Abstract
While immune suppression is a hallmark of head and neck squamous cell carcinoma (HSNCC), the immunological impact of premalignant oral lesions, which often precedes development of HNSCC, is unknown. The present study assessed the changes in splenic and draining lymph node CD4+cell populations and their production of select cytokines that occur in mice with carcinogen-induced premalignant oral lesions and the changes that occur as lesions progress to oral cancer. These studies found skewing toward Th1 and Th17-type phenotypes in the spleen and lymph nodes of mice with premalignant oral lesions and a shift to Treg as lesions progress to cancer. Since the role of Th17 cells in the progression from premalignant lesions to cancer is not clear, studies determined the immunological and clinical effect of treating mice bearing premalignant oral lesions with a TGF-β type 1 receptor inhibitor plus IL-23 as an approach to sustain the Th17 phenotype. These studies showed that the treatment approach not only sustained the Th17 phenotype, but also increased distal spleen cell and regional lymph node cell production of other stimulatory/inflammatory mediators and slowed premalignant lesion progression to cancer.
Keywords: head and neck cancer, HNSCC, IL-17, premalignant oral lesions, Th17, Treg
Patients with select premalignant oral lesions have a high risk of developing squamous cell carcinoma of the head and neck (HNSCC).1 HNSCC is an aggressive malignancy with a five year survival rate of 60%.2 The pathogenesis of HNSCC comprises a multistep process that includes damage from carcinogens such as tobacco and alcohol, and genetic alterations. These alterations are seen in premalignant oral lesions, with leukoplakias being the most common lesion type. The risk of developing into HNSCC has been directly linked to the site and thickness of the leukoplakia, with the highest risk being for those on the tongue, floor of mouth, and lips.1 Despite various treatment approaches including surgical resection, photodynamic therapy and retinoid chemoprevention, leukoplakias in approximately one-third of patients progress to carcinomas. The failure of current therapies to prevent progression and the poor outcome for HNSCC patients supports the need for improved treatments. One model that has been developed for understanding premalignant oral lesions and their progression to cancer is a 4-nitroquinoline-1-oxide (4NQO) carcinogen-induced mouse model.3,4 The 4NQO carcinogen mimics the effects of tobacco products and induces premalignant oral lesions that are predominantly located on the tongue and which progress to oral cancer.
The need to develop alternative treatment approaches to expand the armament that is available against oral cancer provides enthusiasm for evaluating immunological approaches. However, immunotherapeutic approaches are complicated by the profound level of immune suppression in HNSCC patients. This immune suppression is mediate by a variety of cell types including cancer-associated fibroblasts, regulatory T-cells (Treg), and myeloid-derived suppressor cells (MDSC).5–7 Patients with laryngeal squamous cell carcinoma have increased levels of Treg cells and reduced levels of both naïve T-cells and immune stimulatory Th1 cells.8 The impact of immune suppressor cells on immune defenses against cancer is supported by studies showing that treatments to reduce levels of MDSC and Treg in HNSCC patients result in T-cell reactivity against autologous cancer.7 It has also been shown that an increased level of immune inhibitory cells in HNSCC patients predisposes them to development of synchronous squamous cell carcinomas.6 Because of the extensive immune dysfunction in HNSCC patients, it may be more realistic to test immune therapeutic approaches in subjects with premalignant lesions that have a high risk at developing HNSCC so as to limit progression of premalignant oral lesions toward HNSCC. Unfortunately, few studies have assessed the immunological impact of the presence of premalignant oral lesions.
Contrasting to the immune inhibitory milieu of patients and mice with HNSCC, studies have shown an inflammatory response, including increased IL-17 levels, within regional lymph nodes of mice with premalignant oral lesions.9 An increase in IL-17 was also shown in human premalignant lesion tissues as compared to levels in tissues from either control subjects or from patients with HNSCC.10 These shifts from an inflammatory to an inhibitory environment could be due to lymphocyte plasticity that is in part regulated by the cytokine milieu. In a simplified scenario, IL-12 mediates skewing toward Th1, IL-4 toward Th2, TGF-β toward Treg, and IL-6 pus either IL-1 or TGF-β toward Th17, with the Th17 phenotype being stabilized by IL-23.11–13 While Th17 and Treg cells share a common lineage with significant plasticity, they exhibit opposing immunological effects. Th17 cells produce IL-17A, IL-17F, IL-22, IL-21 and IFN-γ. In contrast, Treg cells produce inhibitory mediators and contribute to the immune dysfunction of HNSCC patients.
The role of Th17 cells in promoting or protecting from tumor progression is unsettled and is controversial. Several studies have shown correlations between cancer presence and increased levels of Th17 cells or Th17 cell-associated cytokines, raising suggestions that the Th17 cells contribute to the tumor development and progression. This includes increased levels of Th17 cells or IL-17 in patients with cervical cancer, hypopharyngeal carcinoma, gastric cancer and lung cancer.14–17 The contribution of Th17 cells to inflammation, such as in the gut, has been implicated in the development of colon cancer.18 In addition to studies showing associations between the presence of Th17 cells and cancer, a few interventional studies have been conducted, although results of some of these studies are conflicting. For example, studies in a mouse mammary tumor model showed reduced development of tumor from a tumor cell inoculum in mice treated with IL-17 neutralizing antibody.19 In contrast, murine studies with premalignant epidermal squamous lesions showed increased induction of Th17 cells to coincide with tumor regression.20 These studies have left unanswered whether Th17 cells are beneficial or detrimental in the course of development and progression to cancer.
While premalignant oral lesions are relatively small and localized, the immunological impact of lesions is unknown. The present study measured the changes in CD4+ cell populations and cytokine production that occur distally in the spleen or in regional lymph nodes of mice with 4NQO carcinogen-induced premalignant oral lesions and how these parameters shift as lesions progress to oral cancer. These studies found skewing toward Th1 and Th17-type phenotypes in mice with premalignant oral lesions and a shift to Treg phenotype as lesions progress to cancer. Studies also aimed to determine the immunological and clinical impact of treating mice bearing premalignant oral lesions with a TGF-β type 1 receptor inhibitor plus IL-23 as an approach to sustain the Th17 phenotype. This treatment approach sustained a Th17 phenotype, increased spleen and lymph node cell production of stimulatory/inflammatory mediators, and slowed premalignant lesion progression to cancer.
Methods
4NQO-induced oral premalignant lesion model
A 5 mg/ml 4NQO stock solution in propylene glycol was diluted to 50 μg/ml and administered in the drinking water of 2 month old female C57BL/6 mice (Charles Rivers Laboratory, Wilmington, MA).3,4,21 In this model, premalignant oral lesions appear after approximately 6 weeks of 4NQO treatment and HNSCC is detectable 10 weeks later after approximately 16 weeks of 4NQO treatment. Control mice received propylene glycol diluent. Development of premalignant oral lesions and HNSCC was monitored weekly by sedating mice with inhaled isoflurane (Piramal Healthcare, Bethlehem, PA) and examining the oral cavities by endoscopy using a Stryker 1.9 mm × 30° scope and a Stryker 1088 HD camera (Stryker, Kalamazoo, MI). Clinical assessments of lesion progression were quantitated in a blinded manner by counting the number of visible lesions, and giving lesions a gross pathologic score between 1 and 4 based on their horizontal and vertical size.9 The following criteria were used for scoring: 1: flat macule, 2: raised papule, 3: raised plaque, 4: grossly exophytic.
