Abstract
The pace of discovery of new antiretroviral (ARV) drugs has slowed, although the efficacy and safety of once-daily fixed dose combinations have been extensively investigated. Several traditional ARV drugs remain in phase III clinical trials. This review summarizes current information on ARV drugs in phase III clinical trials and focuses on the development of ARV drugs in the next decade.
KEY WORDS: Antiretroviral drugs, Long-acting formulations, Attachment inhibitors, Maturation inhibitors, Nanomedicine
Graphical abstract
This review summarizes current information on antiretroviral (ARV) drugs in phase III clinical trials and focuses on the development of ARV drugs in the next decade.
1. Introduction
By the end of 2017, United States Food and Administration (US FDA) had approved 43 anti-retroviral drugs for clinical use which include 29 single-tablets and 14 fixed-dose combinations (FDCs). The intensity of the search for novel antiretroviral (ARV) compounds has slowed over the last 10 years and several traditional agents are still in phase III clinical trials. In the next decade, to improve drug safety, adherence and efficacy, the development of new anti-HIV-1 drugs will focus on long-acting formulations, oral attachment inhibitors, maturation inhibitors and new initiatives to cure the disease.
In the first decade of ARV drug therapy, these agents did not fundamentally change the destiny of those with HIV infection, although they could decrease virus load, increase CD4+ cell number and prolong survival over the short term. The major shortcomings were drug toxicity, drug resistance and high drug cost. Combination-based ARV therapy (ART) was introduced in 1996, which led to effectively sustained HIV suppression, significantly recovered immune function, markedly improved clinical symptoms and notably extended lifespan. In the third decade, with further development of ARV drugs and the availability of multiple ART regimens and FDCs, acquired immune deficiency syndrome (AIDS) has become a chronic, manageable and infectious disease. It is noted that in the last several years, instead of the discovery of new ARV drugs, development of effective and well-tolerated once-daily FDCs has been extensively investigated, but several traditional ARV drugs remain in phase III clinical trials. This review introduces current ARV drugs in phase III clinical trials and summarizes the development of ARV drugs over the next decade. All of the 43 FDA-approved drugs are listed here, but additional specialized reviews will be required to address new trends towards the cure of this disease.
2. Anti-retroviral drugs approved by FDA
The first AIDS cases were reported in 1981 in United States1., 2.. The human immunodeficiency virus (HIV) was defined as etiologic microorganism in 19833., 4.. Just four years later, the first HIV medicine Zidovudine was approved by US FDA and quickly opened the new era for anti-retroviral chemotherapy. In the following thirty years, the FDA approved a total of 43 anti-retroviral drugs including 29 single-tablets and 14 FDCs therapeutics. These agents are classified into eight categories of ARV drugs: nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), integrase strand transfer inhibitors (INSTIs), fusion inhibitors (FIs), entry inhibitors (EIs), pharmacokinetic enhancers (PEs) and fixed-dose combinations (FDCs, Table 15., 6., 7.).
Table 1.
US FDA approval of HIV medicines.
| Year | Drug | Year | Drug |
|---|---|---|---|
| 1987 | Zidovudine (NRTI) | 2004 | Epzicom (FDC), Truvada (FDC), Cobicistat (PE), Fosamprenavir (PI) |
| 1991 | Didanosine (NRTI) | 2005 | Tipranavir (PI) |
| 1992 | Zalcitabine (NRTI)a | 2006 | Darunavir (PI), Atripla (FDC) |
| 1994 | Stavudine (NRTI) | 2007 | Maraviroc (EI) |
| 1995 | Lamivudine (NRTI), Saquinavir (PI) | 2008 | Raltegravis ((INSTI) |
| 1996 | Indinavir (PI), Nevirapine (NNRTI), Ritonavir (PI) | 2011 | Complera (FDC), Viramune XR (NNRTI), Rilpivirine (NNRTI) |
| 1997 | Combivir (FDC), Delavirdine (NNRTI), Nelfinavir (PI) | 2012 | Stribild (FDC) |
| 1998 | Abacavir (NRTI), Efavirenz (NNRTI) | 2013 | Dolutegravir (INSTI) |
| 1999 | Amprenavir (PI)a | 2014 | Cobicistat (PE), Triumeq (FDC) |
| 2000 | Didanosine EC (NRTI), Kaletra (FDC), Trizivir (FDC) | 2015 | Evotaz (FDC), Genvoya (FDC), Prezcobix (FDC) |
| 2001 | Tenofovir DF (NRTI) | 2016 | Descovy (FDC), Odefsey (FDC) |
| 2003 | Atazanavir (PI), Emtricitabine (NRTI), Enfuvirtide (FI) |
Drugs are no longer recommended for use in the United States by the HHS HIV/AIDS medical practice guidelines.
