Abstract
Sipuleucel-T, an autologous cellular immunotherapy manufactured from antigen-presenting cells primed to recognize prostatic acid phosphatase, was the first immunotherapy product approved by the US FDA. It was approved for men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer after it was shown to provide a survival advantage. Additional studies have examined its use in other clinical settings and in combination with other approved and investigational immunotherapy agents. This review will discuss the pivotal trials leading to approval, will outline some of the biomarkers associated with its efficacy and will review some of the ongoing combination strategies. Maximizing the efficacy of sipuleucel-T through better patient selection or through combination approaches remains the challenge of the future.
Keywords: : biomarkers, cancer vaccine, castration-resistant prostate cancer, immunotherapy, prostate cancer, sipuleucel-T
Prostate cancer is the most prevalent male cancer and the third most common cause of cancer death in men in the USA [1]. In 2017, it is estimated that approximately 11% of men (one in nine) will be diagnosed with prostate cancer in their lifetime, and >160,000 cases will be diagnosed in the USA this year [2]. At presentation, most of these men will have disease that is localized to the prostate gland [3] which can be managed with active surveillance, radiation therapy or radical surgery with no significant difference in survival [4]. However, up to 50% of men receiving definitive local therapy will subsequently develop biochemical recurrence evidenced by a rising prostate-specific antigen (PSA) level [5,6]. Therapeutic options for biochemical recurrent disease vary based on patient and tumor characteristics, particularly the PSA doubling time [7]. Fewer will go on to develop metastatic disease, which is then incurable [1]. Initially, metastatic prostate cancer is hormone sensitive and treated with androgen deprivation therapy (ADT) or orchiectomy to achieve castrate levels of serum testosterone. Docetaxel and abiraterone with prednisone can be added to the treatment paradigm for some patients in the hormone-sensitive space [8,9]. However, the disease can continue to progress despite castrate levels of testosterone, a state known as castration-resistant prostate cancer (CRPC). For CRPC, there are currently six therapies approved by the US FDA that prolong overall survival [10]. Exactly when and in what sequence to use these drugs is an area of active research [11]. Therapy choice can be affected by the location of disease (i.e., presence of metastatic disease in the bones or presence of visceral/lymph node disease) and the severity of symptoms related to the disease [11]. Sipuleucel-T is the only immune therapy currently approved for prostate cancer by the FDA, and the first therapeutic vaccine to be approved for any cancer [10]. It was FDA approved for asymptomatic or minimally symptomatic metastatic CRPC in 2010, and is manufactured by Dendreon Corporation [12]. This review will focus on its development, current clinical use and future directions.
Background: therapy/pharmacology
Dendritic cells to treat cancer
The basic idea behind immunotherapy is to activate the host immune system, in the case of sipuleucel-T using dendritic cells (antigen-presenting cells), for tumor control. Dendritic cells are the most powerful antigen-presenting cells in the human body and serve to stimulate B- and T-lymphocytes to modulate the body's immune responses [13]. Dendritic cells were first identified in 1973 [14]. It was not until the 1990s that their use in cancer was established when studies in mice showed that dendritic cells could be removed from the host, activated ex vivo with tumor-related antigens and replaced in vivo resulting in protection from tumor inoculation [15]. Similar studies were done with ex vivo priming of autologous dendritic cells with human papillomavirus peptides [16] and with synthetic peptides, with effective tumor response that was mostly driven by T-cell responses [17]. Subsequent research demonstrated that immunity from developing tumors in mice with this method is specific to the tumor antigen used ex vivo to stimulate the dendritic cells [18]. To further increase T-cell activation and subsequent tumor-specific immune response, granulocyte-macrophage colony-stimulating factor (GM-CSF) was used in combination with tumor-specific antigens with success [19,20].
Early pilot studies were performed in humans using ex vivo pulsed dendritic cells to treat follicular B-cell lymphomas [21], multiple myeloma [22], melanoma [23] and carcinoembryonic antigen (CEA)-expressing cancers including colon, breast, ovarian, pancreatic and ampullary cancers [24]. These early trials demonstrated that this approach was safe, produced a tumor-specific immune response and demonstrated tumor activity against the respective tumors [21–24].
Dendritic cell-based therapy for prostate cancer
In prostate cancer, the first step in development was to select a potential tumor-specific target. Prostatic acid phosphatase (PAP) is a prostate-specific enzyme produced by prostatic tissue, first described in association with prostate cancer in the 1930s [25]. It is overexpressed in prostate cancers with very little expression in other tissues or organs [26], and was initially considered as a screening tool [27]. When used as a potential immune target, it was shown in vitro that it could be combined with dendritic cells to induce cytotoxic T-lymphocytes, which then were able to recognize and kill prostate cancer tumor cells [28]. Further animal studies showed that when PAP was conjugated with GM-CSF (the fusion protein, called PA2024), there were greater antigen-specific immune responses [29].
