Abstract
Vitamin D3 has been found to produce therapeutic effects on obesity-associated insulin resistance and dyslipidemia through its potent anti-inflammatory activity, but the precise immunomodulatory mechanism remains poorly understood. In the present study we found that 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D3, significantly attenuated monosodium glutamate (MSG)-induced obesity and insulin resistance as indicated by body weight reduction, oral glucose tolerance improvement, and a glucose infusion rate increase as detected with hyperinsulinemic-euglycemic clamp. Moreover, 1,25(OH)2D3 not only restored pancreatic islet functions but also improved lipid metabolism in insulin-targeted tissues. The protective effects of 1,25(OH)2D3 on glycolipid metabolism were attributed to its ability to inhibit an obesity-activated inflammatory response in insulin secretory and targeted tissues, as indicated by reduced infiltration of macrophages in pancreas islets and adipose tissue while enhancing the expression of Tgf-β1 in liver tissue, which was accompanied by increased infiltration of Treg cells in immune organs such as spleen and lymph node as well as in insulin-targeted tissues such as liver, adipose, and muscle. Together, our findings suggest that 1,25(OH)2D3 serves as a beneficial immunomodulator for the prevention and treatment of obesity or metabolic syndrome through its anti-inflammatory effects.
KEY WORDS: Insulin resistance; Dyslipidemia; MSG-obese rat; Treg cell; Vitamin D3; 1,25-Dihydroxyvitamin D3
Graphical abstract
Obesity is associated with multiple adverse health outcomes collectively summarized as the “metabolic syndrome”, consisting of insulin resistance and dyslipidemia. Administration of 1,25(OH)2D3 protects MSG obese rats from the development of obesity and its related metabolic risks via increasing the infiltration of CD4+CD25+ regulatory T-cells in primary insulin targeted-tissues.
1. Introduction
Obesity is associated with multiple adverse health outcomes collectively summarized as the “metabolic syndrome”, consisting of insulin resistance, dyslipidemia, and cardiovascular diseases1. A linking role of inflammation between obesity and metabolic syndrome has been established which mostly refers to the release of various adipocyte products, such as cytokines, fatty acids, and oxygen free radicals from the white adipose tissue in obese individuals2. Many of these products function as molecules of damage-associated molecular patterns (DAMPs) to initiate chronic low-degree inflammation to promote the infiltration of adipose tissue with activated macrophages through the activation of pattern reorganization receptors (PRRs) such as Toll-like receptors (TLRs) expressed on immune cells and residual cells3, 4. Indeed, accumulated evidence has indicated that both obesity and metabolic syndrome are inflammatory disorders and inflammatory responses are causally involved in insulin resistance by ultimately suppressing the insulin signaling pathway5.
Epidemiological studies indicate that insufficient vitamin D status is a potential contributor to insulin resistance and obesity6, 7, 8. Data from a pilot study examining vitamin D deficiency in type 1 and type 2 diabetes suggests that vitamin D insufficiency is more common in type 2 diabetes than in type 1 diabetes, unrelated to age, sex, or insulin treatment9. However, studies on the administration of vitamin D supplements to vitamin D-sufficient patients with IGT or type 2 diabetes have yielded conflicting results. Some have reported an improvement, others no effect10. One study even showed a worsening of type 2 diabetes: supplementation in three British Asians with vitamin D deficiency and type 2 diabetes led to increased insulin resistance and the deterioration of glycemic control11. Interestingly, Wortsman et al.12 reported that obesity-associated vitamin D deficiency is largely because of the decreased bioavailability of vitamin D3 from cutaneous and dietary sources due to its deposition in body fat compartments. Furthermore, chronic feeding of mice with 1,25(OH)2D3, the biologically active form, suppressed the inflammatory responses in a variety of animal models including experimental asthma, encephalomyelitis, and rheumatoid arthritis13, 14, 15. The potential immune modulating effects of 1,25(OH)2D3 on the immune system have been initially derived from in vitro observations following the treatment of dendritic cells (DC) with 1,25(OH)2D3 which could inhibit the maturation of myeloid DC via reducing secretion of IL-12 as well as the expression of co-stimulatory molecules. 1,25(OH)2D3 also enhances the secretion of CCL22 by DC in vitro, which is a chemokine that attracts T cells into the skin16. IL-10-producing regulatory T cells can be induced in vitro by 1,25(OH)2D3 in the presence of dexamethasone17. In addition, 1,25(OH)2D3 can hamper the secretion of IFN-α from T cells and induce Th2 cell development with increased production of IL-4, IL-5, and IL-1018. Nonetheless, the immunoregulatory mechanism of 1,25(OH)2D3 on obesity-associated insulin resistance and dyslipidemia is not fully understood.
In this study we have investigated the effects and mechanism of 1,25(OH)2D3 in the regulation of insulin sensitivity and glucolipid metabolism in MSG-obese rats. We found that a short course of treatment with 1,25(OH)2D3 improved insulin resistance and glucolipid metabolism disorder of MSG-obese rats by inhibiting the inflammatory responses in primary insulin-targeted tissues including adipose, liver, and muscle, which is largely due to increasing the infiltration of CD4+CD25+FoxP3+ regulatory T-cells in these tissues. Our studies indicate that administration of 1,25(OH)2D3 protects MSG-obese rats from the development of obesity and its related metabolic risks.