3. Classical ARV drugs in phase III clinical trials
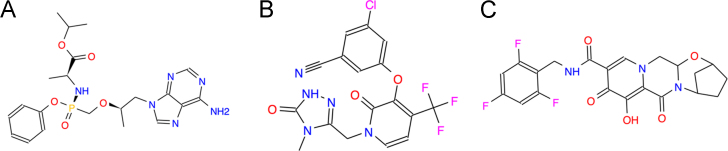
3.1. Tenofovir alafenamide (TAF)
TAF is a nucleotide transcriptase inhibitor (Fig. 1)5., 8.. Tenofovirs disoproxil (TDF), a new prodrug of tenofovir, has a similar structure and is in clinical use. Initially TAF was designed to improve TDF-induced renal and bone toxicity. TAF has achieved 50% effective concentrations (EC50) at 11.0 and 9.7 nmol/L in CD4+ T cells and macrophage, respectively, and decrease of plasma tenofovir by 90%. As compared with TDF, TAF demonstrated several important improvements. The drug can be formulated into small tablets of FDCs, has a low manufacturing cost, and shows highly reduced kidney and bone toxicity. Therefore, TAF is becoming a preferred substitute for TDF with better efficacy. Several clinical studies of TAF combinations, including elvitegrave/cobicistat/emtricitable/TAF (E/C/F/TAF), rilpivirine/emtricitabine/TAF (R/F/TAF) and emtricitabine/TAF (F/TAF) were reported on November 23, 2015, March 1, 2016 and April 25, 2016.
Figure 1.
Classical ARV drugs in phase III clinical trials. (A) Tenofovir alafenamide (GS-7340, Vemlidy, C21H29N6O5P), a nucleotide reverse transcriptase inhibitor and a prodrug of tenofovir. (B) Doravirine (MK-1439, C17H11ClF3N5O3), a non-nucleoside reverse transcriptase inhibitor. (C) Bictegravir (BIC, GS-9883, C21H18F3N3O5), an integrase inhibitor.
Phase III open-label studies have evaluated the safety of E/C/F/TAF in virologically-suppressed adults with mild-to-moderate renal impairment and in treatment-naïve 12—17 year olds9. Whereas E/C/F/TDF is typically reserved for patients with creatinine clearance (CrCl) of at least 70 mL/min, E/C/F/TAF can be used with a pre-treatment estimated CrCl of as low as 30 mL/min9. Phase III randomized, double-blind clinical trials evaluating the safety and efficacy of switching to R/F/TAF in HIV-positive individuals who are virologically suppressed by either R/F/TDF or efavirenz/F/TDF (EVF/F/TDF)are under investigation10. In other phase III studies, 668 and 330 HIV-1 positive patients were recruited in F/TAF and F/TDF groups separately. Through week 48, the success cases of virological inhibition (HIV-1 RNA <50 copies per mL) were maintained in 314 (94%) of patients in the F/TAF group compared with 307 (93%) in the F/TDF group (a difference of 1.3%, 95% CI —2.5 to 5.1), indicating the non-inferiority of F/TAF to F/TDF. Seven patients in F/TAF (2%) and three (1%) in the F/TDF group discontinued treatment due to adverse events. There were no cases of proximal tubulopathy reported in these studies11.