Flow cytometric analysis of surface markers and Foxp3 expression
Spleens and cervical lymph nodes of diluent-treated and 4NQO-treated mice were homogenized to a single cell suspension. Erythrocytes in the spleen cell suspension were lysed. Splenic and lymph node leukocyte cellularity was determined by counting cells excluding trypan blue using a hemocytometer. All reagents for immunostaining of spleen and lymph node cells were from BD Biosciences (San Jose, CA). Conventional (Foxp3−) and regulatory CD4+ and CD8+ cells were identified by washing cells once in Stain Buffer and resuspending them at 1 × 107 cells/ml. Nonspecific staining was blocked by incubation with FBS and anti-CD16/32 monoclonal antibody. Cells were then surface stained with fluorescent-conjugated antibodies against CD4, CD8a, and CD25. Cells were stained intracellularly for Foxp3 after fixation with Foxp3 Fixation Buffer and permeabilization with Foxp3 Permeabilization Buffer. The extent and frequency of positively stained cells was visualized using flow cytometry (FACSCanto, BD Biosciences).
Flow cytometric analysis of spleen and lymph node cell cytokine expression
Reagents for immunostaining CD4+ cells for expression of cytokines were from BD Biosciences. In order to detect intracellular cytokines, single cell suspensions of spleen and lymph node cells were first stimulated for 4 hrs at 37°C with 50 ng/ml phorbol 12-myristate 12-acetate (PMA), 1 μg/ml ionomycin, and brefelden A solution. Nonspecific staining of cells was blocked with FBS and anti-CD16/32 monoclonal antibody prior to cell surface staining with antibodies to CD4. Intracellular cytokine staining was performed by fixation/permeabilization with Cytofix/Cytoperm and then staining with fluorescent-conjugated antibodies against IL-17A, IFN-γ, and IL-4. The intensity of staining and frequency of positively stained cells was visualized by flow cytometry (FACSCanto, BD Biosciences).
Measurement of cytokine secretion by spleen cells and lymph node cells
In order to measure levels of cytokines secreted by spleen and lymph node cells into the culture supernatant, single-cell suspensions were cultured for 3 days in the presence of immobilized anti-CD3. Levels of IL-17A, TNF-α, IL-6, IL-2, IFN-γ, RANTES, IL-4 and IL-10 in culture supernatants were measured using mouse cytometric bead array flex sets according to the instructions of the manufacturer (BD Biosciences). Relative amounts of each cytokine were measured by flow cytometry and analyzed using FCAP Array software (Soft Flow, St. Louis Park, MN). Levels of TGF-β were measured following acid activation by ELISA (eBioscience, San Diego, CA).
Treatment of mice with GW788388 and/or IL-23
The approach used to sustain the Th17 phenotype that is characteristic of mice with premalignant oral lesions and to prevent skewing to the Treg phenotype that is prominent in mice whose lesions have been allowed to progress to oral cancer was to treat mice with IL-23 (R&D Systems, Minneapolis, MN) plus the small molecule TGF-β type I receptor (ALK) inhibitor GW788388 (Selleckchem, Houston, TX). Starting at the time that premalignant oral lesions are detectable, at approximately 6 weeks after initiating 4NQO treatment, mice were divided into groups and treated daily with 400 ng IL-23 by intraperitoneal injection, 50 mg/kg GW788388 by gavage, or both IL-23 plus GW788388.22–24 Control mice and mice receiving treatment with only GS788388 or IL-23 received appropriate placebo treatments with diluent alone.
Statistical analysis
Data were reported using the mean as a measure of central tendency ± standard error of the mean. A one-way ANOVA was used to compare one variable condition between groups. Differences that were identified by the ANOVA analysis were then further analyzed by a two-tailed Student’s t test to determine significance of differences between each of two groups. Significance was reported in the 95% confidence interval.
Results
Increased splenic and lymph node cellularity and levels of CD4+ treg once mice with premalignant oral lesions progress to developing oral cancer
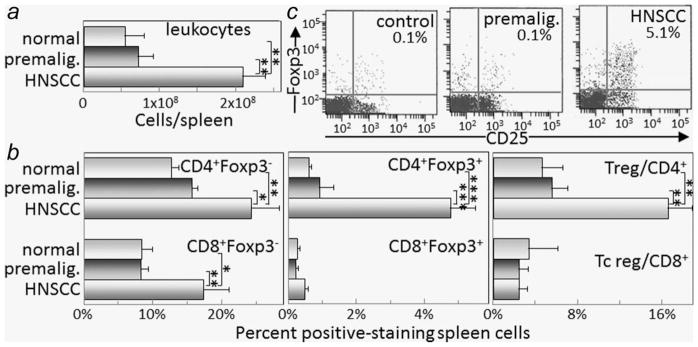
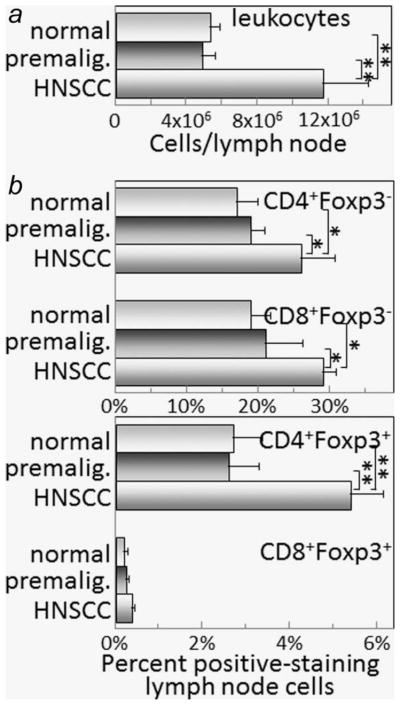
Mice were administered drinking water containing 4NQO to induce the development of premalignant oral lesions that progress to oral cancer. Spleen cells and regional lymph node cells from mice in which either premalignant lesions had developed or from mice whose lesions were allowed to progress to cancer were flow cytometrically analyzed for T-cell subpopulations. While spleen and lymph node sizes and cellularity were similar for healthy control mice and those with 4NQO-induced oral lesions, the spleens and lymph nodes of mice whose lesions had progressed to cancer were enlarged to approximately 3–4-fold and contained approximately three-fold the number of leukocytes compared to the numbers for control mice or mice with lesions (Figs. 1a and 2a).
Figure 1.
