development of PS with regard to their potential application in oncology.
1. Introduction
Cancer is among the leading causes of morbidity and mortality worldwide. In 2012, approximately 14 million cancer cases were newly diagnosed, and the number of cancer-related deaths was 8.2 million, which is projected to rise by about 70% over the next two decades1. Currently, clinical treatments for cancer include surgery, radiation therapy, chemotherapy and, more recently, immunotherapy and other small-molecule targeted therapies, along with a combination of these strategies2. However, these treatments present some important drawbacks. For instance, traditional chemotherapy, as it interferes in cell division, is often associated with severe systemic adverse effects, such as myelosuppression, mucositis, alopecia, and others3. Also, surgical resection of certain tumors cannot avoid a high recurrence rate4, while the cumulative radiation dose extremely limits the radiotherapy5. Thus, although refinement of the conventional anticancer therapy is important, development of new treatment approaches that are safe, potent, and cost-effective seems especially urgent.
2. Photodynamic therapy
2.1. An accidental finding for cancer treatment
In the 1890s, Raab6 accidentally found that the irradiation with visible light was lethal to paramecia previously exposed to acridine, and postulated that the transfer of light energy to the acridine red was the crucial event behind the cytotoxicity observed in paramecia and that this effect was related to the fluorescence of the dye7. Then the first clinical observation of PDT with oral eosin to treat epilepsy was reported by the neurologist Jean Prime in 19008. Later, von Tappeiner and Jesionek9 proposed the use of topical eosin with light exposure to treat skin tumors. This was the first published report on the use of PDT to treat tumors in human patients. Subsequently, von Tappeiner10 observed that O2 was an important component of the events found by Raab, and coined the term “photodynamic action”.
The study of the anticancer potential of PDT was conducted by few researchers up to the 1950s when the interest in this field began to increase. The publication of some seminal reports on the use of porphyrins as both PSs and fluorescence diagnostic tools11 in the 1950s and 1960s was followed by a series of works on the anticancer activity of PDT against different tumors12. Mainly over the last three decades, several types of PSs have been developed and applied in preclinical and clinical trials; some of these molecules reached the market and have shown to be effective against different kinds of cancers13., 14., 15.. The main advantage of PDT over conventional anticancer therapies is the ability to limit toxic effects to the biological tissues exposed to both the PS and light, thus protecting normal tissues.
In addition, PDT has also been used successfully against non-malignant disorders in diverse fields, such as urology16, immunology17, ophthalmology18 dentistry19, dermatology20 and others.
2.2. Photodynamic therapy mechanisms
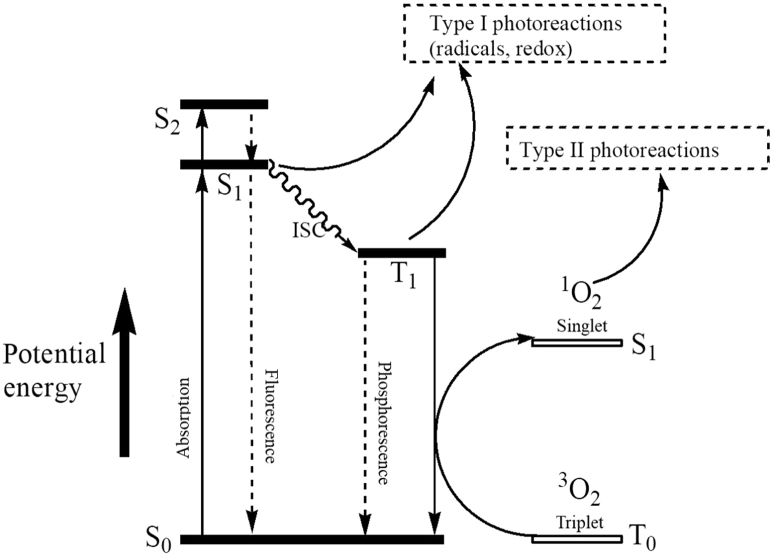
PDT is based on the excitation of PS with light at specific wavelengths, culminating in type I and type II photochemical reactions21. As shown in Fig. 1, a PS can be activated from its ground state to a short-lived excited singlet state (PSEs) by light. Then, either the excited PS may decay back to the ground state by emitting fluorescence, or it can undergo intersystem crossing whereby the spin of its excited electron inverts to form a relatively long-lived triplet state (PSEt). The triplet excited PS can also decay back to the ground state by emitting phosphorescence, but most importantly it can directly interact with surrounding substrates (e.g., cell membrane or other biomolecules) to form radicals, which then react with O2 to produce reactive oxygen species (ROS), such as superoxide anion radicals (O2–·), hydroxyl radicals (·OH), and hydrogen peroxides (H2O2, type I reaction). Alternatively, the energy of the excited PS can be directly transferred to 3O2 (itself a triplet in the ground state) to form 1O2 (type II reaction). It is worth noting that both type I and type II reactions can occur simultaneously, and the ratio between these processes is affected by the nature of the PS, as well as by the concentrations of 3O2 and other substrates. However, most of the experimental studies indicate that the photoactivated production of 1O2, namely type II reaction, plays a dominant role in in vivo PDT.
Figure 1.
Jablonski diagram showing the main events leading to type I and type II photochemical reactions, which eventually may result in oxidative cell damage. S0, ground state of the photosensitizer (PS); S1, first excited singlet state of PS; S2, second excited singlet state of PS; T1, first excited triplet state of PS; ISC, intersystem crossing; 3O2, triplet oxygen; 1O2, singlet oxygen.
In a biological medium the reactive species generated by the photodynamic process can react with a large number of biomolecules, mainly proteins, nucleic acids, and lipids. The damage to biomolecules may (i) irreversibly damage tumor cells resulting in necrosis, apoptosis, or autophagy, (ii) cause tumor ischemia following PDT-induced vascular injury, and (iii) activate the immune response against tumor antigens22., 23., 24., 25.. Therefore, the main downstream targets of PDT include tumor cells, as well as tumor-associated microvasculature, and, indirectly, the host immune system26. Moreover, the combination of PDT with other chemotherapeutic drugs may help to achieve a long-term tumor control, due to their possible synergistic effects resulting from the combination of downstream responses in PDT and the mechanisms of chemotherapeutic drugs27.
3. The photosensitizers for anticancer PDT
3.1. First generation PSs
Hematoporphyrin (Hp), a complex mixture of porphyrinic compounds28, was the first porphyrin used as PS. The purification and chemical modification of Hp led to the discovery of a hematoporphyrin derivative (HpD), which was shown to be more selective for tumor tissues, inducing a less intense skin photosensitization in comparison to Hp29. Later, a mixture of porphyrin dimers and oligomers isolated from HpD was marketed as Photofrin30. Despite Photofrin® was widely used for treating different cancers, the clinical use was limited by its intrinsic drawbacks, including I) poor chemical purity with a mixture of more than 60 molecules; II) its long half-life and intense accumulation in the skin, responsible for the induction of a prolonged skin photosensitization, which sometimes persists for 2 or even 3 months after Photofrin® administration; III) its low molar attenuation coefficient (1.17 × 103 mol/L·cm); and IV) its activation wavelengths being too short for a good tissue penetration30., 31., 32..