3.2. Doravirine
Doravirine is a highly specific non-nucleoside reverse transcriptase inhibitor (Fig. 1)5., 12., 13.. The half maximal inhibitory concentrations (IC50) are only 12, 9.7 and 9.7 nmol/L against the wild type HIV (WT) and 103 N and Y181C reverse transcriptase (RT) mutants, respectively. Doravirine exhibited consistent anti-HIV activities against 10 different HIV-1 subtype viruses, that the resistance suggesting that doravirine is superior overall to that efavirenz and comparable to that of etravirine (ETR) and RPV. A two-drug in vitro combination study reported that doravirine had no antagonistic actions in the antiviral activity of 18 other FDA-licensed anti-HIV drugs. In vivo, doravirine demonstrated robust antiviral activity and good tolerability. Data from a 48 week phase II clinical trial showed that virologic suppression (<40 copies/mL) rates were achieved in 84% of inpatients with viral loads >100,000 copies/ml. In addition, drug-related adverse events, including diarrhea, dizziness, and abnormal dreams, were infrequent in the dorvirine group vs. the EFV group (56.5% versus 31.5%). Doravirine is currently undergoing its phase III clinical development.
3.3. Bictegravir (BIC, GS-9883)
BIC5., 14. is a novel unboosted HIV-1 integrase strand inhibitor (Fig. 1). It inhibits HIV replication in both T-cells lines and in primary human T lymphocytes, with EC50 values ranging from 1.5 to 2.4 nmol/L and selectivity indices of up to 8700. BIC demonstrates synergy of anti-HIV effects in vitro when combined with TAF, emtricitabine and darunavir. BIC is showing an improved resistance profile compared to the INSTI raltegravir and elvitegravir, and was comparable to that of dolutegravir, against nine INSTI-resistant site-directed HIV-1 mutants.
Although the BIC/F/TAF combination is currently being tested in phase III clinical trials, no data from completed phase I or ongoing phase II trials has been reported. Phase III trials of BIC/F/TAF include two head-to-head comparisons with dolutegravir plus F/TAF in treat-naïve adults, with each study enrolling 600 participants in the US, Canada and other countries. Three switch phase III studies are also under evaluation, including the safety and efficacy of switching from dolutegravir plus abacavir/lamivudine (ABC/3BC) to BIC/F/TAF, a switch from boosted atazanavir or darunavir plus either F/TDF or ABC/3TC, and a switch in a cohort comprised of HIV-positive women to all in virologically suppressed participants15.
4. ARV drugs in the pipeline for the next 10 years
4.1. Long-acting ARV formulation
Currently, ARV drugs are administered through lifelong daily oral regimens. However, poor compliance is often observed with ARVs in HIV-1 patients. When suboptimal compliance occurs, circulating ARVs are insufficient to inhibit viral replication, thereby increasing the chances of viral mutation and drug resistance. Thus, long-acting, effective AVRs circumvent the limitations of daily oral regimens, and provide long-lasting ARV efficacy in either pre-exposure or post-exposure prophylaxis.