For the initial human studies, patients had 1.5–2 blood volumes collected via leukapheresis to obtain dendritic cell precursors which comprise about 1% of peripheral cells in whole blood [30]. The blood collection is then sent to Dendreon Corporation where it is depleted of erythrocytes, granulocytes, platelets, low-density monocytes and lymphocytes. What remain is only dendritic cell precursors which are then placed in culture media and incubated with PA2024 for 36–40 h. It is then combined with 250 ml of lactated ringer's solution to prepare for reinfusion back to the patient. Quality measures are checked to ensure sterility and cell viability [29]. It is then sent back to the local oncologist's infusion center and reinfused over 30 min into the patient over 30 min. The entire process from blood volume collection to reinfusion is about 36–48 h long.
Early-phase clinical trials
Small et al. completed the early Phase I/II studies which were done in men with minimally symptomatic or asymptomatic (not requiring narcotic pain medication for treatment) bone-only metastatic CRPC in the Phase I portion and 19 men with castration-resistant biochemical recurrence, without evidence of metastatic disease by standard imaging, in the Phase II portion [29]. Participants were tested and enrolled if negative for HIV, hepatitis B, hepatitis C and human T-cell leukemia virus type-1 (HTLV1). Furthermore, men on systemic corticosteroids or other immunosuppressive agents were not eligible for these trials. Twelve men enrolled on the initial study. Product potency was found to be most strongly correlated with the presence of CD54+ cells, which are not specifically dendritic cells but was associated with the antigen-presenting activity present in the samples so was established as a surrogate measure of number of dendritic cells. T-cells, B-cells, monocytes and natural killer cells were also present in the final product. The total number of nucleated cells per square millimeter was evaluated and the planned dose escalation for the Phase I trial was between 0.2 × 109 cells and 2 × 109 cells/m2. The upper limit was the maximum dose that could be manufactured. One dose was given every 4 weeks for a total of three doses for the Phase I and II trials, with an additional booster dose given at week 24 if the patient had stable disease or at least a partial response [29]. There were no dose-limiting toxicities observed and the maximum dose able to be manufactured was used in later-phase trials [29].
Administration
In the Phase III trials the dosing schedule was amended to once every 2 weeks for a total of three doses, and this is the FDA-approved dose of sipuleucel-T. The product is infused via peripheral intravenous line over 30 min. Diphenhydramine and acetaminophen were also added 30 min prior to each infusion based on earlier trials, and these are the recommended premedications in standard use [31–33].
Immune responses
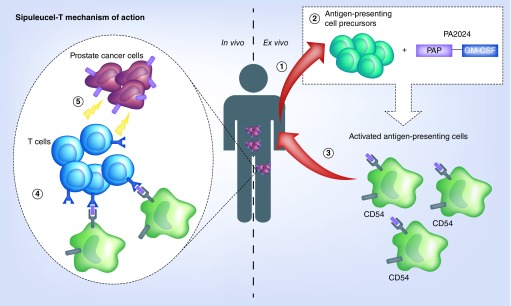
In the initial Phase I and Phase II trials, all men developed T-cell responses to PA2024 (the antigen used in co-culture of the dendritic cells) after a single dose of sipuleucel-T. There was a further increase in antigen-specific T-cell response after the second dose compared with the first, but no further increase after the third dose when compared with the second dose. There was also no difference in the degree of T-cell response dependent on dose received. To evaluate if the immune response was a generalized increase in T-cell responsiveness compared with antigen-specific, responses to influenza were tested and were not seen to be increased after treatment. In addition to the T-cell responses, increases were seen in (IgM and IgG) antibody responses to PAP and GM-CSF [29]. The mechanism of action of sipuleucel-T is illustrated in Figure 1.
Figure 1. . Mechanism of action of sipuleucel-T.
Clinical efficacy
Efficacy was first evaluated in the original Phase I and II trials. Six of the men treated in the Phase I portion of the original study by Small et al. were given the maximum dose which has since been used in all subsequent studies [29]. There were no radiographic responses noted in the six men with metastatic disease; however, three patients had a decrease in PSA of ≥50% and three had a PSA response between 25 and 49% suggesting some anti-cancer activity [29].
There were two subsequent and simultaneous small Phase III trials of sipuleucel-T (D9901 and D9902A), published together as a joint analysis [31]. The primary end point of these trials was disease progression defined as radiographic progression, pain related to bone disease or a clinical event like spinal cord compression. Patients with visceral metastases were excluded from these trials. PSA progression was not included in the primary end point and only 26% of patients had evaluable PSA responses (at least two PSA values at least 4 weeks apart). In both of these studies, there was no difference in median time-to-clinical or radiographic progression between treatment and placebo groups (p = 0.72). Overall survival was a planned analysis, and after all patients were followed for 3 years, there was a 4.3 month improvement in overall survival (p = 0.01) favoring the treatment group [31].