3.2. Second generation PSs
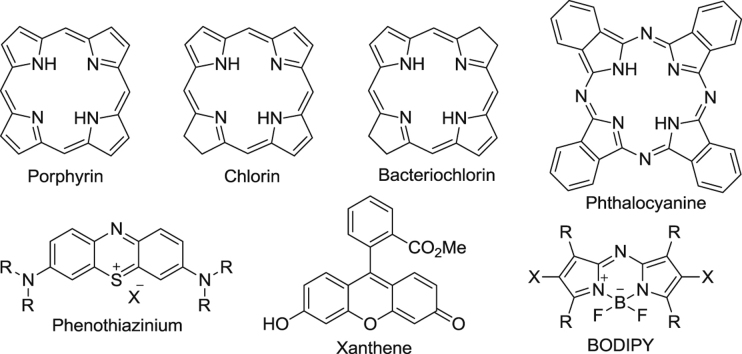
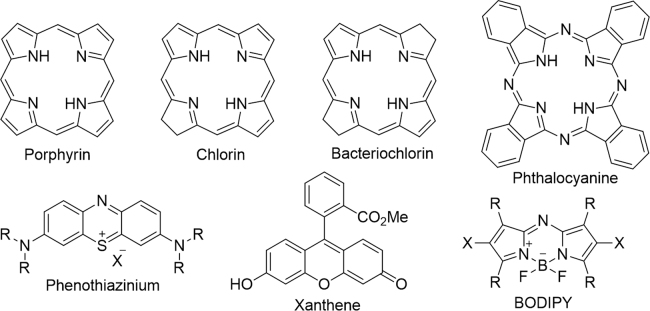
The disadvantages associated with first generation PSs have led to extensive investigation aimed at improving the efficacy of PS molecules via alteration of the peripheral functionality of the porphyrin33, or direct modification of the porphyrin core34, The following the seminal works on the first generation PS have resulted in the production of several new non-porphyrinoid PS molecules (Fig. 2). These have been developed over the decades, including metalloporphyrins (Lutrin® and Lutex®)35., 36., porphycenes37., 38., pheophorbides (Tookad®)39., 40., purpurins (Purlytin®)41, phthalocyanines42., 43., 44., 45., 46., chlorins (Foscan®)47, protoporphyrin IX precursors (Hexvix®, Metvix® and Levulan®)48., 49., phenothiazines (methylene blue, and toluidine blue)50., 51., 52., 53., 54., 55., cyanines (merocyanine 540)56., 57., dipyrromethenes58., 59., hypericin60., 61., 62., and xanthenes (Rose Bengal)63.
Figure 2.
Structural skeletons of several anticancer PSs.
3.3. Strategies for designing new generation PSs
Despite the extensive research performed to develop new and improved PS, only a few second generation PSs, such as Levulan®, MetVix®, Photochlor®64 and NPe665, have been approved for the clinical treatment of cancer66. The rational design of novel PS with desirable properties remains a big challenge for the pharmaceutical industry. The latest review article related to the design of PSs for photodynamic therapy, authored by Garland et al.67, dates back to 2009. As stated before, the overall success of PDT mainly depends on the 1O2 yield, molecule stability, the penetration depth of absorbed light and distribution of PSs, so that:
-
1)
The 1O2 generated in type II photoreaction is a key factor for PDT, since it is considered as the major cytotoxic species in PDT68. Generally, the introduction of heavy atoms (such as Br, and I) into the PS molecule or inhibition of the interaction between triplet excited PS and native free radicals can increase the 1O2 production;
-
2)
A disadvantage of many of the current PSs is their tendency to aggregate, resulting in short triplet state lifetimes and decreased 1O2 yields. Therefore, a structural modification that can suppress the aggregation tendency of a PS should be considered carefully to improve the property of PS. Usually, the presence of a central metal ion and the number of charges in the molecule will have an important role for the stability of PSs;
-
3)
The increased energy of light of longer wavelength is also a major motivation for the development of new PSs. Generally, expanding the molecular conjugate system by introducing an electron-rich donor can improve the PS absorption efficiency of red light, and enhance the penetration of light into human tissue;
-
4)
Improving the target distribution of PS is also very important to increase its efficacy and reduce adverse effects. The ligand-mediated targeting strategy in PDT has been explored. Herein, the targeted ligands, such as biotin, folate, peptide, etc., were frequently used for delivery of PS to cancer tissues;
-
5)
In addition, the positions and types of the substituent groups on the molecule can influence the lipophilicity of the molecule, which further influences the tissue location of PS.
4. Recent development in anticancer PSs
A literature survey indicates that there are quite a lot of review articles about PSs and PDT2., 69., 70., 71., but few of them specifically focus on the discovery and development of new PSs as anticancer agents. With the rapid development in PS research area, a number of new, more potent and tumor-specific PSs showing promising clinic potential have been investigated. Herein, this section summarizes the recently reported PSs mainly focusing on porphyrin, chlorin, phthalocyanine and BODIPY derivatives, aiming to provide a better understanding of the factors affecting the efficacy of PS molecules.
