Abstract
The advent of immune checkpoint inhibitors (PD-1, PD-L1 and CTLA-4) has resulted in unprecedented long-term remissions of unresectable cancers. The efficacy of checkpoint inhibitors was recently demonstrated in gastrointestinal malignancies with mismatch repair deficiencies (dMMR). Pembrolizumab became the first tissue-agnostic US FDA-approved drug based on the presence of the predictive biomarker dMMR. In addition, the FDA in 2017 approved pembrolizumab for PD-L1-positive advanced gastric cancer in third-line and second-line hepatocellular therapy. Novel treatment strategies such as using anti-carcinoembryonic antigen (CEA) bispecific T cells have led to remarkable responses in microsatellite instability-low colorectal cancer. Other major breakthroughs in treating upper gastrointestinal malignancies in 2017 are discussed.
Keywords: : immunotherapy, mismatch repair deficiencies, upper gastrointestinal malignancies
Greater insights into immunoncology and driver mutations have revolutionized cancer treatment. Tissue of origin has become less relevant as the underlying pathophysiology of cancer biology and actionable aberrations have been elucidated. Three treatment paradigms (surgery, chemotherapy and radiation therapy) have dominated therapy for upper gastrointestinal (GI) malignancies during the last three decades, with surgical resection the only hope for cure. With the advent of immune checkpoint inhibitors (PD-1, PD-L1 and CTLA-4), this model has been challenged by unprecedented long-term remissions in patients with unresectable disease. These agents have become standard of care for different solid tumors. The efficacy of checkpoint inhibitors was recently demonstrated in GI malignancies with mismatch repair deficiencies. Tumors with DNA mismatch and repair (dMMR) are typically characterized as having a high mutation burden that increases the likelihood of expressing a neoantigen. These neoantigens can be recognized by the host immune system leading to a cytotoxic immune response against malignant cells. Pembrolizumab recently became the first tissue-agnostic US FDA-approved drug based on the presence of a predictive biomarker, dMMR deficiencies. In addition, the FDA in 2017 approved pembrolizumab for PD-L1-positive advanced gastric cancer in third-line and second-line hepatocellular carcinoma (HCC) therapy (Figure 1). The addition of novel immunotherapies such as inhibitors of IDO have led to increased responses in other tumor types, such as melanoma. Combination immunotherapy has also been explored in GI malignancies. In addition, novel treatment strategies such as using anti-CEA bispecific T cells have led to remarkable responses in microsatellite instability (MSI)-low colorectal cancer and may open new venues for treating upper GI malignancies for which a biomarker has been identified, in other words, HER2-positive gastroesophageal cancers.
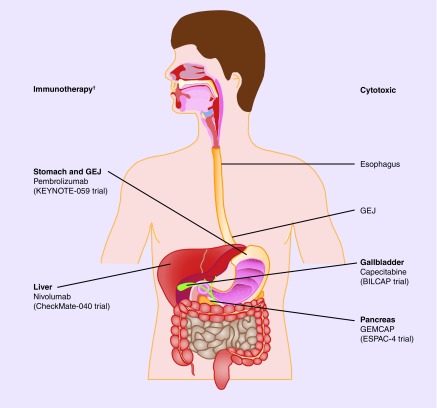
Figure 1. . Breakthrough trials of upper gastrointestinal malignancies.
†Tissue agnostic-MSI high pembrolizumab [106].
GEJ: Gastroesophageal junction.
Targeted therapies are better tolerated than cytotoxic chemotherapy and often provide greater response rates (RRs). Approved targeted therapies in upper GI malignancies include monoclonal antibodies like ramucirumab and trastuzumab (gastroesophageal cancer) as well as tyrosine kinase inhibitors such as sorafenib or regorafenib (HCC) and erlotinib (pancreatic cancer). However, only in the case of trastuzumab has a predictive biomarker of response, HER2 amplification, been identified by immunohistochemistry or fluorescent in situ hybridization (FISH). The generalized use of next-generation sequencing resulted in identifying additional, low prevalence, actionable aberrations in the upper GI malignancies (Table 1).
Table 1. . Incidence of selected actionable aberrations/mutations in upper gastrointestinal malignancy.
| Genetic aberration | Cancer | Incidence (%) | Ref. |
|---|---|---|---|
| HER2 amplification | Gastric Colorectal Gallbladder |
22 6 13 |
[87–89] |
| FGFR fusions | Intrahepatic cholangiocarcinoma | 8–14 | [71,90] |
| IDH mutations | Intrahepatic cholangiocarcinoma | 23 | [69] |
| NTRK fusion | Colorectal Pancreatic |
4 1 |
[91,92] |
| BRCA1–2 mutation | Pancreatic | 1–7/1–3 | [93] |
| MSI-H | Esophagogastric Pancreatic Cholangiocarcinoma |
6 <1–1 9 |
[94–97] |
Other major breakthroughs in treating upper GI malignancies in 2017 are discussed.
Esophagogastric cancer
Esophageal cancer is the sixth cause of cancer mortality in the world. In the US, there were 16,940 diagnoses and 15,690 deaths from esophageal cancer in 2017 [1]. Fewer than 50% of the patients are diagnosed with locoregional disease and are candidates for resection [1]. For patients with high-grade dysplasia or carcinoma in situ, endomucosal resection can be curative [2]. For patients with extensive high-grade dysplasia, ablation decreases the risk of relapse [3,4]. Patients with tumors that invade the submucosa (T1b) or have positive lymph nodes after staging endoscopic ultrasound and/or CT scan are typically treated with neoadjuvant chemoradiation per CROSS trial data. The study showed improved survival with neoadjuvant chemoradiation compared with upfront resection for both adenocarcinoma (43.2 vs 27.1 months; p = 0.038) and squamous cell carcinoma (GEJ; 81.6 vs 21.1 months; p = 0.008) of the esophagus or gastroesophageal junction [5]. The pathologic complete response rate (pCR) for squamous cell carcinoma was 50% (vs 23% for adenocarcinoma). Neoadjuvant chemoradiation did not increase the incidence of postoperative complications. An update from this study demonstrated longer survivals in patients who attained a pCR [6].
Different studies have evaluated intensification of neoadjuvant treatment in an effort to increase the likelihood of attaining a pCR (Table 2). Strategies have included adding targeted therapies, and more recently, using positron emission technology (PET) for early identification of non-PET responders that can be rescued by surgery or crossover to a different chemotherapy backbone [7,8]. CALGB80803 was a randomized Phase II study in patients with cT3–4 or N+ esophageal adenocarcinoma [9]. The study used PET at 5 weeks as an early pharmacodynamic imaging test to evaluate response to induction chemotherapy with FOLFOX or carboplatin plus paclitaxel. PET nonresponders (<35% decrease in standard uptake value) crossed over to the other chemotherapy backbone during chemoradiation. The study's hypothesis was that pCR among PET nonresponders would be 5–20% after crossover. pCR for PET nonresponders who were treated with carboplatin plus paclitaxel (pCR = 19%) and FOLFOX (17%) supported the study hypothesis. Interestingly, the pCR in selected patients (PET responders) in the carboplatin plus paclitaxel arm was lower than expected (pCR 12.5 vs 23% in the CROSS trial). This is an intriguing finding. It is possible that the low number of patients included in the study may account for this. It is also important to note that no randomized Phase III trial has compared induction chemotherapy followed by chemoradiation and surgery versus neoadjuvant chemoradiation in patients with esophageal adenocarcinoma. Therefore, this strategy is not standard of care for these patients. Indeed, Phase II data using this approach have shown that adding chemotherapy to neoadjuvant chemoradiation does not improve pCR compared with CROSS trial data [10,11].