This led to the pivotal trial, IMPACT, which subsequently resulted in FDA approval of sipuleucel-T. The IMPACT trial was a larger Phase III trial with asymptomatic or minimally symptomatic metastatic CRPC (serum testosterone <50 ng/dl). Patients with poor performance status (Eastern Cooperative Oncology Group [ECOG] performance status ≥2), those with visceral metastases, significant skeletal-related events including pathologic long bone fractures or spinal cord compression and those who were recently treated with any chemotherapy or received more than two lines of chemotherapy were excluded from this trial. Overall survival was the primary end point, and was prolonged by 4.1 months in the treatment group when compared with placebo (p = 0.03), although there was no difference in progression-free survival (p = 0.63) or time-to-clinical progression [33].
In all three of the Phase III trials, men who progressed on the placebo arm were allowed to cross over to receive sipuleucel-T. Two-thirds of the cells collected at the initial leukapheresis were cryopreserved at time of collection. After progressive disease, these cells were used for culture with PA2024 and antigen-presenting cell activation, and then administered using the same methodology as sipuleucel-T every 2 weeks for a total of three infusions. A later analysis showed that the men on the placebo arm who went on to receive the previously cryopreserved product (called APC8015F) had a significantly longer median overall survival (20 months compared with a median survival of 9.8 months in the men who never crossed over) [32]. There were significant differences in the groups which may have contributed to these results, including age, extent of disease and ECOG status. However, it raises the possibility that the survival effect seen in the initial IMPACT trial may be underestimated given that over 50% of patients in the IMPACT trial crossed over to receive immunotherapy [34].
Based on the safety data from the early Phase I and II trials, and the efficacy demonstrated by those in addition to the overall survival benefit in the Phase III trials (particularly the IMPACT trial), the FDA approved use of sipuleucel-T in 2010 for asymptomatic or minimally symptomatic men with metastatic CRPC [12].
Sipuleucel-T efficacy has since been studied earlier in the disease course, although use in this setting is not FDA approved. In the neoadjuvant setting, for example, Fong et al. conducted a Phase II trial in men who were scheduled to have radical prostatectomies to determine the direct inflammatory effect of the immunotherapy on the tumor. Prostatectomy specimens in those who received presurgical sipuleucel-T were compared with specimens from participants who did not receive neoadjuvant therapy. Within the tumor, the greatest infiltration of immune cells was found at the interface of the tumor with normal tissue and this included CD3+ T cells, CD4+ Tregs, helper T cells and to a lesser extent B cells [35]. Down staging of the primary tumor was not observed when compared with pre-prostatectomy biopsies, and this highlights that the presence of immune cells alone may not by itself be sufficient for definitive tumor killing [36].
Multiple studies have been done in men with biochemically recurrent disease. A small Phase II trial was conducted with 18 patients who all had biochemical recurrence with rising PSA after definitive surgery or radiation. All 18 men were treated with sipuleucel-T and there was a slowing of PSA doubling time in 13 of the 18 subjects, but treatment did not result in actual declines in absolute PSA levels [37].
A larger Phase II randomized trial was done in men with biochemically recurrent disease after definitive local therapy, where men were randomized (2:1) to receive sipuleucel-T or placebo 3–4 months after the initiation of ADT. The study did not find a difference in the primary end point of time-to-biochemical failure (defined as PSA >3 ng/ml), but did show a significant slowing of PSA doubling time (PSA doubling time of 155 days in the treated arm compared with 105 days in the control arm, p = 0.038). This trial was limited by methodological issues, with no confirmatory PSA after biochemical failure measured, and an arbitrary primary end point of PSA level defining biochemical failure [38].
The most recent data for sipuleucel-T in biochemically recurrent prostate cancer come from the STAND trial [39]. This was a Phase II trial in men with two consecutive PSA values ≥0.2 or two rising values ≥2.0 ng/ml and a PSA doubling time <12 months. The primary end point in this trial was systemic T-cell-specific immune response which is a novel end point in trials using sipuleucel-T, but used given the correlation between immune response and overall survival as described below [39]. In this trial, participants received ADT for 1 year, with sipuleucel-T starting either 6 weeks before the first dose of ADT or 12 weeks after first dose of ADT. Participants who had sipuleucel-T prior to starting ADT had greater peripheral T-cell PA2024-specific immune responses compared with those who had sipuleucel-T after starting ADT, although nearly all (>90%) patients had some measurable immune response. In this study, despite the increase in immune-mediated factors, there was no difference in time-to-next intervention or time-to-metastasis [39]. In addition, since this study did not include an ADT-alone arm, the independent contribution of sipuleucel-T on clinical outcomes could not be evaluated. Therefore, based on the existing data, sipuleucel-T should not be recommended in the biochemical recurrence setting.