4.1. Porphyrin-type PSs
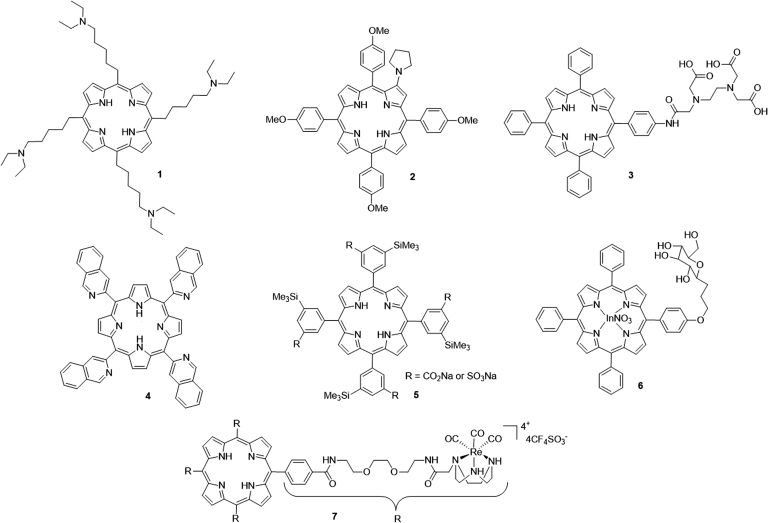
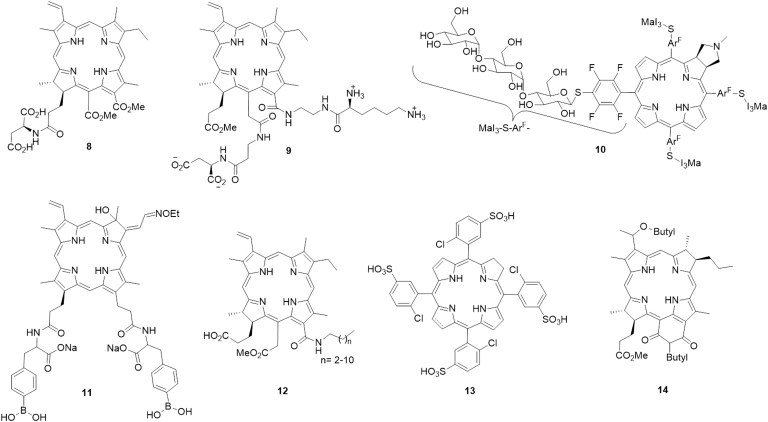
The porphyrin macrocyles were mainly developed and clinically used in the last few decades. Side-chains containing functional groups, such as nitrogen atoms72., 73., carboxylic acid74 and sugar75, are frequently incorporated into the porphyrin skeleton. For example, a novel porphyrin-based PS (5,10,15,20-tetrakis[(5-diethylamino)pentyl] porphyrin, TDPP, 1) with four diethylaminopentyl side-chains recently reported by Li et al.72 showed a high 1O2 yield with the ability to kill human esophageal cancer cell lines (Eca-109) and significantly reduce the growth of Eca-109 xenograft tumors in BABL/c nude mice. Among a series of β-alkylaminoprophyrins reported later by Chen's group73, derivative 2 showed higher phototoxicity than Hp monomethyl ether and the lowest toxicity in the dark. Another new porphyrin derivative 3 bearing ethylenediaminetetraacetic acid showed intense in vitro phototoxicity on HepG2 and BGC823 cell lines, and further inhibited the growth of BGC823 tumors in nude mice74, Mechanism studies indicated that 3 can induce cell death via the mitochondrial apoptotic pathway mainly triggered by lysosomal photodamage. Costa et al.76 recently reported a hydrophobic porphyrin derivative 4 bearing four isoquinoline moieties, which showed a high quantum yield of 1O2 generation, the absence of toxicity in the dark, and significant in vitro phototoxicity against HT29 cells with an IC50 in the micromolar range.
Horiuchi and co-workers77 studied the effect of a silyl group on the photodynamic properties of tetraphenylporphyrin derivative 5. The results indicated that silylation could lead to an improvement in the quantum yield of 1O2 sensitization for derivatives. In addition, there has been recent growth in interest in the preparation of metal-porphyrin conjugates as potential photocytotoxic agents. The porphyrins functionalized with PtII were the first metal-porphyrin derivatives developed for biomedical application78. Later, examples related to other metal-porphyrin conjugates have been described. For example, the glucopyranoside-conjugated porphyrin 6 bearing In+3 synthesized by Nakai et al.75 exhibited strong phototoxicity, correlating with its high abilities of 1O2 yield and cell permeability. Another Re-porphyrin conjugate 7, bearing four [1,4,7]-triazacyclononane units prepared by Mion et al.79, showed remarkable phototoxicity on Hela cells with non-toxicity in the dark (Fig. 3).
Figure 3.
Chemical structures of porphyrin-type PSs 1–7.
4.2. Chlorin-type PSs
Compared with porphyrins, chlorin-type PSs attracted considerable attention due to their intense absorption in relatively harmless NIR region, which can penetrate deeply into biological tissues. However, the development of chlorin derivatives was significantly limited by their poor water solubility. Therefore, chlorin-type PSs have been modified by conjugation with amino acids, peptides, and sugars to improve their solubility for PDT investigations. For example, Meng et al.80 prepared a series of chlorin P6-based water-soluble amino acid conjugates. Among these synthetic derivatives, compound 8 showed strong absorption in the phototherapeutic window, relatively high 1O2 quantum yield, and high phototoxicity against melanoma cells with low toxicity in the dark. Also, compound 8 exhibited better in vivo PDT antitumor efficacy than verteporfin on mice bearing B16F10 tumors. Another photocytotoxic chlorin e6 bis(amino acid) conjugate 9 bearing two different amino acids, lysine at 13 and aspartate at 15 was regioselectively synthesized by Smith and co-workers81. A water-soluble chlorin derivative 10, which was surrounded by four perfluorinated aromatic rings and conjugated with maltotriose (MaI3) molecules, showed excellent biocompatibility, strong photoabsorption in the longer wavelength regions and high photocytotoxicity82.
The use of boron-containing substances in the treatment of cancer has received a great deal of attention due to their high probability of producing particles and lithium-7 nuclei83. Boron-containing chlorin derivatives have also been explored in PDT applications84. Recently, a new chlorin derivative 11 containing phenylboronic acid moieties was synthesized by Tai and co-workers84. This compound could significantly inhibit tumor growth in vivo and showed rapid clearance from normal tissues. To improve the cell permeability of chlorin-type PSs, Gushchina et al.85 introduced hydrophobic carbon chains into chlorin e6 to yield a series of new amide derivatives 12, which exhibited good photoactivity and low toxicity in the dark against P388 and K562 cancer cells. Chlorin and bacteriochlorin derivatives 1386 bearing chloro-5-sulfophenyl fragments showed promising phototherapeutic properties, such as high water solubility, high photostability, high 1O2 quantum yields and negligible dark cytotoxicity. Patel et al.87 recently reported an NIR bacteriochlorin analogue 14 to be a promising dual-function agent for fluorescence-guided surgery with an option for treating cancer in PDT. This compound exhibited higher tumor uptake and long-term cure in BALB/c mice bearing Colon 26 tumors. Most of all, it showed low skin phototoxicity, which provides a significant advantage over the clinically approved HD as well as other porphyrin-based PSs (Fig. 4).
Figure 4.
Chemical structures of chlorin-type PSs 8–14.