Table 2. . Updates in neoadjuvant therapy for esophageal, gastric and gastroesophageal junction cancer.
| Study | Trial | Cancer type | Eligibility | Phase | Number of patients (n) | Timing | Intervention | End point | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Shapiro et al. | CROSS | Esophageal/GEJ | cT12N1M0 or T2–3N0–1M0 | III | 368 | Neoadjuvant | CP + surgery vs surgery alone | Median OS 48.6 vs 24.0 mo (p = 0.003) | [6] |
| Goodman et al. | CALGB80803 | Esophageal/GEJ | cT3–4 or N+ | II | 257 | Adjuvant | FOLFOX-6 or CP, PET nonresponders crossed over to alternate chemotherapy | pCR for PET-nonresponders pCR 15.6% | [9] |
| Al-Batran et al. | FLOT4-AIO | Gastric/GEJ | cT2+ and/or cN+ | II/III | 300 | Adjuvant | FLOT vs ECF/ECX | 3y OS 57 vs 48% | [98] |
CP: Chemotherapy; ECF: Epirubicin, cisplatin, fluorouracil; ECX: Epirubicin, cisplatin, capecitabine; FLOT: Fluorouracil, leucovorin, oxaliplatin, docetaxel; GEJ: Gastroesophageal junction; mo: Month; OS: Overall survival; pCR: Pathologic complete response rate; PET: Positron emission technology.
For patients with resectable distal gastric cancer, perioperative epirubicin, cisplatin and 5-fluoruracil was the standard of care for the last decade based on data from the MAGIC trial [12]. A recent trial adding bevacizumab to epirubicin, cisplatin, fluorouracil (ECF) in the perioperative setting failed to improve outcome [13]. The FLOT4-AIO, an investigator initiated, multicenter, randomized controlled Phase II/III trial was recently presented at ASCO 2017. In this study, patients with gastric (44%) or GEJ cancer (56%), cT2 or higher and/or nodal positive, were randomized to perioperative ECF/ECX × three cycles or FLOT × four cycles (docetaxel 50 mg/m2, intravenous oxaliplatin 85 mg/m2, intravenous leucovorin 200 mg/m2 and fluorouracil 2600 mg/m2 as a 24 h infusion) [14]. Most patients in both arms were able to undergo resection (97 and 94% for FLOT4 vs ECF/ECX). Downstaging was more frequent in the FLOT4 arm as shown by improvement in tumor-free resection margins (R0 84 vs 77%; p = 0.011), smaller tumors at the time of resection (≤T1 25 vs 15%; p = 0.001) and higher incidence of lymph node sterilization (49 vs 41%; p = 0.029). Both groups had similar rates of dose modifications. Patients in the FLOT arm had a higher incidence of grade 3–4 infections (9 vs 1%; p < 0.001) and grade 3–4 diarrhea (10 vs 4%; p = 0.002). However, the overall incidence of treatment-related serious adverse events (35 vs 34%) and toxic deaths (<1%) was similar between both arms. Interestingly, treatment discontinuation per patient request was higher in the ECF arm (13 vs 21%; no statistical significance provided). In subgroup analysis, tumors with intestinal type histology demonstrated the highest benefit from FLOT. These data support FLOT as the new standard when perioperative chemotherapy is considered for patients with gastric cancer. However, in our opinion, neoadjuvant chemoradiation remains the standard of care for patients with GEJ cancers until the pCR in the Phase III trial of FLOT is reported. In Phase II of FLOT, the pCR (15%) was lower than the one reported for adenocarcinoma in the CROSS trial (23%). Given the impact of pCR in survival, we believe that maximizing the opportunity to attain a pCR should influence our treatment choice. The ongoing ESOPEC trial (NCT02509286) is comparing a FLOT versus CROSS schedule and will hopefully elucidate the standard of care in this setting.
Additional lessons can be taken from this study. The rate of completion of postoperative chemotherapy was low in both arms (51 and 44% for FLOT and ECF, respectively), consistent with prior data from the MAGIC trial. Some may argue that all chemotherapy should be given upfront when it is more likely to be better tolerated. However, the MRC-OE02 and RTOG8911 studies provided conflicting results in this setting [15,16]. Also, as discussed above, the use of preoperative chemoradiation has substantially increased the likelihood of attaining a pCR in these patients.
For patients with HER2-positive advanced gastric cancer, the ToGA trial demonstrated a survival improvement when trastuzumab was added to cisplatin and 5-fluorouracil [17]. Retrospective data suggest that the level of HER2 amplification is predictive of response to trastuzumab [18]. However, analysis of samples from ToGA did not confirm this finding [19]. In this HER2 population, efforts to improve outcome have included using an antibody drug conjugate and combination therapy, with disappointing results. The GATSBY trial was a randomized Phase II/III study in patients with HER2-positive gastric or GEJ cancers [20]. Patients who progressed on first-line trastuzumab were randomized to trastuzumab–emtansine (an antibody drug conjugate) versus taxane. There was no benefit with trastuzumab–emtansine in these patients. In addition, dual HER2 blockade failed to improve survival in the first-line setting in the JACOB study [21]. This was a randomized Phase III trial in HER2-positive patients (n = 780) with metastatic gastric (75%) or GEJ cancers (25%) who were randomized to chemotherapy (capecitabine or cisplatin plus 5-fluorouracil) plus trastuzumab, or chemotherapy plus trastuzumab and pertuzumab. Both arms had similar safety profiles including low incidence of left ventricular systolic function but increased incidence of diarrhea with pertuzumab. There was a 3-month improvement in overall survival (OS) with addition of pertuzumab, which was not statistically significant (OS: 17.5 vs 14.2 months; hazard ratio [HR]: 0.84, p = 0.05).
The role of immunotherapy in advanced gastric and GEJ cancer is becoming evident. In a recent retrospective review, PD-L1 expression was increased in 50% of patients with gastric cancer (n = 107) and was associated with worse survival [22]. Different studies have provided evidence of activity with checkpoint inhibitors in heavily pretreated patients with advanced esophageal cancers (Table 3).
Table 3. . Immunotherapy in esophageal, gastric and gastroesophageal junction cancer.
| Trial | Subtype adeno/SCC PD-L1+/any | Phase | n | Line of therapy | Intervention | Outcome RR† (%) | Ref. |
|---|---|---|---|---|---|---|---|
| KEYNOTE-012 Muro et al. |
Adeno | Ib | 39 | >1 | Pembrolizumab | 22 | [99] |
| CheckMate 032 Janjigian et al. |
Both | I/II | 160 | ≥2 | Nivo(3) (n = 59) Nivo(1) + ipi(3) (n = 49) Nivo(3) + ipi(1) (n = 52) |
12 24 8 |
[27] |
| Kudo et al. | SCC | II | 65 | >2 | Nivolumab | 17 | [100] |
| KEYNOTE-059 Fuchs et al. | Adeno | II | 259 25 |
≥3 1 |
Pembrolizumab 3L Pembrolizumab + 5-FU + cisplatin 1l |
11.2 60 |
[24,25] |
| ATTRACTION-2 Kang TS et al. |
Adeno | III | 493 | ≥3 | Nivolumab vs placebo | 5.3 vs 4.1 m HR: 0.63 (p < 0.0001) |
[101] |
| CheckMate 649 Moehler et al. |
Adeno | III | 1266 | 1 | Nivo/ipi vs nivo vs chemotherapy (oxaliplatin + fluoropyrimidine) | Ongoing | [102] |
| CheckMate 649 Janjigian et al. |
Both | III | 870 | 1 | Nivo/ipi vs chemotherapy (oxaliplatin + fluoropyrimidine) | Ongoing | [103] |
| KEYNOTE-061 Ohtu et al. | Adeno (HER2+) | III | 720 | 2 | Pembrolizumab vs paclitaxel | Ongoing | [104] |
| KEYNOTE-062 Tabernero et al. | Adeno carcinoma (PD-L1+/HER2 -) | III | 750 | 1 | Pembrolizumab vs pembro + cisplatin + 5FU vs placebo + cisplatin + 5-FU | Ongoing | [105] |
†RR except for ATTRACTION-2 (overall survival).
RR: Response rate; SCC: Squamous cell carcinoma.