2. Materials and methods
2.1. Animal model
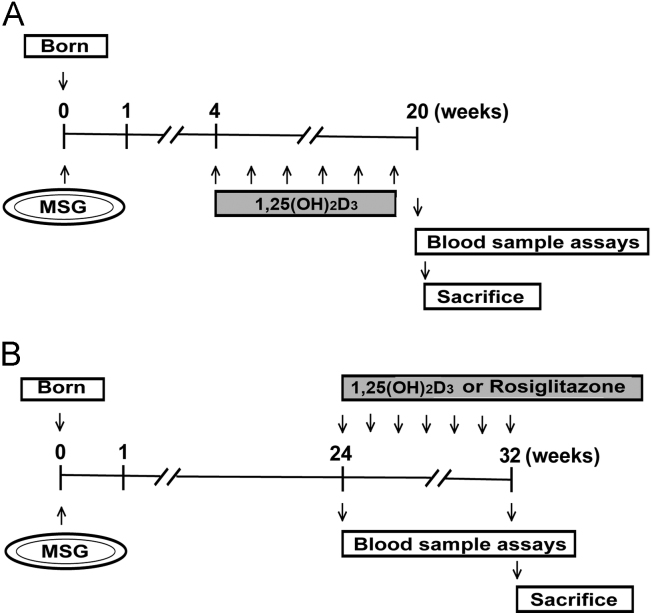
Wistar rats (newborn) were obtained from Vital River Laboratory Animal Technology (Beijing, China). All animal protocols conformed to the Guidelines for the Care and Use of Laboratory Animals prepared and approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences and Peking Union Medical College. Newborn Wistar rats were s.c. injected with monosodium l-glutamate (MSG) at 4 g/kg/day for seven successive days as described previously19. In contrast to the normal rats, the MSG rats developed obesity with increased plasma TG, cholesterol, and free fatty acid contents as well as impaired insulin sensitivity in their adulthood20. Experimental protocols are outlined in Fig. 1. In protocol A (Fig. 1A), the four-week-aged MSG rats were given s.c. injections of 1 μg/kg 1,25(OH)2D3 twice a week for 16 weeks, and insulin tolerance test (ITT) and the euglycemic hyperinsulinemic clamp were assayed at the end of the experiment to evaluate the incidence of insulin resistance. In protocol B (Fig. 1B), obese MSG rats were sorted into three groups according to body weights and fasting plasma glucose values, and then were treated with 1 μg/kg 1,25(OH)2D3 (twice a week), 4 mg/kg/day rosiglitazone or vehicle for 8 weeks.
Figure 1.
Experimental protocol for studying the effects of 1,25(OH)2D3 on the development of insulin resistance in MSG-obese rats. MSG-obese rats were rendered by s.c. injection of MSG (4 g/kg/day) to newborn Wistar rats for seven successive days. (A) In protocol A, four-week-aged MSG rats were given s.c. injections of 1,25(OH)2D3 (1 g/kg, twice a week) for 16 weeks and an insulin tolerance test (ITT) and the euglycemic hyperinsulinemic clamp were conducted at the end of experiment to measure the incidence of insulin resistance. (B) In protocol B, the obese MSG rats were divided into three groups according to body weights and fasting plasma glucose values and were respectively treated with 1,25(OH)2D3 (1 g/kg, twice a week), rosiglitazone (4 mg/kg/day), or vehicle for 8 weeks.
2.2. Oral glucose tolerance test
The OGTT and ITT were carried out as previously described21. In brief, after fasting for 6 h on the last treatment day, the animals were administrated an oral dose of 2 g/kg glucose. Blood samples were collected at 0, 30, 60, and 120 min after glucose loading. Blood glucose was analyzed by the glucose-oxidase method, and the area under the curve (AUC) was generated from the data of OGTT.
2.3. Biochemical analysis
On the final day blood samples were collected. Serum TG (triglyceride), T-CHO (total cholesterol), and LDL-C (low-density lipoproteins cholesterol) were assayed using enzymatic colorimetric methods provided by commercial kits (Biosino Bio-Technology and Science Inc., Beijing, China). Serum insulin was measured using radio-immunoassay kit (Chinese Institute of Atomic Energy, Beijing, China) according to the manufacturer's instructions.
2.4. Clamp experiments in MSG-obese rats
The insulin sensitivity was analyzed using euglycemic hyperinsulinemic clamp technique. Ten rats per group were subjected to this test. The clamp experiments were carried out as previously described22. Briefly, rats fasted for 6 h were anesthetized with 45 mg/kg i.p. of pentobarbital. After tracheotomy, the right jugular vein (infusion of insulin and glucose) and left carotid artery (collecting blood sample) were catheterized. Thirty minutes after catheterization, the basal glucose level was tested, and followed by insulin infusion (8 mU/kg/min). At the same time, a variable rate of glucose (10%) was infused and blood glucose levels were measured at 5-min intervals. The steady state (blood glucose levels at 5–6 mmol/L) was reached about 2 h later and sustained for 30 min with constant glucose infusion. Glucose disappearance was represented by average glucose infusion rate (GIR, mM/kg/min) during steady state (the final 30 min of the clamp).
2.5. Semi-quantitative analysis by RT-PCR
The liver tissues were homogenized in Trizol and total RNA was extracted according to the manufacturer's instructions (Invitrogen, USA). cDNA was synthesized and used for PCR amplification. The RNA content was then analysed by agarose gel electrophoresis using the following specific primer sequences: rat Irs-1 (S) 5′-CACCCACTCCTATCCCG-3′; (A) 5′-CCCTACTCCGTTTGTCCA-3′; rat Ppar-α (S) 5′-GCAAAACTGAAAGCAGAAATTCT-3′; (A) 5′-AGCTCCGTGACGGTCTCCA-3′; rat Il-6 (S) 5′-CCACTGCCTTCCCTACTTCA-3′; (A) 5′-AACGGAACTCCAGAAGACCA-3′; rat Tgf-β1 (S) 5′-CAGACATTCGGCAAGCAGTG-3′; (A) 5′-GTTCATGTCATGGATGGTGC-3′; rat β-actin (S) 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; (A) 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. The value was normalized to the values obtained with β-actin.
2.6. Western blot analysis
Briefly, the homogenized tissue extract (40 mg protein) from the liver was fractionated on a 10% SDS-PAGE gel and the proteins were transferred to PMSF membranes. After blocking with 5% nonfat milk, membranes were incubated with antibodies to pNF-κB, NF-κB, STAT3 and pSTAT3 (Cell Signaling Technology, USA) at 4 °C overnight. The membranes were washed and incubated with HRP-conjugated secondary antibodies at room temperature for 1 h. Enhanced chemiluminescence reagents (Amersham, USA) were employed to visualize the protein bands on the membranes. The densities of specific bands were normalized with the β-actin signal and were quantitated by densitometry using gel-pro software.