Biomarkers to determine clinical efficacy
In the large randomized Phase III trials of sipuleucel-T, a notable finding was no difference in progression-free survival despite an improvement in overall survival [31–33]. This is similar to data that have been seen in other immunotherapy trials, as was seen with nivolumab when used in lung cancer [40] and ipilimumab in melanoma [41]. It may be that classical end points used in prostate cancer, including PSA-related end points, may not be optimal when applied to immune therapies, the way that traditional RECIST criteria needed to be modified after atypical responses seen in immunotherapy trials in other solid tumors [42]. There is also the thought that, unlike chemotherapy which has effects seen close in time to the administration of the drug, the effects of sipuleucel-T may continue to alter disease progression long after treatment is given and additional research is needed to determine who best responds to this therapy [43].
Biomarkers are being researched across many disease specialties to help predict response to immunotherapy. For sipuleucel-T, there are no FDA-approved biomarkers to help determine who is likely to respond or who will have long-term responses after treatment. In general, there are two types of immune parameters that are being studied in sipuleucel-T treatment trials: immune characteristics of the autologous product and host characteristics.
Product parameters
In the original Phase I and II trials by Small et al., within the product there was a correlation between number of cells infused and time to disease progression, with those patients receiving >100 × 106 cells/infusion having a longer median time-to-disease progression compared with those who received fewer than that (31.7 weeks compared with 12.1 weeks) [29]. In the Higano integrated analysis study of the Phase III trials, there was a strong association between increased CD54+ cells – which is actually measured in the product being delivered back to the patient – and overall survival [31]. Sheikh et al. found that the highest product factors: cumulative antigen-presenting cell activation, antigen-presenting cell count and total nucleated cells count, correlated with improved overall survival [44].
Host parameters
In the IMPACT trial, lower PSA was the strongest predictor of response. Participants who had the lowest PSAs at the start of treatment (in this paper, the lowest quartile was defined as ≤22.1 ng/ml) had higher survival rates compared with those participants who had higher baseline PSA values [45].
Sheikh et al. did a secondary analysis of the data from the three Phase III trials of sipuleucel-T [44]. For post-treatment host factors, the development of a detectable antibody peripheral immune response to either the conjugated protein PA2024 or to PAP itself correlated with overall survival. In this study, T-cell proliferation in the host alone was not statistically correlated with the overall survival results [44].
It has also since been found that in addition to immune responses to the components of sipuleucel-T (PAP and PA2024), there are also off-target effects including antibodies to other prostate cancer-associated tumor antigens including PSA, KLK2, K-ras, E-ras, LGALS8 and LGALS3, and these may be important [46]. This so-called phenomenon of ‘antigen spread’ suggests that sipuleucel-T triggers an immune cascade that subsequently results in antibody production against other prostate cancer-associated antigens distinct from the PA2024 target antigen [47].
Safety & tolerability
Overall, sipuleucel-T is well tolerated. In the Phase III combined trials, >97% of patients were able to receive all three infusions [31], and in the IMPACT trial, 92% of patients were able to receive all three infusions with <1% of patients being unable to receive all infusions because of infusion-related adverse events [33]. The integrated Phase III trial analysis showed no increase in grade 3–4 adverse events in the treatment arm [31]. There was, however, an increased rate of grade 3 adverse events in the IMPACT trial (6.8% in treatment vs 1.8% in placebo) [33]. Consistent with earlier studies, almost all patients (>95%) had some inflammatory reaction; however, most of these were mild and resolved within days after each infusion [31,33]. The most common adverse reactions included chills, fatigue, pain and low-grade fever [33,48,49]. A summary of serious adverse events reported in the major clinical trials is shown in Table 1. Anaphylaxis and autoimmune reactions were not seen [33,48,49].
Table 1. . Number of men who experienced grade 3 and higher adverse effects reported in major clinical trials of sipuleucel-T.