The ATTRACTION-2 trial is the first randomized controlled trial comparing nivolumab to placebo in Asian patients with unresectable or recurrent gastric or GEJ adenocarcinomas [23]. All patients had to have received two or more prior lines of therapy. A total of 493 patients were enrolled in 49 centers in Japan, South Korea and Taiwan. Nivolumab improved median OS (5.3 vs 4.1 months; HR: 0.63; p < 0.0001) and objective response rate (ORR: 11.2 vs 0%; p < 0.0001) compared with placebo. The progression-free survival (PFS) was similar between both arms (1.61 vs 1.45 months; p < 0.0001). Rates of grade 3 and 4 adverse events were increased in the nivolumab arm at 10.3% compared with 4.3% in the placebo arm. Ongoing studies will determine if survival benefit from nivolumab is also seen in western populations.
KEYNOTE-059 is a Phase II study that has evaluated single agent pembrolizumab versus a combination of cisplatin and fluoropyrimidine in chemotherapy-naive patients as well as single-agent pembrolizumab in heavily pretreated patients (third or fourth line) [24,25]. ORR among the three cohorts was 11.6% (p-value not provided). The highest ORR in subgroup analysis was in patients with MSI-high tumors at 57.1% compared with non-MSI-high tumors at 9.0%. Of note, a significant decline in response was seen in patients receiving third-line versus fourth-line therapy, at 16.4 and 6.4%, respectively. It is possible that heavily pretreated patients have increased immunosuppression and are less likely to benefit from immunotherapy. This suggests that immunotherapy likely needs to be evaluated at earlier stages, which also opens opportunities to prime responses with chemotherapy or radiation. Results from KEYNOTE-059 led to the recent accelerated FDA approval of pembrolizumab for PD-L1-positive advanced gastric cancer [26].
CheckMate 032 tested the hypothesis that an immunotherapy combination increases RR in patients with metastatic gastroesophageal cancer. This was a Phase I/II study that included esophageal, gastric and GEJ cancer patients treated in three different cohorts (nivolumab 3 mg/kg monotherapy [N3], nivolumab 1 mg/kg and ipilimumab 3 mg/kg [N1 + I3] and nivolumab 3 mg/kg and ipilimumab 1 mg/kg [N3 + I1]) [27]. The trial included 160 patients. Most patients were PD-L1-negative and 45% had received three or more lines of therapy across all arms. Responses were more frequently seen in the N1 + I3 cohort (RR: 24%) compared with 12 and 8% for N3 and N3 + I1, respectively. However, the median duration of response was similar (7.1 months in the N3, 7.9 months in N1 + IPI3). Sixty percent of the patients in any arm progressed within 2 months. Therefore, it will be critical to develop predictive biomarkers to tease out subsets of patients who are likely to benefit. The OS in the N1 + I3 cohort was 6.9 months compared with 6.2 months in N3 and 4.8 months in N3 + I1. Responses were seen regardless of PD-L1 expression, although numerically more responses were seen in PD-L1 high (>1% expression) patients. Toxicities were more frequently seen in either of the combination arms. The N1 + I3 cohort had the highest rate of grade 3 and 4 adverse events at 35% compared with 17% for N3 + I1 and 5% for N3. Interestingly, for PD-L1-negative patients, single-agent nivolumab seemed more effective than any of the combinations (12-month survival rate: 45 vs 32 vs 25%). This could be due to the small number of patients or a true detrimental effect of toxicities related to the combination in the subset of patients less likely to benefit.
Given the relatively small number of patients in each cohort and lack of formal statistical analysis, CheckMate 032 is only hypothesis generating. Consistent with evidence from melanoma, an immunotherapy combination appears to be most active, but at the cost of a significant increase in toxicities [28]. The CheckMate 649 trial will more definitively address the efficacy of combination anti-PD-1 and anti-CTLA-4 therapies for advanced and metastatic esophageal, gastric and GEJ cancers. The role of immunotherapy in earlier lines of therapy is undergoing evaluation (Table 3).
Hepatocellular carcinoma
In the US, liver and intrahepatic bile duct cancers are expected to account for 40,710 new cancer diagnoses and 28,920 deaths in 2017 [1]. In patients with advanced HCC, sorafenib gained regulatory approval based on the results of two randomized Phase III studies that showed modest improvement in survival compared with placebo in Child–Pugh class A patients [29,30]. The high prevalence of hepatitis B virus (HBV) in the Asia Pacific population compared with those in the SHARP study (75 vs 20%) likely contributed to the inferior outcome of both arms in this study. Multiple agents have been directly compared with sorafenib including sunitinib, brivanib and linifanib and all failed to demonstrate improved OS [31–33]. The addition of doxorubicin or erlotinib to sorafenib also did not demonstrate improved OS [34,35]. The REFLECT study recently showed that lenvatinib is not inferior to sorafenib in the first line [36]. Lenvatinib is a multi-tyrosine kinase inhibitor against VEGFR1–3, FGFR1–4, PDGFR, KIT and RET. The study enrolled 907 patients with HCC and Child–Pugh A and was designed to test the noninferiority of lenvatinib with a predefined noninferiority margin of 1.08. The median OS for lenvatinib versus sorafenib was 13.6 versus 12.3 months (HR: 0.92; 95% confidence interval [CI]: 0.79–1.06). Secondary efficacy end points including RR (24 vs 9%; p < 0.001) and PFS (7.4 vs 3.7 months; HR: 0.66; 95% CI: 0.57–0.67) favored the lenvatinib arm. The toxicity profile of both drugs was similar. Lenvatinib is not yet approved by the FDA, and its role in the treatment landscape of HCC is likely to be limited especially if the results of the recently completed CheckMate-459 demonstrate superiority of nivolumab over sorafenib [37].
In the second-line setting, multiple investigational drugs (brivanib, everolimus, ramucirumab and ADI-PEG 20) failed to demonstrate improved OS compared with placebo [38–41]. The RESORCE trial evaluated regorafenib in the second-line setting after progression on sorafenib. In this randomized Phase III study, 573 patients were allocated in a 2:1 fashion to regorafenib (multi-tyrosine kinase inhibitor) versus placebo [42]. The study limited inclusion to patients with Child–Pugh A cirrhosis. A total of 573 patients were recruited, 216 from Asian countries. Regorafenib showed improvement in survival (OS: 10.6 vs 7.8 months; HR: 0.63; p < 0.0001). Treatment-related adverse events in the regorafenib arm were significant (50 vs 16%) despite the patients being preselected after having tolerated first-line treatment with sorafenib. As expected, the most common grade 3 and 4 adverse events in the regorafenib group were hypertension (15%), hand-foot skin reactions (13%), fatigue (9%) and diarrhea (3%). No improvement in health-related quality of life (QoL) was found. Altogether, 2% of deaths of patients in the regorafenib group, compared with 1% in the placebo group, were attributed to drug toxicity. A benefit from regorafenib was seen regardless of hepatitis viral status. Regorafenib is currently a category 1 recommendation by the National Comprehensive Cancer Network after progression on sorafenib [43].
As with esophageal, gastric and GEJ cancers, PD-L1 inhibitors are making inroads in HCC. The results of CheckMate-040, a Phase I/II, open-label, noncomparative, dose escalation and expansion trial of nivolumab in patients with advanced HCC were recently reported [44]. The study enrolled 262 patients with a Child–Pugh (CP) score of 7 or less (CP 6 or less for the dose expansion). The maximum tolerated dose was not reached during dose escalation and nivolumab 3 mg/kg was chosen for the dose expansion. Four cohorts were enrolled in the dose expansion: sorafenib untreated or intolerant without viral hepatitis, sorafenib progressors without viral hepatitis, hepatitis C virus (HCV)-infected and HBV-infected. ORR was 20% in dose expansion and 15% in the dose escalation groups. Across all cohorts of the dose expansion phase, an additional 45% had stable disease (SD). Similar to other malignancies, a trend toward increased response was seen in PD-L1-positive patients. However, a sizeable number of PD-L1 negative patients benefited from nivolumab (ORR PD-L1-positive vs -negative: 26 vs 19%). The ORR of 20% is remarkable, especially because responses to sorafenib in the first line are rarely seen (2%) [29]. Per CheckMate-040 results, the FDA granted accelerated approval of nivolumab at a fixed dose of 240 mg for patients with HCC who progressed on sorafenib [45]. A randomized Phase III study comparing nivolumab to sorafenib in the first line recently completed accrual and results are expected in 2018 (NCT2576509) [46].