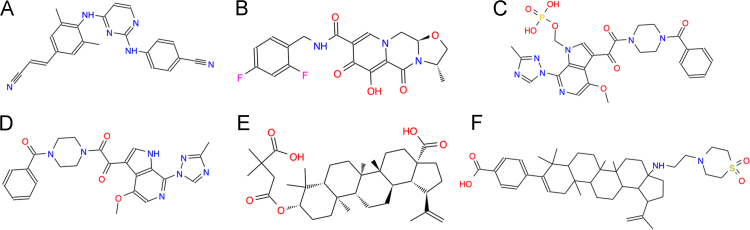
RPV is a second-generation NNRTI that has a longer half-life and reduced toxicity compared with previous NNRTIs (Fig. 2)16. It has been widely used as a single ARV or a combination component for HIV therapy at the oral 25 mg dose. To further extend its half-life, long-acting RPV has been investigated recently17., 18., 19.. Single dose administration of the long-acting RPV achieved constant drug retention in plasma for more than 6 months and 100% drug bioavailability in a pharmacokinetic study. In one phase I study of the long-acting formulation with 60 healthy HIV-negative volunteers, intramuscular or abdominal subcutaneous administered RPV was retained in the plasma for 12—26 weeks at more than 10 ng/mL. Another study of long-acting RPV reported that maximum drug concentrations of 34, 82, and 160 ng/mL at 300, 600 and 1200 mg treatment, respectively, following intramuscular administration. The half-life of this RPV formulation was more than 3 months. All of these reports indicate that the long-acting formulation of RPV is devoid of adverse drug events20. More importantly, no hypersensitivity reactions or EGG abnormalities were observed. Currently, there is an ongoing phase II clinical trial for RPV.
Figure 2.
ARV drugs in the pipeline for the next 10 years. (A) Rilpivirine hydrochloride (Edurant; TMC278 hydrochloride, C22H19ClN6), a second-generation NNRTI. (B) Cabrotegravir (S/GSK1265744 or GSK744, C19H17F2N3O5), an integrase inhibitor. (C) Fostemsavir, BMS-663068, C25H26N7O8P), the phosphonooxymethyl prodrug of BMS-626529, a novel small-molecule attachment inhibitor. (D) Temsavir (BMS-626529, C24H23N7O4), a novel small-molecule attachment inhibitor. (E) Bevirimat, BVM (MPC-4326, PA-457, C36H56O6), the first maturation inhibitor. (F) BMS-955176 (C42H62N2O4S), the second generation HIV maturation inhibitor.
4.1.1. Cabotegravir
Cabotegravir (Fig. 2) is an HIV-1 integrase strand transfer inhibitor. The long-acting injectable formulations of cabotegravir are being studied for clinical development21., 22.. It is known that carbotegravir has superior ARV activity with an IC50 of 0.22 and 0.34 nmol/L against HIV-1Bal and HIV-1NL-43. This novel integrase inhibitor specifically binds to plasma albumin protein leading to a protein-adjusted IC90 of 166 ng/mL with an oral plasma half-life of 40 h. In a phase II study, once daily treatment with 5 or 30 mg cabotegravir for 10 days demonstrated excellent antiviral efficacy, with a decrease of plasma HIV-1 RNA for 2.2—2.3 log10 copies/mL. Interestingly, the long-acting formulation of cabotegravir is packaged into crystal nanoparticles. Following subcutaneous or intramuscular administration, long-acting cabotegravir is slowly absorbed into blood from tissue injection sites. In a phase I trial, the long-acting injectable nanosuspension of cabotegravir were evaluated following 100—800 mg intramuscular and 100—400 mg subcutaneous injections. The therapeutically relevant plasma concentrations were achieved within 3 days after drug injection and remained in circulation from 21—50 days. Up to now, no severe injectable site reactions (ISRs), or ISR-related withdrawals have been reported for this long-acting cabotegravir formulation.
In addition, a combination of cabotegravir with RPV is being tested as a double-drug regimen for the HIV therapy23. The phase II investigation was performed to evaluate the efficacy and safety with the oral combination of cabotegravir+RPV in HIV-1-infected patients. At 48 week, 82% of participants across the three cabotegravir+RPV arms achieved the primary endpoint, with similar response rates across cabotegravir doses, relative to a 71% response rate in the EFV control arm. Although follow-up viral load data are not yet available, cabotegravir+RPV was generally well tolerated with no drug-related serious adverse events and few adverse event-related withdrawals.