4.3. Phthalocyanine-type PSs
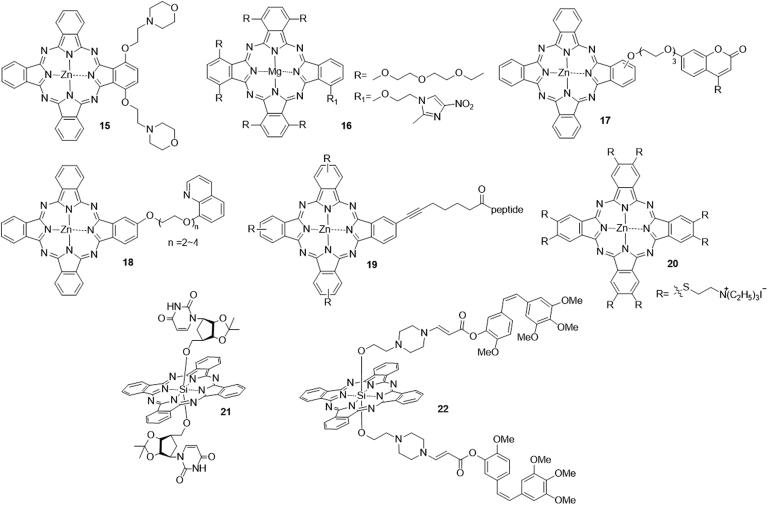
Phthalocyanine derivatives were shown to be most promising PSs. However, the low solubility and π—π stacking in these molecules limited their further clinical application. Strategies to overcome these disadvantages can involve incorporations of cationic88 or anionic groups89, peptides90, β-cyclodextrins91, crown ethers92, glycerinum93 and so on. Recently, 2-(morpholin-4-yl)ethoxy-substituted phthalocyanine 15 was synthesized by Kucinska et al.94 Biological test results indicated that 15 showed potent cytotoxicity against PC3 and A375 under irradiation, while its cytotoxicity in the dark was very low. Another novel Mg(II)-phthalocyanine 16, bearing (2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy substituent at a non-peripheral position, was found to show strong photocytotoxicity at 1 mol/L with 100% photokilling of the human oral squamous cell carcinoma cell lines, HSC-395.
The development of multifunctional molecules has also been considered for overcoming drug resistance and low therapeutic efficacy. For example, Zhou et al.96 reported a derivative 17 bearing a cytostatic coumarin moiety, zinc(II) phthalocyanine and a tri(ethyleneglycol) linkage showed dual photodynamic and chemotherapeutic activities. These conjugates exhibit high photocytotoxicity against HepG2 cells (IC50≈14—44 nmol/L), low aggregation tendency and high cellular uptake. Other similar examples are the phthalocyanine-8-hydroxyquinoline conjugates 1897. Ranyuk et al.98 reported a series of water-soluble zinc phthalocyanine-peptide conjugates 19, which targeted the gastrin-releasing peptide receptor. Novel far-red-absorbing Zn(II) phthalocyanine derivative 20 bearing [(triethylammonio)ethyl]sulfanyl substituents in the peripheral or nonperipheral positions were synthesized by Machacek et al.99 The bioassay results indicated that the Zn complex exhibited photocytotoxicity against 3T3, Hela, SK-MEL-28, and HCT116 cancer cell with IC50 values in a submicromolar range, and low toxicity in the dark (TC50≈1500 mol/L). Shen et al.100 reported a series of first silicon(IV) phthalocyanine nucleoside (uridine, 5-methyluridine, cytidine, and 5-N-cytidine) conjugates. Among them, the uridine-containing complex 21 exhibited the highest photocytotoxicity against HepG2 cells (IC50 = 6 nmol/L), with high cellular uptake and non-aggregated nature in the biological media. Bio et al.101 developed a multifunctional prodrug 22 composed with Si phthalocyanine, a SO-labile aminoacrylate linker and the cytotoxic drug combretastatin A-4 (CA4). Once illuminated, 22 showed improved toxicity, but reduced toxicity in the dark compared with CA4 (Fig. 5).
Figure 5.
Chemical structures of phthalocyanine-type PSs 15–22.
4.4. BODIPY-type PSs
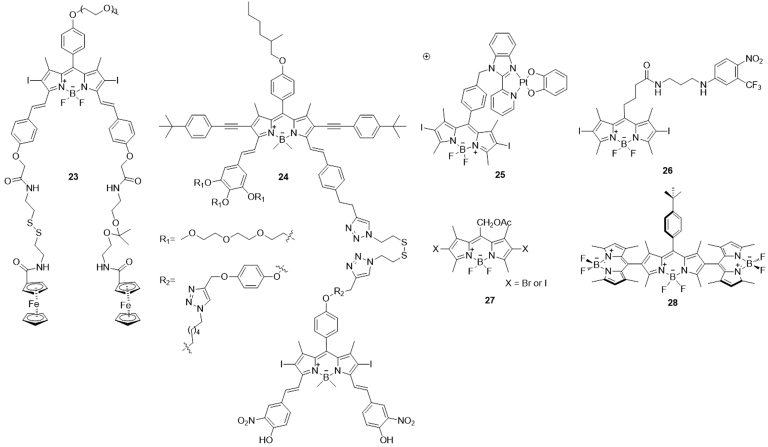
Another promising class of PS suitable for PDT is the distyryl boron dipyrromethene (BODIPY) dyes, which have proven to be a valuable family of compounds with diverse applications rivaling that of porphyrin. The BODIPY PSs are equipped with heavy halogen atoms, such as Br and I, in the organic chromophore to make compounds reach the triplet excited state by quenching the fluorescence and facilitating intersystem crossing.
Erbas-Cakmak et al.102 designed a water-soluble pH and GSH responsive distyryl-BODIPY PS 23, which could be activated by protonation at neutral pH and reductive cleavage of the disulfide linker at elevated GSH concentration. Recently, Lo and coworkers103 reported another new class of pH/thiol responsive BODPY PSs that contained either the ketal or disulfide linker. Noteworthy, the unsymmetrical complex 24 exhibited the greatest enhancement in the 1O2 generation and fluorescence intensity upon activation, which was considered to be a promising theranostic agent for targeted imaging and PDT of cancer. Platinum (II) complex 25 synthesized by Mitra et al.104 could achieve mitochondria-targeted photocytotoxicity via disruption of the mitochondrial membrane potential and apoptosis. This complex generated excellent photocytotoxicity against HaCaT cells but remained non-toxic in the dark (IC50>100 mol/L). A novel photoactivatable bichromophoric conjugate 26 was developed by Fraix and coworkers105. This compound combined BODIPY and aniline derivative as nitric oxide photodonor, which had an amplified photomortality on melanoma cancer cells. Lincoln et al.106 prepared two small meso-acetoxymethyl BODIPY dyes 27, which showed improved photostability against singlet oxygen compared to the BODIPY PSs lacking the acetoxymethyl group. Compounds 27 can readily embed in the lipid membranes of Hela cells and efficiently induced light-dependent apoptosis at nanomolar concentration. An orthogonal BODIPY trimer 28 without halogen atom substituent was shown to have strong absorption in the visible region and high 1O2 generation capability107 (Fig. 6).
Figure 6.
Chemical structures of BODIPY-type PSs 23–28.
5. Conclusions
The discovery of novel PS molecules with desired pharmaceutical properties and the application of novel PS in clinical trials are challenging tasks. During the last several years, most research work is based on the modification and optimization of old-style PSs. Therefore, molecules considered as the second generation PS mainly have been derived from porphyrin and porphyrin-related structures. The most recent activity in the PS field for PDT of cancer has been considerable, and the design of non-porphyrin PSs, which possess shorter periods of photosensitization, longer activation wavelengths and higher singlet oxygen yield, still attracts more attention in the field of anticancer PDT.