| Name of study | Phase I/II trial | Integrated analysis of Phase III trials | IMPACT Phase III trial |
|---|---|---|---|
| Year of publication (reference) Number of men who received sipuleucel-T |
2000‡ n = 31 |
2009§ n = 147 |
2010¶ n = 127 |
| Any grade 3 or greater toxicity | 2 | 37 | 69 |
| Pain (includes back, limb, musculoskeletal pain, myalgias) | –† | 10 | 39† |
| Fatigue | – | 2† | 10† |
| Chills | – | 7† | 4† |
| Anemia | – | 6 | 5 |
| Fever | 2† | 3† | 1 |
| Dyspnea | – | 5 | |
| GI upset including nausea/vomiting/diarrhea | – | 2 | 3 |
| Headache | – | 2 | 1 |
| Hypertension | – | – | 2 |
| Weight loss | – | – | 2 |
| Anorexia | – | – | 1 |
| Depression | – | – | 1 |
The Phase III integrated analysis was the first to report a possible increased risk of cerebrovascular events due to sipuleucel-T treatment [31]. This was because the incidence of cerebrovascular events – including hemorrhagic, ischemic or embolic stroke, transient ischemic attack or bleeding from a metastatic lesion – was seen in 7.5% of those in the treatment arm but only 2.6% of participants in the placebo arm [31]. The IMPACT trial did not demonstrate an increased risk of cerebrovascular events (2.4% in treatment arm vs 1.8% in placebo arm) [33]. However, based on these results, the FDA required a postmarketing registry to determine the rate of cerebrovascular events with sipuleucel-T [12]. One study has since been published including the three Phase III trials as well as the Phase II trial in hormone-sensitive biochemical relapse [38]. The combined data of all four trials showed an incidence of cerebrovascular events of 3.5% after treatment with sipuleucel-T compared with 2.6% in control patients which was not significantly different [49]. Results of the registry (called the PROCEED trial – NCT01306890) have not yet been reported but are expected in late 2017 [50].
Current status of immunotherapy in prostate cancer
Great strides have been made in the field of immuno-oncology, yet besides sipuleucel-T, there have been disappointing results in prostate cancer. Large Phase III trials of the CTLA-4 inhibitor, ipilimumab, did not show any improvement in overall survival compared with placebo in patients with metastatic castration resistant prostate cancer either before [51] or after treatment with chemotherapy [52]. Additionally, a basket trial including men with metastatic CRPC showed no responses after treatment with the single-agent PD-1 antibody, nivolumab [53]. However, some PSA responses as well as objective responses have been observed using both CTLA-4 and PD-1 inhibitors together, suggesting that a subset of patients may benefit. In a recent study adding pembrolizumab to metastatic CRPC patients progressing on enzalutamide, dramatic PSA and objective responses were seen in a significant number of men [54]. In another small trial combining ipilimumab and nivolumab, PSA and objective responses appeared to be more frequent than with each agent used alone, and there was a suggestion that patients with DNA repair gene mutations derived the greatest benefit [55]. Finally, with the recent FDA approval in May 2017 of pembrolizumab for mismatch repair-deficient or microsatellite-unstable cancers of any histology, this will clearly have applicability to a small subset of prostate cancers [56].
Why has prostate cancer not been as responsive as other diseases to immunotherapy? For one, prostate cancer has fewer somatic mutations compared with other tumors that are responsive to immunotherapy, like lung cancer and melanoma, and it is thought this yields fewer neoantigens that would stimulate immune responses [57]. Additionally, the prevalence of mismatch repair inactivating genes, a predictive marker of response to PD-I inhibitors [58], is low in prostate cancer, about 2–3% overall [59]. Additionally, there may be other pathways and targets that provide resistance to immunotherapy, with one such molecule, VISTA, recently described as an inhibitory molecule important in the pathway inhibiting response to ipilimumab in prostate cancer patients [60].
However, there are opportunities to build on the success of sipuleucel-T by combining it with other approved cancer therapies which could increase the immune response generated by sipuleucel-T. A small Phase II trial (22 patients) with biochemically recurrent prostate cancer gave patients sipuleucel-T and concurrent bevacizumab with continuation of bevacizumab until toxicity or disease progression. This study of 22 men showed an improvement in PSA doubling time from a median of 6.9–12.7 months (p = 0.01) [61]. There are no other trials currently ongoing with this combination, probably due to the failure of the Phase III bevacizumab prostate cancer trial [62]. Another trial compared sipuleucel-T with concurrent versus sequential abiraterone, and no difference was found in the product or host immune effects when administered with abiraterone. PSA end points in that trial included percentage of patients with at least a 50% PSA decline and maximal percentage decrease of PSA, and there was no difference between the two arms [63]. Enzalutamide was also studied in combination or in sequence with sipuleucel-T in men with asymptomatic or minimally symptomatic metastatic CRPC. Preliminary data from that study suggest increased product immune activation, measured by CD54+ upregulation in the product, in the arm with concurrent enzalutamide which was started 2 weeks prior to infusion of sipuleucel-T [64]. Additional clinical results are pending from the full trial which was set to include data for 50 patients [64].