4.1.2. Nanoformulated ART (NanoART)
NanoART is another long-acting strategy for extending the half-life of ARV drugs for HIV treatment24., 25.. In the past ten years, nanotechnology has been employed to manipulate the formulation of the traditional ARV drug at the level of drug size, shape, and surface charge26. Several studies have shown that solid crystal nanoparticles can remarkably increase drug level in blood circulation and lymphoid tissue27., 28.. This nanoART, which selectively targets mononuclear macrophage as a cellular drug depot, delivers ARV drugs such as atazanavir to tissue reservoirs. This macrophage-mediated drug delivery facilitates long-acting drug release and achieves viral suppression for more than two weeks or even one month after single dose regimen29. It was reported that the advanced nanoART, which is coated with folic acid receptors, can improve drug concentration tenfold (compared to the first generation of nanoART) in serum and lymph node over two weeks with one injection28. Moreover, the novel mixed-lineage kinase 3 inhibitor (URMC-099) was observed to slow down the release of nanoART, leading to significant increases in the long-acting efficacy of nanoART30., 31.. URMC-099 was originally discovered as an anti-neuroinflammation drug for the treatment of HIV-1 associated neurocognitive disorders (HAND) and Alzheimer's disease32., 33., 34.. However, the combination of anti-neuroinflammation drug URMC-099 and ARV drug nanoART has created a novel long-acting strategy for HIV eradication and further improves the cell-based drug delivery platform direct for translational application.
4.2. New attachment inhibitors
HIV-1 entry into target cells involves viral attachment, co-receptor-engagement and fusion. New attachment inhibitors which target each step of the entry process have been developed recently (Table 2). Fostemsavir (BMS-663068, Fig. 2)35., 36. is the phosphonooxymethyl prodrug of BMS-626529 (Fig. 2), a novel small-molecule attachment inhibitor that targets HIV-1 gp120 and prevent its binding to CD4+ T cells. Fostemsavir inhibits HIV-1LAI infection at EC 50 of 0.7 nmol/L. In a study of a cohort of laboratory strains, it exhibited an EC50 of 0.01 nmol/L against the most susceptible HIV and an EC50 value of 2 μmol/L against the least susceptible HIV. In addition, the virus was found to be resistant to other HIV entry inhibitors, such as enfuvirtide (ENF) and ibalizumab, and maintained susceptibility to BMS-62652937. A phase III study of fostemsavir was initiated in February 2015.
Table 2.
Entry inhibitors that have reached late stage clinical trials.
| Inhibitor | Development status | Drug class | Action against entry stage |
|---|---|---|---|
| BMS-663068 | Phase III | Small molecular | Binds to region with CD4 |
| Ibalizumab | Phase III | Post-attachment inhibitor (mAb) | Binds to CD4 |
| Aplaviroc | Phase IIb and III | Small molecular CCR5 antagonist | Binds to CCR5 |
| Cenicriviroc | Phase IIb and III | Small molecular CCR5 and CCR2 antagonist | Binds to CCR5 and CCR2 |
| Sifuvirtide | Early phase | Peptides fusion inhibitor | Binds to gp41 |
Ibalizumab (TMB-355, also known as TNX-355) is a non-immunosuppressive monoclonal antibody that targets membrane protein CD4, the primary receptor for HIV entry. This MAb is a potent inhibitor of HIV-1 in vitro and shows synergy when combined with HIV gp120 antibodies or the fusion inhibitor enfuvirtide. It does not interfere with antigen presentation of immune cells23., 38., 39.. In phase I studies39, intravenous injection of ibalizumab decrease the plasma HIV-1 RNA for up to 1.5 log10-fold at Day 14—21 after single dose treatment. A phase II study40 of ibalizumab reported that ibalizumab plus an optimized background regimen resulted in further decrease in plasma HIV-1 RNA compared to the background regimen alone. A subsequent phase II study also demonstrated that intravenous infusion of ibalizumab every two weeks, leading to significant viral load reduction over 24 weeks. Currently, heavily treatment-experienced patients have been enrolled in the nonrandomized arm of the phase III study to evaluate the combination treatment of fostemsavir and ibalizumab41.
