Abstract
Background
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system and is one of the leading causes of disability in young adults. Cell therapy is emerging as a therapeutic strategy to promote repair and regeneration in patients with disability associated with progressive MS.
Methods
We conducted a phase I open-label clinical trial investigating the safety and tolerability of autologous bone marrow mesenchymal stem cell-derived neural progenitor (MSC-NP) treatment in 20 patients with progressive MS. MSC-NPs were administered intrathecally (IT) in three separate doses of up to 1 × 107 cells per dose, spaced three months apart. The primary endpoint was to assess safety and tolerability of the treatment. Expanded disability status scale (EDSS), timed 25-ft walk (T25FW), muscle strength, and urodynamic testing were used to evaluate treatment response. This trial is registered with ClinicalTrials.gov, number NCT01933802.
Findings
IT MSC-NP treatment was safe and well tolerated. The 20 enrolled subjects completed all 60 planned treatments without serious adverse effects. Minor adverse events included transient fever and mild headaches usually resolving in <24 h. Post-treatment disability score analysis demonstrated improved median EDSS suggesting possible efficacy. Positive trends were more frequently observed in the subset of SPMS patients and in ambulatory subjects (EDSS ≤ 6.5). In addition, 70% and 50% of the subjects demonstrated improved muscle strength and bladder function, respectively, following IT MSC-NP treatment.
Interpretation
The possible reversal of disability that was observed in a subset of patients warrants a larger phase II placebo-controlled study to establish efficacy of IT MSC-NP treatment in patients with MS.
Funding source
The Damial Foundation.
Keywords: Multiple sclerosis, Mesenchymal stem cells, Intrathecal, Clinical trial
Highlights
-
•
In this phase I study repeated intrathecal dosing of autologous MSC-NPs in patients with progressive MS was well tolerated.
-
•
Dose of up to 10 million cells was safe and not associated with any significant adverse events.
-
•
The treatment was associated with functional neurological improvement, particularly in ambulatory patients.
Multiple sclerosis is one of the leading causes of disability in young adults. Our study is the first evidence suggesting that a cell-based therapeutic approach is capable of reversing disability in multiple sclerosis. Results of our study add to existing evidence demonstrating the safety and tolerability of intrathecal administration of autologous mesenchymal stem cell-derived neural progenitors. Our study was uniquely associated with repeated administrations of cells freshly harvested from culture, as opposed to cryopreserved cells thawed at the bedside, which may have contributed to the observed efficacy of the treatment. The continued development of this therapeutic approach will likely have implications for treating other neurological diseases.
1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune demyelinating disease of the central nervous system (CNS). Although the cause of the MS is unknown, the disease is characterized pathologically by early acute lesions made up of discrete areas of inflammatory demyelination that either resolve by remyelination or evolve into chronic lesions with associated axonal loss, oligodendroglial cell loss and glial scarring. Lesions of inflammatory demyelination may be visualized by MRI imaging of the CNS. Clinically, most patients have disease onset in young adulthood with characteristic symptom relapses and remissions (RRMS). Over time, RRMS may evolve to a secondary progressive (SPMS) phase with accrual of permanent disability. In about 10–15% of patients the disease course is primarily progressive from onset (PPMS). Available disease modifying therapies may prevent or delay disease progression through immunosuppression and immunomodulation (Comi et al., 2017). Once progressive disability is established, however, there are no therapies currently available to protect, repair, or regenerate neural tissue in order to restore neurological function (Ontaneda et al., 2017). Common clinical manifestations of patients with SPMS or PPMS include motor weakness with progressive paralysis, sensory dysfunction, bladder/bowel dysfunction, coordination difficulties and cognitive decline. Cell-based therapies are currently under investigation to alleviate some of these clinical symptoms as a strategy to target progressive decline, which remains a major unmet need in MS.
Mesenchymal stem cells (MSCs) extracted from various tissues including bone marrow have multipotent mesodermal differentiation potential, but more importantly have demonstrated ability to promote tissue repair through the release of paracrine factors (Meirelles Lda et al., 2009). Intravenous administration of MSCs or conditioned media derived from MSCs is protective against experimental autoimmune encephalomyelitis (EAE) in mice through immunomodulatory mechanisms (Bai et al., 2012; Payne et al., 2013; Rafei et al., 2009; Rajan et al., 2016; Zappia et al., 2005), thus forming the preclinical basis for clinical safety testing of intravenous (Cohen et al., 2017; Connick et al., 2012; Llufriu et al., 2014) or intrathecal (Karussis et al., 2010; Mohyeddin Bonab et al., 2007; Yamout et al., 2010) transplantation of autologous MSCs in patients with MS. MSCs are also capable of neuroprotection, promotion of oligodendrogenesis, and inhibition of gliosis, and thus may impact multiple aspects of MS pathology in the CNS (Chen et al., 2001; Li et al., 2005; Rivera et al., 2006; Shen et al., 2011; Steffenhagen et al., 2012; Zhang et al., 2006).
MSC-derived neural progenitors (MSC-NPs) are a subpopulation of MSCs that exhibit neuroectodermal lineage characteristics with reduced capacity to undergo mesodermal differentiation (Fu et al., 2008; Harris et al., 2012a; Harris et al., 2012b; Hermann et al., 2004; Mareschi et al., 2006). These properties are theorized to minimize the risk of ectopic differentiation after CNS transplantation (Grigoriadis et al., 2011). Similar to MSCs MSC-NPs exhibit immunoregulatory and trophic properties both in vitro and in vivo along with upregulation of candidate trophic factors including hepatocyte growth factor (HGF) (Harris et al., 2012b; Harris et al., 2012a; Cristofanilli et al., 2011). Intrathecal (IT) delivery of MSC-NPs during the chronic phase of EAE resulted in neurological recovery associated with increased spinal cord myelination decreased immune infiltration in the CNS and increased recruitment of endogenous progenitor cells (Harris et al., 2012b). Importantly multiple doses rather than a single dose were necessary to demonstrate improvement in neurological function (Harris et al., 2012b)
The clinical feasibility of IT MSC-NP treatment in MS was initially investigated in six patients with advanced MS treated with two to five injections of escalating doses of autologous MSC-NPs (Harris et al., 2016). Patients were followed an average of 7.4 years after initial injection. There were no serious adverse events or safety concerns noted, and the treatments were well-tolerated (Harris et al., 2016). Four of the six patients showed a measurable clinical improvement following MSC-NP treatment. Based on these pre-clinical and early clinical studies, we initiated an open-label phase I trial using IT autologous MSC-NPs to establish safety and tolerability and to determine efficacy trends in 20 patients with progressive MS. The outcomes of this study support the overall safety and tolerability of this therapeutic approach, in addition to revealing possible evidence of efficacy.