Finally, a Phase I trial of nine patients was recently completed with sipuleucel-T followed by ipilimumab. Patients were treated with one, two or three doses of ipilimumab dosed at 1 mg/kg (approved dose is 3 mg/kg in melanoma) [65]. This study showed an increase in immunoglobulins after dosing with ipilimumab that was above what is historically seen with sipuleucel-T alone, suggesting that it enhances the immune response and may provide additional clinical benefit without significant side effects [66]. Additional combination studies to test if the immune response of sipuleucel-T can be increased are underway (listed in Table 2).
Table 2. . Selected ongoing clinical trials of sipuleucel-T in the treatment of prostate cancer.
| Trial identifier | Phase | Title and sample size | Description of trial |
|---|---|---|---|
| NCT02793219 | II | Sipuleucel-T followed by docetaxel in castration-resistant prostate cancer n = 32 |
Effect of docetaxel chemotherapy when given immediately following sipuleucel-T on immune biomarkers and clinical end points |
| NCT02793765 | II | Docetaxel followed by Sipuleucel-T in metastatic prostate cancer n = 32 |
Effect of docetaxel chemotherapy when given immediately prior to sipuleucel-T on immune biomarkers and clinical end points |
| NCT01804465 | II | A randomized Phase II trial combining sipuleucel-T with immediate vs delayed CTLA-4 blockade for prostate cancer n = 54 |
Effect of different timing of ipilimumab on immune responses and clinical end points |
| NCT03024216 | Ib | Clinical study of atezolizumab (Anti-PD-L1) and sipuleucel-T in patients with asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer n = 34 |
Safety effect of combination immunotherapy with sipuleucel-T with anti-PD-L1 drug atezolizumab |
| NCT01560923 | II | Phase II study of sipuleucel-T and indoximod (IDO inhibitor) for patients with refractory metastatic prostate cancer n = 47 |
Randomized study of sipuleucel-T followed by the IDO inhibitor indoximod to evaluate immune response and clinical end points |
| NCT01706458 | II | Sipuleucel-T with or without pTVG-HP DNA booster vaccine in prostate cancer n = 18 |
Randomized trial to evaluate effect of a PAP-directed plasma DNA vaccine on immune response to sipuleucel-T |
| NCT02463799 | II | Phase II randomized study of sipuleucel-T with or without radium-223 in men with asymptomatic or minimally symptomatic bone-metastatic CRPC n = 34 |
Randomized trial to evaluate effect of combination of sipuleucel-T with radium-223 on immune response and clinical outcomes |
|
NCT02232230 NCT01833208 NCT01818986 |
II | Sipuleucel-T and external-beam radiation for metastatic castrate-resistant prostate cancer n = variable |
Sipuleucel-T given in combination with various forms of radiation to metastatic lesion to see if response to sipuleucel-T is altered |
| NCT01881867 | II | CYT107 after vaccine treatment (Sipuleucel-T) in patients with metastatic castration-resistant prostate cancer n = 54 |
Randomized trial to study immune response to sipuleucel-T when recombinant glycosylated human interleuken-7 (CYT 107) is given versus not given after sipuleucel-T |
IDO: Indoleamine 2,3 dioxygenase; PAP: Prostatic acid phosphatase; pTVG-HP: Plasmid DNA vaccine encoding human prostatic acid phosphatase.
Regulatory affairs
Based on the Phase III clinical trial results, the FDA approved sipuleucel-T monotherapy in 2010 for the treatment of metastatic CRPC with minimal or no symptoms (i.e., no narcotics for cancer-related pain) [12]. The EMA initially approved sipuleucel-T in 2013 for men with minimal or no symptoms who have bone-only metastatic CRPC [67]; however, it was later withdrawn in 2015 at the request of the manufacturer, Dendreon, because no further European development or marketing was planned [68]. It is currently not available in any other country although Dendreon was recently acquired by the Chinese company Sanpower Group with possible plans to promote use in China and Southeast Asia according to a company press release [69].
Conclusion
Sipuleucel-T is still the only agent in its class for the treatment of advanced prostate cancer. It is an autologous cellular vaccine that activates the host immune system and prolongs survival in men with asymptomatic and minimally symptomatic metastatic CRPC. There are many other clinical trials, and more to be completed soon, that suggest it may be useful in earlier stages of disease, and its efficacy may be improved through combinations with other therapies. However, outside of these clinical trials, sipuleucel-T should not currently be used in earlier prostate cancer disease states, nor should it be combined with other agents. That being said, discovering sipuleucel-T-based combination strategies that may result in serological and/or objective clinical responses must be our goal moving forward.
Executive summary.
Sipuleucel-T is the only approved cellular immunotherapy in prostate cancer
Sipuleucel-T was US FDA approved in 2010 for the treatment of metastatic castration-resistant prostate cancer.
It is approved for use in men with asymptomatic disease and no visceral metastases.
Mechanism of action of sipuleucel-T
Autologous cellular vaccine – peripheral blood containing dendritic cells are removed, activated ex vivo with PA2024 (prostatic acid phosphatase and granulocyte-stimulating factor fusion protein) and then reinfused into the patient.