4.3. The maturation inhibitors
The maturation inhibitors have been developed as a novel category of ARV drugs which targets the final step of HIV virion assembly and packaging. Unlike the PI, HIV maturation inhibitors directly bind to the HIV capsid protein and inhibit viral protein assembly into infectious HIV particle. Maturation inhibition leads to the production of noninfectious, immature virus particles. No other class of drugs shares this mechanism of action. Thus, maturation inhibitors retain inhibitory activity against HIV strains that are resistant to other classes of drugs. Several clinical trials with maturation inhibitors are underway.
Bevirimat (BVM, Fig. 2)42 is the first maturation inhibitor, which preferentially inhibits cleavage of SP1 from the C-terminus of HIV capsid (CA) protein. BVM has strong anti-HIV-1 IIIB activity with IC50 of 1.3 nmol/L. Several studies have demonstrated the following: 1. BVM induces the accumulation of the unspliced CA-SP1 intermediate in both intracellular space and virus-associated protein fractions from HIV-1-infected cells; 2. BVM has no effect on divergent sequences at the CA-SP1 junction; 3. The majority of BVM drug-resistance mutations are found on the CA-PS1 junction or within SP1.
BMS-955176 (Fig. 2)43 and its backup candidate BM986173 represent second generation maturation inhibitors. BMS-955176 has a maximum reduction of viral load of 1.7 log10 on a 40-mg once daily dose. This compound, unlike the first generation of maturation inhibitors, exhibits potent ARV activity against wild type HIV or HIV with baseline Gag polymorphisms. In a ten-day study of BMS-955176 monotherapy, similar antiviral activity was found in subjects with either wild type HIV or HIV with Gag polymorphisms, and in subjects with either HIV-1 subtype B or subtype C. In another study, evaluation of BMS-95166 in combination with atazanavir (with or without ritonavir boosting) showed similar maximum median declines in viral loads through Day 42. Until now, it has been found that BMS-955176 is generally well tolerated, with no adverse events requiring discontinuation. Phase II studies are under investigation including a safety and efficacy comparison with EFV (with both BMS-176 and EFV combined with TDF/FTC) and an open-label evaluation of BMS-955176 combined with dolutegravir and atazanavir in treatment-experienced adults. Pharmacokinetics and additional safety trials are also ongoing.
5. Conclusions
In the last ten years, ART research has switched from a focus on new drug development to optimization of current fixed dose combinations44. To approach the 90-90-90 goals of World Health Organization (WHO) by 2020 (90% of HIV-1 infected people diagnosed; 90% of those who are diagnosed started on ART; 90% of those on ART virally suppressed), current FDCs are still considered as the major therapeutic agents to fight against HIV infection. Thus, the development of long-acting formulations, oral attachment inhibitors and maturation inhibitors is urgently needed and essential for improvement of HIV therapy in the coming decade.
Acknowledgments
The author acknowledge Dr. Robert Schooley for the editorial assistance.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Centers for Disease Control and Prevention Pneumocystis pneumonia—Los Angeles. Mmwr Morb Mortal Wkly Rep. 1981;30:250–252. [PubMed] [Google Scholar]
- 2.Centers for Disease Control Kaposi's sarcoma and pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 3.Klatzmann D., Barre-Sinoussi F., Nugeyre M.T., Danquet C., Vilmer E., Griscelli C. Selective tropism of Lymphadenopathy Associated Virus (LAV) for helper-inducer T lymphocytes. Science. 1984;225:59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- 4.Gallo R.C., Salahuddin S.Z., Popovic M., Shearer G.M., Kaplan M., Haynes B.F. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 5.Horn T., Collins S. TAG; New York: 2016. The antiretroviral pipeline. [Google Scholar]
- 6.Kiyota T., Morrison C.M., Tu G., Dyavarshetty B., Weir R.A., Zhang G. Presenilin-1 familial Alzheimer's disease mutation alters hippocampal neurogenesis and memory function in CCL2 null mice. Brain Behav Immun. 2015;49:311–321. doi: 10.1016/j.bbi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AIDSinfo Fact Sheet. FDA-Approved Hiv Medicines. 2017. Available from: 〈https://aidsinfo.nih.gov/understanding-hiv-aids/infographics/25/fda-approval-of-hiv-medicines〉
- 8.Gallant J.E., Daar E.S., Raffi F., Brinson C., Ruane P., DeJesus E. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3:e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 9.Pozniak A., Arribas J.R., Gathe J., Gupta S.K., Post F.A., Bloch M. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a Single-Arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr. 2016;71:530–537. doi: 10.1097/QAI.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical Trials. Gov. Study to evaluate switching from a regimen consisting of efavirenz/emtricitabine/tenofovir disoproxil fumarate (Efv/FTC/TDF) Fixed Dose Combination (FDC) to emtricitabine/rilpivirine/tenofovir alafenamide (FTC/RPV/TAF) FDC in virologically-suppressed, HIV-1 infected adults. [Updated 11.12.17]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02345226〉.