2. Methods
2.1. Study Design and Oversight
The study was an open-label, single-arm, phase I clinical trial to evaluate safety and tolerability of repeated IT administration of autologous MSC-NPs in 20 patients with progressive MS (Fig. 1). All study activities were conducted at the Tisch MS Research Center of New York. The study was conducted as an FDA investigational new drug, and is registered with ClinicalTrials.gov, number NCT01933802. The study was approved by Western Institutional Review Board and was conducted in accordance with the Helsinki Declaration. All patients gave written informed consent. An independent external data and safety monitoring board evaluated all safety data.
Fig. 1.
CONSORT flowchart for single-arm, open-label, phase 1 clinical trial of intrathecal autologous MSC-NP in patients with progressive MS.
The treatment phase of the study consisted of three separate IT injections of up to 1 × 107 autologous MSC-NPs spaced three months apart. The dose and dosing schedule were based on a preclinical study in mouse EAE, as well as observed safety and tolerability in an early clinical dose escalation study (Harris et al., 2016; Harris et al., 2012b). All patients were assessed on the day of treatment, and one day, one week, and one month following each dose. Post-treatment assessments occurred three and six months following the third dose. Long-term safety assessment was scheduled 24 months following the third dose. Although the study was not blinded, all treatments were administered by a single neurologist, and all assessments by a separate neurologist.
Eligible patients had clinically definite SPMS or PPMS with significant disability (EDSS ≥ 3.0) that was not acquired within the 12 months prior to enrollment. The inclusion of patients with a relatively stable disease state was designed to allow better discernment between natural disease progression and treatment-related events. Disease stability was determined by less than a 1.0 point change in EDSS in the 12 months preceding entry into the treatment phase of the study, and lack of gadolinium-enhancing lesions on an MRI and by a stable MRI disease burden (number and size of T2 lesions) over the same period. Exclusion criteria included patients with cognitive impairment (as determined by mini-mental and PASAT testing), which might impact fully informed consent, and with existing comorbidities such as cancer history that might complicate safety outcomes of the experimental treatment. To minimize additional variables, patients who were already receiving disease-modifying therapies (DMT) upon entering the study continued as a concomitant treatment through the course of the study (Table 1). In subjects receiving concomitant intrathecal methotrexate (IT-MTX), methotrexate and MSC-NPs were administered at least four weeks apart to minimize any effects of this treatment on the cells.
Table 1.
Patient demographics and dosing.
| Study subject ID | Age/gender | MS subtype | Baseline EDSS | Disease duration (years) | Concomitant DMT (time started)a | Number of MSC-NPs per IT dose |
||
|---|---|---|---|---|---|---|---|---|
| 1st dose | 2nd dose | 3rd dose | ||||||
| 01 | 65/M | SPMS | 3.5 | 14 | IT-MTX (5 yrs prior) | 9.6 × 106 | 10 × 106 | 10 × 106 |
| 02 | 54/F | SPMS | 5.5 | 13 | DMF (2 yrs prior), IVIG (1 yr prior) | 9.8 × 106 | 10 × 106 | 10 × 106 |
| 03 | 58/M | SPMS | 6.0 | 17 | IT-MTX (7 yrs prior) | 10 × 106 | 10 × 106 | 10 × 106 |
| 04 | 59/M | SPMS | 6.0 | 18 | IVIG (4 wks post) | 10 × 106 | 10 × 106 | 10 × 106 |
| 05 | 39/F | SPMS | 6.0 | 16 | NAT (8 yrs prior) | 7.4 × 106 | 10 × 106 | 10 × 106 |
| 06 | 51/F | SPMS | 6.0 | 25 | RTX (3 yrs prior) | 8.0 × 106 | 8.6 × 106 | 5.3 × 106 |
| 07 | 55/F | SPMS | 6.5 | 18 | RTX (2 yrs prior) | 7.2 × 106 | 9.2 × 106 | 10 × 106 |
| 08 | 53/F | SPMS | 6.5 | 27 | IFN-β (6 yrs prior) | 10 × 106 | 10 × 106 | 10 × 106 |
| 09 | 56/M | PPMS | 6.5 | 22 | None | 9.7 × 106 | 9.2 × 106 | 8.9 × 106 |
| 10 | 37/F | PPMS | 6.5 | 14 | IT-MTX (2 yrs prior) | 10 × 106 | 10 × 106 | 10 × 106 |
| 11 | 27/F | SPMS | 7.0 | 10 | RTX (2 wks post) | 10 × 106 | 10 × 106 | 10 × 106 |
| 12 | 52/F | SPMS | 7.5 | 32 | IT-MTX (11 yrs prior) | 7.2 × 106 | 7.0 × 106 | 10 × 106 |
| 13 | 45/F | SPMS | 7.5 | 11 | None | 9.6 × 106 | 9.8 × 106 | 9.7 × 106 |
| 14 | 34/F | SPMS | 7.5 | 12 | RTX (5 yrs prior) | 7.0 × 106 | 10 × 106 | 9.6 × 106 |
| 15 | 50/F | SPMS | 7.5 | 19 | RTX (6 yrs prior) | 8.9 × 106 | 10 × 106 | 10 × 106 |
| 16 | 63/F | SPMS | 8.0 | 32 | IT-MTX (6 yrs prior) | 10 × 106 | 9.5 × 106 | 8.9 × 106 |
| 17 | 61/F | SPMS | 8.0 | 32 | None | 9.3 × 106 | 9.0 × 106 | 10 × 106 |
| 18 | 50/M | PPMS | 8.0 | 10 | RTX (2 yrs prior) | 8.5 × 106 | 10 × 106 | 9.9 × 106 |
| 19 | 36/F | SPMS | 8.0 | 20 | IT-MTX (11 yrs prior) | 10 × 106 | 9.6 × 106 | 7.1 × 106 |
| 20 | 35/M | PPMS | 8.5 | 13 | None | 10 × 106 | 10 × 106 | 7.8 × 106 |
Abbreviations: DMT, disease-modifying therapy; IT, intrathecal; SPMS, secondary progressive multiple sclerosis; PPMS, primary progressive multiple sclerosis; IT-MTX, intrathecal methotrexate; RTX, rituximab; NAT, Natalizumab; DMF, dimethyl fumarate; IVIG, intravenous immunoglobulin; IFN-β, interferon-β-1a.
Time in number of years (yrs) or weeks (wks) the concomitant DMT was started either prior to or following (post) the first IT MSC-NP treatment.