The in vivo response is mostly antigen-specific T-cell immune stimulation.
Outcomes
Prolongs median overall survival by approximately 4 months compared with placebo but does not prolong progression-free survival.
Major side effects
Most common: chills, fatigue, pain and fever.
More than 95% of patients will have inflammatory reaction around time of infusion that goes away within days.
Autoimmune and anaphylactic reactions have not been seen in clinical trials with sipuleucel-T.
Ongoing registration study to determine if there is an increased risk of cerebrovascular events.
Future perspective
Sipuleucel-T is currently being tested earlier in the disease course, including in the neoadjuvant and biochemically recurrent disease state.
It is also being tested in combination with other immunotherapies to try and enhance immune response.
Conclusion
Sipuleucel-T is an autologous cellular vaccine, first-in-class in prostate cancer, used for the treatment of asymptomatic, metastatic, bone-only and castration-resistant prostate cancer.
Finding new ways to enhance the immune response in prostate cancer is an active and promising area of new research.
Footnotes
Financial & competing interests disclosure
This work was partially supported by NIH grant P30 CA006973 (ES Antonarakis) and Conquer Cancer Foundation/Bristol-Meyers Squibb Young Investigator Award (CE Handy). ES Antonarakis is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation, ESSA, AstraZeneca, Clovis and Merck; he has received research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol Myers-Squibb, AstraZeneca, Clovis and Merck; and he is the co-inventor of a biomarker technology that has been licensed to Tokai and Qiagen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program. Cancer statistics. 2017. www.cancer.gov/about-cancer/understanding/statistics
- 3.Herget KA, Patel DP, Hanson HA, Sweeney C, Lowrance WT. Recent decline in prostate cancer incidence in the United States, by age, stage, and Gleason score. Cancer Med. 2016;5(1):136–141. doi: 10.1002/cam4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 5.Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology. 2006;68(3):593–598. doi: 10.1016/j.urology.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 6.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J. Urol. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 7.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. HO. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2012;13(10):983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Drugs approved for prostate cancer. 2014. www.cancer.gov/about-cancer/treatment/drugs/prostate
- 11.Handy CE, Antonarakis ES. Sequencing treatment for castration-resistant prostate cancer. Curr. Treat. Options Oncol. 2016;17(12):64. doi: 10.1007/s11864-016-0438-9. [DOI] [PubMed] [Google Scholar]
- 12.Malarkey MA, Witten CM. Approval letter – Provenge. 2015. www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210215.htm
- 13.Nair-Gupta P, Blander JM. An updated view of the intracellular mechanisms regulating cross-presentation. Front. Immunol. 2013;4:401. doi: 10.3389/fimmu.2013.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flamand V, Sornasse T, Thielemans K, et al. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo . Eur. J. Immunol. 1994;24(3):605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 16.Ossevoort MA, Feltkamp MC, van Veen KJ, Melief CJ, Kast WM. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J. Immunother. Emphas. Tumor Immunol. 1995;18(2):86–94. doi: 10.1097/00002371-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 18.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 1996;183(1):283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc. Natl Acad. Sci. USA. 1996;93(20):10972–10977. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat. Med. 1999;5(10):1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 21.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat. Med. 1996;2(1):52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma – a feasibility study. Blood. 1999;93(7):2411–2419. [PubMed] [Google Scholar]
- 23.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4(3):328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 24.Morse MA, Deng Y, Coleman D, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin. Cancer Res. 1999;5(6):1331–1338. [PubMed] [Google Scholar]
- 25.Gutman EB, Sproul EE, Gutman AB. Significance of increased phosphatase activity of bone at the site of osteoplastic metastases secondary to carcinoma of the prostate gland. Am. J. Cancer. 1936;28(3):485–495. [Google Scholar]
- 26.Cunha AC, Weigle B, Kiessling A, Bachmann M, Rieber EP. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett. 2006;236(2):229–238. doi: 10.1016/j.canlet.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Muniyan S, Chaturvedi NK, Dwyer JG, Lagrange CA, Chaney WG, Lin MF. Human prostatic acid phosphatase: structure, function and regulation. Int. J. Mol. Sci. 2013;14(5):10438–10464. doi: 10.3390/ijms140510438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate. 1998;36(2):129–138. doi: 10.1002/(sici)1097-0045(19980701)36:2<129::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin. Oncol. 2000;18(23):3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]; • Early phase trials (Phase I and II trial) which were first in man study of sipuleucel-T, and demonstrated safety and signal of efficacy.
