Abstract
In order to clarify regions of production and to discriminate processing methods, quantitative and qualitative analyses for saccharides and terpenes in 35 batches of Alismatis Rhizoma were performed. Methodologies included HPLC—PDA, HPLC—VWD and UHPLC—MSn, combined with principal component analysis (PCA) and partial least squares regression techniques (PLSR). The inhibitory effects of triterpenes and Alismatis Rhizoma extracts on lipase activity were evaluated in vitro. PLSR analysis revealed significant positive correlations (R2 = 0.5795) between the contents of triterpenes 10, 14, 15, 18 and 22 and the inhibitory effects of Alismatis Rhizoma. The present study establishes an effective method for simultaneous determination of multiple components, and identifies key bioactive triterpenes. These results can be used for systematic and novel analytical strategies for the quality control of Alismatis Rhizoma production.
KEY WORDS: Alismatis Rhizoma, Qualitative analysis, Saccharide, Terpene, Lipase
Graphical abstract
With the application of 1H-NMR-based metabolomics, a new processing method was proposed for medicinal slices of Cistanche deserticola. Different parts of C. deserticola were also differentiated, indicating that 1H-NMR-based metabolomics was a versatile analytical platform.
1. Introduction
Alismatis Rhizoma is the tuber of Alisma orientale (Sam.) Juz. (Alismataceae), an aquatic plant, which is widely distributed in China, Korea, Japan, North America, and Europe1. In China, Alismatis Rhizoma is a well-known traditional Chinese medicine and functional food (Zexie), such as Jian Zexie (Alismatis Rhizoma from Fujian Province, China) and Chuan Zexie (Alismatis Rhizoma from the Sichuan Province, China)—based on the regions of origin. On the basis of processing methods, the Zexie can be classified as either Sheng Zexie (air-dried Alismatis Rhizoma) or Yan Zexie (stir-baked Alismatis Rhizoma with salt solution), which have been used as a folk diuretic and hypolipidemic agents in China, Korea and Japan2. Pharmacological research revealed the diuretic, hypopietic, hypolipaemic, and anti-atherosclerotic activities of the extracts of Alismatis Rhizoma1., 3., 4.. Previous chemical investigations of Alismatis Rhizoma reported its primary constituents to be polysaccharides, protostane-type triterpenes together with other trace constituents guaiane-type sesquiterpenes, and kaurane-type diterpenes5., 6., 7., 8., 9., 10., 11.. Pharmacological research has indicated that the protostane-type triterpenes, such as alisols A, F and H possess the hypolipemic activity concerned with the inhibition of lipase, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, fatty acid synthase, and acyl-CoA-cholesterol acyltransferase12., 13., 14., 15., 16., 17..
Current standards for medical material quality control require an understanding of the bioactivities of natural products and their chemical components18., 19., 20.. For Alismatis Rhizoma, only the triterpenes have been identified with few bioactivities evaluation21., 22., 23., 24.. In the present study, multiple technologies including HPLC, UHPLC—MSn, chemical derivatization, biological evaluation and chemomerics analysis have been applied to investigate the quality control methods for analyzing the materials form different origins and processing methods. Finally, the qualitative analysis of 30 protostane triterpenes and quantitative analysis of 3 saccharides and 11 triterpenes have been conducted for 35 batches of Alismatis Rhizoma. Principal component analysis (PCA) has been applied for the discrimination of Alismatis Rhizoma from different origins and processing methods. The inhibitory effects against lipase of 11 triterpene standards together with 35 batches of Alismatis Rhizoma were also evaluated. The correlation between bioactivity and the content of triterpenes has been analyzed by partial least squares regression techniques PLSR, which identified key, potent triterpenes for quality control of Alismatis Rhizoma. The aim of the present study was to establish qualitative chemical analysis methods for Alismatis Rhizoma, and to suggest the key bioactive components which will improve qualitative control methodology for this important medicine and food.
2. Materials and methods
2.1. Materials and reagents
Thirty-five batches of Alismatis Rhizoma were collected from Sichuan Province (No. CY1−CY7, CS1−CS11) and Fujian provinces (No. JY1−JY6, JS1−JS11) in China. These batches included air-dried crude drug (Sheng Zexie, No. CS1−CS11, JS1−JS11) and stir-baked Alismatis Rhizoma with salt solution (Yan Zexie, No. CY1−CY7, JY1−JY6).
Twenty-six protostane triterpenes and seven sesquiterpenes were obtained from Alismatis Rhizoma in our laboratory and identified by spectroscopic data with the purity not less than 98%. d-Galactose (Gal), d-glucose anhydrous (Glu), d-lyxose (Lyx) and arabinose (Ara), and trifluoroacetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (SCRC, Shanghai, China). Phosphoric acid, hydrochloric acid, ammonium acetate and sodium hydroxide were obtained from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). Acetonitrile (HPLC) and methanol (HPLC) and lipase (from Candida rugosa) were obtained from Sigma—Aldrich Co. LLC. (China). Ethylenediaminetetraacetic acid (EDTA), 1-phenyl-3-methyl-5-pyrazolone (PMP), 3-(N-morpholino)propanesulfonic acid (MOPS) and butyl 4-nitrobenzoate were purchased from J&K Scientific Ltd. (China).
2.2. Quantitative analysis of monosaccharides in polysaccharides with HPLC—VWD
2.2.1. Reference solutions and sample solutions
Standard stock solutions with concentrations of 1 mg/mL were prepared by dissolving accurately weighed reference standards arabinose, glucose, galactose, lyxose (internal standard) in water, respectively. Then 0.25 mL solution and 0.30 mL of 0.15 mol/L sodium hydroxide, and 0.50 mL of 0.1 mol/L methanolic solution of 1-phenyl-3-methyl-5-pyrazolone (PMP) was stirred at 70 °C for 30 min. Then 0.30 mL of 0.15 mol/L hydrochloric acid was added. Finally, the solution was transferred to a 2-mL of volumetric flask, diluted to volume and passed through a nylon filter having a 0.45 μm porosity25., 26., 27..
The mixed standards solution for linearity experiments was prepared by 1 mg lyxose, 15 mg glucose, 5 mg galactose and 4.5 mg arabinose dissolved in 1 mL water, which was prepared to be PMP derivative solution as mentioned previously.