Additional to the synthesis of new types of PS molecules, the association of classical PSs to different carriers has also been explored to improve their photophysical properties and/or their targeting to tumors. On one hand, antibodies, receptor ligands, and other targeting molecules have been used to actively increase the accumulation of PSs in tumors. On the other hand, different nanostructures have been used to enhance or to maintain the activity of PSs in aqueous media, and to actively and/or passively deliver these molecules to tumors. Both of these systems, and even combinations of them, have been referred to as the third generation PS, and some encouraging results have been reported in the literature regarding the use of this strategy in anticancer PDT. With the significant successes on the developments of new PSs for PDT, it is expected that PDT will gain more widespread use in clinical practice.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21672082), Shandong Key Development Project (No. 2016GSF201209), Young Taishan Scholars Program (No. tsqn20161037), Shandong Talents Team Cultivation Plan of University Preponderant Discipline (No. 10027), Shandong Natural Science Foundation for Distinguished Young Scholars (JQ201721), and by the Brazilian Government Agencies FAP/DF (0193.001020/2015) and CNPq (447.628/2014-3).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Hua Zhang, Email: bio_zhangh@ujn.edu.cn.
Luis Alexandre Muehlmann, Email: luismuehlmann88@gmail.com.
References
- 1.World Health Organization. Cancer. [Cited 30 March 2017]. Available from: 〈http://www.who.int/mediacentre/factsheets/fs297/en/〉.
- 2.Lucky S.S., Soo K.C., Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 3.De Angelis C. Side effects related to systemic cancer treatment: are we changing the Promethean experience with molecularly targeted therapies? Curr Oncol. 2008;15:198–199. doi: 10.3747/co.v15i4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uramoto H., Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G., Zhang S., Ma Y., Wang Q., Chen X., Zhang L. Effects of error on dose of target region and organs at risk in treating nasopharynx cancer with intensity modulated radiation therapy. Pak J Med Sci. 2016;32:95–100. doi: 10.12669/pjms.321.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raab O. Uber die Wirkung fluoreszierender Stoffe auf Infusorien. Z Biol. 1900;39:524–546. [Google Scholar]
- 7.Ackroyd R., Kelty C., Brown N., Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–669. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.PrimeJ. Des Accidents Toxiques Prodult par l’Eosinate se Sodium. 2nd ed. Paris: Jouve et Boyer; 1900.
- 9.Von Tappeiner H., Jesionek A. Therapeutische Versuche mit fluoreszierenden Stoffen. Muench Med Wochenschr. 1903;47:2042–2044. [Google Scholar]
- 10.Tappeiner H., Jodlbauer A. Über die Wirkung der photodynamischen (fluoreszierenden) Stoffe auf Infusorien. Dtsch Arch Klin Med. 1904;80:427–487. [Google Scholar]
- 11.Lipson R.L., Baldes E.J., Olsen A.M. The use of a derivative of hematoporphyrin in tumor detection. J Natl Cancer Inst. 1961;26:1–11. [PubMed] [Google Scholar]
- 12.Dougherty T.J., Kaufman J.E., Goldfarb A., Weishaupt K.R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 13.Lovell J.F., Liu T.W.B., Chen J., Zheng G. Activatable photosensitizers for imaging and therapy. Chem Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 14.Cerman E., Çekiç O. Clinical use of photodynamic therapy in ocular tumors. Surv Ophthalmol. 2015;60:557–574. doi: 10.1016/j.survophthal.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Benov L. Photodynamic therapy: current status and future directions. Med Princ Pract. 2015;24:14–28. doi: 10.1159/000362416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozzini G., Colin P., Betrouni N., Nevoux P., Ouzzane A., Puech P. Photodynamic therapy in urology: what can we do now and where are we heading? Photodiagn Photodyn Ther. 2012;9:261–273. doi: 10.1016/j.pdpdt.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Cotter T.G. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 18.Silva J.N., Filipe P., Morlière P., Mazière J.C., Freitas J.P., Gomes M.M. Photodynamic therapy: dermatology and ophthalmology as main fields of current applications in clinic. Biomed Mater Eng. 2008;18:319–327. [PubMed] [Google Scholar]
- 19.Konopka K., Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 20.Babilas P., Schreml S., Landthaler M., Szeimies R.M. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118–132. doi: 10.1111/j.1600-0781.2010.00507.x. [DOI] [PubMed] [Google Scholar]
- 21.Ding H.Y., Yu H.J., Dong Y., Tian R.H., Huang G., Boothman D.A. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276–280. doi: 10.1016/j.jconrel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah J., Park S., Aglyamov S., Larson T., Ma L., Sokolov K. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J Biomed Opt. 2008;13:034024. doi: 10.1117/1.294036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solban N., Rizvi I., Hasan T. Targeted photodynamic therapy. Lasers Surg Med. 2006;38:522–531. doi: 10.1002/lsm.20345. [DOI] [PubMed] [Google Scholar]
- 25.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 26.Longo J.P.F., Muehlmann L.A., Miranda-Vilela A.L., Portilho F.A., De Souza L.R., Silva J.R. Prevention of distant lung metastasis after photodynamic therapy application in a breast cancer tumor model. J Biomed Nanotechnol. 2016;12:689–699. doi: 10.1166/jbn.2016.2208. [DOI] [PubMed] [Google Scholar]
- 27.Castano A.P., Mroz P., Wu M.X., Hamblin M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci U S A. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dougherty T.J., Grindey G.B., Fiel R., Weishaupt K.R., Boyle D.G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975;55:115–121. doi: 10.1093/jnci/55.1.115. [DOI] [PubMed] [Google Scholar]
- 29.Juzeniene A., Peng Q., Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci. 2007;6:1234–1245. doi: 10.1039/b705461k. [DOI] [PubMed] [Google Scholar]