- 30.Van Voorhis W, Hair LS, Steinman RM, Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J. Exp. Med. 1982;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, Phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 32.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled Phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 33.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 34.George DJ, Nabhan C, DeVries T, Whitmore JB, Gomella LG. Survival outcomes of sipuleucel-T Phase III studies: impact of control-arm cross-over to salvage immunotherapy. Cancer Immunol. Res. 2015;3(9):1063–1069. doi: 10.1158/2326-6066.CIR-15-0006. [DOI] [PubMed] [Google Scholar]
- 35.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju268. pii:dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. 2010;10(8):580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beinart G, Rini BI, Weinberg V, Small EJ. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin. Prostate Cancer. 2005;4(1):55–60. doi: 10.3816/cgc.2005.n.013. [DOI] [PubMed] [Google Scholar]
- 38.Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011;17(13):4558–4567. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 39.Antonarakis ES, Kibel AS, Yu EY, et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a Phase II randomized trial. Clin. Cancer Res. 2017;23(10):2451–2459. doi: 10.1158/1078-0432.CCR-16-1780. [DOI] [PubMed] [Google Scholar]; • STAND trial; Phase II trial of sipuleucel-T prior to or after androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer which showed greater PA2024-specific immune responses when given prior to androgen deprivation therapy.
- 40.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 43.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized Phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. CII. 2013;62(1):137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Combined analysis of the three Phase III trials showing association between overall survival and host immune correlates including detectable antibody immune response to PAP or PA2024, antigen-presenting cell activation, antigen-presenting cell count and total nucleated cells.
- 45.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–1302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 46.GuhaThakurta D, Sheikh NA, Fan LQ, et al. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin. Cancer Res. 2015;21(16):3619–3630. doi: 10.1158/1078-0432.CCR-14-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drake CG. The potential role of antigen spread in immunotherapy for prostate cancer. Clin. Adv. Hematol. Oncol. 2014;12(5):332–334. [PMC free article] [PubMed] [Google Scholar]; • Sipuleucel-T also causes ‘antigen spread’ meaning off-target immune effects and antibodies to other prostate cancer-associated antigens.
- 48.Higano CS, Small EJ, Schellhammer P, et al. Sipuleucel-T. Nat. Rev. Drug Discov. 2010;9(7):513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 49.Hall SJ, Klotz L, Pantuck AJ, et al. Integrated safety data from 4 randomized, double-blind, controlled trials of autologous cellular immunotherapy with sipuleucel-T in patients with prostate cancer. J. Urol. 2011;186(3):877–881. doi: 10.1016/j.juro.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 50.https://clinicaltrials.gov/ct2/show/NCT01306890 Clinical trails database: NCT01306890.
- 51.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, Phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 52.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, Phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–52817. doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boudadi K, Suzman DL, Luber B, et al. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2017;35(15 Suppl.):5035–5035. [Google Scholar]
- 56.Schweizer MT, Cheng HH, Tretiakova MS, et al. Mismatch repair deficiency may be common in ductal adenocarcinoma of the prostate. Oncotarget. 2016;7(50):82504–82510. doi: 10.18632/oncotarget.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guedes LB, Antonarakis ES, Schweizer MT, et al. MSH2 loss in primary prostate cancer. Clin. Cancer Res. 2017;23(22):6863–6874. doi: 10.1158/1078-0432.CCR-17-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017;23(5) doi: 10.1038/nm.4308. nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107(1):67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 62.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled Phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 2012;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small EJ, Lance RS, Gardner TA, et al. A randomized Phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2015;21(17):3862–3869. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 64.Quinn DI, Petrylak DP, Pieczonka CM, et al. A randomized Phase II, open-label study of sipuleucel-T with concurrent or sequential enzalutamide in metastatic castration-resistant prostate cancer (mCRPC) JCO. 2014;32(15):e16071–e16071. [Google Scholar]
- 65.Food and Drug Administration. FDA approval for ipilimumab. 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2011/125377s0000lbl.pdf
- 66.Scholz M, Yep S, Chancey M, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. ImmunoTargets Ther. 2017;6:11–16. doi: 10.2147/ITT.S122497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.European Medicines Agency. Press Release; 2013. Provenge.www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002513/WC500151157.pdf [Google Scholar]
- 68.European Medicines Agency. Provenge. 2015. www.ema.europa.eu/ema
- 69.Sanpower Group. Sanpower Group completes the acquisition of Dendreon from Valeant. PRNewswire. 2017. www.prnewswire.com/news-releases/sanpower-group-completes-the-acquisition-of-dendreon-from-valeant-300481852.html
- 70. Integrated analysis – *Two simultaneous trials of 225 men with mCRPC demonstrating overall survival benefit compared to placebo.
- 71. IMPACT trial – *Pivotal randomized Phase III trial of 127 men with mCRPC demonstrating overall survival benefit compared to placebo.