2.7. Immunolabelling and confocal microscopy
At the end of experiment the liver, pancreas and infrarenal adipose tissues were removed from MSG rats, fixed with 4% polyformalin in phosphate-buffered saline and embedded in paraffin. Liver and adipose tissue sections were stained with hematoxylin-eosin (H&E) and fat cell sizes were measured. The pancreas was fixed in bourin for paraffin embedding and stained with gomori and H&E for analysis of pancreatic β cell mass and islet morphology. Immunostaining was performed using GLUT2 (Bioss, China) and CD68 (BD, USA) antibody. An ABC kit and 3,3-diaminobenzidine (DAB) were used for amplification and staining, respectively. Quantitative analysis of the positive area was performed by image analysis using a computer with Image analyzer (Image Pro-Plus 5.1). Twenty fields were randomly selected from the each section, and the positively stained area was measured and expressed as IOD for each field. Liver, fat and muscle sections were subjected to immunofluorescence for identifying CD4+CD25+FoxP3+ Tregs. Tissue sections were incubated with CD4+, CD25+ and FoxP3+ primary antibodies (Cell Signaling Technology) at 4 °C overnight. The sections were washed thrice and then incubated with Alexa488-, Alexa647- or Alexa350-conjugated secondary antibodies (Invitrogen) for 30 min. The colocalization of CD4 (green), CD25 (red) and FoxP3 (blue) were acquired using a confocal microscope FV1000 (Olympus Microsystems) and the Tregs were identified by the coexpression of CD4, CD25 and FoxP3 (white dots).
2.8. Flow cytometry analysis
CD4+CD25+FoxP3+ Tregs were identified by methods described previously23. Briefly, single-cell suspensions from spleens and mesentery lymph nodes were stained with antibodies that recognize the following antigens: CD4, CD25 and FoxP3, which were labeled with FITC, APC or PE (eBioscience, USA), respectively. More than 10,000 events were recorded through a CD4+ cell gate, and then CD5+FoxP3+ Tregs were determined using a PARTEC CyFlow (Munich, Germany) and data were analyzed using FCS EXPRESS.
2.9. Statistical analyses
All values are presented as mean±standard error of mean (SEM). Student's t-test was used for two-group comparisons. Multiple comparisons among three or more groups were performed by one-way ANOVA. P<0.05 or P<0.01was considered statistically significant.
3. Results
3.1. 1,25(OH)2D3 protected mice from developing MSG-induced insulin resistance
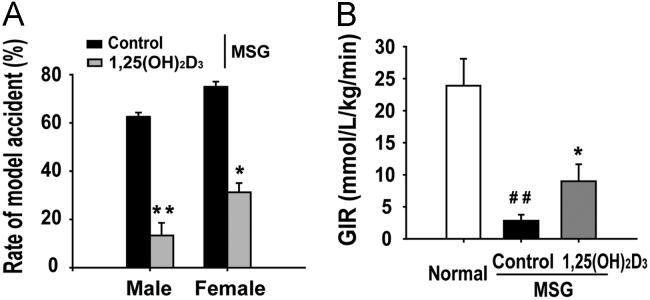
As obesity is the most important determinant of metabolic syndrome which is characterized by insulin resistance, hypertriglyceridaemia, hypo-HDL-cholesterolaemia and hypertension, we are firstly interested in analyzing whether 1,25(OH)2D3 protected MSG-induced obese rats from insulin resistance. The male and female MSG rats were given an early-life treatment with 1 μg/kg of 1,25(OH)2D3 twice a week from 4 to 20 weeks of age, corresponding to early childhood in humans (Fig. 1A). Using the oral glucose tolerance test, we found that the incidence of insulin resistance in vehicle-treated controls was 61% in male rats and 78% in females. However, only 16% of male rats and 27% of female rats treated with 1,25(OH)2D3 developed insulin resistance (Fig. 2A). Moreover, 1,25(OH)2D3 increased the glucose infusion rate (GIR) of MSG rats with early-life treatment (Fig. 2B). These data suggest that 1,25(OH)2D3 protects against the development of MSG-induced obesity.
Figure 2.
1,25(OH)2D3 decreased the MSG-induced incidence of insulin resistance. Male and female MSG rats were treated with 1,25(OH)2D3 (1 g/kg, every other day) from weaning to 5-month age. Insulin resistance was evaluated by ITT and the euglycemic hyperinsulinemic clamp. (A) The incidence of insulin resistance was identical in male and female rats. (B) 1,25 (OH)2D3 treatment significantly increased the decreased glucose infusion rate (GIR) in MSG obese rats. Data are expressed as mean±SEM, n=10. ##P<0.01 vs. normal group; *P<0.05, **P<0.01 vs. MSG group.