To prepare sample solutions, 2 g of sample powder (capable of passing a 4-mm sieve) was accurately weighed and extracted with 50 mL of water under reflux for three times (2 h for each time). After the evaporation of water in vacuum, the residue was dissolved in 5 mL water and 75 mL methanol. After standing for 12 h at 4 °C, the solution was centrifuged (4000 rpm × 30 min, BIO-DL Mini-7K, Shanghai, China), and the precipitate was evaporated to dryness and dissolved in 10 mL of hot water. The solution was centrifuged (4000 rpm × 10 min), and the supernatant was transferred to a pressure vial respectively, added with 0.25 mL 4 mol/L trifluoroacetic acid, and hydrolyzed at 110 °C for 4 h. Then, 0.5 mL of methanol was added for four times and evaporated to remove the trifluoroacetic acid. Then, the residue was dissolved in 0.5 mL water, and 0.25 mL aqueous solution was prepared to be PMP derivative solution as described previously.
2.2.2. Quantitative analysis of monosaccharides in polysaccharides using HPLC—VWD
The quantitative analysis of monosaccharides in polysaccharides was performed using an Agilent 1260 HPLC system with a VWD detector and Waters XBridge C18 column (150 mm × 4.6 mm, 2.5 μm). The column temperature was maintained at 30 °C. The mobile phase consisted of acetonitrile (A) and 10 mmol/L ammonium acetate aqueous solution (B) with the gradient program as 0/21/60 min, 16%/16%/24% (A) as well as the flow rate of 0.45 mL/min. The injection volume was 10 μL and the detection wavelength was 250 nm. On the basis of reference standards, the retention time was used for the identification of monosaccharides in samples. The contents of each component were determined by an external standard method.
2.3. Qualitative analysis of sesquiterpenes and triterpenes with HPLC—PDA
2.3.1. Reference and sample solutions
Standard stock solutions with known concentrations were prepared by dissolving accurately weighed reference standards (sesquiterpenes and triterpenes for qualitative and quantitative analysis) in methanol. Reference standard solutions were prepared by dilution of stock solutions.
For preparation of sample solutions, 1 g of sample powder (capable to passing a 4-mm sieve) was accurately weighed, extracted with 20 mL acetonitrile ultrasonically (70 Hz) for 30 min. After the evaporation of acetonitrile, the residue was dissolved with methanol and diluted to the volume of 25-mL using volumetric flask.
2.3.2. HPLC—PDA analysis of sesquiterpenes and triterpenes
Qualitative analysis of sesquiterpenes and triterpenes was performed on a Waters e2695 Separation Module equipped with a Waters 2998 PDA detector, and Empower Chemistation. A Merck Lichrospher RP-18 endcapped column (250 mm × 4.6 mm, 5 μm) was used for the separation with the flow rate of 0.8 mL/min. The column temperature was maintained at 30 °C. The optimized mobile phase consisted of acetonitrile (A) and aqueous buffer solution of 0.025% phosphate (v/v) with the gradient program as 0/5/12/22/42/52/62/72/75 min, 38%/38%/50%/55%/55%/77%/77%/80%/80% (A). The injection volume was 10 μL. On basis of the structures of reference standards, the detection wavelengths were set as 210, 254, 280 nm. On the basis of reference standards, the retention time and UV spectra were applied to identify the components in samples.
2.4. Quantitative analysis of triterpenes
2.4.1. Reference standard solutions and sample solutions
Standard stock solutions of 11 triterpenes were obtained with 1 mg standard triterpenes dissolved in 1 mL methanol, which was diluted to 100 ng/mL. Working reference solutions were prepared by dilution of stocks.
To prepare sample solutions, 10 mg of sample powder (capable to passing a 4-mm sieve) were accurately weighed, extracted with 10 mL acetonitrile untrosonicly (70 Hz) for 20 min. The solutions were replenished weight, passed through a nylon filter having a 0.45 μm porosity, and analyzed as described below.
2.4.2. Quantitative analysis of triterpenes with LC—ESI—MSn
Quantitative analysis was performed on a tandem mass spectrometer which was connected to Shimadzu Prominenece UHPLC system. Separation experiment was performed using Shimadzu Prominenece UHPLC with Waters XBridge C18 column (150 mm × 4.6 mm, 2.5 μm) and the optimized chromatographic conditions. Mobile phase: acetonitrile (A) and aqueous buffer solution of 0.1% methanoic acid (v/v) with the gradient program as 0/10/12/20/25/31 min, 50%/50%/65%/65%/94%/94% (A). The flow rate was 0.5 mL/min and the injection volume was 10 μL. The column temperature was maintained at 30 °C. Quantitative mass spectrometry was performed using a AB Sciex Qtrap® 4500 tandem mass spectrometer (Foster City, CA, USA) with an electrospray ionization source (ESI) in the positive ion mode. The dynamic multiple reaction monitoring (DMRM) method was used for the multiple components quantification28. The optimized conditions were as follows: ion spray voltage, 4.5 kV (Positive mode); gas source (1), 30 psi; gas source (2), 40 psi; turbo temperature, 500 °C; entrance potential (EP), 10 V; and collision cell exit potential (CXP), 13 V; the curtain gas pressure at 20 mPa. And the information for DMRM parameters together with the related optimized declustering potential (DP), and collision energy (CE) for the different analytes were shown in the quantitative analysis results section (Table 1).
Table 1.
Retention time, λmax of UV, and MS characteristics of reference standards.