- 11.Sax P.E., Zolopa A., Brar I., Elion R., Ortiz R., Post F. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67:52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 12.Lai M.T., Feng M., Falgueyret J.P., Tawa P., Witmer M., DiStefano D. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014;58:1652–1663. doi: 10.1128/AAC.02403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales-Ramirez J.O., Gatell J.M., Hagins D.P., Thompson M., Arastéh K., Hoffmann C., et al. Safety and antiviral effect of MK-1439, A novel NNRTI (+FTC/TDF) in ART-naive HIV-infected patients. In: Proceedings of Conference on Retroviruses and Opportunistic Infections (CROI); Mar 3-6; Boston, Massachusetts: CROI, 2014.
- 14.Tsiang M., Jones G.S., Goldsmith J., Mulato A., Hansen D., Kan E. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60:7086–7097. doi: 10.1128/AAC.01474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Trials. Gov. Safety and efficacy of switching to A FDC of B/F/TAF from E/C/F/TAF, E/C/F/TDF, or ATV+RTV+FTC/TDF in virologically suppressed HIV-1 infected women. [Updated 06.11.17]. Available from: 〈https://www.clinicaltrials.gov/ct2/show/NCT02652624〉.
- 16.Goebel F., Yakovlev A., Pozniak A.L., Vinogradova E., Boogaerts G., Hoetelmans R. Short-term antiviral activity of TMC278--a novel NNRTI--in treatment-naive HIV-1-infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- 17.Baert L., Van'T Klooster G., Dries W., François M., Wouters A., Basstanie E. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Verloes R., Deleu S., Niemeijer N., Crauwels H., Meyvisch P., Williams P. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med. 2015;16:477–484. doi: 10.1111/hiv.12247. [DOI] [PubMed] [Google Scholar]
- 19.Jackson A.G., Else L.J., Mesquita P.M., Egan D., Back D.J., Karolia Z. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther. 2014;96:314–323. doi: 10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 20.Clinical Trials. Gov. Switch Study to evaluate the safety and efficacy of emtricitabine/rilpivirine/tenofovir alafenamide (FTC/RPV/TAF) fixed dose combination (FDC) in HIV-1 positive adults who are virologically suppressed on emtricitabine/rilpivirine/tenofovir disoproxil fumarate (FTC/RPV/TDF). [Updated 08.12.17]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02345252〉.