3.2. 1,25(OH)2D3 improved insulin sensitivity of MSG-obese rats
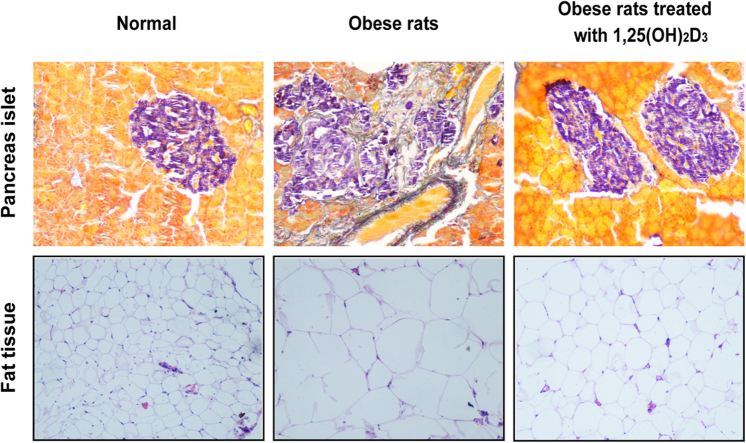
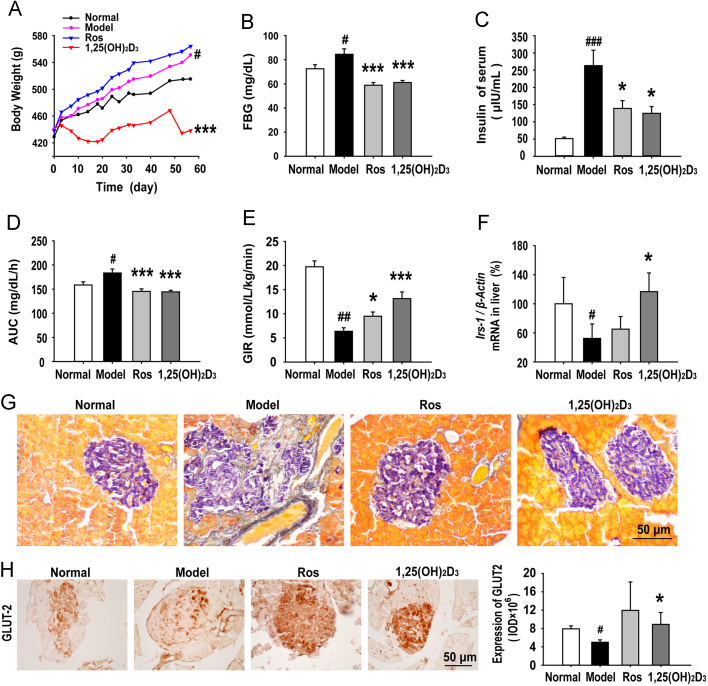
To determine the potential therapeutic significance of 1,25(OH)2D3 on established obesity, the body weight, glucose/lipid metabolism, and insulin sensitivity were then chosen to evaluate the efficacy of 1,25(OH)2D3 in the insulin resistant adult MSG rats (Fig. 1B). After an eight-week treatment, the body weight of the 1,25(OH)2D3-treated MSG rats was significantly lower than that of the vehicle- or rosiglitazone- (a positive control) treated rats (Fig. 3A), and there was no change in serum calcium and serum phosphorus levels (data not shown). As expected, the blood glucose (Fig. 3B) and plasma insulin levels (Fig. 3C) of 1,25(OH)2D3-treated MSG rats were dramatically decreased compared to the vehicle-treated MSG rats. We further examined the effects of 1,25(OH)2D3 on the improvement of insulin resistance in the MSG-obese rats by conducting OGTT and euglycemic-hyperinsulinemic clamp tests. In the OGTT assay, the integrated blood glucose (AUC) levels of both 1,25(OH)2D3 and rosiglitazone-treated MSG rats significantly decreased at 2 h after glucose loading (Fig. 3D). Using the euglycemic-hyperinsulinemic clamp, we observed that there was significant insulin resistance in MSG-obese rats, characterized by decreased GIR. However, both 1,25(OH)2D3 and rosiglitazone treatment increased the GIR of MSG rats by 107% and 49%, respectively, compared with the vehicle-treated MSG rats (Fig. 3E). Corresponding to decreased insulin sensitivity, the mRNA expression of Irs-1, which plays an important biological function for glucose uptake and augmenting insulin signaling, was dramatically up-regulated in the 1,25(OH)2D3-treated MSG rat livers (Fig. 3F). As accumulated evidence has shown that obesity is characterized by both peripheral insulin resistance and impaired insulin secretion, we are interested to know whether 1,25(OH)2D3 could be beneficial to improve MSG-induced dysfunction of pancreatic islets. In the MSG rats the islets showed substantial fibrosis, whereas there was little fibrosis in the 1,25(OH)2D3-treated MSG rats and the overall morphology of the islets was more regular and rounded (Fig. 3G). GLUT2 is the most important glucose transporter ensuring glucose uptake for most tissues. The suppression of GLUT2 in β-cells is associated with glucose-sensitive insulin secretion and the loss of high-Km glucose transport. We found GLUT2 loss in MSG rats and 1,25(OH)2D3 normalized the expression of GLUT2 (Fig. 3H). All together, these results indicate that 1,25(OH)2D3 administration leads to an improvement of glucose metabolism and insulin sensitivity in MSG-induced obese rats.
Figure 3.
1,25(OH)2D3 reduced obesity and improved insulin sensitivity and the metabolism of glucose. (A) 1,25(OH)2D3 reduced the MSG-gained body weight. (B) 1,25(OH)2D3 decreased the level of fasting blood glucose (FBG) measured by the glucose-oxidase method. (C) 1,25(OH)2D3 decreased the concentration of serum insulin as measured by radioimmunoassay. (D) The area under the curve (AUC) was calculated from the glucose concentrations at 0, 30, 60, 120 min after glucose load and was plotted on the graph. (E) The glucose infusion rate (GIR) in euglycemic hyperinsulinemic clamp studies. (F) The mRNA expression of Irs-1 in liver was tested by RT-PCR. (G) Pancreatic islet sections were stained with gomori as described in Methods. 1,25(OH)2D3 reduced fibroblast infiltration in pancreatic islets. (H) The expression of GLUT2 in pancreas was determined by immunochemical staining. 1,25(OH)2D3 augmented the GLUT2 expression in pancreatic islets. Data are expressed as mean±SEM, n=10. #P<0.05, ##P<0.01, ###P<0.001 vs. normal group; *P<0.05, ***P<0.001 vs. MSG group.