| No. | Compd. | UV λmax (nm) | MW | MS1 | Base peak (MS1) | Retention time (min) |
|---|---|---|---|---|---|---|
| 1 | Orientalol B | 200 | 254 | 272.1 | [M+NH4]+ | 7.080 |
| 2 | Alismoxide | 200 | 238 | 221.0 | [M+H—H2O]+ | 11.206 |
| 3 | 11-Oxo-13-nor-alisnol | 236.6 | 220 | 221.0 | [M+H]+ | 14.680 |
| 4 | 17-Epoalisol A | 200; 286.4 | 506 | 507.3 | [M+H]+ | 18.247 |
| 5 | Guaianediol | 200 | 238 | 221.3 | [M+H—H2O]+ | 19.012 |
| 6 | Alisol P | 200 | 520 | 538.8 | [M+NH4]+ | 23.816 |
| 7 | Alisol F | 200 | 488 | 471.5 | [M+H—H2O]+ | 27.113 |
| 8 | 16β-Hydroxy alisol A | 200 | 506 | 507.8 | [M+H]+ | 31.827 |
| 9 | Alismaketone B 23-acetate | 200 | 530 | 553.5 | [M+Na]+ | 32.364 |
| 10 | Alisol A | 200 | 490 | 473.4 | [M+H—H2O]+ | 34.172 |
| 11 | Alisol E 23-acetate | 200 | 532 | 515.7 | [M+H—H2O]+ | 37.66 |
| 12 | 25-Anhydroalisol F | 200 | 470 | 453.3 | [M+H—H2O]+ | 39.198 |
| 13 | Alismol | 200 | 220 | 221.0 | [M+H]+ | 49.952 |
| 14 | 25-Anhydroalisol A | 200 | 472 | 490.5 | [M+NH4]+ | 54.054 |
| 15 | Alisol B | 200 | 472 | 473.5 | [M+H]+ | 56.043 |
| 16 | 11-Deoxyalisol A | 200 | 474 | 475.3 | [M+H]+ | 58.525 |
| 17 | Alismanol F | 200 | 560 | 578.0 | [M+NH4]+ | 61.569 |
| 18 | Alisol B 23-acetate | 200 | 514 | 532.5 | [M+NH4]+ | 64.183 |
| 19 | Alisol B monoacetate | 200 | 514 | 532.4 | [M+NH4]+ | 68.969 |
| 20 | 11-Deoxy-25-anhydroalisol E | 200 | 456 | 457.7 | [M+H]+ | 71.611 |
| 21 | Alismanol O | 239 | 502 | 503.5 | [M+H]+ | 12.429 |
| 22 | 16-Oxo-alisol A | 247.3 | 504 | 505.7 | [M+H]+ | 13.305 |
| 23 | Alismanol M | 248.5 | 504 | 522.7 | [M+NH4]+ | 15.203 |
| 24 | Ligucyperonol | 250.8 | 234 | 235.5 | [M+H]+ | 20.540 |
| 25 | Alismanol M | 200;259.1 | 488 | 506.3 | [M+NH4]+ | 28.477 |
| 26 | 24-Deacetyl alisl O | 249.6 | 470 | 471.1 | [M+H]+ | 58.030 |
| 27 | Alismanol E | 254.4 | 468 | 469.7 | [M+H]+ | 58.530 |
| 28 | Alisol O | 249.6 | 512 | 513.0 | [M+H]+ | 62.059 |
| 29 | Alismanol D | 286.4 | 500 | 501.4 | [M+H]+ | 12.937 |
| 30 | 1β-Hydroxy-β-cyperone | 303.1 | 234 | 235.3 | [M+H]+ | 19.905 |
| 31 | 16-Oxo-11-anhydroalisol A | 288.8 | 486 | 487.6 | [M+H]+ | 20.911 |
| 32 | Alismanol B | 285.3 | 468 | 469.5 | [M+H]+ | 28.276 |
| 33 | Alismanol G | 226;300.7 | 368 | 369.6 | [M+H]+ | 30.122 |
2.5. Inhibitory activity of Alismatis Rhizoma extracts on lipase activity
100 mg of 35 batches of Alismatis Rhizoma were extracted with 100 mL acetonitrile untrosonicly for 20 min. The CH3CN extracts were dissolved with DMSO to get a working concentration corresponding to 0.5 g of sample per litter. Eleven triterpene standards were dissolved in DMSO to obtain different working concentration for the bioassay of lipase activity29. Orlistat was used as the positive control to assess the bioassay method.
The bioassay solution contained 6 μL Lipase solution (1 U Lipase, 10 mmol/L 3-morpholinopropanesulfonic acid, 1 mmol/L EDTA) and 154 μL Tris buffer (100 mmol/L Tris–HCl, 5 mmol/L CaCl2, pH 7.0). After the addition of 20 μL different concentrations of samples, the bioassay solution was incubated for 15 min at 37 °C. Then, 20 μL substrate (4-nitrophenyl butyrate) solution was used to start the enzymatic reaction, which was incubated for an additional 15 min at 37 °C. The production of p-nitrophenol was then determined using microplate reader at 405 nm.
2.6. PCA of triterpenes and monosaccharides on the basis of quantitative determination
PCA was conducted on the basis of content determination for 11 triterpenes and 4 monosaccharides using SIMCA-P+12.0 software25., 30..
2.7. PLSR analysis of triterpenes contents and lipase activity
On the basis of content determination of triterpenes and bioassay of CH3CN extracts of 35 batches Alismatis Rhizoma, the PLSR was carried out using Matlab 7.0.
3. Results and discussion
3.1. Qualitative and quantitative analysis of monosaccharide components in polysaccharides with HPLC—VWD
3.1.1. Qualitative analysis of monosaccharide components
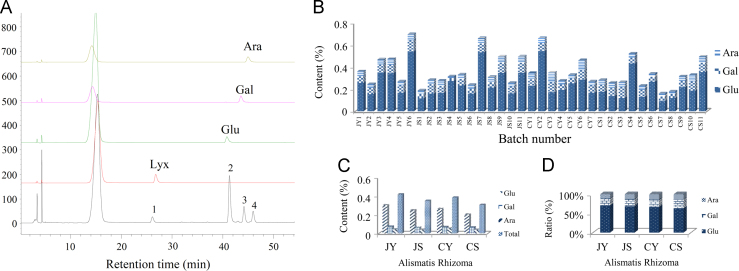
After the PMP (1-phenyl-3-methyl-5-pyrazolone) derivatives were prepared for monosaccharide components in samples and reference standards, HPLC—VWD was applied to analyze the components. As shown in the HPLC chromatograms of Alismatis Rhizoma sample and the reference standards (Fig. 1A), visible peaks 2, 3, 4 in chromatogram of Alismatis Rhizoma were identified as glucose, galactose, and arabinose, respectively (peak 1 was lyxose used as internal standard). The other components below LOD have not been identified.
Figure 1.
(A) Qualitative analysis of monosaccharides components in polysaccharides of Alismatis Rhizoma. (B) Contents of Glu, Gal and Ara for polysaccharides in 35 batches. (C) Content of each monosaccharide and the total saccharides constituting polysaccharides in four different types Alismatis Rhizoma: JY, JS, CY, CS. (D) Average ratio of each monosaccharide in total content of saccharides on the basis of four different types Alismatis Rhizoma: JY, JS, CY, CS.
3.1.2. Method validation for the quantitative analysis of monosaccharides components
Precision for the determination of glucose, galactose, and arabinose was assessed with six samples. The individual RSDs for the contents of three mononsaccharides were in the range from 0.58% to 2.76%; the RSD for the total content of monosaccharides was 0.35% (Supplementary Information Table S1).
Intermediate precision for the determination of individual monosaccharides and total content was performed with the studies of three different days and three different analysts. The RSDs of intra-day variation were from 0.19% to 3.14% for the individual monosaccharide, and 0.04–0.83% for total content (Supplementary Information Table S2).
The linearity ranges for three monosaccharides were determined as glucose (0.09375−1.875 mg/mL), galactose (0.03125—0.625 mg/mL), arabinose (0.028125−0.5625 mg/mL) with correlations 0.9999−1.0000 (Supplementary Information Table S3).