2.2. Clinical Assessments
All study subjects underwent baseline exams that included EDSS, T25FW, nine-hole peg test, paced auditory serial addition test (PASAT), multiple sclerosis quality of life questionnaire, physical exam, neurological exam, and headache pain scale. Additional safety testing was performed as suggested by the FDA. Follow-up exams and neurological assessments were conducted at a pre-designated frequency after each dose in order to assess adverse events, and three and six months after the third dose to compare with pre-treatment baseline. The numerical headache pain scale from 0 (no pain at all) to 10 (pain as bad as it can be) was completed pre-treatment, and 24 h, 3 days, 5 days, 1 week, 2 weeks, and 1 month following each treatment. Headaches were classified as “mild” for pain rated between 0 and 3, “moderate” for pain rated between 4 and 7, and “severe” for pain rated between 8 and 10.
Brain MRI scans with and without gadolinium enhancement were performed at baseline, two months after the first dose, and three months after the third dose to assess safety and document any change. All scans were read by visual inspection by independent neuro-radiologists who were blinded to the sequence of MRIs in relation to treatment. Changes in T2 lesion burden, T1 black holes, brain volume, and gadolinium-enhancing lesions were documented.
To address bladder function, a pertinent history of symptoms was taken and any use of medications affecting bladder function was noted. All subjects underwent urodynamic testing at baseline and three months after the third dose. All urodynamic testing was performed in the same laboratory and results interpreted by a single neuro-urologist. The following parameters were recorded: PVR, cystometry with simultaneous measurements of bladder, urethral and subtracted rectal pressures for first sensation, maximum bladder capacity, urethral pressure profile with functional length measurement and maximum closing pressure. Bladder pressure was assessed with filling and sphincter relaxation and synergic voiding assessed. Urinary peak flow rate was also measured.
Muscle strength was graded by a single neurologist using Medical Research Council (MRC) scale as follows: 0, no contraction (plegic); 1, trace of contraction; 2, active movement, gravity eliminated; 3, active movement against gravity; 4, active movement against gravity and resistance; 5, normal strength (Medical Research Council, 2000). Muscles that were scored included deltoid, biceps brachii, triceps, wrist extensors, digit extensors and flexors in the upper limb and iliopsoas, hip abductors/adductors, quadriceps, hamstrings, ankle dorsi and planter flexors, and toe flexors and extensors in the lower limbs. Marked improvement was defined as improvement in multiple muscle groups and increased muscle function. Moderate improvement defined as increased strength in at least one muscle with functional change. Mild improvement defined as increased strength in at least one muscle without functional change.
2.3. Preparation of Autologous MSC-NP Cells
Clinical-grade autologous MSC-NPs for each subject were isolated, expanded, and analyzed under a current good manufacturing practice (cGMP)-compliant process in a clean-room facility at the Tisch MS Research Center of New York. As previously described (Harris et al., 2012a; Harris et al., 2016), MSCs were isolated based on plastic adherence from the mononucleated cell fraction from a 20 ml sternal bone marrow aspirate, and expanded ex vivo in MSC growth medium (Lonza) supplemented with 2 mM GlutaMAX™I CTS™ (Thermo Fisher Scientific) and 10% double-filtered autologous serum collected from peripheral blood of each study participant. Cells were incubated in a humidified 37 °C incubator at 5% CO2 and 5% O2. MSCs were cryopreserved after two and three passages, generating a stock of cells sufficient for multiple subsequent expansions. For each treatment, a portion of MSCs were thawed, expanded for two to three more passages, then cultured for two to three weeks in neural progenitor maintenance medium (Lonza) supplemented with 20 ng/ml each of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) to generate neural progenitors (MSC-NPs). Just prior to the injection, MSC-NPs were collected, washed, counted, and resuspended in preservative-free saline for immediate intrathecal administration. The target cell number was between 5 × 106 and 1 × 107 cells. The maximum dose of 1 × 107 cells per treatment was not always attained due to the individual growth characteristics of each batch of MSC-NPs (Table 1).
Quality and safety testing was conducted at multiple steps during MSC-NP manufacturing to ensure quality, sterility and batch-to-batch consistency. Bone marrow-derived MSCs were tested for MSC characteristics including growth, morphology, cell surface expression (CD105+/CD73+/CD90+/CD45−/CD34−/CD14−/CD20−/HLA-DR−), and in vitro osteogenic and adipogenic differentiation potential as determined by alizarin red or Oil Red O positivity, respectively, as described previously (Harris et al., 2012a). After each MSC expansion, chromosome analysis was performed on a minimum of 20 DAPI-banded metaphases and all metaphases were fully karyotyped (Molecular Cytogenetics Core Facility, Memorial Sloan Kettering Cancer Center). MSC-NPs generated from MSCs were assessed based on neurosphere morphology, cell number, and cell viability by trypan blue. Sterility was assessed by qPCR with Microbial DNA qPCR Assays Pan Bacteria 3 and Pan Aspergillus/Candida (Qiagen) using DNA extracted from MSC-NP conditioned media. Final product sterility was confirmed by gram stain and by 14-day liquid culture.
Confirmation of neural lineage differentiation of MSC-NPs was performed by TaqMan® Gene Expression Assays (Thermo Fisher Scientific) as previously described (Harris et al., 2012a) to confirm neural lineage gene upregulation of C-X-C motif chemokine receptor 4 (CXCR4) (Assay ID: Hs00607978_s1), Sry-box 2 (SOX2) (Assay ID: Hs01053049_s1), toll like receptor 2 (TLR2) (Assay ID: Hs01872448_s1), and leukemia inhibitory factor (LIF) (Assay ID: Hs01055668_m1) and mesodermal lineage gene downregulation of smooth muscle isoform of alpha 2 actin (ACTA2) (Assay ID: Hs00426835_g1) and CD90 (THY1) (Assay ID: Hs00264235_s1) in MSC-NPs compared to the MSCs from which they were derived. Gene expression was normalized to IPO8 endogenous control (Hs00183533_m1) and relative quantification was determined by delta delta Ct analysis of MSC-NPs compared to the MSCs using RQ Manager software (Applied Biosystems). Final product testing specified >2-fold up- or down-regulation of at least 4 of the 6 genes on the panel for product release. Additional gene targets analyzed included hepatocyte growth factor (HGF) (Assay ID: Hs00300159_m1), nestin (NES) (Assay ID: Hs00707120_s1), neurofilament medium (NEFM) (Assay ID: Hs00193572_m1), and glial fibrillary acidic protein (GFAP) (Assay ID: Hs00909238_g1).