- 30.Triesscheijn M., Baas P., Schellens J.H.M., Stewart F.A. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- 31.Bellnier D.A., Greco W.R., Loewen G.M., Nava H., Oseroff A.B., Dougherty T.J. Clinical pharmacokinetics of the PDT photosensitizers porfimer sodium (photofrin), 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (photochlor) and 5-ALA-induced protoporphyrin IX. Lasers Surg Med. 2006;38:439–444. doi: 10.1002/lsm.20340. [DOI] [PubMed] [Google Scholar]
- 32.Sharman W.M., Allen C.M., Van Lier J.E. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999;4:507–517. doi: 10.1016/s1359-6446(99)01412-9. [DOI] [PubMed] [Google Scholar]
- 33.Josefsen L.B., Boyle R.W. Photodynamic therapy: novel third-generation photosensitizers one step closer? Br J Pharmacol. 2008;154:1–3. doi: 10.1038/bjp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall M.H., Jr, Basu P., Buranda T., Wicks B.S., Findsen E.W., Ondrias M. Photoinduced electron transfer in covalently linked oxomolybdenum(V) porphyrin systems. Inorg Chem. 1997;36:5676–5677. doi: 10.1021/ic9705711. [DOI] [PubMed] [Google Scholar]
- 35.Magda D., Miller R.A. Motexafin gadolinium: a novel redox active drug for cancer therapy. Semin Cancer Biol. 2006;16:466–476. doi: 10.1016/j.semcancer.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Young S.W., Woodburn K.W., Wright M., Mody T.D., Fan Q., Sessler J.L. Lutetium texaphyrin (PCI-0123): a near-infrared, water-soluble photosensitizer. Photochem Photobiol. 1996;63:892–897. doi: 10.1111/j.1751-1097.1996.tb09647.x. [DOI] [PubMed] [Google Scholar]
- 37.Vogel E., Köcher M., Schmickler H., Lex J. Porphycene-α novel porphin isomer. Angew Chem Int Ed. 1986;25:257–259. [Google Scholar]
- 38.Guardiano M., Biolo R., Jori G., Schaffner K. Tetra-n-propylporphycene as a tumour localizer: pharmacokinetic and phototherapeutic studies in mice. Cancer Lett. 1989;44:1–6. doi: 10.1016/0304-3835(89)90100-6. [DOI] [PubMed] [Google Scholar]
- 39.Trachtenberg J., Weersink R.A., Davidson S.R.H., Haider M.A., Bogaards A., Gertner M.R. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008;102:556–562. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]
- 40.Taneja S.S., Bennett J., Coleman J., Grubb R., Andriole G., Reiter R.E. Final results of a phase I/II multicenter trial of WST11 vascular targeted photodynamic therapy for hemi-ablation of the prostate in men with unilateral low risk prostate cancer performed in the United States. J Urol. 2016;196:1096–1104. doi: 10.1016/j.juro.2016.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsyth T.P., Nurco D.J., Pandey R.K., Smith K.M. Synthesis and structure of a 5,15-bis(4-pyridyl)purpurin. Tetrahedron Lett. 1995;36:9093–9096. [Google Scholar]
- 42.De Moraes M., De Vasconcelos R.C., Longo J.P.F., Muehlmann L.A., De Azevedo R.B., Lemos T.M. Effects of photodynamic therapy mediated by nanoemulsion containing chloro-aluminum phthalocyanine: a histologic and immunohistochemical study in human gingiva. Photodiagn Photodyn Ther. 2015;12:592–597. doi: 10.1016/j.pdpdt.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Muehlmann L.A., Rodrigues M.C., Longo J.P.F., Garcia M.P., Py-Daniel K.R., Veloso A.B. Aluminium-phthalocyanine chloride nanoemulsions for anticancer photodynamic therapy: development and in vitro activity against monolayers and spheroids of human mammary adenocarcinoma MCF-7 cells. J Nanobiotechnol. 2015;13:36. doi: 10.1186/s12951-015-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller J.D., Baron E.D., Scull H., Hsia A., Berlin J.C., McCormick T. Photodynamic therapy with the phthalocyanine photosensitize Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borgatti-Jeffreys A., Hooser S.B., Miller M.A., Thomas R.M., De Gortari A., Lucroy M.D. Preclinical evaluation of zinc phthalocyanine tetrasulfonate-based PDT. Proc SPIE. 2005;5686:624–630. [Google Scholar]
- 46.Wainwright M. Photodynamic therapy: the development of new photosensitisers. Anti-Cancer Agents Med Chem. 2008;8:280–291. doi: 10.2174/187152008783961888. [DOI] [PubMed] [Google Scholar]
- 47.Biel M.A. Photodynamic therapy of head and neck cancers. In: Gomer C., editor. Photodynamic Therapy. Methods in Molecular Biology. Humana Press; Totowa, NJ: 2010. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy J.C., Pottier R.H., Pross D.C. Photodynamic therapy with endogenous protoporphyrin. IX: basic principles and present clinical experience. J Photochem Photobiol B Biol. 1990;6:143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 49.Ormond A.B., Freeman A.B. Dye sensitizers for photodynamic therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tardivo J.P., Del Giglio A., De Oliveira C.S., Gabrielli D.S., Junqueira H.C., Tada D.B. Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagn Photodyn Ther. 2005;2:175–191. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- 51.Graciano T.B., Coutinho T.S., Cressoni C.B., de Paula Freitas C., Pierre M.B.R., De Lima Pereira S.A. Using chitosan gels as a toluidine blue O delivery system for photodynamic therapy of buccal cancer: in vitro and in vivo studies. Photodiagn Photodyn Ther. 2015;12:98–107. doi: 10.1016/j.pdpdt.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Harris F., Chatfield L.K., Phoenix D.A. Phenothiazinium based photosensitisers-photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6:615–627. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 53.Harris F., Sayed Z., Hussain S., Phoenix D.A. An investigation into the potential of phenothiazinium-based photo-sensitisers to act as PDT agents. Photodiagn Photodyn Ther. 2004;1:231–239. doi: 10.1016/S1572-1000(04)00046-8. [DOI] [PubMed] [Google Scholar]
- 54.Cincotta L., Foley J.W., Cincotta A.H. Phototoxicity, redox behavior, and pharmacokinetics of benzophenoxazine analogues in EMT-6 murine sarcoma cells. Cancer Res. 1993;53:2571–2580. [PubMed] [Google Scholar]
- 55.Cincotta L., Foley J.W., MacEachern T., Lampros E., Cincotta A.H. Novel photodynamic effects of a benzophenothiazine on two different murine sarcomas. Cancer Res. 1994;54:1249–1258. [PubMed] [Google Scholar]
- 56.Delaey E., Van Laar F., De Vos D., Kamuhabwa A., Jacobs P., De Witte P. A comparative study of the photosensitizing characteristics of some cyanine dyes. J Photochem Photobiol B Biol. 2000;55:27–36. doi: 10.1016/s1011-1344(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 57.Atzpodien J., Gulati S.C., Clarkson B.D. Comparison of the cytotoxic effects of merocyanine-540 on leukemic cells and normal human bone marrow. Cancer Res. 1986;46:4892–4895. [PubMed] [Google Scholar]