Arterial-based therapy remains an appropriate option for unresectable, multinodular HCC. Multiple trials have demonstrated improved survival compared with supportive care with this modality [47–49]. In addition, two recent trials demonstrated no difference in median OS for arterial selective internal radiation therapy (SIRT) versus sorafenib (Table 4). The decision about which agent is best used to start, sorafenib or arterially directed therapy, in unresectable HCC remains at the discretion of the physician. Both are category 2A recommendations per National Comprehensive Cancer Network guidelines [43].
Table 4. . Updates in hepatocellular carcinoma selective internal radiation.
| Study | Trial | Phase | Number of patients | Child–Pugh class | Line of therapy | Intervention | Median OS | RR (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Vilgrain et al. | SARAH | III | 459 | A/B | Failed 2 + rounds of chemoembolization | SIRT (Y-90) vs sorafenib | 8.0 vs 9.9 (months) (p = 0.179) | 19.0 vs 11.6 (p = 0.042) | [50] |
| Pierce et al. | SIRveNIB | III | 360 | A/B | ≤2 rounds of chemoembolization | SIRT (Y-90) vs sorafenib | 8.8 vs 10.0 (months) (p = 0.203) | 16.5 vs 1.7 (p < 0.001) | [51] |
| Meyer et al. | TACE 2 | III | 313 | A | 1 | TACE + sorafenib vs TACE + placebo | 631 vs 598 (days) (p = 0.57) | 36 vs 31 (p-value not provided) | [53] |
OS: Overall survival; RR: Response rate; SIRT: Selective internal radiation therapy; TACE: Transarterial chemoembolization.
The SARAH trial is a randomized controlled, open-label, Phase III study of systemically untreated HCC that is locally advanced or recurrent but not amenable to other treatments or that has failed two or more rounds of chemoembolization [50]. Patients were randomized 1:1 to SIRT with Y-90 resin microspheres or oral sorafenib 800 mg daily. A total of 459 patients were recruited and an intention to treat (ITT) analysis revealed no statistically significant difference in median OS (8.0 months in the SIRT group vs 9.9 months in the sorafenib group; p = 0.179). Similarly, no statistical difference in PFS was seen (4.1 months SIRT vs 3.7 months sorafenib groups; p = 0.727). However, the cumulative incidence of radiological progression in the liver as first event was significantly lower in SIRT patients than in sorafenib patients, with a 27% reduction (p = 0.015). RRs were also statistically improved in patients treated with SIRT compared with sorafenib, 19.0 versus 11.6% (p = 0.042), respectively. Treatment-related serious adverse events were similar between the two groups with 11.7% in the SIRT group compared with 16.5% in the sorafenib group.
The SIRveNIB study is a randomized controlled trial comparing SIRT with Y-90 resin microspheres to sorafenib [51]. This trial included patients who had locally advanced HCC, Child–Pugh A or B and had received two or fewer prior administrations of hepatic arterial-directed therapy. A total of 360 patients were enrolled. The study enrolled an exclusively Asian population (HBV prevalence: 58%). No statistical difference was seen in OS with a median OS of 8.8 months in the SIRT group versus 10.0 months (p = 0.203) in the sorafenib group. Like the SARAH trial, the ITT population tumor RRs were higher in the SIRT cohort compared with the sorafenib cohort (16.5 vs 1.7%; p < 0.001). No difference was seen in time to progression in the ITT groups. An important limitation of this study was that up to 30% of patients in the SIRT arm did not receive the allocated treatment intervention per protocol (vs 9% in the sorafenib arm). Needless to say, it is difficult to demonstrate a benefit from an experimental arm when a third of patients did not receive such treatment. In a survival analysis of treatment received, SIRT showed a 1-month improvement in survival (6.4 vs 5.3 months; HR: 0.73; p < 0.019). SIRT was also better tolerated than sorafenib as shown by a decreased incidence of treatment-related adverse events (13% in the SIRT arm vs 37% in the sorafenib arm). Based on results from the SARAH and SIRveNIB studies, SIRT is a reasonable option for patients with multinodular HCC who do not wish to deal with the considerable side effects of sorafenib.
It has been hypothesized that transarterial chemoembolization (TACE)-induced hypoxia promotes angiogenesis and may drive cancer progression [52]. Therefore, the addition of angiogenesis inhibitors to TACE may benefit patients with HCC. This hypothesis was recently tested in the TACE 2 trial [53]. Patients with unresectable liver-confined HCC, Child–Pugh A, were randomized 1:1 to TACE or TACE plus sorafenib. Sorafenib was started within 24 hours of randomization. TACE was started 2–5 weeks postrandomization. Interestingly, the most common cause of cirrhosis in this UK study was alcohol intake rather than HCV infection (42 vs 24%). The trial was terminated early after a planned interim futility analysis showed no improvement in OS or PFS with the addition of sorafenib. ORRs were similar between the two cohorts, 36% in the sorafenib group and 31% (no p-value provided) in the placebo group. Increased toxicity was seen in the sorafenib cohort with higher rates of grade 3 and 4 fatigue (18 vs 13%), abdominal pain (13 vs 8%), diarrhea (10 vs 3%) and GI disorders (11 vs 8%). At least one serious adverse event was seen in 41% of patients in the sorafenib group versus 32% in the placebo group. The SELECT and STAH trials are currently testing the same hypothesis as the TACE 2 study in Asian patients [54,55].
Biliary cancer
Cholangiocarcinoma is a heterogeneous group of rare cancer types including intrahepatic cholangiocarcinoma (IHCC) and extrahepatic cholangiocarcinoma (EHCC) as well as gallbladder cancer (GBCA) [56]. These tumors have different patterns of relapse as well as unique molecular portraits [57]. They are often lumped together in clinical trials which make interpretation of study results difficult. Fewer than 11,740 patients will be diagnosed with EHCC or GBCA in the USA in 2017 [1]. The incidence of IHCC is less well reported as these patients are often misdiagnosed as having carcinoma of unknown primary metastatic to the liver. A recent analysis of Surveillance Epidemiology and End Results (SEER) data showed a 128% increase in the incidence of cholangiocarcinoma in the last four decades. The annual incidence of IHCC in USA is still fewer than 4000 new cases [58]. Most patients are unresectable at the time of initial presentation. Even in the minority of patients who are eligible for potentially curative surgical resection, the 5-year survival rate is 32.5% [59]. Until 2017, there was little evidence to support adjuvant treatment in these patients. A randomized study in Japan that included patients with bile duct cancer, pancreatic adenocarcinomas as well as ampullary cancers showed a survival benefit with mitomycin plus 5-FU compared with placebo (5-year survival rate [5ySR]: 28 vs 14%; p = 0.0367) [60]. However, a large percentage of patients enrolled in this study had suboptimal surgeries (40% for bile duct and 55% for GBCA).