To determine the extraction recoveries of monosaccharides and the preparation of PMP derivatives, three different levels of reference standards were spiked before hydrolysis. The recoveries of different levels of three monosaccharides ranged from 98.9% to 101.15% (Supplementary Information Table S4).
With regard to sample specificity, the HPLC retention times of three monosaccharides in samples were consistent with those of the reference standards (Fig. 1A), with no interfering peaks being observed in the blank sample.
Stability of sample solutions was investigated through determination of three components after room temperature storage for 0, 2, 4, 6, 8, 10, 12, 24 h. The RSDs for three monosaccharides were evaluated as glucose (24 h, 1.24%), galactose (24 h, 3.09%), arabinose (24 h, 2.05%), which suggested stability of monosaccharides within 24 h (Supplementary Information Table S5).
3.1.3. Contents of polysaccharides in 35 batches Alismatis Rhizoma
35 batches of Alismatis Rhizoma were collected from Sichuan Province (No. CY1−CY7, CS1−CS11) and Fujian provinces (No. JY1−JY6, JS1−JS11) in China. These batches included air-dried crude drug (Sheng Zexie, No. CS1−CS11, JS1−JS11) and stir-baked Alismatis Rhizoma with salt solution (Yan Zexie, No. CY1−CY7, JY1−JY6). On the basis of qualitative analysis, three monosaccharides Glu, Gal and Ara were determined to constitute polysaccharides of Alismatis Rhizoma. Therefore, the monosaccharide content of polysaccharides were determined for 35 batches of Alismatis Rhizoma (Fig. 1B). The average contents of glucose, galactose and arabinose for 35 batches were 0.264%, 0.066%, 0.044% with the average total content of 0.356% (Supplementary Information Table S6). The average ratios of three monosaccharides constituting polysaccharides in 35 batches Alismatis Rhizoma were analyzed (Supplementary Information Fig. S1). On the basis of 35 batches of samples, the average ratios of individual components in total saccharides were established as glucose (67.0%), galactose (19.9%), arabinose (13.1%). Thus, glucose was the major monosaccharide for the polysaccharides from Alismatis Rhizoma.
As shown in Fig. 1C and D, no significant difference was observed for Alismatis Rhizoma based on different producing regions and processing methods. However some tiny distinctions could be found in the Fig. 1C and D.
Total content of saccharides in Fujian province was higher than Sichuan province and stir baked crude drugs with salt solution were found to contain more saccharides than air-dried crude drugs. These differences corresponded to the difference in the content of glucose, the major component. Additionally, the ratio of glucose in total content of saccharides was higher in Alismatis Rhizoma from Fujian province than those from Sichuan province (Fig. 1D).
3.2. Qualitative analysis of terpenes in Alismatis Rhizoma with HPLC—PDA
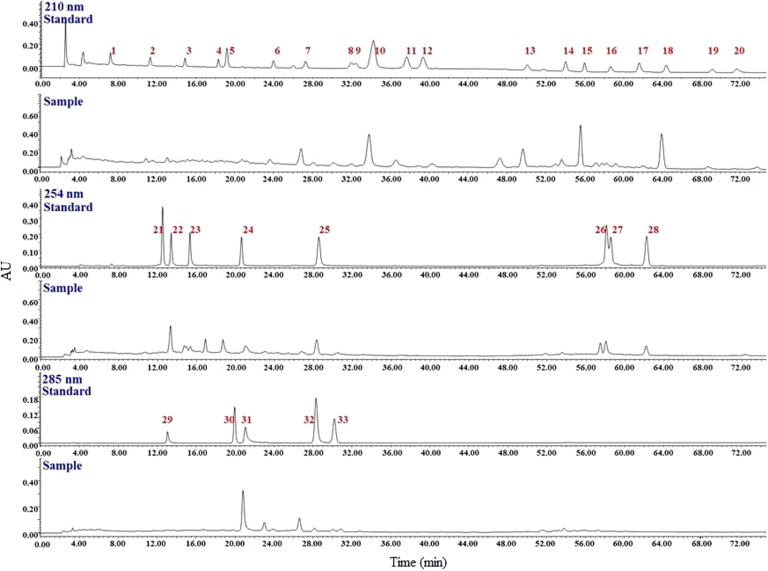
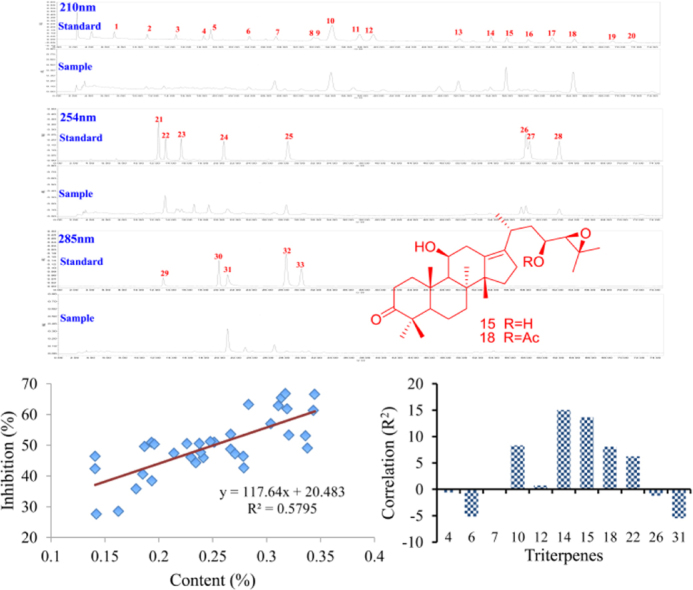
The CH3CN extract of Alismatis Rhizoma was analyzed by use of HPLC—PDA with an optimized chromatographic method, resulting in good separation for most of the main peaks. Chromatograms of terpenes in Alismatis Rhizoma and reference standards are shown in Fig. 2. Thirty-three terpenes including 7 sesquiterpenes and 26 triterpenes were identified for Alismatis Rhizoma on the basis of retention times, UV spectra and MS characteristic (Table 1).
Figure 2.
Chromatograms of the CH3CN extract of Alismatis Rhizoma and 33 reference standard terpenes using HPLC—PDA with three detected UV wavelengths.