- 58.Awuah S.G., You Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012;2:11169–11183. [Google Scholar]
- 59.Lim S.H., Thivierge C., Nowak-Sliwinska P., Han J.Y., Van Den Bergh H., Wagnières G. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J Med Chem. 2010;53:2865-–28674. doi: 10.1021/jm901823u. [DOI] [PubMed] [Google Scholar]
- 60.Kitanov G.M. Hypericin and pseudohypericin in some Hypericum species. Biochem Syst Ecol. 2001;29:171–178. doi: 10.1016/s0305-1978(00)00032-6. [DOI] [PubMed] [Google Scholar]
- 61.Kubin A., Wierrani F., Burner U., Alth G., Grunberger W. Hypericin-the facts about a controversial agent. Curr Pharm Des. 2005;11:233–253. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- 62.Garg A.D., Krysko D.V., Vandenabeele P., Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother. 2012;61:215–221. doi: 10.1007/s00262-011-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panzarini E., Inguscio V., Fimia G.M., Dini L. Rose bengal acetate photodynamic therapy (RBAc-PDT) induces exposure and release of damage-associated molecular patterns (DAMPs) in human HeLa cells. PLoS One. 2014;9:e105778. doi: 10.1371/journal.pone.0105778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pandey R.K., Herman C.K. Shedding some light on tumours. Chem Ind. 1998;18:739–743. [Google Scholar]
- 65.O'Connor A.E., Gallagher W.M., Byrne A.T. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 66.Yoon I., Li J.Z., Shim Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin Endosc. 2003;46:7–23. doi: 10.5946/ce.2013.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garland M.J., Cassidy C.M., Woolfson D., Donnelly R.F. Designing photosensitizers for photodynamic therapy: strategies, challenges and promising developments. Future Med Chem. 2009;1:667–691. doi: 10.4155/fmc.09.55. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez J.M., Bilgin M.D., Grossweiner L.I. Singlet oxygen generation by photodynamic agents. J Photochem Photobiol B Biol. 1997;37:131–140. [Google Scholar]
- 69.Xodo L.E., Cogoi S., Rapozzi V. Photosensitizers binding to nucleic acids as anticancer agents. Future Med Chem. 2016;8:179–194. doi: 10.4155/fmc.15.180. [DOI] [PubMed] [Google Scholar]
- 70.Rajendran M. Quinones as photosensitizer for photodynamic therapy: ros generation, mechanism and detection methods. Photodiagn Photodyn Ther. 2016;13:175–187. doi: 10.1016/j.pdpdt.2015.07.177. [DOI] [PubMed] [Google Scholar]
- 71.Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J.W., Wu Z.M., Magetic D., Zhang L.J., Chen Z.L. Antitumor effects evaluation of a novel porphyrin derivative in photodynamic therapy. Tumour Biol. 2015;36:9685–9692. doi: 10.1007/s13277-015-3745-z. [DOI] [PubMed] [Google Scholar]
- 73.Liao P.Y., Wang X.R., Gao Y.H., Zhang X.H., Zhang L.J., Song C.H. Synthesis, photophysical properties and biological evaluation of β-alkylaminoporphyrin for photodynamic therapy. Bioorg Med Chem. 2016;24:6040–6047. doi: 10.1016/j.bmc.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 74.Chen J.J., Hong G., Gao L.J., Liu T.J., Cao W.J. In vitro and in vivo antitumor activity of a novel porphyrin-based photosensitizer for photodynamic therapy. J Cancer Res Clin Oncol. 2015;141:1553–1561. doi: 10.1007/s00432-015-1918-1. [DOI] [PubMed] [Google Scholar]
- 75.Nakai M., Maeda T., Mashima T., Yano S., Sakuma S., Otake E. Syntheses and photodynamic properties of glucopyanoside-conjugated indium(III) porphyrins as a bifunctional agent. J Porphyrins Phthalocyanies. 2013;17:1173–1182. [Google Scholar]
- 76.Costa L.D., Silva Jde A E., Fonseca S.M., Arranja C.T., Urbano A.M., Sobral A.J. Photophysical characterization and in vitro phototoxicity evaluation of 5,10,15,20-tetra(quinolin-2-yl)porphyrin as a potential sensitizer for photodynamic therapy. Molecules. 2016;21:439. doi: 10.3390/molecules21040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horiuchi H., Hosaka M., Mashio H., Terata M., Ishida S., Kyushin S. Silylation improves the photodynamic activity of tetraphenylporphyrin derivatives in vitro and in vivo. Chemistry. 2014;20:6054–6060. doi: 10.1002/chem.201303120. [DOI] [PubMed] [Google Scholar]
- 78.Brunner H., Obermeier H. Platinum(II) complexes with porphyrin ligands-additive cytotoxic and photodynamic effect. Angew Chem Int Engl. 1994;33:2214–2215. [Google Scholar]
- 79.Mion G., Gianferrara T., Bergamo A., Gasser G., Pierroz V., Rubbiani R. Phototoxic activity and DNA interactions of water-soluble porphyrins and their rhenium(I) conjugates. ChemMedChem. 2015;10:1901–1914. doi: 10.1002/cmdc.201500288. [DOI] [PubMed] [Google Scholar]
- 80.Meng Z., Yu B., Han G.Y., Liu M.H., Shan B., Dong G.Q. Chlorin P6-based water-soluble amino acid derivatives as potent photosensitizers for photodynamic therapy. J Med Chem. 2016;59:4999–5010. doi: 10.1021/acs.jmedchem.6b00352. [DOI] [PubMed] [Google Scholar]
- 81.Jinadasa R.G.W., Zhou Z.H., Vicente M.G., Smith K.M. Syntheses and cellular investigations of di-aspartate and aspartate-lysine chlorin e6 conjugates. Org Biomol Chem. 2016;14:1049–1064. doi: 10.1039/c5ob02241j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narumi A., Tsuji T., Shinohara K., Yamazaki H., Kikuchi M., Kawaguchi S. Maltotriose-conjugation to a fluorinated chlorin derivative generating a PDT photosensitizer with improved water-solubility. Org Biomol Chem. 2016;14:3608–3613. doi: 10.1039/c6ob00276e. [DOI] [PubMed] [Google Scholar]
- 83.Kahl S.B., Koo M.-S. Synthesis of tetrakis-carborane-carboxylate esters of 2,4-bis(α,β-dihydroxyethyl)-deuteroporphyrin IX. J Chem Soc Chem Commun. 1990;0:1769–1771. [Google Scholar]