More recently, the SWOG 0809 study demonstrated the feasibility of delivering adjuvant gemcitabine followed by concurrent capecitabine and radiation for patients with resected bile duct cancer [61]. The BILCAP trial sought to test the hypothesis that adjuvant capecitabine would improve OS in resectable bile duct cancers [62]. Patients (n = 447) were randomized 1:1 to capecitabine (1250 mg/m2 D1–14 every 21 days for eight cycles) or observation. The most common tumor type was distal EHCC (36%), followed by hilar cholangiocarcinoma (28%) and IHCC and GBCA (both at 18%). The study included Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2 patients but fewer than 3% of patients had PS 2. Nearly 50% of patients had positive lymph nodes and 40% had positive margins. Median OS of the capecitabine arm in the ITT population was 53 versus 36 months in the observation arm (HR: 0.80; 95% CI: 0.63–1.04; p = 0.097). Although the study was not statistically significant, there was a clear separation between survival curves for each arm starting at around 10–12 months. The difference in OS between arms is clinically meaningful. In addition, a sensitivity analysis adjusting for nodal status, disease grade and gender showed the OS difference was statistically significant (HR: 0.71; 95% CI: 0.55–0.92; p < 0.01). The analysis per protocol also showed a statistically significant improvement in OS with adjuvant capecitabine (52.7 vs 36.1 months; 95% CI: 0.58–0.97; p = 0.028). The benefit was seen across all subgroups regardless of gender, age, node and margin status with the exception of hilar cholangiocarcinoma. Most grade 3–4 adverse events had an incidence rate of less than 10% except for hand-foot syndrome (21%). A QoL study showed no difference between arms, and the authors concluded that treatment was well tolerated. However, only 55% of patients could complete eight cycles of capecitabine and dose reductions are likely to be needed, especially in the USA where patients typically experience greater toxicities with higher doses of capecitabine compared with European patients [63]. Given the small percentage of PS 2 patients included in this study, we do not believe these results can be extrapolated to frail patients.
Two clinical trials have demonstrated a statistical improvement in OS with gemcitabine and oxaliplatin (GEMOX) versus best supportive care in cases of advanced GBCA [64,65]. UNICANCER GI, a Phase III clinical trial, sought to evaluate the benefit of GEMOX compared with surveillance in patients with localized biliary tract cancer [66]. Relapse-free survival favored the GEMOX arm at 30.4 months versus 22.0 months; however, this was not statistically significant (HR: 0.83; p = 0.31). GEMOX was generally well tolerated with at least one serious adverse event in the GEMOX arm of 21.3 versus 10.1% in the surveillance arm. No improvement in QoL was found between the cohorts. The negative results of the UNICANCER study could possibly be driven by the larger population of IHCC patients compared with those in the BILCAP study (44 vs 18%).
Unfortunately, progress in treating patients with advanced biliary cancer has been modest. Gemcitabine in combination with cisplatin continues to be the standard of care per ABC trial results. This doublet improved survival compared with single-agent gemcitabine (OS of 11.7 vs 8.1 months; HR: 0.64; p < 0.001) [67]. There are no randomized data to support second-line therapy. A retrospective, single-center cohort analysis of metastatic, recurrent or inoperable biliary tract cancer, showed a 12-month OS of 53% for those who received second-line chemotherapy versus 21% for patients who received only best supportive care, with an HR of 0.36 (p = 0.001) [68]. These results were possibly confounded as the group that received second-line therapy had statistically improved prognostic variables (a higher Karnofsky Index; p = 0.0001), lower serum bilirubin (p = 0.03), higher hemoglobin (p = 0.002) and higher serum albumin (p = 0.0007). Using inverse probability of treatment weighting, researchers found no difference in OS when controlling for positive prognostic factors.
There are currently no approved targeted therapies for MSI-low cholangiocarcinoma. IDH1 mutations have an unusually high predilection for IHCC, with one case series finding 23% possessed this mutation [69]. A Phase I 3 + 3 designed trial testing the safety of an oral inhibitor of mIDH1 (AG-120) in IDH-positive recurrent or progressive cholangiocarcinoma was found to be well-tolerated with no dose-limiting toxicities seen [70]. In this biomarker selected population, the RR was disappointing at 6%, albeit 56% had SD and 6-month PFS was 40%. A follow-up Phase III trial is ongoing (ClarIDHy) to determine AG-120's efficacy, with plans to recruit 170 patients (NCT02073994).
FGFR mutations are found in 15–20% of IHCC [71]. FGFR appears to be an independent prognostic factor for cholangiocarcinoma. In a retrospective analysis of patients with cholangiocarcinoma and FGFR mutations who received FGFR inhibitors, an improved OS was found of 33 versus 17 months (p = 0.010) [72]. Early results from an ongoing open-label, Phase II study (NCT02150967) of BGJ398, a selective pan-FGFR inhibitor, were reported in abstract form (n = 47) [73]. This trial recruited patients with cholangiocarcinoma who did not respond or were intolerant to platinum-based chemotherapy and harbored a FGFR2 gene fusion or other FGFR genetic alterations. The primary end point was ORR. At data cut-off, among 36 patients evaluable for response, 22% of patients had a response per investigator assessment. Disease control rate was seen in 75% of patients and SD in 53%. Grade 3 or 4 adverse events were seen in 40% of patients, but were generally manageable with only two patients having to completely discontinue the medication (n = 47).
Pancreatic cancer
In the US, an estimated 53,670 new cases of pancreatic cancer will be diagnosed in 2017. Despite pancreatic cancer's relatively low incidence rate, it is the fourth leading cause of cancer death in the US with an estimated 43,090 deaths predicted in 2017 [1]. Surgery remains the only curative treatment for pancreatic cancer although less than 20% are resectable at the time of diagnosis [74]. Five year mortality for resectable disease remains poor even with adjuvant chemotherapy (Table 5). The ESPAC-4 study recently provided the best 5ySR following resection among western populations (5ySR = 29%) [75]. In this study, patients (n = 732) with macroscopically resected adenocarcinoma of the pancreas (R0 or R1) within 12 weeks of resection were randomly assigned 1:1 to single-agent gemcitabine versus gemcitabine (1000 mg/m2 days 1, 8 and 15) plus capecitabine (825 mg/m2 p.o. b.i.d. days 1–21; GEMCAP). This was a very poor prognosis patient population with high R1 resection rate (61%) and high percentage of positive lymph nodes (80%). In addition, patients with elevated Ca19.9 were eligible. R1 resection in this protocol was defined as any cancer cell within 1 mm of resection margin (CONKO-001, which showed a survival benefit with single-agent gemcitabine, limited enrollment to Ca19.9 <92 IU) [76]. Patients in the GEMCAP arm had improved median OS (28.0 vs 25.5 months; HR: 0.82; p = 0.032) [75]. A subset analysis in patients with R1 resection, showed no difference in median OS compared with single agent (23.7 vs 23.0 months; HR: 0.90; 95% CI: 0.72–1.13). However, the survival curves start to separate after 50% of events have occurred in each arm, and there seems to be a clear trend toward improved survival with the combination (5ySR: 24 vs 17%). In addition, patients were not randomized according to resection margins but only country of enrollment; therefore, in our opinion, the doublet is the new standard of care for patients with good PS regardless of margin status. Toxicities were manageable. It is important to note that 40% of patients in GEMCAP had grade 3–4 neutropenia. Although rates of febrile neutropenia were reportedly low in both groups (exact percentages not provided), dose reductions are often needed when using GEMCAP. Indeed, median dose intensity in the GEMCAP arm was around 80% for both gemcitabine and capecitabine. The most common grade 3–4 adverse events in the GEMCAP arm other than neutropenia were hand-foot syndrome (7%) and diarrhea (5%).
Table 5. . Updates in neoadjuvant therapy for pancreaticobiliary cancer.
| Study | Trial | Cancer type | Eligibility | Phase | Number of patients (n) | Timing | Intervention | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Primrose et al. | BILCAP | Cholangiocarcinoma or gallbladder cancer | Macroscopically complete resection (R0 and R1) | III | 447 | Adjuvant | Capecitabine vs observation | Median OS 51.1 vs 36.5 mo (p = 0.097) | [62] |
| Edeline et al. | UNICANCER | Biliary tract | Complete resection (R0 and R1) | III | 196 | Adjuvant | GEMOX vs observation | Median RFS 30.4 vs 22.0 mo (p = 0.31) | [66] |
| Neoptolemos et al. | ESPAC-4 | Pancreatic | Resected adenocarcinoma of the pancreas (R0 or R1) | III | 730 | Adjuvant | GEMCAP vs GEM | Median OS 28.0 vs 25.5 mo (p = 0.032) | [75] |
mo: Month; OS: Overall survival; RFS: Relapse-free survival.