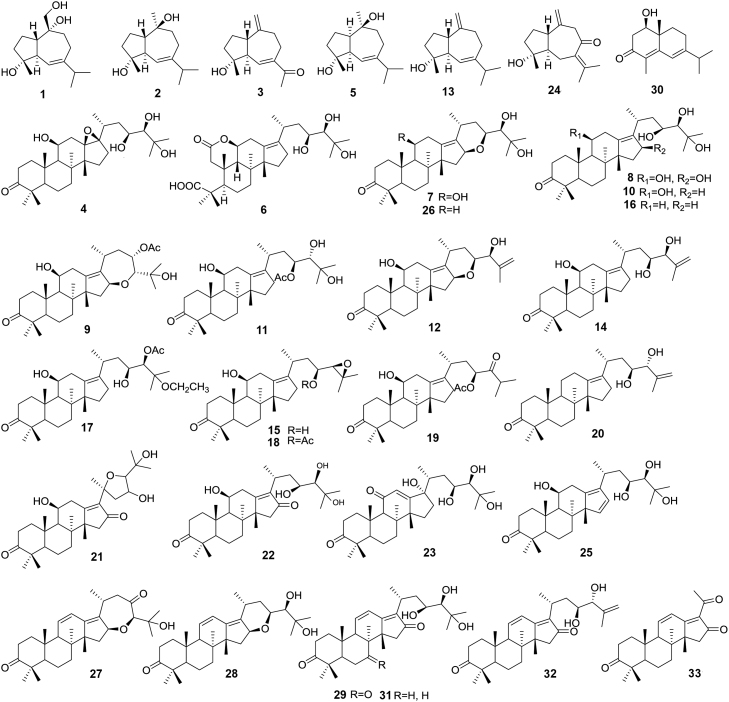
As shown in Fig. 3, chromophores for 33 terpenes (which gave various UV spectra in HPLC—PDA) were identified as either unconjugated olefinic bond (such as 1−20), α,β-unsaturated ketone (21−24), conjugated diene (25, 27, 28), or conjugated diene ketone (29−32). Thus, three UV wavelengths 210, 254, and 280 nm were monitored to detect 33 terpenes.
Figure 3.
Triterpenes and sesquiterpenes for the qualitative analysis of Alismatis Rhizoma.
Under the optimized mobile phase, most of the 33 terpenes were well separated in 72 min, except for peaks 8 and 9, 26 and 27 (Fig. 2). Analysis of the structures and retention times of 33 terpenes indicated that sesquiterpenes have shorter retention times than those of triterpenes. The quantity of hydroxyl groups in triterpenes affected the polarity significantly, which resulted in the shorter retention time corresponding to more hydroxyl groups for triterpenes.
The qualitative analysis experiments were performed in 35 batches Alismatis Rhizoma. Results suggested a high degree of similarity for the chromatograms with 33 terpenes detected. No significant difference was observed for the discrimination of Alismatis Rhizoma harvested from various producing regions or produced by different processing methods. Thus, the chemical similarities between different Alismatis Rhizoma samples prevented the discriminatioin of different crude drugs from Alismatis Rhizoma.
3.3. Quantitative analysis of triterpenes in Alismatis Rhizoma with UHPLC—MSn
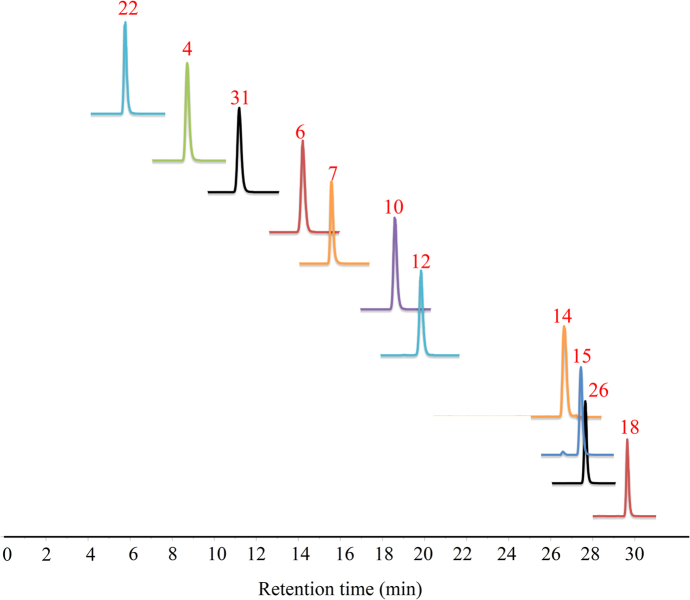
On the basis of the qualitative analysis, protostane triterpenes have been chosen to perform quantitative experiment using UHPLC—MSn. Accession of the separation, specificity of ion peaks, and stability of triterpenes resulted in the content determination of 11 protostane triterpenes for 35 batches Alismatis Rhizoma using UHPLC—MSn with reference standards. Rather than using a conventional mass spectrometry (MS) method with multiple reaction monitoring (MRM), the present work applied dynamic multiple reaction monitoring (DMRM) to determine the contents of 11 triterpenes in order to get a rapid, sensitive MS method. The DMRM transitions and the related optimized declustering potential (DP), and collision energy (CE) for the different analytes are shown in Table 2. The DMRM results from analysis of 11 triterpenes are displayed in Fig. 4.
Table 2.
UHPLC—MSn method for the content determination of 11 triterpenes.
| No. | Name | MW | MS1 | MS2 | Base peak (MS1) | Ret time (min) | Delta ret time (min) | DP | CE |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 17-Epoalisol A | 506 | 507.3 | 489.5 | [M+H]+ | 8.45 | 1.5 | 70 | 11 |
| 6 | Alisol P | 520 | 538.8 | 431.4 | [M+NH4]+ | 13.65 | 1.5 | 70 | 23 |
| 7 | Alisol F | 488 | 471.5 | 339.2 | [M+H—H2O]+ | 15.34 | 1.5 | 110 | 20 |
| 10 | Alisol A | 490 | 473.4 | 383.2 | [M+H—H2O]+ | 18.32 | 1.5 | 110 | 18 |
| 12 | 25-Anhydroalisol F | 470 | 453.3 | 339.4 | [M+H—H2O]+ | 19.51 | 1.5 | 90 | 16 |
| 14 | 25-Anhydroalisol A | 472 | 490.5 | 473.2 | [M+NH4]+ | 26.46 | 1.5 | 60 | 14 |
| 15 | Alisol B | 472 | 473.5 | 365.1 | [M+H]+ | 27.35 | 1.5 | 90 | 12 |
| 18 | Alisol B 23-acetate | 514 | 532.5 | 437.4 | [M+NH4]+ | 29.65 | 1.5 | 70 | 22 |
| 22 | 16-Oxo-alisol A | 504 | 505.7 | 415.3 | [M+H]+ | 5.67 | 1.5 | 86 | 29 |
| 26 | 24-Deacetyl alisl O | 470 | 471.1 | 339.1 | [M+H]+ | 27.46 | 1.5 | 110 | 18 |
| 31 | 16-Oxo-11-anhydroalisol A | 486 | 487.6 | 397.4 | [M+H]+ | 10.77 | 1.5 | 80 | 26 |
Figure 4.