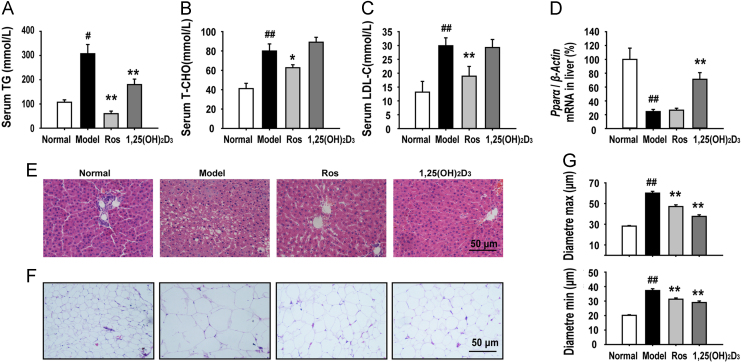
3.3. 1,25(OH)2D3 ameliorated lipid metabolism of MSG-obese rats
As abnormal lipid metabolism is an important consequence and contributing factor in insulin resistance in the pathogenesis of obesity, we investigated the effect of 1,25(OH)2D3 on the regulation of lipid metabolism. We found that 1,25(OH)2D3 treatment significantly reduced the serum TG level of MSG rats (Fig. 4A), but did not change the level of the serum T-CHO and LDL-C (Fig. 4B and C). We further examined the effect of 1,25(OH)2D3 in liver and adipose tissues, both of which are the most important insulin-targeted tissues. PPAR-α is believed to participate in the regulation of fatty acid uptake and β-oxidation in the liver tissue. The mRNA expression of Ppar-α was significantly down-regulated in the vehicle or rosiglitazone-treated MSG rat liver, which was significantly up-regulated by 1,25(OH)2D3 (Fig. 4D). Histomorphological analysis of liver tissues using H&E staining revealed severe hepatic steatosis in the MSG rats, characterized by excessive accumulation of fat in hepatic intracellular vesicles. Treatment of the MSG rats with 1,25(OH)2D3 completely eliminated the steatosis. Liver slices from the 1,25(OH)2D3 treated MSG rats were found to be histologically comparable to those from normal rats (Fig. 4E). Additionally, it has been proved that the accumulation of “dysfunctional” adipose tissue characterized by the presence of “large” lipid-laden adipocytes contributes to the insulin resistance of obese individuals. For adipose tissue, MSG induced a dramatic increase in the fat mass and adipocyte size, while this adipocyte hypertrophy was attenuated by 1,25(OH)2D3 treatment (Fig. 4F and G). All of these data suggest that 1,25(OH)2D3 has a critical role in the regulation of lipid metabolism in liver and adipose tissues, both of which are the primary insulin-targeted tissues involved in the regulation of lipid metabolism.
Figure 4.
1,25(OH)2D3 regulated lipid metabolism in MSG rats. (A)–(C) 1,25(OH)2D3 treatment decreased the level of serum TG, but did not change the levels of serum CHO and LDL-C. (D) The mRNA expression of Pparα in liver was tested by RT-PCR. (E) Liver sections were stained with H&E as described in methods. 1,25(OH)2D3 treatment inhibited MSG-induced fatty infiltration in liver. (F) Representative H&E staining of fat tissue sections. (G) 1,25(OH)2D3 treatment reduced adipocyte diameters (max and min) as measured with H&E staining. Data are expressed as mean±SEM, n=10. #P<0.05, ##P<0.01 vs. normal group; *P<0.05, **P<0.01 vs. MSG group.
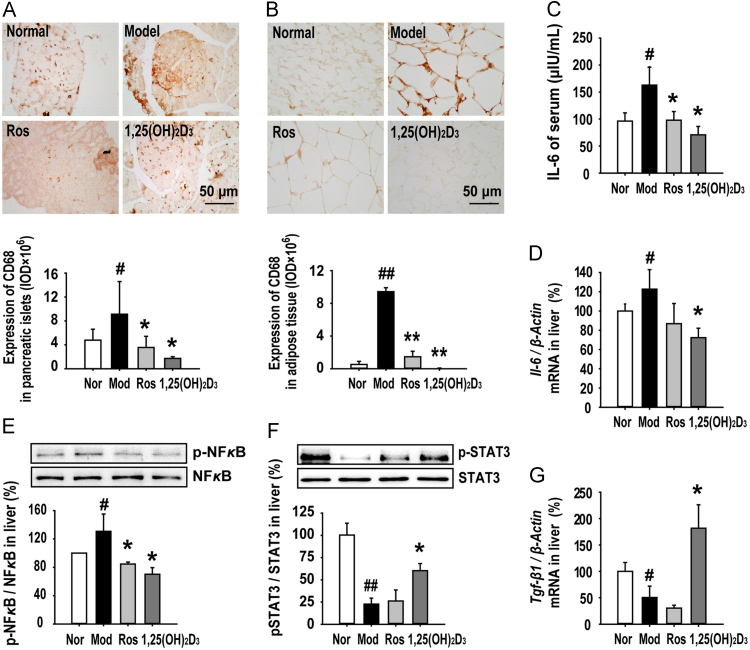
3.4. 1,25(OH)2D3 exerted anti-inflammatory effects in MSG-obese rats
The macrophage is a major cell-type involved in chronic inflammation, and studies in obese humans and rodents have shown a dramatic activation of macrophages that produce inflammatory factors in pancreatic islets and adipose tissues. MSG-induced dysregulation of glucose and lipid led to high-level expression of CD68, a marker of activated macrophages, in pancreatic islets (Fig. 5A) and adipose tissues (Fig. 5B), indicating more pronounced macrophage infiltration in the insulin-secretory organ and insulin-targeted tissues. In comparison to the MSG rat, treatment with 1,25(OH)2D3 resulted in significant suppression of macrophage infiltration in both tissues (Fig. 5A and B). Interleukin-6 (IL-6), a typical inflammatory cytokine that is secreted by macrophages and Th2 cells, can alter insulin sensitivity by mediating different steps in the insulin signaling pathway. We found that serum IL-6 was increased in MSG rats (Fig. 5C), and a corresponding increase was also observed on the mRNA expression of Il-6 in MSG rat livers (Fig. 5D). Furthermore, we found that phosphorylation of NF-κB, a nuclear factor playing a central role in inflammatory disease, was increased in MSG rat livers (Fig. 5E). 1,25(OH)2D3 elicited a distinct anti-inflammatory role by decreasing the IL-6 serum level, as well as the infiltration of IL-6 and activation of NF-κB in MSG rat livers (Fig. 5C-E). Additionally, we tested the activity of STAT3, a transcription factor which can suppress the production of pro-inflammatory cytokines. In the livers of MSG rats the phosphorylation of STAT3 was lower than normal rats, which was partly recovered in the livers of MSG rats treated with 1,25(OH)2D3, but not rosiglitazone (Fig. 5F). Transforming growth factor-β1 (TGF-β1) is an inhibitory inflammatory factor secreted by Treg cells. We found a reduction of Tgf-β1 in MSG rat livers and 1,25(OH)2D3 normalized its expression (Fig. 5G). These results indicate that treatment with 1,25(OH)2D3 inhibits the MSG-enhanced pro-inflammatory response, but restores the inhibitory inflammatory response in adipose and liver tissues of obese rats.