Increased spleen cellularity, T-cell and Treg levels when premalignant lesions have progressed to oral cancer. Spleen cellularity and T-cell content were assessed in healthy control mice or mice receiving 4NQO treatment for 6 and 16 weeks when premalignant lesions or oral cancer, respectively, were established. Spleen cell mononuclear cell counts were determined (a). Spleen cells were then immunostained for Foxp3 and either CD4 or CD8. Shown are percent spleen cells staining positive as conventional CD4 + Foxp3− or CD8 + Foxp3− cells, regulatory CD4 + Foxp3+ or CD8 + Foxp3+ cells, and the ratio of regulatory to conventional CD4+ or CD8+ cells (b). Also shown are typical dot blots of cells from control mice or mice with either premalignant oral lesions or oral cancer staining as CD25 + Foxp3+ Treg cells (c). * = p <0.05, ** = p <0.01, *** = p <0.001.
Figure 2.
Increased lymph node cellularity, T-cell and Treg levels when premalignant lesions have progressed to oral cancer. Lymph node cellularity and T-cell content were assessed in healthy control mice or mice receiving 4NQO treatment for 6 and 16 weeks when premalignant lesions or oral cancer, respectively, were established. Lymph node cell mononuclear cell counts were determined (a). Lymph node cells were then immunostained for Foxp3 and either CD4 or CD8. Shown are percent lymph node cells staining positive as conventional CD4 + Foxp3− or CD8 + Foxp3− cells, or as regulatory CD4 + Foxp3+ or CD8 + Foxp3+ cells (b). * = p <0.05, ** = p <0.01.
Flow cytometric analysis of spleen cell subpopulations showed similar percentages of conventional (Foxp3−) CD4+ cells and CD8+ cells in spleens of control mice and those with premalignant oral lesions, and a prominent increase in the proportion of conventional T-cells in spleens of mice with oral cancer (Fig. 1b). The proportions of Treg cells (Foxp3+) in healthy control mice and mice with premalignant oral lesions were similarly low, but the splenic Treg levels in mice with oral cancer were significantly increased (Figs. 1b and 1c). While the levels of CD4+ cells expressing Foxp3 were increased in mice with oral cancer, the levels of CD8+ cells expressing Foxp3 remained at the same low levels as seen in control mice. Even though the percentages of both Treg and conventional CD4+ T-cells were increased, the ratio of CD4+ Treg to conventional CD4+ T-cells remained significantly increased in tumor-bearing mice as compared to the Treg to conventional T-cell ratio in control mice or mice with premalignant oral lesions.
The increase in spleen cellularity in tumor-bearing mice further compounded the increase in the absolute number of splenic Treg. When calculating the absolute splenic number of CD4+ Treg in tumor-bearing mice by multiplying spleen cellularity by the percent CD4 + Foxp3+ cells, the absolute number of Treg was increased 28-fold as compared to the number of splenic CD4+ Treg in control mice. The absolute number of splenic CD4+ Treg in mice with premalignant oral lesions was slightly increased over levels in control mice, although this increase was not statistically significant.
As was seen for spleen cells, the percentage of conventional CD4+ and CD8+ lymph node cells was comparable for control mice and mice with premalignant oral lesions, but was increased in regional lymph nodes from mice whose lesions were allowed to progress to oral cancer (Fig. 2b). However, the increase in conventional T-cell in lymph nodes of mice with oral cancer was not as prominent as in the spleen. Also similar to the spleen, there were no differences in the percentages of CD8+ lymph node cells expressing Foxp3 among the experimental groups, but the percentage of CD4+ cells expressing Foxp3 was increased in the lymph nodes of mice with oral cancer.
Shifts to an inflammatory and then immune suppressive phenotype in mice with premalignant oral lesions that progress to cancer
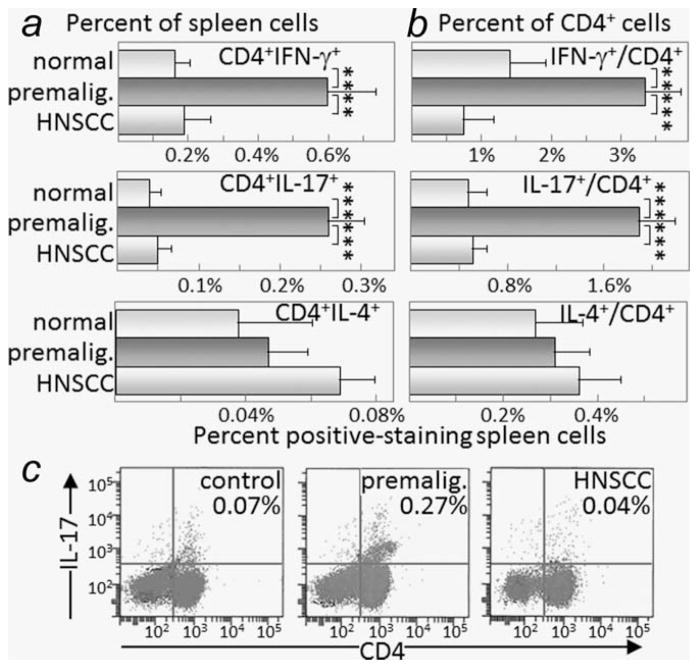
Mice with premalignant oral lesions and those whose lesions were allowed to progress to oral cancer were examined for splenic levels of cytokine-expressing CD4+ T-cells. Mice with premalignant oral lesions had an increased percentage of splenic CD4+ cells expressing the Th1 cytokine IFN-γ and the inflammatory cytokine IL-17. Shown in Figure 3a are summaries of the percentages of splenic CD4 + IFN-γ+ or CD4 + IL-17+ T-cells. These data are also expressed as the percent of CD4+ cells that express IFN-γ or IL-17 (Fig. 3b). Representative flow cytometric analyses for CD4+ cell expression of IL-17 are shown in Figure 3c. In contrast to the increased levels of CD4+ cells expressing IFN-γ and IL-17 among spleen cells from mice with premalignant oral lesions, the levels of CD4+ cells expressing the Th2 cytokine IL-4 was very low (Figs. 3a and 3b), with the level of IL-4-expressing CD4+ cells being no different between control mice or mice with premalignant oral lesions. The levels of CD8+ cells expressing either IFN-γ, IL-17 or IL-4 were low to undetectable in the spleen of either control mice or mice with premalignant oral lesions (not shown).
Figure 3.
Increased levels of IFN-γ- and IL-17-staining CD4+ cells in spleens of mice with premalignant oral lesions. Spleen cells from healthy control mice, mice with premalignant oral lesions (6 weeks of 4NQO treatment) or mice with oral cancer (16 weeks of 4NQO treatment) were surface immunostained for CD4 and stained intracellularly for cytokines. Shown are percentages of spleen cells staining positive for CD4 plus either IFN-γ, IL-17 or IL-4 (a) and the percentages of CD4+-gated cells staining positive for these cytokines (b). Also shown are typical dot blots of cells from control mice or mice with either premalignant oral lesions or oral cancer staining as CD4 + IL-17+ Th17 cells (c). ** = p <0.01, *** = p <0.001.