The benefit of chemotherapy in patients with advanced pancreatic cancer is modest. Cancer stem cells may play a role in resistance to therapy [77]. Treatment strategies targeting stem cell population are of interest. The YOSEMITE study recently failed to show a survival benefit when demcizumab, a Notch inhibitor, was added to nab-paclitaxel and gemcitabine in a randomized Phase II trial [78]. Napabucasin (BBI608), an oral first-in-class cancer stemness inhibitor, showed a promising RR (51%) when added to nab-paclitaxel plus gemcitabine in a recent early Phase I/II study [79]. It is important to remember that RRs are typically overestimated in early phase studies (for instance RR in the Phase I trial with nab-paclitaxel and gemcitabine was 48% but only 23% in the MPACT trial) [80]. A randomized Phase III trial is currently ongoing to test benefit from adding napabucasin to standard chemotherapy (NCT02993731).
Targeting cancer cell metabolism is also an area of great interest in pancreatic cancer [81]. To meet high metabolic demands from a rapid proliferation rate, cancer cells can metabolize glucose through aerobic mitochondrial metabolism of pyruvate and also through anaerobic conversion of pyruvate to lactate (Warburg effect). A number of drugs targeting cancer cell metabolism are undergoing development. CPI-613 is a first-in-class lipoate analog. PDH and α-KGDH of the tricarboxylic acid (TCA) cycle need lipoate as a catalytic co-factor. CPI-613 binds to PDH and α-KGDH and disrupts their activity [82]. A small Phase I study recently showed a RR of 61% when CPI-613 was added to mFOLFIRINOX [83]. Unfortunately, on-treatment biopsies were not obtained and the study provided no insight into biomarkers of response. A randomized Phase III study with CPI-613 in combination with chemotherapy is currently being planned.
Targeting the tumor microenviroment has emerged as a potential target in solid tumors. PEGPH20, a recombinant human hyaluronidase enzyme, directly degrades hyaluronic acid (HA). It is hypothesized that HA accumulation contributes to increased interstitial pressure and inhibits delivery of cytotoxic agents. A randomized Phase II study evaluated the benefit of adding PEGPH20 to nab-paclitaxel plus gemcitabine in chemonaive metastatic pancreatic cancer (n = 279) [84]. The study was negative in the overall population. However, for patients with high HA expression on baseline biopsies, the combination arm with PEGPH20 had improved PFS (HR: 0.73; 95% CI: 0.53–1.00; p = 0.048), and a trend toward improved OS (11.5 vs 8.5 months; HR: 0.96; 95% CI: 0.57–1.61) [84].
Conclusion
A major breakthrough in 2017 for the treatment of all cancers, but specifically advanced upper GI malignancies, was the approval of pembrolizumab for patients with MSI-high tumors regardless of tissue of origin. More recently, the FDA also granted accelerated approval of nivolumab for patients with HCC that had progressed on sorafenib and pembrolizumab for PD-L1-positive advanced gastric cancer [26,45]. The recently completed CheckMate-047 results will be reported in the next months and can potentially lead to regulatory approval of nivolumab in the first-line treatment of HCC. It is also likely that additional approvals will follow in patients with gastroesophageal cancers.
It is evident that despite initial skepticism, immunoncology will play an important role in the treatment of upper GI malignancies. There will be many challenges on the horizon. First, only a small subset of patients with upper GI malignancies benefits from immunotherapy; and therefore, there is an urgent need to identify biomarkers of response. Responses are generally enriched in patients with high tumor mutation burden and/or high PD-L1 expression; however, these are still suboptimal biomarkers of response. Ongoing efforts looking at predictive gene signatures, T-cell clonality and frame-shift mutations leading to neoantigen expression will be critical. Combination immunotherapy including vaccines, different checkpoint inhibitors (TIGIT, LAG-3, TIM-3 and CD47) and IDO inhibitors are of great interest but are likely to increase immune related toxicities. In addition, the best assessment method to evaluate response to immunotherapy has yet to be defined.
The treatment landscape for patients with pancreaticobiliary malignancies has also gone through significant changes in 2017. Capecitabine has become a new standard of care for patients with resected bile duct cancer and PS 0–1. Of note, in the BILCAP study, a survival benefit was not seen in patients with hilar EHCC. These patients have a higher risk of locoregional relapse and could potentially benefit from intensification of local treatment including addition of chemoradiation. Similarly, for patients with resected pancreatic cancer, the ESPAC-4 study established GEMCAP as the new standard. The results of the recently completed APACT study are eagerly awaited and, if positive, could provide an additional treatment option for these patients. If the APACT study results in OS improvement, we could envision a scenario where GEMCAP could be offered to patients who do not desire to deal with neuropathy or hair loss associated with nab-paclitaxel. Furthermore, nab-paclitaxel plus gemcitabine would be a better option for patients on warfarin or with decreased kidney function. In addition, the use of next-generation sequencing has led to the identification of actionable genetic aberrations in patients with advanced pancreatic cancer that can potentially be targeted [85,86].
In summary, the treatment landscape for upper GI malignancies has entered an exciting era of rapid advances. Strategies to select patients likely to respond to immunotherapy and combination strategies with immunotherapy and cytotoxic agents or targeted therapies that modify the tumor microenvironment to enhance response to immunotherapy are warranted in the future.
Future perspective
Immunotherapy has emerged as a novel treatment paradigm for a subset of patients with upper GI malignancies. Ongoing research will contribute to identify novel biomarkers of response to immunotherapy beyond PD-L1 expression and tumor mutation burden. The use of mass spectrometry and novel bioinformatic algorithms may facilitate the identification of neoepitopes likely to trigger an immune response. These tools may be critical in the future to enrich clinical trials with subsets of patients likely to benefit from immunotherapy. In addition, targeted therapy will continue to expand as novel actionable aberrations (i.e., NTRK fusions) expressed at low prevalence across different tumor types continue to be identified. Combinations of different immunotherapies or immunotherapy with targeted therapy or chemotherapy are currently undergoing evaluation in patients with GI malignancies and may contribute to change the treatment landscape for these groups of diseases in the next years.
Summary points.
FLOT4-AIO included gastric/gastroesophageal junction (GEJ) cancers cT2 or higher and/or nodal-positive and were randomized to perioperative ECF/ECX × three cycles or FLOT × four cycles. Perioperative FLOT4 demonstrated superior tumor-free resection margins, lower T-stage at resection and high incidence of lymph node sterilization.
The ATTRACTION-2 trial demonstrated improved overall survival (OS) of nivolumab compared with placebo for unresectable or recurrent gastric or GEJ adenocarcinomas refractory to two or more lines of therapy.
The KEYNOTE-059 trial demonstrated high response rates for microsatellite instability-high gastric/GEJ tumors compared with nonmicrosatellite instability-high (57 vs 9%) with pembrolizumab.
The CheckMate 032 trial suggests improved response rates with combination immunotherapy (nivolumab and ipilimumab) compared with monotherapy (nivolumab) but with comparable OS rates in gastroesophageal cancers and was limited due to small recruitment numbers.
The REFLECT study demonstrated that lenvatinib is not inferior to sorafenib in the first-line setting for hepatocellular carcinoma (HCC) with equivalent median OS.
The RESORCE trial evaluated regorafenib in the second-line setting after progression on sorafenib and showed improvement in OS compared with placebo.
The CheckMate-040 trial demonstrated safety and response rates of nivolumab for treatment of HCC leading to its US FDA approval for second-line therapy.