UHPLC-MSn results of 11 reference standards with DMRM.
3.3.1. Method validation of triterpenes: precision, linearity, accuracy, specificity, stability and ruggedness
Precision was assessed on the determination of 11 triterpenes in samples as triplicates. The RSDs of inner-day variation ranged from 1.0% to 3.2% for individual triterpenes and 0.82% for total content (Supplementary Information Table S7). Intermediate precision was performed for the content determination of 11 triterpenes in the quantitative analysis experiments with three days and different analysts and triplicates. The average RSDs of the content of each triterpene were in the range of 0.29–3.75% and 0.78–0.99% for total content (Supplementary Information Table S8). Accuracy was tested with percentage of recovery for 11 triterpene standards. Three different concentrations (low, medium and high) of reference triterpenes were spiked to sample. Recovery rates of 11 triterpene standards ranged from 97.90% to 101.71% with the RSDs in the range of 0.15–3.04% (Supplementary Information Table S9). Stability of sample solution was investigated with storage at room temperature for 0, 2, 4, 6, 8, 12, 24 h. And the content determination results suggested that 11 triterpenes solutions were stable within 24 h (RSDs 0.66–3.60%, Supplementary Information Table S10). Standard curves with 11 standards displayed good linearity with correlation coefficients in the range from 0.9967 to 0.9999. The linearity ranges for 11 standards are listed in Supplementary Information Table S11 along with the LOD and LOQ values. Retention times and ion pairs of 11 triterpenes in sample solutions corresponded well with comparable values from the respective standard triterpene. Good specificity was obtained by use of UHPLC—MSn as the quantitative experiment with DMRM method.
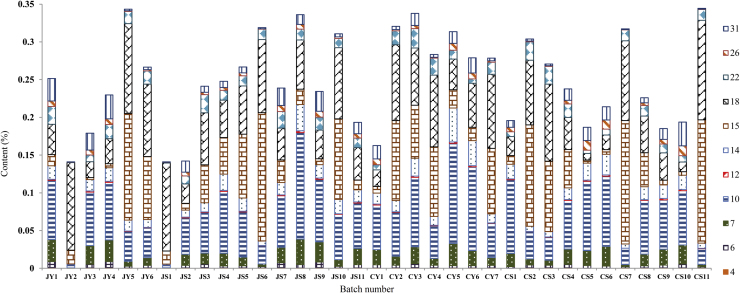
3.3.2. Contents of 11 triterpenes in 35 batches of Alismatis Rhizoma
Contents of 11 triterpenes in 35 batches of Alismatis Rhizoma were determined (Fig. 5 and Supplementary Information Table S12). On the basis of content of individual triterpenes or total content of triterpenes, no differences were detected from the quantitative analysis experiment between samples of Alismatis Rhizoma from different producing regions or different processing methods. The total contents of main triterpenes in Alismatis Rhizoma ranged from 0.14%−0.34% (Supplementary Information Fig. S2). Triterpenes 10, 15 and 18 were the major components with the ratios of 25%, 22% and 26% in total triterpenes (Supplementary Information Fig. S3). Additionally, the ratio analysis of individual triterpenes in 35 batches samples also confirmed the 10 (alisol A), 15 (alisol B) and 18 (alisol B 23-acetate) as the major triterpenes, except that four samples (JY2, JS1, CS7, CS11) contained low level of 10, and seven samples (JY1, JY3, JS9, CS5, CS6, CS9, CS10) had low content of 15 (Supplementary Information Fig. S4). And, all of the samples had high content about triterpene 18 with the highest content of 82% (Supplementary Information Fig. S4).
Figure 5.
Content of main triterpenes in 35 batches of Alismatis Rhizoma.
Differences in processing methods of Alismatis Rhizoma may alter the triterpenes content of these preparations, although no such differences were found between sample sources from various producing regions. For example, the content of triterpenes may differ between air dried Alismatis Rhizoma and stir-baked Alismatis Rhizoma with salt solution. Structure analysis indicated that triterpenes 12 and 31 were the dehydrated derivatives of 7 and 22, respectively. And triterpene 15 was a deacetylated analogue of 18. So, the relative ratios about 12/7, 31/22 and 15/18 were analyzed between JY+CY samples and JS+CS samples, which were clarified by the processing methods (Supplementary Information Fig. S5). Different samples from both processing methods had similar relative ratio about 12/7. The relative ratio about 31/22 suggests that stir baking with salt solution may promote the dehydration of 22 to get triterpene 31. Furthermore, the acetylation of triterpenes was not affected by salt processing. Therefore, the air-dried or salt-baked processing methods displayed similar effects on the structures of triterpene contents.
3.4. Inhibitory effects of 11 triterpenes and 35 batches of Alismatis Rhizoma on lipase activity
Alismatis Rhizoma is well known for its hypolipidemic effects, and protostane triterpenes (major components) have been reported to mimic such hypolipidemic effects. In our previous study, the total triterpenes displayed association with the triglyceride level in mouse. In the present work, the hypolipidemic effects of 11 triterpene standards studied in vitro (Table 3). Most of the triterpenes displayed moderate inhibitory effect on lipase with the IC50 values<200 μmol/L, except for compounds 4, 6 and 22. Noteworthy were the inhibitory effects of alisol B (15) and alsiol B 23-acetate (18) on lipase activity with the IC50 values of 9.37 and 7.83 μmol/L, respectively. This bioassay indicated that protostane triterpenes from Alismatis Rhizoma may function as the active hypolipidemic agents in these preparations.
Table 3.
Inhibitory effects on Lipase of triterpene standardsa.
| Compd. | IC50 (μmol/L) | SD |
|---|---|---|
| 4 | >200 | – |
| 6 | >200 | – |
| 7 | 64.17 | 3.32 |
| 10 | 21.60 | 0.72 |
| 12 | 21.40 | 1.04 |
| 14 | 11.37 | 0.21 |
| 15 | 9.37 | 0.21 |
| 18 | 7.83 | 0.15 |
| 22 | >200 | – |
| 26 | 50 | 0.33 |
| 31 | 164.03 | 10.28 |
| Orlistatb | 0.33 | 0.03 |
All of the bioassay experiments were conducted in triplicates.
The positive control.