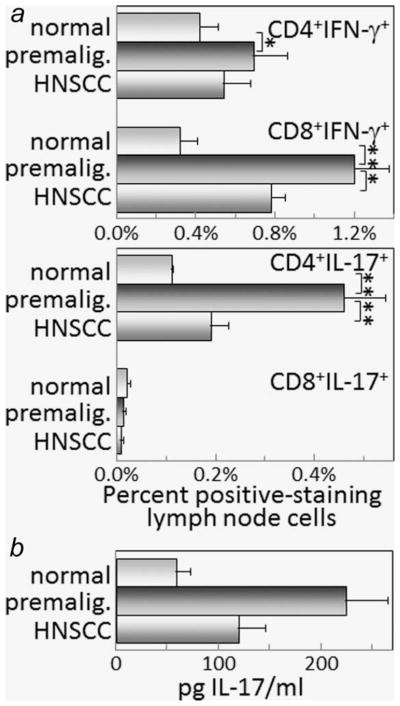
Cytokine expression by lymph node cells of mice with premalignant oral lesions differed in some respects from what was seen for spleen cells. While the percentage of CD4+ lymph node cells expressing IFN-γ was greater for mice with premalignant oral lesions compared to levels in healthy control mice, the increase was not as prominent as what was seen for spleen cells (Fig. 4a). Differing from spleen cells was the increase in IFN-γ-expressing CD8+ cells in lymph nodes from mice with premalignant oral lesions compared to levels in control mice. As was seen for the spleen, the percentage of CD4+ cells expressing IL-17 was increased in lymph nodes of mice with premalignant oral lesions compared to levels in control mice (Fig. 4a). Also similar to the spleen, expression of IL-17 by CD8+ lymph node cells was minimal to undetectable. These changes in levels of CD4+ lymph node cells expressing IL-17 were mirrored by changes in secretion of IL-17 by the lymph node cells (Fig. 4b).
Figure 4.
Increased levels of IFN-γ- and IL-17-staining lymph node cells in mice with premalignant oral lesions. Lymph node cells from healthy control mice, mice with premalignant oral lesions (6 weeks of 4NQO treatment) or mice with oral cancer (16 weeks of 4NQO treatment) were surface immunostained for CD4 or CD8 and stained intracellularly for cytokines. Shown are percentages of spleen cells staining positive for CD4 or CD8 plus either IFN-γ or IL-17 (a). Also shown are levels of IL-17 that are secreted by lymph node cells from control mice or mice with either premalignant oral lesions or oral cancer (b). ** = p <0.01, *** = p <0.001.
While mice with premalignant oral lesions had an increase in splenic and lymph node levels of CD4+ cells expressing IFN-γ or IL-17, once the lesions progressed to oral cancer, the levels of these cytokine-expressing cells declined to the levels that were more comparable to levels in control mice (Figs. 3a and 3b, and Fig. 4a). The levels of splenic CD4+ cells expressing IL-4 remained at the same low levels in mice with oral cancer as in mice with premalignant oral lesions and in control mice (Figs. 3a and 3b), and were not detectable in lymph nodes of mice with oral cancer (not shown).
Shifts in levels of cytokines that can regulate CD4+ cell plasticity
The above studies showing an increase in Th17 cells in mice bearing premalignant oral lesions and a shift to Treg cells in mice whose lesions were allowed to progress to oral cancer prompted assessment of spleen cell secretion of IL-17 as well as cytokines that regulate Th17 cell levels. Consistent with the increased levels of splenic Th17 cells in mice with premalignant oral lesions, production of IL-17 by spleen cells from lesion-bearing mice was elevated (Fig. 5). When lesions are prominent, but before the detectable presence of oral cancer, IL17 levels peak and then subside as lesions reach a more advanced stage. At the time that oral cancer becomes detectable, spleen cell production of IL-17 is low to undetectable.
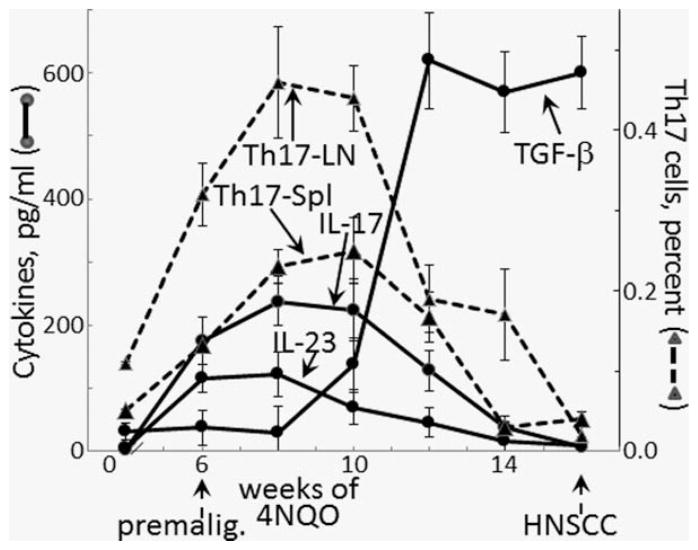
Figure 5.
Increased spleen cell production of IL-17 and IL-23 and the concurrent increase in splenic and lymph node Th17 cells in mice with premalignant oral lesions, which decline as mice progress to developing oral cancer. Spleen cells from healthy control mice or mice treated for various durations with 4NQO were collected and cultured for 3 days on anti-CD3. Culture supernatants were then collected for measurement of secreted cytokines. Also, both spleen and lymph node levels of Th17 cells were measured by flow cytometrically analyzing cells staining positive for CD4 and IL-17. Secretion of IL-17 was significantly increased after 6*, 8***, 10** and 12* weeks of 4NQO treatment. Secretion of IL-23 was increased after 6*, 8** and 10* weeks of 4NQO treatment. TGF-β secretion was increased after 10*, 12***, 14*** and 16*** weeks of 4NQO treatment. Splenic Th17 cell levels were increased after 6*, 8**, 10* and 12* weeks of 4NQO treatment. Lymph node Th17 cell levels were increased after 6***, 8***, 10*** and 12* weeks of 4NQO treatment. * = p <0.05, ** = p <0.01, *** = p <0.001.
The relative proportions of IL-23 and TGF-β influence the stability of Th17 cells.11–13 Because spleen cell production of IL-17 increased when lesions appeared but then declined as lesions became fully developed, studies were conducted to determine if this decline in IL-17 and the above-demonstrated shift to a Treg phenotype were associated with changes in spleen cell production of IL-23 and TGF-β. Similar to the production timeline pattern of IL-17, spleen cell production of IL-23 increased in mice with premalignant lesions and then diminished as lesions became more advanced (Fig. 5). However, in contrast to the IL-17 pattern, the decline in IL-23 secretion was more rapid as lesions progressed and its production was insignificant by the time that oral cancer could be detected. As production of IL-23 was declining, secretion of TGF-β steeply increased. TGF-β production peaked well before any oral cancer could be detected and remained at the peak levels into the time period when oral cancers became visible. These studies showing a shift in the balance of TGF-β and IL-23 to favor a decline in Th17 cells and to promote development of Treg cells are consistent with the decline in spleen cell Th17 levels and production of IL-17 as premalignant oral lesions progress to oral cancer.