The SARAH trial demonstrated similar median OS and serious adverse events between selective internal radiation therapy (SIRT) and sorafenib for treatment of locally advanced and recurrent HCC. The SIRveNIB trial demonstrated marginally improved OS of SIRT compared with sorafenib (6.4 vs 5.3 months) and with significantly fewer adverse events in the SIRT arm.
The BILCAP trial demonstrated a trend toward improved median OS with adjuvant capecitabine compared with placebo after resection of bile duct cancers.
FGFR inhibitors have shown activity in patients with cholangiocarcinoma harboring FGFR fusions.
The ESPAC-4 trial demonstrated improved median OS of gemcitabine plus capecitabine compared with single agent gemcitabine in the adjuvant setting for resected pancreatic cancer.
Targeting cancer stem cells, tumor microenvironment and cancer cell metabolism are novel targets for ongoing clinical trials.
Footnotes
Author contributions
J Aaron provided scientific editing.
Financial & competing interests disclosure
Funding was provided by P30CA042014-23 National Cancer Institute grant to HCI. We support deposit of this paper in the NIHMS system/PMC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as: • of interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett's eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39(12):1086–1091. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N. Engl. J. Med. 2009;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 4.Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Holscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann. Surg. 2011;254(1):67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomized controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 7.Ruhstaller T, Pless M, Dietrich D, et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter Phase IB/II trial (SAKK 75/06) J. Clin. Oncol. 2011;29(6):626–631. doi: 10.1200/JCO.2010.31.9715. [DOI] [PubMed] [Google Scholar]
- 8.Mariette C, Piessen G, Monterymard C, et al. Efficacy and safety of perioperative chemotherapy with 5FU-cisplatine-cetuximab in gastric and gastroesophageal junction adenocarcinomas (GGOJA): a single-arm multicenter Phase II trial (FFCD 0901) J. Clin. Oncol. 2015;33(Suppl. 3):154–154. [Google Scholar]
- 9.Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): a randomized Phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J. Clin. Oncol. 2017;35(Suppl. 4):1. doi: 10.1200/JCO.20.03611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera F, Galan M, Tabernero J, et al. Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(5):1430–1436. doi: 10.1016/j.ijrobp.2008.12.087. [DOI] [PubMed] [Google Scholar]
- 11.Ilson DH, Minsky BD, Ku GY, et al. Phase II trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer. 2012;118(11):2820–2827. doi: 10.1002/cncr.26591. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicenter, open-label, randomized Phase II–III trial. Lancet Oncol. 2017;18(3):357–370. doi: 10.1016/S1470-2045(17)30043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Batran S-E, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil and leucovorin versus epirubicin, cisplatin and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the Phase II part of a multicenter, open-label, randomized Phase II/III trial. Lancet Oncol. 2016;17(12):1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]; • FLOT4-AIO established FLOT as new standard for perioperative chemotherapy for any >cT2 and/or nodal-positive gastric cancer.
- 15.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009;27(30):5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 16.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J. Clin. Oncol. 2007;25(24):3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 17.Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase III, open-label, randomized controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Martin C, Plaza JC, Pazo-Cid R, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol. 2013;31(35):4445–4452. doi: 10.1200/JCO.2013.48.9070. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomized, open-label, adaptive, Phase II/III study. Lancet Oncol. 2017;18(5):640–653. doi: 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 21.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab (P) 1 trastuzumab (H) 1 chemotherapy (CT) for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (mGC/GEJC): final analysis of a Phase III study (JACOB) Ann. Oncol. 2017;28(Suppl. 5):v209–v268. [Google Scholar]
- 22.Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des. Devel. Ther. 2015;9:901–909. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, Phase III trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs CS, Ohtsu A, Tabernero J, et al. Preliminary safety data from KEYNOTE-059: pembrolizumab plus 5-fluorouracil (5-FU) and cisplatin for first-line treatment of advanced gastric cancer. J. Clin. Oncol. 2016;34(Suppl. 15):4037. [Google Scholar]; • KEYNOTE-059 trial of interest for microsatellite instability-high gastric cancers.
- 25.Fuchs CS, Doi T, Jang RW-J, et al. KEYNOTE-059 cohort 1: efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J. Clin. Oncol. 2017;35(Suppl. 15):4003. [Google Scholar]; • KEYNOTE-059 trial of interest for microsatellite instability-high gastric cancers.
- 26.Keytruda®, prescribing information. Merck & Co., Inc.; NJ, USA: 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s024lbl.pdf [Google Scholar]
- 27.Janjigian YY, Ott PA, Calvo E, et al. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. J. Clin. Oncol. 2017;35(Suppl. 15):4014. [Google Scholar]
- 28.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 31.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized Phase III trial. J. Clin. Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized Phase III BRISK-FL study. J. Clin. Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 33.Cainap C, Qin S, Huang W-T, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized Phase III trial. J. Clin. Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance) J. Clin. Oncol. 2016;34(Suppl. 4):192. [Google Scholar]
- 35.Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a Phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015;33(6):559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AL, Finn RS, Qin S, et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2017;35(Suppl. 15):4001. [Google Scholar]
- 37.Sangro B, Park JW, Dela Cruz CM, et al. A randomized, multicenter, Phase III study of nivolumab versus sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J. Clin. Oncol. 2016;34(Suppl. 15) [Google Scholar]
- 38.Llovet JM, Decaens T, Raoul J-L, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized Phase III BRISK-PS study. J. Clin. Oncol. 2013;31(28):3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 39.Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the evolve-1 randomized clinical trial. JAMA. 2013;312(1):57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 40.Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomized, double-blind, multicenter, Phase III trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 41.Abou-Alfa GK, Qin S, Ryoo B-Y, et al. Phase III randomized study of second line ADI-peg 20 (A) plus best supportive care versus placebo (P) plus best supportive care in patients (pts) with advanced hepatocellular carcinoma (HCC) J. Clin. Oncol. 2016;34(Suppl. 15):4017–4017. doi: 10.1093/annonc/mdy101. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]; • RESORCE trial showed benefit of regorafenib in hepatocellular carcinoma beyond progression on sorafenib.
- 43.National Comprehensive Cancer Network. Hepatobiliary cancers version 3.2017. 2017. www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 44.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, noncomparative, Phase I/II dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • CheckMate-040 trial showed benefit of nivolumab in the second line in advanced hepatocellular carcinoma.
- 45.Opdivo®, prescribing information. Bristol-Myers Squibb Co.; NJ, USA: 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s034lbl.pdf [Google Scholar]
- 46.NIH Clinical Trials. An investigational immuno-therapy study of nivolumab compared with sorafenib as a first treatment in patients with advanced hepatocellular carcinoma. https://clinicaltrials.gov/show/NCT02576509
- 47.Huang YH, Chen CH, Chang TT, et al. The role of transcatheter arterial embolization for patients with unresectable hepatocellular carcinoma: a nationwide, multicenter study evaluated by cancer stage. Aliment. Pharmacol. Ther. 2005;21(6):687–694. doi: 10.1111/j.1365-2036.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- 48.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 49.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 50.Vilgrain V, Bouattour M, Sibert A, et al. SARAH: a randomized controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma. Int. J. Hepatol. 2017;66(1):S85–S86. [Google Scholar]
- 51.Chow PHW, Gandhi M. Phase III multicenter open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: the SIRveNIB study. J. Clin. Oncol. 2017;35(Suppl. 15):4002. [Google Scholar]
- 52.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 53.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomized placebo-controlled, double-blind, Phase III trial. Lancet Gastroenterol. Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 54.NIH Clinical Trials. Sorafenib chemoembolization evaluation controlled trial (SELECT) https://clinicaltrials.gov/ct2/show/NCT01906216
- 55.NIH Clinical Trials. A randomized, controlled Phase III trial of sorafenib with or without cTACE in patients with advanced HCC. https://clinicaltrials.gov/ct2/show/NCT01829035
- 56.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat. Rev. Gastroenterol. Hepatol. 2016;13(5):261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015;47(9):1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 58.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the USA.: intrahepatic disease on the rise. Oncologist. 2016;21(5):594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang J-Y, Kim S-W, Park DJ, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann. Surg. 2005;241(1):77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A Phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: a Phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J. Clin. Oncol. 2015;33(24):2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J. Clin. Oncol. 2017;35(Suppl. 15):4006. [Google Scholar]; • BILCAP trial is the first randomized Phase III trial in patients with biliary malignancies to show survival benefit with adjuvant chemotherapy.