2.4. Treatment Procedure
Injections of autologous cell suspensions were performed intrathecally via standard lumbar puncture at the L3-L4 level using a 24 gauge non-traumatic spinal needle. Cerebrospinal fluid (CSF) (3–5 ml) was withdrawn, and MSC-NPs were resuspended in 2 ml of sterile saline and injected into the intrathecal space, followed by a flush of 2 ml of saline. In eight of the subjects, cells were administered intrathecally via the in-dwelling access port of their implanted baclofen pump. For all IT-MSC-NP treatments, prophylactic IV infusion of antibiotics (80 mg of tobramycin and 500 mg of vancomycin) was co-administered to minimize any risk of meningitis. Tobramycin or vancomycin had no effect on MSC-NP cell viability or proliferation in vitro (data not shown).
2.5. Statistical Analysis
The Wilcoxon Signed-Rank test was used to compare median EDSS from baseline (at the time of the first dose) to the final time point 6 months post-3rd dose. The Wilcoxon Rank Sum test was used to assess the change from baseline to 6 months between ambulatory patients (baseline EDSS ≤ 6.5) and non-ambulatory patients (baseline EDSS ≥ 7.0). The Fisher's exact test was used to assess the association between EDSS improvement (i.e. at least a 0.5 point decrease in EDSS or more at six months post-treatment vs. no change or increase in EDSS) and baseline ambulatory status, as well as the association between EDSS improvement and disease subtype (i.e., SPMS vs. PPMS). All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. Statistical analysis was performed in R Version 3.3.1 (R Core Team, Vienna, Austria).
3. Results
Demographics of the 20 study subjects are shown in Table 1. The study cohort had a mean EDSS of 6.8 (range 3.5 to 8.5) and mean disease duration of 18.8 years (range 10–32 years). Only six of the twenty patients were male and 80% of the patients had SPMS. Half of the study subjects were non-ambulatory at the time of enrollment (EDSS ≥ 7.0), eight subjects required assistance for ambulation (EDSS 6.0 to 6.5), and two subjects were able to ambulate without assistance (EDSS 3.5 to 5.5). There were no significant associations observed between ambulatory status and patient demographics (age, gender, disease subtype, or disease duration).
The primary objective of the phase I study was to test safety and tolerability of autologous MSC-NPs administered intrathecally in MS patients. MSC-NPs were administered every three months for a total of three doses of up to 1 × 107 cells per dose. Variation in the final dose, which averaged 9.4 × 106 cells (range 5.3 × 106 to 1 × 107 cells) was dependent on the individual growth characteristics of each batch of MSC-NPs (Table 1). There was no correlation between the number of cells administered or cell viability with age or disease type. All patients received the target dose range of five to ten million cells, and 70% received the maximum dose. Each batch of MSC-NPs was confirmed to have neural lineage characteristics prior to injection (Supplementary Fig. 1).
There were no serious adverse events or hospitalizations associated with IT MSC-NP treatment (Table 2). Specifically, we did not observe any cases of chemical or infectious meningitis. The most frequently observed minor adverse events consisted of transient headaches and fever occurring within 24 h after the treatment in 17 out of 20 patients (85%). Of the 60 total treatments, the majority (34/60, or 57%) were associated with mild to moderate headaches, and 15% (9/60) were associated with severe headaches. All headaches resolved within 1 week after over-the-counter treatment with acetaminophen, ibuprofen, naproxen, or no treatment. The exception was subject #17 who experienced headache of mild to moderate severity that persisted for one month that was determined to be a consequence of the lumbar puncture procedure (spinal headache). In addition, 5 patients experienced low-grade fever (below 100 °F) 24 h after treatment that resolved in all cases within 48 h without any treatment. Overall, fever was associated with 9 out of 60 treatments (15%). The incidence of headache or fever did not differ among the 3 IT administrations. Upon specific questioning, none of the adverse events were severe enough for any study subject to choose to discontinue participation in the trial.
Table 2.
Adverse events during or after IT MSC-NP treatment.
| Events | Number out of 20 participants (% of participants) |
|---|---|
| Any event | 18 (90%) |
| Any severe event, hospitalization or death | 0 (0%) |
| Any headache | 17 (85%) |
| Mild/moderate headache | 11 (55%) |
| Severe headache | 6 (30%) |
| Fever | 5 (25%) |
| Urinary tract infection | 9 (45%) |
| Fatigue | 2 (10%) |
| MS exacerbation | 0 (0%) |
| Post lumbar puncture headache | 1 (5%) |
| Miscellaneous (renal calculus) | 1 (5%) |
| Depression | 1 (5%) |
The safety data was further supported by a lack of any change in brain MRI scans during the study. Specifically, no new T2 lesions or changes in disease burden were observed. These data demonstrate that multiple IT administration of MSC-NPs was well tolerated and was associated with short-term safety.
The study design finalized with FDA guidance was not blinded, and there were no placebo controls. All study subjects were monitored for changes in clinical status. The primary post-treatment clinical assessments were conducted at three and six months following the third treatment and compared to baseline (pre-treatment) in order to determine trends in efficacy. Of the 20 study subjects, 15 (or 75%) demonstrated neurological improvement associated with IT MSC-NP treatment. Improvements were documented in the following areas: EDSS, MRC muscle strength scale, timed 25-ft walk (T25FW), and/or bladder function. Of the remaining subjects, two showed disease worsening despite the treatment, and three subjects showed no change.
Muscle strength testing by MRC muscle strength scale showed that 14 of the 20 subjects (70%) showed objective increase in muscle strength (Table 3) with most of the subjects (13 out of 14) demonstrating improvement in lower limb (LL) strength. Only one of the 14 patients had increased strength limited to the upper limbs (UL). In addition to greater muscle strength, four out of the ten ambulatory subjects showed a >20% improvement in T25FW speed compared to pre-treatment baseline (Table 4). Two additional study subjects (#11 and #12) who were non-ambulatory at baseline were able to perform the walk test post-treatment, albeit with bilateral use of assistive devices.
Table 3.