In addition to tracking spleen cell cytokine production while premalignant oral lesions progress to oral cancer, levels of CD4+ cells expressing IL-17 in the spleen and in regional lymph nodes was measured (Fig. 5). Similar to the increase in spleen cell production of IL-17 in mice with premalignant oral lesions, levels of Th17 cells were increased in the spleens and lymph nodes of these mice. Also similar to the decline in IL-17 production as lesions neared the time when oral cancer becomes detectable, levels of Th17 in the spleen and regional lymph nodes declined. These results show that levels of Th17 cells in the spleen and in the lymph nodes coincide with changes in the levels of cytokines that sustain Th17 cells or mediate skewing to a Treg phenotype.
Impact of treating mice bearing premalignant oral lesions to sustain a Th17 cell phenotype on spleen and lymph node cell cytokine production
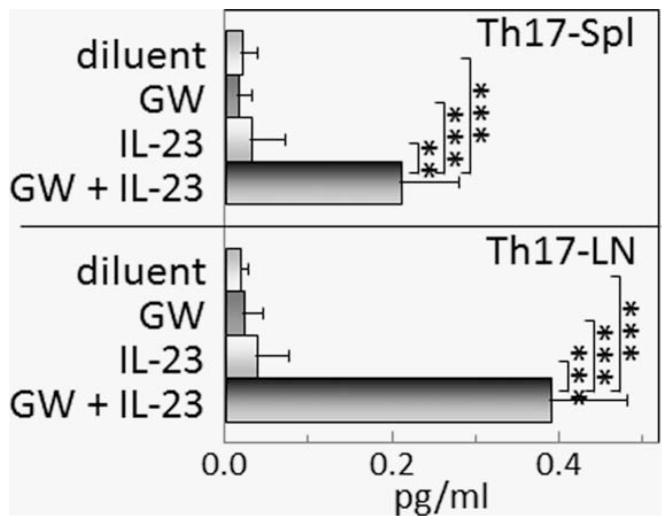
The role of Th17 cells in tumor development versus anti-tumor defenses is unresolved. The shift from a prominent Th17 phenotype and secretion of Th17-supporting cytokines by spleen and lymph node cells of mice with premalignant oral lesions to a Treg phenotype with increased TGF-β production as lesions progress to cancer prompted studies to sustain the Th17-supporting cytokine balance and determine how that impacts on cytokine production. Starting from Week 6 of 4NQO treatment, when premalignant oral lesions were detectable by endoscopic examination, mice received treatment with either diluent, IL-23, the small molecule TGFβ type I receptor inhibitor GW788388, or both Il-23 and GW788388. After 2 months of treatment, spleen cell production of inflammatory (Fig. 6a), immune stimulatory (Fig. 6b), and inhibitory (Fig. 6c) mediators was measured. Lymph node cell production of some of these same cytokines was also measured (Figs. 7ac). The combination of IL-23 and GW788388 sustained spleen and lymph node cell production of increased levels of IL-17 (Figs. 6a and 7a) similar to peak levels produced when premalignant lesions become fulling established (Figs. 4 and 5). Consistent with the increase in production of IL-17, levels of CD4+ cells expressing IL-17 were increased in both the spleen and lymph nodes from mice receiving the combination treatment, with the individual treatments having no effect on Th17 levels (Fig. 8). In addition to sustaining production of IL-17, spleen cell production of the inflammatory mediator TNF-α was increased by the combination treatment, although TNF-α production by lymph node cells was minimal to undetectable. The individual treatments with either IL-23 or GW788388 did not sustain production of IL-17, although production of TNF-α by spleen cells of mice treated with IL-23 alone was increased over levels that were produced by spleen cells of mice that were treated with either diluent or GW788388 alone. The effects of treatments on spleen cell production of IL-6 differed from what was seen for the other inflammatory mediators that were measure as there were no effects of the treatments, with the exception of treatment with GW788388. Treatment with GW788388 alone diminished spleen cell production of IL-6, although this decline was not evident for spleen cells from mice that were treated with both GW788388 and IL-23. In contrast, lymph node cell production of IL-6 was minimal and was not affected by either the individual or combination treatments.
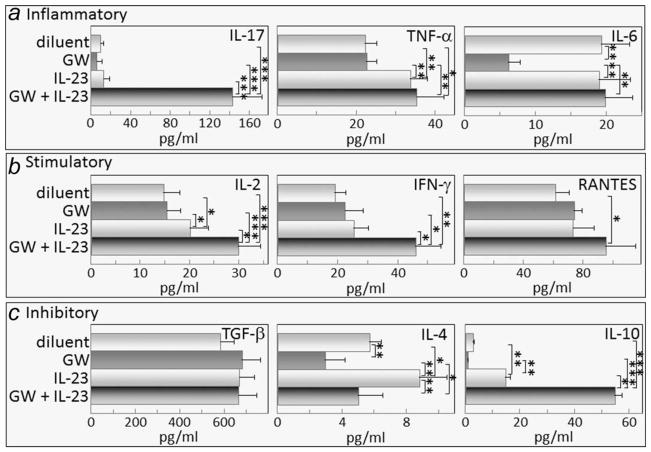
Figure 6.
Combination treatment with GW788388 and IL-23 aiming to sustain Th17 cell levels increases spleen cell production of the inflammatory mediators IL-17 and TNF-α, the stimulatory mediators IL-2, IFN-γ and RANTES, and the inhibitory mediator IL-10. Starting from Week 6 of 4NQO administration, when premalignant oral lesions were detectable on the tongue, mice were initiated on treatment with diluent, GW788388, IL-23 or both GW788388 and IL-23. After 2 months of these treatments, spleens were collected and cultured on anti-CD3 for 3 days. Supernatants were collected and used for measurement of the inflammatory mediators IL-17, TNF-α and IL-6 (a); stimulatory mediators IL-2, IFN-γ and RANTES (b); and inhibitory mediators, TGF-β, IL-4 and IL-10 (c). * = p <0.05, ** = p <0.01, *** = p <0.001.