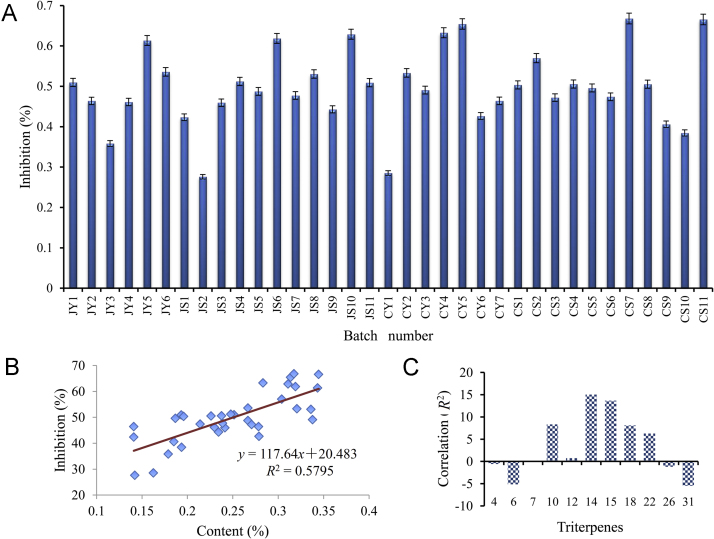
In the present work, the content of prostane triterpenes was determined on CH3CN extract of Alismatis Rhizoma. Therefore, the lipase inhibitory effects of CH3CN extracts of 35 batches of Alismatis Rhizoma were evaluated in vitro with the concentrations corresponding to the equal quantity of Alismatis Rhizoma. All of the samples displayed inhibitory effects on lipase at the concentration of 0.5 g/L of the Alismatis Rhizoma material with the inhibition in the range of 27%–67% (Fig. 6A). In consideration that triterpenes were the main components of CH3CN extracts, the biological effect of extracts corresponded to those of triterpenes. And the correlation between biological effects and contents of triterpenes were investigated subsequently.
Figure 6.
(A) Inhibitory effects on lipase of 35 batches of Alismatis Rhizoma. (B) Linearity correlation about the total content of triterpenes and inhibition on lipase of Alismatis Rhizoma. (C) PLSR about the content of each triterpene and the inhibitory effects on lipase.
3.5. Chemometric analysis of the contents of triterpenes, saccharides, and biological effect
3.5.1. PCA of triterpenes and polysaccharides in 35 samples
PCA was efficient in summarizing multivariate variation into a few principle components remaining maximum possible variability. Content of 11 triterpenes was simultaneously calculated and score plot of first two principle components is shown in Supplementary Information Fig. S6A. The content of 11 triterpenes and 3 monosaccharide components in polysaccharides was simultaneously calculated and score plot of first two principle components is shown in Supplementary Information Fig. S6B. As discussed previously, 35 batches of Alismatis Rhizoma were classified into four groups as JY, JS, CY, CS according to the producing regions (Fujian, Sichuan provinces of China) and processing method (air-dried, stir-baked with salt). The analysis results of PCA were designed to discriminate Alismatis Rhizoma from different producing regions or processing methods. However, Supplementary Information Fig. S6A and B displayed overlapping dots, which indicated that the geographical origins and processing methods were not the exclusive factors influencing the contents of triterpenes and polysaccharides. Thus, classification with data of triterpenes and polysaccharides was unsuccessful.
3.5.2. Correlation between the triterpenes contents and lipase inhibitory effects
On the basis of the contents of total triterpenes in Alismatis Rhizoma and the inhibitory effects on lipase of Alismatis Rhizoma, the linearity correlation was analyzed using the data of 35 batches samples. As shown in Fig. 6B, a good linearity was observed between contents of total triterpenes and inhibitory effects on lipase (R2 = 0.5795). This correlation indicated that triterpenes were the key biological components of Alismatis Rhizoma for the lipase inhibition. Additionally, partial least squares regression (PLSR) between the contents of each triterpene and inhibitory effects on lipase were performed using Matlab 7.0 (Fig. 6C). Significant correlations have been observed for triterpenes 6, 10, 14, 15, 18, 22, and 31. In addition, positive correlative triterpenes 10, 14, 15, 18, 22 displayed positive key roles in the inhibitory effects of Alismatis Rhizoma, indicating that higher contents of these triterpenes corresponded to more potent biological effects. However, negative correlations were also observed between the content of triterpenes 6, 31 and biological effects. This result could be explained by the hypothesis that the content of 6 and 31 may influence the content of other positive correlation triterpenes, such as 14, 15 and 18. Thus, the PLSR suggests that these relevant triterpenes should be subject to additional study in development of quality control testing of Alismatis Rhizoma.
4. Conclusions
In the present study, the quantitative and qualitative analysis of saccharides, sesquiterpenes and triterpenes in 35 batches of Alismatis Rhizoma was performed using multiple-optimized chromatographic methods. The potential discrimination of samples between various producing regions and/or distinct processing methods of Alismatis Rhizoma was analyzed systematically based on the contents of components, structural analysis and PCA. In further consideration of the medicinal usage of Alismatis Rhizoma, the inhibitory effects on lipase activity of crude extracts of Alismatis Rhizoma and various triterpenes extensively evaluated in vitro. The significant positive correlations between contents of triterpenes 10, 14, 15, 18 and 22 and inhibitory effects of Alismatis Rhizoma were observed in the PLSR experiment, which indicated that these chemical components are vital factors for quality control of Alismatis Rhizoma in the future. Therefore, the present study not only established an effective method for simultaneous determining multiple components, but also identified key triterpenes according to the biological effect of Alismatis Rhizoma, all of which indicating that the quantitative and qualitative analyses in the chemical components and bioactivities would be a useful approach for controlling Chinese medicines.
Acknowledgments
This research program was supported financially by the National Natural Science Foundation of China (Nos. 81622047, 81503201 and 81473334), and Dalian Outstanding Youth Science and Technology Talent awards (2015J12JH201), and the Distinguished Professor of Liaoning Province, Liaoning Bai Qian Wan Talents Program, and by the Innovation Team of Dalian Medical University.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2017.09.004.
Contributor Information
Chao Wang, Email: wach_edu@sina.com.