Muscle strength at baseline and three months post-third IT MSC-NP treatment.
| Study Subject ID | Upper limb (UL) Lower limb (LL) |
Muscle strength (weakest-strongest)a |
Description | |||
|---|---|---|---|---|---|---|
| Baseline |
Post-treatment |
|||||
| Left | Right | Left | Right | |||
| 01 | UL | 5 | 5 | 5 | 5 | Marked improvement in right LL with normalization of motor strength. |
| LL | 5 | 3–4 | 5 | 5 | ||
| 02 | UL | 5 | 5 | 5 | 5 | Marked improvement bilaterally in LL with normalization of motor strength. |
| LL | 4–5 | 3–5 | 5 | 5 | ||
| 03 | UL | 4–5 | 5 | 4–5 | 5 | Marked improvement in left LL. |
| LL | 1–5 | 5 | 3–5 | 5 | ||
| 04 | UL | 5 | 2–5 | 5 | 3–5 | Marked improvement bilaterally in LL with modest improvement in right UL. |
| LL | 4–5 | 3–5 | 5 | 4–5 | ||
| 05 | UL | 5 | 5 | 5 | 5 | Moderate improvement bilaterally in hip flexors. |
| LL | 4–5 | 4–5 | 4–5 | 4–5 | ||
| 06 | UL | 4–5 | 5 | 4–5 | 5 | Moderate improvement in bilateral hip flexors. |
| LL | 2–4 | 4–5 | 3–4 | 4–5 | ||
| 07 | UL | 5 | 4–5 | 5 | 4–5 | No change in motor strength. |
| LL | 2–5 | 2–4 | 2–5 | 2–4 | ||
| 08 | UL | 4–5 | 5 | 5 | 5 | Moderate improvement in left UL and bilaterally in LL. |
| LL | 2–4 | 3–5 | 2–5 | 5 | ||
| 09 | UL | 5 | 4–5 | 4–5 | 5 | Mild improvement in right UL and LL. Weaker left digit extensors. |
| LL | 4–5 | 2–5 | 4–5 | 3–5 | ||
| 10 | UL | 4 | 5 | 4–5 | 5 | No overall change in motor strength. |
| LL | 1 | 3–5 | 2 | 3–4 | ||
| 11 | UL | 5 | 5 | 5 | 5 | Moderate improvement bilaterally in LL. |
| LL | 2–4 | 2–3 | 3–5 | 2–4 | ||
| 12 | UL | 5 | 3–5 | 5 | 3–5 | Moderate improvement in right LL. |
| LL | 2–4 | 1–2 | 2–4 | 1–2 | ||
| 13 | UL | 3–4 | 5 | 2–3 | 5 | Increased weakness of left UL and right LL. |
| LL | 1–2 | 4–5 | 1–2 | 3–5 | ||
| 14 | UL | 4–5 | 4–5 | 4–5 | 4–5 | No change in motor strength. |
| LL | 2–4 | 2–4 | 2–4 | 2–4 | ||
| 15 | UL | 4–5 | 4–5 | 4–5 | 4–5 | No change in motor strength. |
| LL | 2–4 | 2–5 | 2–4 | 2–5 | ||
| 16 | UL | 4–5 | 4–5 | 4–5 | 4–5 | No change in motor strength. |
| LL | 0–2 | 2–5 | 0–2 | 2–5 | ||
| 17 | UL | 4 | 5 | 3–4 | 5 | Mild improvement in right quadriceps. |
| LL | 0 | 0 | 0 | 0–2 | ||
| 18 | UL | 4–5 | 5 | 4–5 | 5 | Mild improvement in right quadriceps. |
| LL | 1–2 | 1–2 | 1–2 | 1–4 | ||
| 19 | UL | 5 | 5 | 5 | 5 | Moderate improvement bilaterally in LL. |
| LL | 0 | 2 | 1–2 | 2–4 | ||
| 20 | UL | 2–3 | 2–4 | 4–5 | 2–4 | Moderate improvement in left UL. |
| LL | 0 | 0 | 0 | 0 | ||
Muscle strength graded MRC scale as follows: 0, no contraction (plegic); 1, trace of contraction; 2, active movement, gravity eliminated; 3, active movement against gravity; 4, active movement against gravity and resistance; 5, normal strength. Muscle strength indicated as weakest to strongest muscle.
Table 4.
Timed 25-ft walk results at baseline and three months post-third IT MSC-NP treatment.
| Study subject ID | T25FW time at baseline (seconds) | T25FW time post-treatment (seconds)a | % Improvement of T25FW | |
|---|---|---|---|---|
| Ambulatory at baseline | 01 | 6.0 | 5.0 | 17% |
| 02 | 10.8 | 5.9 | 46% | |
| 03 | 12.7 | 9.6 | 24% | |
| 04 | 8.4 | 6.1 | 26% | |
| 05 | 12.2 | 12.6 | -3% | |
| 06 | 18.3 | 18.6 | −2% | |
| 07 | 28.1 | 27.0 | 4% | |
| 08 | 53.4 | 23.9 | 55% | |
| 09 | 25.3 | 22.0 | 13% | |
| 10 | 97.4 | – | n/a | |
| Non-ambulatory at baselineb | 11 | – | 39.1 | n/a |
| 12 | – | 165.8 | n/a |
Abbreviations: T25FW, timed 25-ft walk; “-”, test not performed because subject not ambulatory; n/a, not applicable.
Pre- and post-walk times were determined using the same assistive device (if any) for each individual study subject.
Remaining non-ambulatory study subjects (ID# 13–20) not shown due to lack of change in ambulation post-treatment.
EDSS was assessed every three months starting at baseline, at the time of second and third dosing, and three and six months post third dose (Fig. 2). Overall, there was a decrease in median EDSS from 6.8 at baseline to 6.5 six months post-third treatment (p = .058). It was notable that the minimum EDSS score was lower at the final time-point (minimum EDSS of 1.5) than at baseline (minimum EDSS of 3.5). Eight study subjects (40%) demonstrated at least a 0.5 point improvement in EDSS six months post-treatment, with four of the eight subjects showing an improvement of 2.0 or greater positive change compared to baseline. Ten study subjects showed continued stable EDSS throughout the course of treatment, and two study subjects had evidence of disease progression as determined by a decline in EDSS. There was a statistically non-significant change in EDSS score from baseline to six months post-treatment in subjects who were ambulatory at baseline (median difference 0.5 (0.0;2.0)) compared to non-ambulatory patients (median difference 0.0 (0.0;0.0)) (p = .097). Similarly, a higher proportion of ambulatory patients (60%) had an EDSS improvement of 0.5 or more compared to non-ambulatory patients (20%) (p = .170). There was also a statistically non-significant trend for a higher percentage of improvement (≥0.5 on EDSS vs. no improvement) in SPMS patients (50%) compared to patients with PPMS (0%) (p = .117).
Fig. 2.