Figure 5.
1,25(OH)2D3 protected MSG obese rats against inflammatory injury in pancreatic islet, adipose, and liver tissues. (A) and (B) 1,25(OH)2D3 treatment reduced the infiltrating macrophages in pancreas islets and adipose tissue. (C) 1,25(OH)2D3 decreased the level of serum IL-6. (D) The mRNA expression of Il-6 in the liver was tested by RT-PCR as described in methods. (E) and (F) The expression of NF-κBp65, NF-κB, p-STAT3 and STAT3 in liver tissues was analyzed by Western blot. Data are presented as folds of the normal group in three independent experiments. (G) 1,25(OH)2D3 treatment increased expression of Tgf-β1 mRNA in liver. Data are expressed as mean±SEM, n=10. #P<0.05, ##P<0.01 vs normal group; *P<0.05, **P<0.01 vs. MSG group.
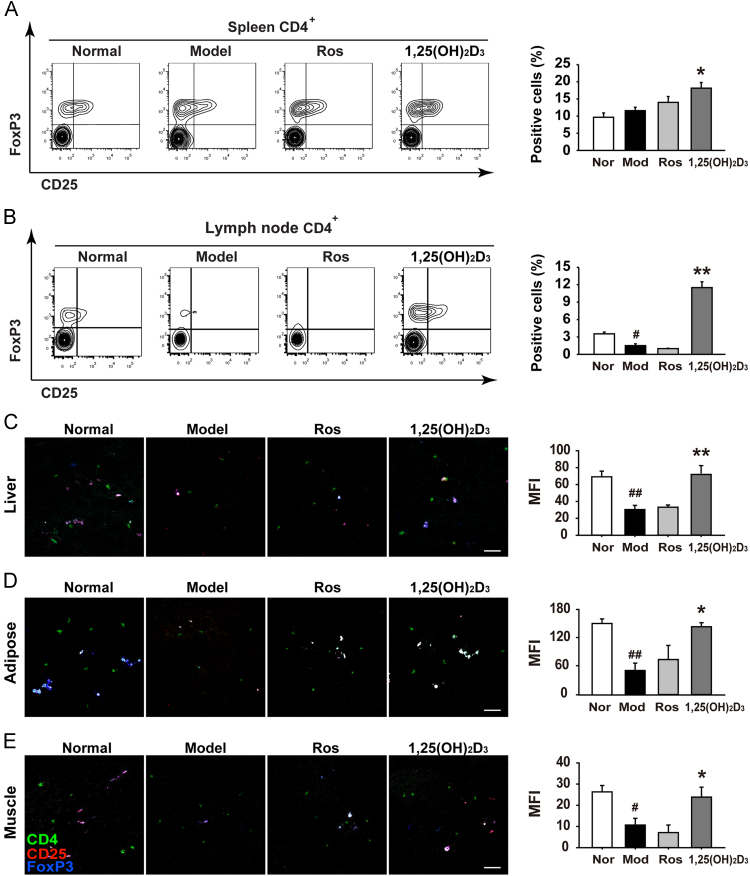
3.5. 1,25(OH)2D3 increased the number of Tregs in spleen and lymph node
Accumulated evidence suggests that regulatory T cells play a key role in controlling the inflammation status. To confirm our above findings that the decreased TGF-β1 in MSG rat livers was markedly restored by treatment of 1,25(OH)2D3, we performed further studies using flow cytometry to detect the number of Treg cells in secondary immune organs such as spleen and lymph node. We found that the number of CD4+CD25+FoxP3+Tregs did not change in spleen (Fig. 6A) but significantly decreased in the lymph node (Fig. 6B) of MSG rats. However, 1,25(OH)2D3-treated MSG rats revealed a significant increase of Tregs in both spleen and lymph node, compared to vehicle-treated MSG rats (Fig. 6A and B). Because nonresolving inflammation is known to drive insulin resistant in insulin-targeted tissues, we further examined whether the treatment of 1,25(OH)2D3 could impact the infiltration of Treg in these tissues. Using confocal microscopy, we observed that 1,25(OH)2D3 could dramatically enhance the frequency of CD4+CD25+FoxP3+ Tregs in primary insulin-targeted tissues such as liver (Fig. 6C), adipose (Fig. 6D) and muscle (Fig. 6E). These findings indicate that Tregs have a protective role in the pathogenesis of obesity as well as insulin resistant and that 1,25(OH)2D3 may have general therapeutic efficacy for obesity by increasing the secretion or infiltration of Tregs in immune- or insulin-dependent tissues.
Figure 6.
1,25(OH)2D3 enhances the tissue-infiltration of Treg cells in MSG obese rats. (A) and (B) 1, 25(OH)2D3 treatment increased the numbers of Treg in the spleen and lymph node. Treg was detected with flow cytometry as indicated. Moreover, 1,25(OH)2D3 treatment increased the numbers of Treg in the liver (C), adipose tissue (D), and skeleton muscle (E). Treg was detected with the confocal microscope. Data are representative of three independent experiments with identical results. #P<0.05, ##P<0.01 vs. normal group; *P<0.05, **P<0.01 vs. MSG group.