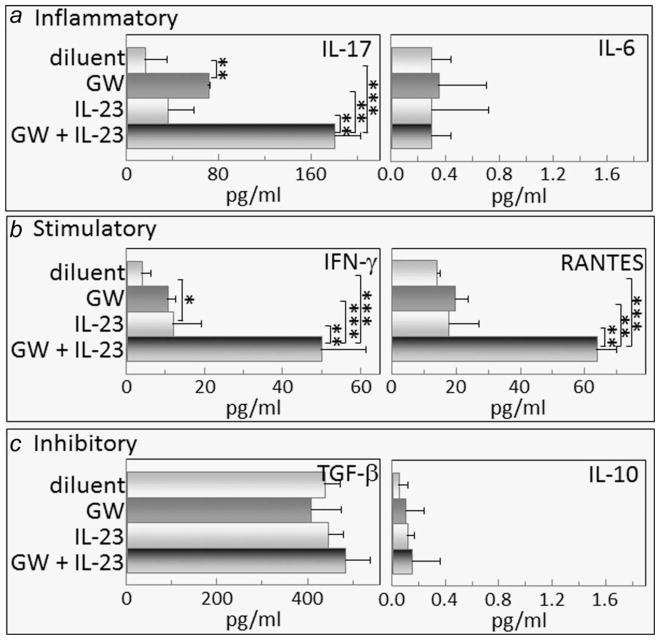
Figure 7.
Combination treatment with GW788388 and IL-23 aiming to sustain Th17 cell levels increases lymph node cell production of the inflammatory mediator IL-17, the stimulatory mediators IFN-γ and RANTES, but has not effect on levels of the inhibitory mediators TGF-β and IL-10. Starting from Week 6 of 4NQO administration, when premalignant oral lesions were detectable on the tongue, mice were initiated on treatment with diluent, GW788388, IL-23 or both GW788388 and IL-23. After 2 months of these treatments, lymph nodes were collected and cultured on anti-CD3 for 3 days. Supernatants were collected and used for measurement of the inflammatory mediators IL-17 and IL-6 (a); stimulatory mediators IFN-γ and RANTES (b); and inhibitory mediators, TGF-β, and IL-10 (c). * = p <0.05, ** = p <0.01, *** = p <0.001.
Figure 8.
Combination treatment with GW788388 and IL-23 sustains increased Th17 cell levels in the spleen and lymph nodes of mice with premalignant oral lesions. Starting from Week 6 of 4NQO administration, when premalignant oral lesions were detectable on the tongue, mice were initiated on treatment with diluent, GW788388, IL-23 or both GW788388 and IL-23. After 2 months of these treatments, spleens and lymph nodes were collected and were surface immunostained for CD4 and stained intracellularly for IL-17. Shown are percentages of spleen cells and lymph node cells staining positive for CD4 plus IL-17. ** = p <0.01, *** = p <0.001.
In addition to affecting production of inflammatory mediators, the combination GW788388 plus IL-23 treatment increased spleen cell production of each of the stimulatory mediators measured: IL-2, IFN-γ and RANTES (Fig. 6b). Production of IFN-γ and RANTES by lymph node cells of mice receiving the combination treatment was also increased, although production of IL-2 was not measured (Fig. 7b). Treatment with IL-23 alone stimulated spleen cell, but not lymph node cell, production of IL-2, but not to the extent stimulated by the combination treatment and had no effect on production of the other stimulatory mediators. GW788388 treatment alone had no effect of production of either IL-2, IFN-γ or RANTES.
There were no consistent patterns in the effects of treating mice bearing premalignant lesions with IL-23 and/or GW788388 on their production of the inhibitory mediators, TGF-β, IL-4 and IL-10 (Fig. 6c). Production of TGF-β was unaffected by any of the treatments, whereas treatment with the combination of IL-23 and GW788388 stimulated IL-10 production. IL-4 production was reduced when mice were treated with GW788388, increased when mice were treated with IL-23 alone, and unaffected by the combination treatment. In contrast, the effects of treatment with either diluent, GS788388 or IL-23, either individually or in combination, on lymph node cell production of inhibitory mediators was more consistent that the effect on spleen cell cytokine production (Figs. 6c and 7c). None of the treatments had any effect on lymph node cell production of the inhibitory mediators TGF-β and IL-10, and levels of IL-4 were undetectable regardless of the treatment group. These results show that the combination treatment of IL-23 plus GW788388 sustains increased production of IL-17 and also stimulates production of inflammatory and stimulatory mediators, but has variable effects on production of inhibitory mediators.
Reduced progression of premalignant oral lesions in mice treated with IL-23 plus GW788388
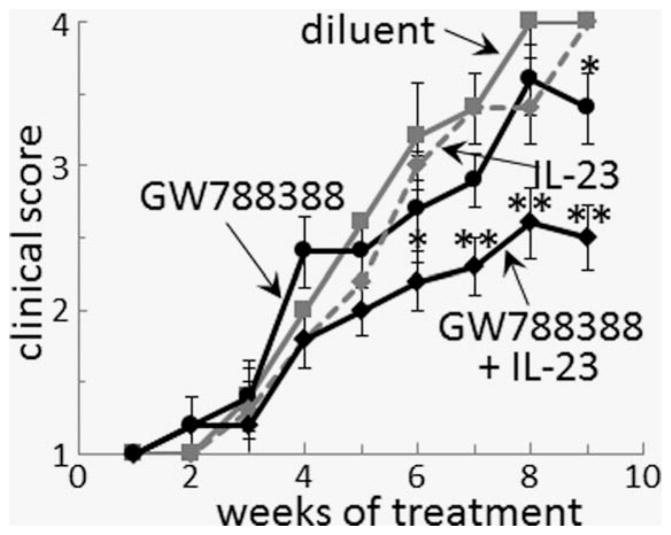
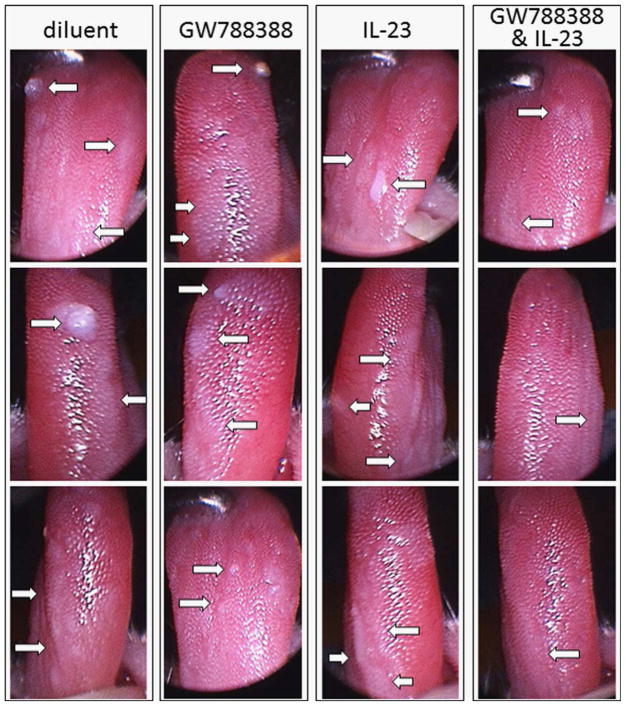
Endoscopic analyses were used to determine the clinical impact of treatments to sustain the Th17 phenotype that is characteristic of mice with premalignant oral lesions, but which shifts to an immune suppressive phenotype as lesions progress to cancer. Since treatments were started after premalignant oral lesions appeared, the clinical assessments were scored in a blinded manner from 1 indicating smaller earlier lesions to 4 for a cancerous appearance (Fig. 9). For most of the time points when mice were examined endoscopically, treatment with either GW788388 alone or IL-23 alone had no impact on progression of lesions to cancer, with cancer development being similar to that seen for diluent-treated mice. The only exception was at the last time point, when diluent-treated mice and mice treated with IL-23 alone all showed progression to tumor, several mice that were treated with GW788388 showed a less pronounced clinical score. Treatment with the combination of GW788388 plus IL-23 slowed progression of the premalignant lesions and seemed to result in a plateau in the progression. The study was terminated when the cancer that developed in the other treatment groups necessitated euthanasia of the experimental mice. Shown in Figure 10 are endoscopic tongue images of 3 representative mice from each group taken after 7 weeks of treatment. While all of these images show presence of premalignant lesions, the lesions were more extensive, with some mice also showing tumors, in diluent-treated mice and, to varying extents, mice treated with GW788388 or IL-23 alone. Lesions were also detectable in mice receiving the combination treatment, but they were less apparent and tumors were not seen at the time that the study was terminated.