- 63.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J. Clin. Oncol. 2008;26(13):2118–2123. doi: 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J. Clin. Oncol. 2010;28(30):4581–4586. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]
- 65.Dwary AD, Sharma A, Mohanti BK, et al. A randomized controlled trial (RCT) comparing best supportive care (BSC), 5-FU plus folinic acid (FUFA) and, gemcitabine plus oxaliplatin (Gem-Ox) in management of unresectable gallbladder cancer (GBC) J. Clin. Oncol. 2009;27(Suppl. 15):4521. [Google Scholar]
- 66.Edeline J, Bonnetain F, Phelip JM, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) Phase III trial. J. Clin. Oncol. 2017;35(Suppl. 4):225. [Google Scholar]
- 67.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 68.Moik F, Riedl JM, Winder T, et al. Benefit of second-line chemotherapy for advanced biliary tract cancer. J. Clin. Oncol. 2017;35(Suppl. 15):e15621. [Google Scholar]
- 69.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J. Clin. Oncol. 2017;35(Suppl. 15):4015. [Google Scholar]
- 71.Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19(3):235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain A, Shroff RT, Kelley RK, et al. FGFR pathway genetic aberrations in cholangiocarcinoma: demographics and experience with targeted therapy. J. Clin. Oncol. 2016;34(Suppl. 15):109. [Google Scholar]
- 73.Javle M, Shroff R, Zhu A, et al. A Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J. Clin. Oncol. 2018;36(3):276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J. Gastrointest. Oncol. 2012;3(4):326–334. doi: 10.3978/j.issn.2078-6891.2012.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicenter, open-label, randomized, Phase III trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]; • ESPAC-4 trial established gemcitabine plus capecitabine as a new standard for resected pancreatic cancer plus.
- 76.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the conko-001 randomized trial. JAMA. 2013;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 77.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cubillo Gracian A, Dean A, Muñoz A, et al. YOSEMITE: a three arm double-blind randomized Phase II study of gemcitabine, paclitaxel protein-bound particles for injectable suspension and placebo (GAP) versus gemcitabine, paclitaxel protein-bound particles for injectable suspension and either 1 or 2 truncated courses of demcizumab (GAD) Ann. Oncol. 2017;28(Suppl. 5) [Google Scholar]
- 79.Bekaii-Saab T, Starodub A, El-Rayes B, et al. LBA-002A Phase Ib/II study of cancer stemness inhibitor napabucasin in combination with gemcitabine (gem) & nab-paclitaxel (nabptx) in metastatic pancreatic adenocarcinoma (mpdac) patients (pts) Ann. Oncol. 2017;28(Suppl. 3) [Google Scholar]
- 80.Hoff DDV, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a Phase I/II trial. J. Clin. Oncol. 2011;29(34):4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michalski CW, Hackert T, Büchler MW. Targeting metabolism in pancreatic cancer. Lancet Oncol. 2017;18(6):699–700. doi: 10.1016/S1470-2045(17)30304-2. [DOI] [PubMed] [Google Scholar]
- 82.Rafael Pharmaceuticals, Inc. CPI-613: a first-in-class therapeutic agent targeting cancer cell metabolism. http://rafaelpharma.com/wp-content/uploads/2017/05/Rafael-Website-CPI-613-Fact-Sheet-2.pdf
- 83.Alistar A, Morris BB, Desnoyer R, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-center, open-label, dose-escalation, Phase I trial. Lancet Oncol. 2017;18(6):770–778. doi: 10.1016/S1470-2045(17)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hingorani SR, Bullock AJ, Seery TE, et al. Randomized Phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) vs AG in patients (Pts) with untreated, metastatic pancreatic ductal adenocarcinoma (mPDA) J. Clin. Oncol. 2017;35(Suppl. 15):4008–4008. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 85.Hyman DM, Laetsch TW, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. J. Clin. Oncol. 2017;35(Suppl. 18) [Google Scholar]
- 86.Singhi AD, Ali SM, Lacy J, et al. Identification of targetable ALK rearrangements in pancreatic ductal adenocarcinoma. J. Natl Compr. Cancer Netw. 2017;15(5):555–562. doi: 10.6004/jnccn.2017.0058. [DOI] [PubMed] [Google Scholar]
- 87.He C, Bian XY, Ni XZ, et al. Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J. Gastroenterol. 2013;19(14):2171–2178. doi: 10.3748/wjg.v19.i14.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo AN, Kwak Y, Kim D-W, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE. 2014;9(5):e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest. Cancer Res. 2014;7(2):42–48. [PMC free article] [PubMed] [Google Scholar]
- 90.Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 2014;45(8):1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 91.Créancier L, Vandenberghe I, Gomes B, et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 2015;365(1):107–111. doi: 10.1016/j.canlet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 92.Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin. Cancer Res. 2017;23(7):1638–1646. doi: 10.1158/1078-0432.CCR-16-2411. [DOI] [PubMed] [Google Scholar]
- 93.cBioPortal: For Cancer Genomics. www.cbioportal.org/
- 94.Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (magic) trial. JAMA Oncol. 2017;3(9):1197–1203. doi: 10.1001/jamaoncol.2016.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Humphris JL, Patch AM, Nones K, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152(1):68–74. doi: 10.1053/j.gastro.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 96.Laghi L, Beghelli S, Spinelli A, et al. Irrelevance of microsatellite instability in the epidemiology of sporadic pancreatic ductal adenocarcinoma. PLoS ONE. 2012;7(9):e46002. doi: 10.1371/journal.pone.0046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goyal L, Deshpande V, Chung DC, et al. Mismatch repair protein loss and microsatellite instability in cholangiocarcinoma. J. Clin. Oncol. 2014;32(Suppl. 3):237–237. [Google Scholar]
- 98.Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized Phase 3 trial. J. Clin. Oncol. 2017;35(Suppl. 15):4004. [Google Scholar]
- 99.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicenter, open-label, Phase Ib trial. Lancet Oncol. 2016;17(6):717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 100.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicenter, Phase II trial. Lancet Oncol. 2017;18(5):631–639. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 101.Kang Y-K, Satoh T, Ryu M-H, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, Phase III trial. J. Clin. Oncol. 2017;35(Suppl. 4):2. [Google Scholar]
- 102.Moehler MH, Janjigian YY, Adenis A, et al. CheckMate 649: a randomized, multicenter, open-label, Phase III study of nivolumab (nivo)+ipilimumab (ipi) or nivo+chemotherapy (CTX) vs CTX alone in pts with previously untreated advanced (adv) gastric (G) or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 2017;35(Suppl. 15) [Google Scholar]
- 103.Janjigian YY, Adenis A, Aucoin J-S, et al. Checkmate 649: a randomized, multicenter, open-label, Phase III study of nivolumab (nivo) plus ipilimumab (ipi) versus oxaliplatin plus fluoropyrimidine in patients (pts) with previously untreated advanced or metastatic gastric (G) or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 2017;35(Suppl. 4) [Google Scholar]
- 104.Ohtsu A, Tabernero J, Bang Y-J, et al. Pembrolizumab versus paclitaxel as second-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Phase III KEYNOTE-061 study. J. Clin. Oncol. 2016;34(Suppl. 15):TPS4137. [Google Scholar]
- 105.Tabernero J, Bang Y-J, Fuchs CS, et al. KEYNOTE-062: Phase III study of pembrolizumab (MK-3475) alone or in combination with chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2016;34(Suppl. 4):TPS185. [Google Scholar]
- 106.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]