4. Discussion
It has become clear that obesity is one of the major risk factors for the onset of metabolic syndrome and type 2 diabetes mellitus. Recent studies have shown that obesity patients and animals appear to be in a chronic low-grade inflammation status characterized by increased plasma level of TNF-α, IL-6, IL-1, IL-8, MCP-1, and PAI-124, 25, 26. The accumulation of lipids and the expansion of the fat mass initiate the production of cytokines and chemokines. The proinflammatory factors inducing and maintaining the chronic inflammatory response are the critical inhibitors of insulin action on target organs, especially liver and adipose tissue. It is attributed to their architectural organization in which metabolic cells (hepatocytes or adipocytes) are in close proximity to immune cells (Kupffer cells or macrophages). For instance, co-cultured 3T3-L1 adipocytes and macrophages alter the expression of GLUT4 and insulin receptor substrate-127. Studies in MCP-1 receptor-deficient mice suggest that these mice developed obesity after high-fat feeding but had less macrophage infiltration and weaker insulin resistance than control mice28. Macrophages in adipose tissue are suspected to be the major source of inflammatory factors such as TNF-α and IL-6. Corresponding with the Th1/Th2 concept of T-cell activation, a concept of M1/M2 polarization has recently been advanced for macrophages. In adipose tissue macrophages normally are of M2-like phenotype, characterized by expression cytokines involved anti-inflammation including IL-10, TGF-β, IL-4 and IL-1329. Consumption of high-fat diet shifts cytokine expression of adipose tissue macrophages from M2- to M1-patterns by decreasing expression of IL-10 and increasing TNF-α and IL-630. Islets isolated from high-fat-fed mice produce much more inflammatory factors, including IL-6, IL-8, chemokine KC, granulocyte colony-stimulating factor, and MIP-1α31. In our studies, newborn rats were subjected to monosodium L-glutamate (MSG) s.c. injection which caused chemical ablation of the hypothalamic arcuate nucleus, and as a result induced progressive obesity, insulin resistance, and dyslipidemia. In MSG rats, several cellular lesions have been associated with insulin resistance, including decreased expression of insulin signal protein such as IRS-1 in liver and GLUT2 in pancreas, adipocyte hypertrophy and ECM deposition in pancreas islets. These lesions are concomitant with a chronic inflammatory status involving enhanced macrophage infiltration in adipose tissue and pancreas, an up-regulated IL-6 level and down-regulated TGF-β expression.
Numerous evidence has suggested that inflammation promotes energy expenditure in liver, adipose and skeletal muscle tissues to fight against energy surplus. Over-expression of NF-κB p65, the most crucial pro-inflammatory transcription factor, protects mice from high fat diet-induced obesity and insulin resistance through up-regulation of the expression of TNF-α and IL-6 in adipose tissue and macrophages32. In addition, inflammatory cytokines, such as TNF-α, IL-1 and IL-6, can also serve as anti-obesity signals by modifying both energy intake and energy expenditure33, 34, 35. However, both obesity and inflammation could be reciprocal causative factors, and breaking the obesity–inflammation cycle with anti-inflammation reagents seems to be a more effective strategy against obesity from metabolic syndrome and type 2 diabetes. Indeed, IL-1R antagonist and salicylates, a treatment that inhibits the activity of NF-κB, have been used in animal models to improve insulin sensitivity36. Agents such as thiazolidinedione (TZD) have been proven to have anti-inflammatory activities that partially contributes to their insulin-sensitizing effects37. In this work, we found that rosiglitazone exhibited anti-inflammatory properties by reducing macrophage infiltration in adipose tissue and pancreas. In recent years, 1,25(OH)2D3 has been shown not only to be important for insulin secretion from the pancreatic beta-cell but also for apoptosis of adipose cells, both of which are closely associated with 1,25(OH)2D3-triggered intracellular uptake of calcium38, 39, 40, 41. Furthermore, most of the known biological effects of 1,25(OH)2D3 are mediated through the vitamin D3 receptor (VDR), which can be found in a wide range of immune cells, including lymphocytes, dentritic cells and macrophages. Accumulating research suggest that the immune properties of 1,25(OH)2D3 include inhibition of DC differentiation and maturation, suppression of T cell activation, and enhancement of phagocytosis in monocytes or macrophages.
It has been shown that 1,25(OH)2D3 could reshape immune homeostasis involving a shift of production of T-cell cytokines from predominantly Th1 (IL-2, IFNγ) to Th2 (IL-4, IL-10). This shift probably is due to a direct interference of 1,25(OH)2D3 with the antigen-presenting dendritic cells. Indeed, 1,25(OH)2D3 induces a transformation of dendritic cells towards a tolerogenic phenotype42. In vitro treatment with 1,25(OH)2D3 inhibited IFNγ-induced phosphorylation of STAT1 and IL-12-triggered phosphorylation of TYK2, JAK2, STAT3, and STAT4 in association with a decrease in T cell proliferation43. In another study Gregori et al.44 found that the analog of 1,25(OH)2D3 inhibited IL-12 production, blocked pancreatic infiltration of Th1 cells, and enhanced CD4+CD25+ Tregs. In our studies, we found exogenous 1,25(OH)2D3 induced the infiltration of Treg cells not only in secondary immune organ such as spleen and lymph node, but also in primary insulin-targeted tissues such as liver, adipose and muscle, which provide straightforward in vivo evidence that 1,25(OH)2D3 can be used as an immunomodulator for the therapy of obesity via enhanced Tregs-mediated inhibition of the inflammatory response in both immune organs and insulin-dependent tissues.