Xiaochi Ma, Email: maxc1978@163.com.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Tian T., Chen H., Zhao Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: a review. J Ethnopharmacol. 2014;158:373–387. doi: 10.1016/j.jep.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Pharmacopoeia Commission . Medical Science and Technology Press; Beijing: 2010. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- 3.Li Q., Qu H. Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma. Fitoterapia. 2012;83:1046–1053. doi: 10.1016/j.fitote.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Qu S.Y., Su Q., Peng K.L., Liu J.H., Yin C.P. Effects of the total triterpenoids extract of Alismatis Rhizoma on calcium oxalate urinary stone formation in rats. Acta Med Univ Sci Tchnol Huazhong. 2011;40:634–639. [Google Scholar]
- 5.Hu X.Y., Guo Y.Q., Gao W.Y., Zhang T.J., Chen H.X. Two new triterpenes from the rhizomes of Alisma orientalis. J Asian Nat Prod Res. 2008;110:481–484. doi: 10.1080/10286020801948441. [DOI] [PubMed] [Google Scholar]
- 6.Hu X.Y., Guo Y.Q., Gao W.Y., Chen H.X., Zhang T.J. A new triterpenoid from Alisma orientalis. Chin Chem Lett. 2008;19:438–440. [Google Scholar]
- 7.Oshima Y., Iwakawa T., Hikino H. Alismol and alismoxide, sesquiterpenoids of Alisma Rhizomes. Phytochemistry. 1983;22:183–185. [Google Scholar]
- 8.Nakajima Y., Satoh Y., Katsumata M., Tsujiyama K., Ida Y., Shioji J. Terpenoids of Alisma orientale rhizome and the crude drug Alismatis rhizoma. Phytochemistry. 1994;36:119–127. [Google Scholar]
- 9.Xu W., Zheng C.S., Wu S.S. Screening lipid-reducing active components from Rhizoma Alismatis by computer simulation. J Fujian Univ Tradit Chin Med. 2010;20:34–36. [Google Scholar]
- 10.Li S., Jin S., Song C., Chen C., Zhang Y., Xiang Y. The metabolic change of serum lysophosphatidylcholines involved in the lipid lowering effect of triterpenes from Alismatis Rhizoma on high-fat diet induced hyperlipidemia mice. J Ethnopharmacol. 2016;177:10–18. doi: 10.1016/j.jep.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z.J., Pei P. Application and development of lipid-lowing drugs. China Mod Med. 2013;20:14–15. [Google Scholar]
- 12.Chen J.C., Lu X.F., He G.Q. Research progress of screening methods for hypolipidemic components in vitro. Food Sci. 2010;31:287–291. [Google Scholar]
- 13.Xiao S., Yu R., Ai N., Fan X. Rapid screening natural-origin lipase inhibitors from hypolipidemic decoctions by ultrafiltration combined with liquid chromatography–mass spectrometry. J Pharm Biomed Anal. 2015;104:67–74. doi: 10.1016/j.jpba.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Zheng C.D., Duan Y.Q., Gao J.M., Ruan Z.G. Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J Chin Med Assoc. 2010;73:319–324. doi: 10.1016/S1726-4901(10)70068-X. [DOI] [PubMed] [Google Scholar]
- 15.Chen L.L., Hu Z.F., Ding X.P., Qi J., Zhu D.N., Yu B.Y. Identification and determination of the major triterpenes in Rhizoma Alismatis by HPLC-evaporative light scattering detection and HPLC/electrospray ionization-MS. J AOAC Int. 2013;96:260–264. doi: 10.5740/jaoacint.11-012. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y.W., Li Q., Lv C.X., Liu X.J., Chen X.H., Bi K.S. Simultaneous determination of four active components in Alisma orientale (Sam.) Juz. by HPLC–DAD using a single reference standard. J Pharm Anal. 2015;5:85–92. doi: 10.1016/j.jpha.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S.M., Kang J.S., Hwang G.S., Kim Y.H., Lee C.G., Yeo W.H. Quality evaluation of Alismatis Rhizoma by high performance liquid chromatography. Arch Pharm Res. 2004;27:460–464. doi: 10.1007/BF02980090. [DOI] [PubMed] [Google Scholar]
- 18.Li T., Zhuang S., Wang Y., Wang Y., Wang W., Zhang H. Flavonoid profiling of a traditional Chinese medicine formula of Huangqin Tang using high performance liquid chromatography. Acta Pharm Sin B. 2016;6:148–157. doi: 10.1016/j.apsb.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian Z., Li S. Analysis of Cordyceps by multi-column liquid chromatography. Acta Pharm Sin B. 2017;7:202–207. doi: 10.1016/j.apsb.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Zhang Y, Wu W, Huang L, Guo D, Liu C. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm Sin B 2017. Available from:<10.1016/j.apsb.2017.04.012> [DOI] [PMC free article] [PubMed]
- 21.Mai Z.P., Zhou K., Ge G.B., Wang C., Huo X.K., Dong P.P. Protostane triterpenoids from the Rhizome of Alisma orientale exhibit inhibitory effects on human Carboxylesterase 2. J Nat Prod. 2015;78:2372–2380. doi: 10.1021/acs.jnatprod.5b00321. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W., Huang X., Li X., Zhang F., Chen S., Ye M., Huang M. Qualitative and quantitative analysis of major triterpenoids in Alismatis Rhizoma by high performance liquid chromatography/diode-array detector/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. Molecules. 2015;20:13958–13981. doi: 10.3390/molecules200813958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin X.L., Mai Z.P., Wang X., Chen L., Deng S., Zhang B. Protostane alisol derivatives from the rhizome of Alisma orientale. Phytochem Lett. 2016;16:8–11. [Google Scholar]
- 24.Mai Z.P., Xin X.L., Sun Z., Zhang N., Huang S.S., Wang C. Biotransformation of alisol G by Penicillium janthinellum and the hCE2 inhibitory effects of its metabolites. Phytochem Lett. 2015;13:228–233. [Google Scholar]
- 25.Da J., Wu W.Y., Hou J.J., Long H.L., Yao S., Yang Z. Comparison of two official Chinese pharmacopoeia species of Ganoderma based on chemical research with multiple technologies and chemometrics analysis. J Chromatogr A. 2012;1222:59–70. doi: 10.1016/j.chroma.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Fu D.T., O’Neill R.A. Monosaccharide composition analysis of oligosaccharides and glycoproteins by high-performance liquid chromatography. Anal Biochem. 1995;227:377–384. doi: 10.1006/abio.1995.1294. [DOI] [PubMed] [Google Scholar]
- 27.Wu X., Jiang W., Lu J., Yu Y., Wu B. Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 2014;145:976–983. doi: 10.1016/j.foodchem.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Liang J., Wu W.Y., Sun G.X., Wang D.D., Hou J.J., Yang W.Z. A dynamic multiple reaction monitoring method for the multiple components quantification of complex traditional Chinese medicine preparations: Niuhuang Shangqing pill as an example. J Chromatogr A. 2013;1294:58–69. doi: 10.1016/j.chroma.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Jang D.S., Lee G.Y., Kim J., Lee Y.M., Kim J.M., Kim Y.S., Kim J.S. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta. Arch Pharm Res. 2008;31:666–670. doi: 10.1007/s12272-001-1210-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Zhu S.B., Xie M.Y., Nie S.P., Liu W., Li C. Quality control and original discrimination of Ganoderma lucidum based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal Chim Acta. 2008;623:146–156. doi: 10.1016/j.aca.2008.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material