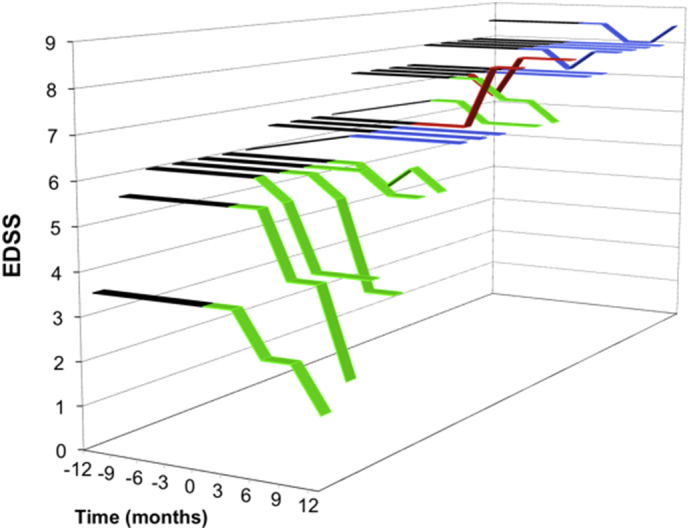
Changes in EDSS score after MSC-NP administration. EDSS scores are depicted for each of the 20 study subjects over the duration of the study (months). IT MSC-NP treatments were initiated at time 0 months. Black bars indicate EDSS scores during the one-year interval prior to receiving the first dose (months −12 to 0). Eight subjects showed ≥0.5 point reduction in EDSS (yellow bars), 10 subjects showed no change in EDSS (blue bars) and 2 subjects showed disease worsening (red bars). Overall, in the 20 study subjects there was a decrease (p = 0.058) in median value from baseline (month 0) EDSS 6.8 to final post-treatment (month 12) EDSS of 6.5 as determined by Wilcoxon Signed-Rank test.
To determine whether bladder function was affected by IT MSC-NP treatment, we conducted urodynamic testing in all study subjects at baseline and three months post-third treatment, in addition to taking a urinary function history and noting use of bladder medications. One subject had normal bladder function at baseline, and a second subject neglected to complete baseline urodynamics so they were not included in the bladder function analysis. Of the remaining 18 study subjects, nine subjects (50%) demonstrated either symptomatic and/or urodynamic improvement in bladder function post treatment (Table 5). Improvement was evident either by a >20% improvement in bladder capacity at three-month follow-up compared to baseline, and/or by discontinuation of bladder medication and documented improvement in bladder function upon questioning. We hypothesize that improvement of bladder function may reflect MSC-NP-mediated repair and regeneration in spinal cord areas proximal to the area of injection.
Table 5.
Bladder function improvements following IT MSC-NP treatment.
| Study subject IDa | Baseline bladder Function | Post-treatment bladder function |
|---|---|---|
| 01 | Micturition urgency treated with tolterodine. UD consistent with mild BPH. | Improved urgency and D/C tolterodine. No change in urodynamics. |
| 02 | Incomplete emptying with mild urgency. PVR of 274 ml. | Asymptomatic. PVR normalized to <70 ml. |
| 03 | Urgency and hesitancy with incomplete emptying. Treated with tamsulosin. UD testing showed neurogenic bladder with outlet obstruction and PVR of 200 ml. | Improved urgency and emptying with D/C tamsulosin. UD showed improved detrusor-sphincter dysfunction with PVR of 100 ml. |
| 05 | Urgency and retention requiring IC ×3 daily. Treated with oxybutynin and tamsulosin. UD showed detrusor overactivity with PVR of 400 ml. | Improved urgency and able to void without IC. D/C medications. Improved detrusor contractility on UD with 40% reduction of PVR. |
| 07 | Urgency treated with mirabegron. UD showed neurogenic bladder with detrusor overactivity and bladder capacity of 100 ml. | Improved urgency. UD showed improved detrusor overactivity and increased bladder capacity to 300 ml. |
| 11 | Urgency with incontinence. Treated with mirabegron. UD showed severe detrusor sphincter dyssynergia with detrusor overactivity. Bladder capacity of 200 ml. | Improved urgency with no incontinence. UD confirmed improved detrusor function with bladder capacity increased to 600 ml. |
| 12 | Urgency, incomplete emptying, and double voiding. UD showed PVR of 270 ml. | Asymptomatic with no double voiding. UD showed improved PVR by 20%. |
| 15 | Urgency with incontinence requiring diapers. Treated with tamsulosin. UD showed severe detrusor overactivity with bladder capacity of 177 ml. | Improved urgency and incontinence. D/C diaper use. UD showed decreased bladder pressures and detrusor activity with increased bladder capacity by 70%. |
| 16 | Urgency with incontinence and incomplete emptying. Treated with tamsulosin. UD showed detrusor overactivity and reduced bladder capacity of 107 ml. | Improved urgency and bladder emptying with absence of incontinence. D/C tamsulosin. UD showed normalized detrusor activity with increased bladder capacity to 667 ml. |
Abbreviations: UD, urodynamics; IC, intermittent catheterization; D/C, discontinued; PVR, post-void residual volume; BPH, benign prostatic hypertrophy.
Bladder function details shown only for the 9 study subjects who demonstrated bladder function improvement post-treatment.
As intact cognition function was a requirement to participate in this study, we did not observe any changes on PASAT testing. There were no efficacy trends in upper limb function as determined by the nine-hole peg test, although one patient who could not perform the test pre-treatment showed measurable improvement in the non-dominant limb (Supplementary Table 1). Overall, we observed that subjects with improved EDSS after IT MSC-NP treatment also showed consistent improvement in muscle strength, T25FW, and/or bladder function (Supplementary Table 2).
4. Discussion
This single center, open-label phase I study in patients with MS shows that repeated administration of intrathecally-injected bone marrow derived MSC-NPs is safe and well tolerated. Consistent with previous reports, intrathecal cell therapy is associated with transient headaches and mild febrile reactions in the immediate post-injection period (Bonab et al., 2012; Harris et al., 2016; Karussis et al., 2010; Mohyeddin Bonab et al., 2007). Importantly, there were no serious adverse events associated with this treatment. Despite having the option to discontinue treatment at will, all 20 of the study subjects tolerated and completed the 60 planned treatments.
Although this open-label single arm study was not designed to establish efficacy, we report our observations. Improvement of established disability was seen in 40% of patients as assessed by a decrease in post-treatment EDSS scores compared to baseline. This possible efficacious therapeutic effect is confounded by a lack of comparator placebo arm. However, improvement was also observed in muscle strength testing in at least one muscle group in 70% of patients, which was associated with improved ambulatory speed and less measurable disability in 40% of all patients. Consistent with our previous observations (Harris et al., 2016), post-treatment improvements in bladder function were observed in 50% of study subjects as determined by decreased symptomology, decreased medication use or improved urodynamic values. These efficacy trends were particularly encouraging given that the patient selection was not particularly suited to determine efficacy as the majority of patients had advanced disability (mean EDSS at baseline was 6.8) and had long-standing disease (mean disease duration over 18 years). Statistical trends in the data suggest that the therapeutic response to IT MSC-NP treatment may be most evident in ambulatory patients who have SPMS, in contrast to those who are non-ambulatory or who have PPMS. However, we acknowledge that the open-label study design of this phase I trial was not primarily setup to determine efficacy but to ensure safety. The overriding safety concerns with the use of multiple IT cell injections, including the possible development of acute hydrocephalus by the infusion of IT cells or risk of tumor development, resulted in close initial monitoring and a staggered initial treatment of patients. Despite the shortcomings of the efficacy assessments, our positive results are noteworthy for a phase I study in a population of advanced MS patients, and potentially represent a new therapeutic option for MS patients with established disability.