5. Conclusions
In summary, our studies demonstrate that 1,25(OH)2D3 treatment can reduce body weight, improve glucose and lipid metabolism, decrease the incidence of obesity-induced insulin resistance and improve established insulin resistance in MSG-obese rats. Moreover, the chronic inflammatory status of insulin target tissues was ameliorated and was clearly linked with enhanced CD4+CD25+FoxP3+ regulatory T cells. These findings add substance to the inflammatory theory behind the pathogenesis of obesity and insulin resistance. Furthermore, our study indicates that 1,25(OH)2D3 has potential immunoregulatory efficacy on obesity and insulin resistance, both of which are becoming the leading risk factors for morbidity and mortality in metabolic syndrome and diabetic patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81773800 and 81471070); and the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-010 to Xiao-wei Zhang and 2016-I2M-1-012 to Wen Jin).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Alberti K.G., Zimmet P., Shaw J., IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Jin C., Flavell R.A. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol. 2013;132:287–294. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odegaard J.I., Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belenchia A.M., Tosh A.K., Hillman L.S., Peterson C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97:774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 7.Cediel G., Corvalán C., López de Romaña D., Mericq V., Uauy R. Prepubertal adiposity, vitamin D status, and insulin resistance. Pediatrics. 2016;138:e20160076. doi: 10.1542/peds.2016-0076. [DOI] [PubMed] [Google Scholar]
- 8.Liu X.J., Wang B.W., Zhang C., Xia M.Z., Chen Y.H., Hu C.Q. Vitamin D deficiency attenuates high-fat diet-induced hyperinsulinemia and hepatic lipid accumulation in male mice. Endocrinology. 2015;156:2103–2113. doi: 10.1210/en.2014-2037. [DOI] [PubMed] [Google Scholar]
- 9.Di Cesar D.J., Ploutz-Snyder R., Weinstock R.S., Moses A.M. Vitamin D deficiency is more common in type 2 than in type 1 diabetes. Diabetes Care. 2006;29:174. doi: 10.2337/diacare.29.1.174. [DOI] [PubMed] [Google Scholar]
- 10.Pittas A.G., Lau J., Hu F., Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor A.V., Wise P.H. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74:365–366. doi: 10.1136/pgmj.74.872.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 13.Zhen C., Feng X., Li Z., Wang Y., Li B., Li L. Suppression of murine experimental autoimmune encephalomyelitis development by 1,25-dihydroxyvitamin D3 with autophagy modulation. J Neuroimmunol. 2015;280:1–7. doi: 10.1016/j.jneuroim.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Gorman S., Judge M.A., Burchell J.T., Turner D.J., Hart P.H. 1,25-dihydroxyvitamin D3 enhances the ability of transferred CD4+ CD25+ cells to modulate T helper type 2-driven asthmatic responses. Immunology. 2010;130:181–192. doi: 10.1111/j.1365-2567.2009.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelajo C.F., Lopez-Benitez J.M., Miller L.C. Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev. 2010;9:507–510. doi: 10.1016/j.autrev.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Penna G., Amuchastegui S., Giarratana N., Daniel K.C., Vulcano M., Sozzani S. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 17.Urry Z., Xystrakis E., Richards D.F., McDonald J., Sattar Z., Cousins D.J. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Investig. 2009;119:387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahon B.D., Wittke A., Weaver V., Cantorna M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 19.Li P.P., Shan S., Chen Y.T., Ning Z.Q., Sun S.J., Liu Q. The PPARα/γ dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats. Br J Pharmacol. 2006;148:610–618. doi: 10.1038/sj.bjp.0706745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirata A.E., Andrade I.S., Vaskevicius P., Dolnikoff M.S. Monosodium glutamate (MSG)-obese rats develop glucose intolerance and insulin resistance to peripheral glucose uptake. Braz J Med Biol Res. 1997;30:671–674. doi: 10.1590/s0100-879x1997000500016. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 22.Sørensen H., Winzell M.S., Brand C.L., Fosgerau K., Gelling R.W., Nishimura E. Glucagon receptor knockout mice display increased insulin sensitivity and impaired β-cell function. Diabetes. 2006;55:3463–3469. doi: 10.2337/db06-0307. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 24.Ferrante A.W., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 26.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Stephens J.M., Lee J., Pilch P.F. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 28.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K.I., Kitazawa R. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Investig. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeyda M., Farmer D., Todoric J., Aszmann O., Speiser M., Györi G. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 30.Zeyda M., Stulnig T.M. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 32.Tang T.Y., Zhang J., Yin J., Staszkiewicz J., Gawronska-Kozak B., Jung D.Y. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H., Hirosumi J., Uysal K.T., Guler A.D., Hotamisligil G.S. Exclusive action of transmembrane TNFα in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–1511. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 34.García M.C., Wernstedt I., Berndtsson A., Enge M., Bell M., Hultgren O. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 35.Wallenius V., Wallenius K., Ahrén B., Rudling M., Carlsten H., Dickson S.L. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 36.Larsen C.M., Faulenbach M., Vaag A., Vølund A., Ehses J.A., Seifert B. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 37.Ohga S., Shikata K., Yozai K., Okada S., Ogawa D., Usui H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am J Physiol Ren Physiol. 2007;292:F1141–F1150. doi: 10.1152/ajprenal.00288.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sergeev I.N. Vitamin D–cellular Ca2+ link to obesity and diabetes. J Steroid Biochem Mol Biol. 2016;164:326–330. doi: 10.1016/j.jsbmb.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Sergeev I.N., Song Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res. 2014;58:1342–1348. doi: 10.1002/mnfr.201300503. [DOI] [PubMed] [Google Scholar]
- 40.Song Q., Sergeev I.N. Calcium and vitamin D in obesity. Nutr Res Rev. 2012;25:130–141. doi: 10.1017/S0954422412000029. [DOI] [PubMed] [Google Scholar]
- 41.Sergeev I.N., Rhoten W.B. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136:2852–2861. doi: 10.1210/endo.136.7.7789310. [DOI] [PubMed] [Google Scholar]
- 42.Mathieu C., Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 43.Muthian G., Raikwar H.P., Rajasingh J., Bright J.J. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 44.Gregori S., Giarratana N., Smiroldo S., Uskokovic M., Adorini L. A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]