Figure 9.
Combination treatment with GW788388 and IL-23 slows the progression of premalignant oral lesions to cancer. Starting from Week 6 of 4NQO administration, when premalignant oral lesions were detectable on the tongue, mice were initiated on treatment with diluent, GW788388, IL-23 or both GW788388 and IL-23. At weekly intervals, their oral cavity was examined and photographed in a blinded manner by endoscopy. The number of visible lesions was counted, and lesions were given a gross pathologic score based on their horizontal and vertical size (1: flat macule, 2: raised papule, 3: raised plaque, 4: grossly exophytic). Differences in clinical score between mice receiving the combination GW788388 plus IL-23 treatments and the other three groups of mice are indicated as follows: * = p <0.05, ** = p <0.01.
Figure 10.
Examples of endoscopic images of 4NQO-treated mice receiving either diluent, GW778388, IL-23 or both GW788388 and IL-23 treatments. Starting from Week 6 of 4NQO administration, when premalignant oral lesions were detectable on the tongue, mice were initiated on treatment with diluent, GW788388, IL-23 or both GW788388 and IL-23. Shown are endoscopic images of three representative mice from each group taken after 7 weeks of onset of these treatments. Arrows point to examples of abnormal areas of the tongue.
Discussion
The immunological impact of cancer presence has been studied for a variety of cancer types, including oral cancer. In contrast, the immunological impact of the presence of premalignant lesions has not been well defined. This is particularly the case for premalignant oral lesions. This study showed that, although carcinogen-induced premalignant oral lesions are localized and relatively small, they have a distant immunological impact. This was seen both distally in the spleen and locally in regional lymph nodes. The spleens and lymph nodes of mice bearing premalignant oral lesions had an increase in CD4+ cells expressing the inflammatory mediator, IL-17, and in cells expressing the immune stimulatory mediator, IFN-γ. In contrast, mice whose lesions were allowed to progress to oral cancer showed a shift to CD4+ spleen cells expressing Foxp3, a Treg phenotype. The increase in spleen cellularity in mice with oral cancer further accentuated the magnitude of the increase in Treg.
The shift from increased levels of cells with a Th17 phenotype in mice with premalignant lesions to a Treg phenotype in mice with oral cancer prompted assessment of spleen cell secretion of mediators that are supportive of a Th17 phenotype or a Treg phenotype during the course of progression from lesions to cancer. These studies showed increased secretion of IL-23 and low levels of TGF-β secretion during the period of when levels of Th17 and secretion of IL-17 were increased. However, as lesions progressed to oral cancer, levels of the Th17-supportive cytokine, IL-23, declined and this decline was then followed by a decline in secretion of IL-17. As levels of IL-23 declined, production of TGF-β increased, suggesting a shift in cytokine production that favored skewing from a Th17 to a Treg phenotype.
The role of Th17 cells in cancer development has been conflicting and may depend on whether the role is being evaluated in the context of inflammation-induced tumor promotion or their role in later stages of tumor progression. Assessments of Th17 and IL-17 levels in the peripheral blood of gastric cancer patients showed levels to be increased as compared to levels in healthy controls.16 Also, expression of Th17-associated cytokines in patients with hypopharyngeal carcinoma was increased with more advanced-stage cancer.15 In contrast, while IL-17 and other Th17-associated cytokine levels were elevated in non-small cell lung cancer patients, levels were lower in subjects with more advanced cancer.17 Such studies differ in part from the results of the present study which showed that the Th17 phenotype is low in mice with oral cancer, but they share with the results in this study the decline in the Th17 phenotype as cancer advance. Not yet determined is whether these correlations of increases in Th17 cells or Th17-associated cytokines with cancer presence suggest their contributions to cancer progress or if it represents an attempted, but failed, immune response.
Studies to determine the impact of modulating the Th17 phenotype on tumor development or progression are far fewer than studies showing correlations between tumor and Th17 cells. Studies in a mouse mammary tumor model showed that treating mice with anti-IL-17 neutralizing antibody slowed growth of the inoculated tumors.19 In contrast, murine studies with premalignant epidermal squamous lesions showed that induction of Th17 cells led to tumor regression.20 Also, a murine glioma tumor model showed that the anti-tumor effects of a tumor vaccine strategy were associated with increased splenic Th17-type immunity.25 In a clinical tumor vaccine study with melanoma patients, those who had higher Th17 cell levels at the time of vaccination had an improved rate of survival.26
Because of the paucity of interventional studies to determine the role of the Th17 phenotype in cancer development, and in particular development of oral cancer, studies were conducted in the 4NQO model to determine if treatment aiming to maintain the Th17 phenotype that is seen in mice with premalignant oral lesions would have a positive or negative immunological effect and whether it would reduce versus enhance progression of oral lesions to cancer. The interventional approach was specifically selected to be amenable to being used clinically for patients having premalignant oral lesions with a high likelihood of progressing to cancer. Treatment of mice bearing premalignant oral lesions for approximately 2 months with the combination of the TGF-β type I receptor inhibitor GW788388 and the Th17-stabilizing cytokine IL-23 sustained increased production of IL-17. However, it also resulted in increased production of other inflammatory and stimulatory mediators. Surprisingly, in the midst of the combination treatment increasing spleen cell production of stimulatory/inflammatory mediators, production of the inhibitory mediator IL-10 was also increased. How this increased production of L-10 impacted on overall immune competence is not clear. In contrast, production of IL-10 by lymph node cells of mice receiving the combination treatment was not increased. In most cases, the effect of the treatment with GW788388 plus IL-23 on cytokine production was similar for spleen cells and lymph node cells, indicating that the sustainment of a Th17 and an inflammatory/stimulatory phenotype occurred locally as well as distally. Treatment with GW788388 alone or with IL-23 alone typically had no effect on cytokine production. Of clinical significance, this study showed an improved clinical response to the combination treatment, as seen by a reduction in the progression of premalignant oral lesions to oral cancer.