- 84.Asano R., Nagami A., Fukumoto Y., Miura K., Yazama F., Ito H. Synthesis and biological evaluation of new boron-containing chlorin derivatives as agents for both photodynamic therapy and boron neutron capture therapy of cancer. Bioorg Med Chem Lett. 2014;24:1339–1343. doi: 10.1016/j.bmcl.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 85.Gushchina O.I., Larkina E.A., Nikolskaya T.A., Mironov A.F. Synthesis of amide derivatives of chlorin e6 and investigation of their biological activity. J Photochem Photobiol B. 2015;153:76–81. doi: 10.1016/j.jphotobiol.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Dąbrowski J.M., Arnaut L.G., Pereira M.M., Monteiro C.J., Urbańska K., Simões S. New halogenated water-soluble chlorin and bacteriochlorin as photostable PDT sensitizers: synthesis, spectroscopy, photophysics, and in vitro photosensitizing efficacy. ChemMedChem. 2010;5:1770–1780. doi: 10.1002/cmdc.201000223. [DOI] [PubMed] [Google Scholar]
- 87.Patel N., Pera P., Joshi P., Dukh M., Tabaczynski W.A., Siters K.E. Highly effective dual-function near-infrared (NIR) photosensitizer for fluorescence imaging and photodynamic therapy (PDT) of cancer. J Med Chem. 2016;59:9774–9787. doi: 10.1021/acs.jmedchem.6b00890. [DOI] [PubMed] [Google Scholar]
- 88.Arslanoğlu Y., Nyokong T. Synthesis and photophysical studies of mono-carboxy phthalocyanines containing quaternizable groups. Polyhedron. 2011;30:2733–2739. [Google Scholar]
- 89.de Oliveira K.T., De Assis F.F., Ribeiro A.O., Neri C.R., Fernandes A.U., Baptista M.S. Synthesis of phthalocyanines-ALA conjugates: water-soluble compounds with low aggregation. J Org Chem. 2009;74:7962–7965. doi: 10.1021/jo901633a. [DOI] [PubMed] [Google Scholar]
- 90.Bıyıklıoğlu Z., Kantekin H. Synthesis and spectroscopic properties of a series of octacationic water-soluble phthalocyanines. Synth Met. 2011;161:943–948. [Google Scholar]
- 91.Silva A.R., Simioni A.R., Tedesco A.C. Photophysical and complexation studies of chloro-aluminum phthalocyanine with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J Nanosci Nanotechnol. 2011;11:4046–4055. doi: 10.1166/jnn.2011.3823. [DOI] [PubMed] [Google Scholar]
- 92.Durmus M., Yaman H., Göl C., Ahsen V., Hyokong T. Water-soluble quaternized mercaptopyridine-substituted zinc-phthalocyanines: synthesis, photophysical, photochemical and bovine serum albumin binding properties. Dyes Pigm. 2011;91:153–163. [Google Scholar]
- 93.Do Nascimento F.B., manieri T.M., Cerchiaro G., Ribeiro A.O. Synthesis of unsymmetrical phthalocyanine derivatives and their interaction with mammary MCF7 cells. Dyes Pigm. 2013;99:316–322. [Google Scholar]
- 94.Kucinska M., Skupin-Mrugalska P., Szczolko W., Sobotta L., Sciepura M., Tykarska E. Phthalocyanine derivatives possessing 2-(morpholin-4-yl)ethoxy groups as potential agents for photodynamic therapy. J Med Chem. 2015;58:2240–2255. doi: 10.1021/acs.jmedchem.5b00052. [DOI] [PubMed] [Google Scholar]
- 95.Wierzchowski M., Sobotta L., Skupin-Mrugalska P., Kruk J., Jusiak W., Yee M. Phthalocyanines functionalized with 2-methyl-5-nitro-1H-imidazolylethoxy and 1,4,7-trioxanonyl moieties and the effect of metronidazole substitution on photocytotoxicity. J Inorg Biochem. 2013;127:62–72. doi: 10.1016/j.jinorgbio.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 96.Zhou X.Q., Meng L.B., Huang Q., Li J., Zheng K., Zhang F.L. Synthesis and in vitro anticancer activity of zinc(II) phthalocyanines conjugated with coumarin derivatives for dual photodynamic and chemotherapy. ChemMedChem. 2015;10:304–311. doi: 10.1002/cmdc.201402401. [DOI] [PubMed] [Google Scholar]
- 97.Jia X., Yang F.F., Li J., Liu J.Y., Xue J.P. Synthesis and in vitro photodynamic activity of oligomeric ethylene glycol-quinoline substituted zinc(II) phthalocyanine derivatives. J Med Chem. 2013;56:5797–5805. doi: 10.1021/jm400722d. [DOI] [PubMed] [Google Scholar]
- 98.Ranyuk E., Cauchon N., Klarskov K., Guérin B., Van Lier J.E. Phthalocyanine-peptide conjugates: receptor-targeting bifunctional agents for imaging and photodynamic therapy. J Med Chem. 2013;56:1520–1534. doi: 10.1021/jm301311c. [DOI] [PubMed] [Google Scholar]
- 99.Machacek M., Cidlina A., Novakova V., Svec J., Rudolf E., Miletin M. Far-red-absorbing cationic phthalocyanine photosensitizers: synthesis and evaluation of the photodynamic anticancer activity and the mode of cell death induction. J Med Chem. 2015;58:1736–1749. doi: 10.1021/jm5014852. [DOI] [PubMed] [Google Scholar]
- 100.Shen X.M., Zheng B.Y., Huang X.R., Wang L., Huang J.D. The first silicon(IV) phthalocyanine-nucleoside conjugates with high photodynamic activity. Dalton Trans. 2013;42:10398–10403. doi: 10.1039/c3dt50910a. [DOI] [PubMed] [Google Scholar]
- 101.Bio M., Rajaputra P., Nkepang G., You Y. Far-red light activatable, multifunctional prodrug for fluorescence optical imaging and combinational treatment. J Med Chem. 2014;57:3401–3409. doi: 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erbas-Cakmak S., Cakmak F.P., Topel S.D., Uyar T.B., Akkaya E.U. Selective photosensitization through an AND logic response: optimization of the pH and glutathione response of activatable photosensitizers. Chem Commun (Camb) 2015;51:12258–12261. doi: 10.1039/c5cc01261a. [DOI] [PubMed] [Google Scholar]
- 103.Jiang X.J., Lau J.T., Wang Q., Ng D.K., Lo P.C. pH- and thiol-responsive BODIPY-based photosensitizers for targeted photodynamic therapy. Chemistry. 2016;22:8273–8281. doi: 10.1002/chem.201600452. [DOI] [PubMed] [Google Scholar]
- 104.Mitra K., Gautam S., Kondaiah P., Chakravarty A.R. BODIPY-appended 2-(2-pyridyl)benzimidazole platinum(II) catecholates for mitochondria-targeted photocytotoxicity. ChemMedChem. 2016;11:1956–1967. doi: 10.1002/cmdc.201600320. [DOI] [PubMed] [Google Scholar]
- 105.Fraix A., Blangetti M., Guglielmo S., Lazzarato L., Marino N., Cardile V. Light-tunable generation of singlet oxygen and nitric oxide with a bichromophoric molecular hybrid: a bimodal approach to killing cancer cells. ChemMedChem. 2016;11:1371–1379. doi: 10.1002/cmdc.201500396. [DOI] [PubMed] [Google Scholar]
- 106.Lincoln R., Durantini A.M., Greene L.E., Martínez S.R., Knox R., Becerra M.C. meso-Acetoxymethyl BODIPY dyes for photodynamic therapy: improved photostability of singlet oxygen photosensitizers. Photochem Photobiol Sci. 2016;16:178–184. doi: 10.1039/c6pp00166a. [DOI] [PubMed] [Google Scholar]
- 107.Ozdemir T., Bila J.L., Sozmen F., Yildirim L.T., Akkaya E.U. Orthogonal bodipy trimers as photosensitizers for photodynamic action. Org Lett. 2016;18:4821–4823. doi: 10.1021/acs.orglett.6b02418. [DOI] [PubMed] [Google Scholar]