A number of key factors may have contributed to our observations using IT MSC-NP treatment. First, the experimental design included multiple rather than single dosing of MSC-NPs, as a result of our previous work in the EAE animal model of MS (Harris et al., 2012b). Likewise, in other neurological diseases such as amyotrophic lateral sclerosis (ALS), multiple dosing of intrathecal MSCs has been incorporated into early phase I/II study designs as supported by pre-clinical models (Oh et al., 2015; Staff et al., 2016; Zhang et al., 2009a). Based on the proposed trophic mechanism of action of MSC-NPs, we hypothesize that multiple doses of cells may be necessary for the sustained production of immunomodulatory and trophic factors in order to exceed a therapeutic threshold (Harris et al., 2012b; Zhang et al., 2009a). We found that MSC-NPs express and secrete a number of trophic factors including HGF, GDNF, IGF, and LIF (Harris et al., 2012a), all of which have been shown previously to mediate various aspects of neural repair including recruitment of endogenous progenitors (Bai et al., 2012; Deverman and Patterson, 2012; Huang and Dreyfus, 2016; Zhang et al., 2009b; Zhao et al., 2008). In addition, the release of immunomodulatory factors by intrathecally transplanted MSC-NPs may specifically target the compartmentalized inflammation such as astrocyte and microglial activation characteristic of progressive MS (Mahad et al., 2015).
Another important aspect of this study was the use of the intrathecal route of administration for cell delivery. Although intravenous administration of bone marrow MSCs was capable of suppressing EAE through immunomodulatory mechanisms, it has not been established that a sufficient number of cells cross the blood-brain barrier to directly impact reparative mechanisms in the CNS (Abramowski et al., 2016; Gerdoni et al., 2007; Kassis et al., 2008). Indeed, early clinical trials testing intravenous autologous MSCs have shown limited therapeutic benefit in MS (Cohen et al., 2017; Connick et al., 2012; Llufriu et al., 2014). Because physical disability in MS is likely a manifestation of spinal cord disease, the IT route of administration maximizes the therapeutic potential for effect in the spinal cord. The safety and feasibility of this approach is supported by initial open-label clinical studies investigating autologous MSCs or MSC-NPs in MS administered via the IT route (Bonab et al., 2012; Harris et al., 2016; Karussis et al., 2010; Mohyeddin Bonab et al., 2007; Yamout et al., 2010). Although some side effects associated with meningeal irritation were initially reported, particularly in one patient who received a very high cell dose (Yamout et al., 2010), IT cell delivery is generally well tolerated. Lastly, and perhaps most importantly, MSC-NPs were freshly harvested from cell culture and immediately administered to the patient, thus addressing a recent concern regarding impaired therapeutic functionality of cryopreserved cells thawed at bedside (Francois et al., 2012; Moll et al., 2014).
In conclusion, this study shows that intrathecal therapy with MSC-NPs is safe and well tolerated in patients with MS. Improvement of neurological disability was noted in a number of patients. These findings have led to the initiation of a FDA-approved randomized, placebo controlled and blinded phase II study in a larger group of patients to determine efficacy.
Funding Source
The study was supported by funding from the Damial Foundation. The funding source had no involvement in the study, manuscript preparation, or decision to submit the study for publication.
Conflicts of Interests
The authors indicate no potential conflicts of interest.
Contributors
VKH and SAS wrote the manuscript and were involved in study design, figures, literature search and data gathering, analysis, and interpretation. JS, TV, and VS were involved in data gathering, analysis and interpretation. LB and GJ were involved in study coordination, data gathering, and figures. GS was involved in data gathering. All authors have seen and approved the final version of the manuscript for publication.
Acknowledgements
The authors thank Mason Diamond, DDS for regulatory consultation, Gouri Nanjangud, PhD, FACMG from the Molecular Cytogenetics Core Facility at Memorial Sloan-Kettering Cancer Center for assistance with cytogenetics, and Linda M. Gerber, PhD, and Gülce Askin, MPH, from the Department of Healthcare Policy & Research, Division of Biostatistics and Epidemiology at Weill Cornell Medicine for statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.02.002.
Appendix A. Supplementary data
Supplementary figure 1 and supplementary tables 1 and 2.
References
- Abramowski P., Krasemann S., Ernst T., Lange C., Ittrich H., Schweizer M., Zander A.R., Martin R., Fehse B. Mesenchymal stromal/stem cells do not ameliorate experimental autoimmune encephalomyelitis and are not detectable in the central nervous system of transplanted mice. Stem Cells Dev. 2016;25:1134–1148. doi: 10.1089/scd.2016.0020. [DOI] [PubMed] [Google Scholar]
- Bai L., Lennon D.P., Caplan A.I., Dechant A., Hecker J., Kranso J., Zaremba A., Miller R.H. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat. Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonab M.M., Sahraian M.A., Aghsaie A., Karvigh S.A., Hosseinian S.M., Nikbin B., Lotfi J., Khorramnia S., Motamed M.R., Togha M., Harirchian M.H., Moghadam N.B., Alikhani K., Yadegari S., Jafarian S., Gheini M.R. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr. Stem Cell Res. Ther. 2012;7:407–414. doi: 10.2174/157488812804484648. [DOI] [PubMed] [Google Scholar]
- Chen J., Li Y., Wang L., Lu M., Zhang X., Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Cohen J.A., Imrey P.B., Planchon S.M., Bermel R.A., Fisher E., Fox R.J., Bar-Or A., Sharp S.L., Skaramagas T.T., Jagodnik P., Karafa M., Morrison S., Reese Koc J., Gerson S.L., Lazarus H.M. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult. Scler. 2017 doi: 10.1177/1352458517703802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi G., Radaelli M., Soelberg Sorensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389:1347–1356. doi: 10.1016/S0140-6736(16)32388-1. [DOI] [PubMed] [Google Scholar]
- Connick P., Kolappan M., Crawley C., Webber D.J., Patani R., Michell A.W., Du M.Q., Luan S.L., Altmann D.R., Thompson A.J., Compston A., Scott M.A., Miller D.H., Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M., Harris V.K., Zigelbaum A., Goossens A.M., Lu A., Rosenthal H., Sadiq S.A. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev. 2011;20:2065–2076. doi: 10.1089/scd.2010.0547. [DOI] [PubMed] [Google Scholar]