While the present studies were terminated and mice were euthanized when tumors became advanced in groups receiving control treatment or treatment with either GW788388 or IL-23 alone, future studies are needed to determine the feasibility of maintaining a longer-term sustainment of a Th17 phenotype. Specifically, studies are needed to determine the longer-term duration of the clinical effect of the combination treatment, including assessment of whether the combination treatment slows or prevents progression to cancer, and whether it might induce regression. Also needed is determination of whether the sustained Th17 reactivity exhibits specificity toward premalignant lesions or oral cancer. Treatment of mice having premalignant oral lesions with GW788388 plus IL-23 to sustain a Th17 phenotype also triggered other inflammatory/stimulatory cytokines, but it has yet to be determined whether these other cytokines are stimulated secondary to a Th17 response. Also yet to be determined are the mechanisms involved in the slowing of the progression to cancer. It is possible that the combination treatment reduced premalignant lesion progression to cancer either through the sustainment of a Th17 phenotype or, alternatively, it might be completely independent on the effects on Th17 cells. For example, it may be that tumor progression is delayed as a result of GW788388 inhibition of TGF-β signaling. However, the lack of clinical response in mice receiving treatment with GW788388 alone would suggest that this would not be the only explanation for the effectiveness of the combination treatment. Clearly, the mechanisms that lead to the reduced progression need to be defined. The present study is one of a handful of interventional studies to assess the role of Th17 cells in progression of lesions to cancer and the only study that the authors are aware of that used an interventional approach to show that treatment to sustain a Th17 phenotype limits progression of premalignant oral lesions to oral cancer.
What’s new?
While premalignant oral lesions generally are small and localized, their immunological impact on distant tissues is unknown. Here, the spleens and regional lymph nodes of mice with carcinogen-induced premalignant oral lesions were found to trend toward Th1- and Th17-type phenotypes. As lesions advanced to oral cancer, phenotypes transitioned to Treg and TGF-β production. In mice with premalignant lesions, Th17 was sustained following treatment with a TGF-β type 1 receptor inhibitor and IL-23. Treatment also elevated the production of other immunological mediators by the spleen and regional lymph nodes and decreased the rate of progression of premalignant oral lesions.
Acknowledgments
Grant sponsor: Clinical Sciences Research Program of the Department of Veterans Affairs; Grant number: I01-CX000851; Grant sponsor: National Institutes of Health; Grant number: R01-CA128837 (M.R.I. Young)
References
- 1.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. [Accessed on December 18, 2015];Surveillance Epidemiology and End Results. 2015 Available at: http://seer.cancer.gov/statfacts/html/oralcav.html.
- 3.Tang XH, Knudsen B, Bemis D, et al. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 4.De Costa AM, Justis DN, Schuyler CA, Young MR. Administration of a vaccine composed of dendritic cells pulsed with premalignant oral lesion lysate to mice bearing carcinogen-induced premalignant oral lesions stimulates a protective immune response. Int Immunopharmacol. 2012;13:322–30. doi: 10.1016/j.intimp.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi H, Sakakura K, Kawabata-Iwakawa R, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2015;64:1407–17. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang WL, Chang WL, Yang HB, et al. Quantification of tumor infiltrating Foxp3+ regulatory T cells enables the identification of high-risk patients for developing synchronous cancers over upper aerodigestive tract. Oral Oncol. 2015;51:698–703. doi: 10.1016/j.oraloncology.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Weed DT, Vella JL, Reis IM, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W, Li WJ, Fu QL, et al. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. 2015;33:354–62. doi: 10.3892/or.2014.3553. [DOI] [PubMed] [Google Scholar]
- 9.De Costa AM, Schuyler CA, Walker DD, et al. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2011;61:927–39. doi: 10.1007/s00262-011-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodford D, Johnson SD, De Costa A-MA, et al. An inflammatory cytokine milieu is prominent in premalignant oral lesions, but subsides when lesions progress to squamous cell carcinoma. J Clin Cell Immunol. 2014;5:1–17. doi: 10.4172/2155-9899.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Sun X, Zhao J, et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–301. doi: 10.1136/annrheumdis-2013-204228. [DOI] [PubMed] [Google Scholar]
- 12.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–12. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moudgil KD. Interplay among cytokines and T cell subsets in the progression and control of immune-mediated diseases. Cytokine. 2015;74:1–4. doi: 10.1016/j.cyto.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Tian X, Mumtahana F, et al. The existence of Th22, pure Th17 and Th1 cells in CIN and cervical cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer. 2015;15:717. doi: 10.1186/s12885-015-1767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Wang J, Wang R, et al. Th1-, Th2-, and Th17-associated cytokine expression in hypopharyngeal carcinoma and clinical significance. Eur Arch Otorhinolaryngol. doi: 10.1007/s00405-015-3779-2. in press. [DOI] [PubMed] [Google Scholar]
- 16.Zhong F, Cui D, Tao H, et al. IL-17A-producing T cells and associated cytokines are involved in the progression of gastric cancer. Oncol Rep. 2015;34:2365–74. doi: 10.3892/or.2015.4246. [DOI] [PubMed] [Google Scholar]
- 17.Liao C, Yu ZB, Meng G, et al. Association between Th17-related cytokines and risk of non-small cell lung cancer among patients with or without chronic obstructive pulmonary disease. Cancer. 2015;121:3122–9. doi: 10.1002/cncr.29369. [DOI] [PubMed] [Google Scholar]
- 18.Deng Z, Mu J, Tseng M, et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat Commun. 2015;6:6956. doi: 10.1038/ncomms7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benevides L, da Fonseca DM, Donate PB, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75:3788–99. doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed J, Ryscavage A, Perez-Lorenzo R, et al. β1-induced inflammation in premalignant epidermal squamous lesions requires IL-17. J Invest Dermatol. 2010;130:2295–303. doi: 10.1038/jid.2010.92. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SD, De Costa AM, Young MR. Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer. Cancers. 2014;6:756–70. doi: 10.3390/cancers6020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. IL-23 Provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–93. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 23.Mensah-Brown EP, Shahin A, Al Shamisi M, et al. IL-23 leads to diabetes induction after subdiabetogenic treatment with multiple low doses of streptozotocin. Eur J Immunol. 2006;36:216–23. doi: 10.1002/eji.200535325. [DOI] [PubMed] [Google Scholar]
- 24.Tan SM, Zhang Y, Connelly KA, et al. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:H1415–25. doi: 10.1152/ajpheart.01048.2009. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, Fang M, Xuan W, et al. The therapeutic potency of HSP65-GTL in GL261 glioma-bearing mice. J Immunother. 2015;38:341–9. doi: 10.1097/CJI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 26.Kollgaard T, Ugurel-Becker S, Idorn M, et al. Pre-vaccination frequencies of Th17 cells correlate with vaccine-induced T-cell responses to survivin-derived peptide epitopes. PLoS One. 2015;10:e0131934. doi: 10.1371/journal.pone.0131934. [DOI] [PMC free article] [PubMed] [Google Scholar]