- Deverman B.E., Patterson P.H. Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J. Neurosci. 2012;32:2100–2109. doi: 10.1523/JNEUROSCI.3803-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M., Copland I.B., Yuan S., Romieu-Mourez R., Waller E.K., Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Zhu L., Huang Y., Lee T.D., Forman S.J., Shih C.C. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17:1109–1121. doi: 10.1089/scd.2008.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdoni E., Gallo B., Casazza S., Musio S., Bonanni I., Pedemonte E., Mantegazza R., Frassoni F., Mancardi G., Pedotti R., Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Grigoriadis N., Lourbopoulos A., Lagoudaki R., Frischer J.M., Polyzoidou E., Touloumi O., Simeonidou C., Deretzi G., Kountouras J., Spandou E., Kotta K., Karkavelas G., Tascos N., Lassmann H. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp. Neurol. 2011;230:78–89. doi: 10.1016/j.expneurol.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Harris V.K., Faroqui R., Vyshkina T., Sadiq S.A. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl. Med. 2012;1:536–547. doi: 10.5966/sctm.2012-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris V.K., Yan Q.J., Vyshkina T., Sahabi S., Liu X., Sadiq S.A. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J. Neurol. Sci. 2012;313:167–177. doi: 10.1016/j.jns.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Harris V.K., Vyshkina T., Sadiq S.A. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18:1476–1482. doi: 10.1016/j.jcyt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gastl R., Liebau S., Popa M.O., Fiedler J., Boehm B.O., Maisel M., Lerche H., Schwarz J., Brenner R., Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J. Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- Huang Y., Dreyfus C.F. The role of growth factors as a therapeutic approach to demyelinating disease. Exp. Neurol. 2016;283:531–540. doi: 10.1016/j.expneurol.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., Bulte J.W., Petrou P., Ben-Hur T., Abramsky O., Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis I., Grigoriadis N., Gowda-Kurkalli B., Mizrachi-Kol R., Ben-Hur T., Slavin S., Abramsky O., Karussis D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch. Neurol. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen J., Zhang C.L., Wang L., Lu D., Katakowski M., Gao Q., Shen L.H., Zhang J., Lu M., Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Llufriu S., Sepulveda M., Blanco Y., Marin P., Moreno B., Berenguer J., Gabilondo I., Martinez-Heras E., Sola-Valls N., Arnaiz J.A., Andreu E.J., Fernandez B., Bullich S., Sanchez-Dalmau B., Graus F., Villoslada P., Saiz A. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D.H., Trapp B.D., Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- Mareschi K., Novara M., Rustichelli D., Ferrero I., Guido D., Carbone E., Medico E., Madon E., Vercelli A., Fagioli F. Neural differentiation of human mesenchymal stem cells: evidence for expression of neural markers and eag K+ channel types. Exp. Hematol. 2006;34:1563–1572. doi: 10.1016/j.exphem.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Medical Research Council . 4th edition. Elsevier Saunders; London: 2000. Aids to the Examination of the Peripheral Nervous System. [Google Scholar]
- Meirelles Lda S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Mohyeddin Bonab M., Yazdanbakhsh S., Lotfi J., Alimoghaddom K., Talebian F., Hooshmand F., Ghavamzadeh A., Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J. Immunol. 2007;4:50–57. [PubMed] [Google Scholar]
- Moll G., Alm J.J., Davies L.C., Von Bahr L., Heldring N., Stenbeck-Funke L., Hamad O.A., Hinsch R., Ignatowicz L., Locke M., Lonnies H., Lambris J.D., Teramura Y., Nilsson-Ekdahl K., Nilsson B., Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.W., Moon C., Kim H.Y., Oh S.I., Park J., Lee J.H., Chang I.Y., Kim K.S., Kim S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 2015;4:590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontaneda D., Thompson A.J., Fox R.J., Cohen J.A. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. doi: 10.1016/S0140-6736(16)31320-4. [DOI] [PubMed] [Google Scholar]
- Payne N.L., Sun G., Mcdonald C., Layton D., Moussa L., Emerson-Webber A., Veron N., Siatskas C., Herszfeld D., Price J., Bernard C.C. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2013;22:1409–1425. doi: 10.3727/096368912X657620. [DOI] [PubMed] [Google Scholar]
- Rafei M., Campeau P.M., Aguilar-Mahecha A., Buchanan M., Williams P., Birman E., Yuan S., Young Y.K., Boivin M.N., Forner K., Basik M., Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- Rajan T.S., Giacoppo S., Diomede F., Ballerini P., Paolantonio M., Marchisio M., Piattelli A., Bramanti P., Mazzon E., Trubiani O. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci. Rep. 2016;6 doi: 10.1038/srep38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera F.J., Couillard-Despres S., Pedre X., Ploetz S., Caioni M., Lois C., Bogdahn U., Aigner L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006;24:2209–2219. doi: 10.1634/stemcells.2005-0614. [DOI] [PubMed] [Google Scholar]
- Shen L.H., Xin H., Li Y., Zhang R.L., Cui Y., Zhang L., Lu M., Zhang Z.G., Chopp M. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke. 2011;42:459–464. doi: 10.1161/STROKEAHA.110.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff N.P., Madigan N.N., Morris J., Jentoft M., Sorenson E.J., Butler G., Gastineau D., Dietz A., Windebank A.J. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 2016;87:2230–2234. doi: 10.1212/WNL.0000000000003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffenhagen C., Dechant F.X., Oberbauer E., Furtner T., Weidner N., Kury P., Aigner L., Rivera F.J. Mesenchymal stem cells prime proliferating adult neural progenitors toward an oligodendrocyte fate. Stem Cells Dev. 2012;21:1838–1851. doi: 10.1089/scd.2011.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamout B., Hourani R., Salti H., Barada W., El-Hajj T., Al-Kutoubi A., Herlopian A., Baz E.K., Mahfouz R., Khalil-Hamdan R., Kreidieh N.M., El-Sabban M., Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Zappia E., Casazza S., Pedemonte E., Benvenuto F., Bonanni I., Gerdoni E., Giunti D., Ceravolo A., Cazzanti F., Frassoni F., Mancardi G., Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Y., Lu M., Cui Y., Chen J., Noffsinger L., Elias S.B., Chopp M. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J. Neurosci. Res. 2006;84:587–595. doi: 10.1002/jnr.20962. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhou C., Teng J.J., Zhao R.L., Song Y.Q. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2009;11:299–306. doi: 10.1080/14653240902806986. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ma Z., Smith G.M., Wen X., Pressman Y., Wood P.M., Xu X.M. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhang N., Prestwich G.D., Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol. Biosci. 2008;8:836–842. doi: 10.1002/mabi.200700334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1 and supplementary tables 1 and 2.