- 21.Margolis D.A., Boffito M. Long-acting antiviral agents for Hiv treatment. Curr Opin HIV AIDS. 2015;10:246–252. doi: 10.1097/COH.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis D.A., Brinson C.C., Smith G.H., de Vente J., Hagins D.P., Eron J.J. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 23.Henrich T.J., Kuritzkes D.R. HIV-1 entry inhibitors: recent development and clinical use. Curr Opin Virol. 2013;3:51–57. doi: 10.1016/j.coviro.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gendelman HE, Balogh LP, Bawa R, Bradbury M, Chang EH, Chiu W, et al. Proceedings of the 4th annual meeting of the American society for nanomedicine. J Neuroimmune Pharm 2014; 9 Suppl 1:S1-S3. [DOI] [PubMed]
- 25.Gorantla S., Zhang G., Dash P.K. Nanomedicines and antiretroviral therapy. J Neuroimmune Pharm. 2014;9:21–22. [Google Scholar]
- 26.Nowacek A., Zhang G., McMillan J., Kiyota T. Nanomedicine and neurodegenerative disorders. In: Preedy V.R., Hunter R.J., editors. Nanomedicine in health and disease. CRC Press; Boca Raton: 2011. p. 377. [Google Scholar]
- 27.Li T., Gendelman H.E., Zhang G., Puligujja P., McMillan J.M., Bronich T.K. Magnetic resonance imaging of folic acid-coated magnetite nanoparticles reflects tissue biodistribution of long-acting antiretroviral therapy. Int J Nanomed. 2015;10:3779–3790. doi: 10.2147/IJN.S83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puligujja P., Balkundi S.S., Kendrick L.M., Baldridge H.M., Hilaire J.R., Bade A.N. Pharmacodynamics of long-acting folic acid-receptor targeted ritonavir-boosted atazanavir nanoformulations. Biomaterials. 2015;41:141–150. doi: 10.1016/j.biomaterials.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo D., Zhang G., Wysocki T.A., Wysocki B.I., Gelbard H.A., Liu X.M. Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance. J Virol. 2014;88:9504–9513. doi: 10.1128/JVI.01557-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang G., Dash P.K., Wiederin J.L. Urmc-099: a mixed-lineage kinase-3 (MLK3) inhibitor with the potential to eradicate human immunodeficiency virus infection. J Neuroimmune Pharm. 2013;8:437. [Google Scholar]
- 31.Zhang G., Guo D., Dash P.K., Araínga M., Wiederin J.L., Haverland N.A. The mixed lineage kinase-3 inhibitor URMC-099 improves therapeutic outcomes for long-acting antiretroviral therapy. Nanomedicine. 2016;12:109–122. doi: 10.1016/j.nano.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong W., Embury C.M., Lu Y., Whitmire S.M., Dyavarshetty B., Gelbard H.A. The mixed-lineage kinase 3 inhibitor URMC-099 facilitates microglial amyloid-β degradation. J Neuroinflammation. 2016;13:184. doi: 10.1186/s12974-016-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J., Hu D., Xia J., Liu J., Zhang G., Gendelman H.E. Enhancement of NMDA receptor-mediated excitatory postsynaptic currents by gp120-treated macrophages: implications for HIV-1-associated neuropathology. J Neuroimmune Pharmacol. 2013;8:921–933. doi: 10.1007/s11481-013-9468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiyota T., Gendelman H.E., Weir R.A., Higgins E.E., Zhang G., Jain M. CCL2 affects β-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowicka-Sans B., Gong Y.F., McAuliffe B., Dicker I., Ho H.T., Zhou N. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56:3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nettles R.E., Schürmann D., Zhu L., Stonier M., Huang S.P., Chang I. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068, an oral HIV-1 attachment inhibitor in HIV-1-infected subjects. J Infect Dis. 2012;206:1002–1011. doi: 10.1093/infdis/jis432. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Zhou N., Sun Y., Ray N., Lataillade M., Hanna G.J. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother. 2013;57:4172–4180. doi: 10.1128/AAC.00513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X.Q., Sorensen M., Fung M., Schooley R.T. Synergistic in vitro antiretroviral activity of a humanized monoclonal Anti-CD4 antibody (TNX-355) and enfuvirtide (T-20) Antimicrob Agents Chemother. 2006;50:2231–2233. doi: 10.1128/AAC.00761-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuritzkes D.R., Jacobson J., Powderly W.G., Godofsky E., DeJesus E., Haas F. Antiretroviral activity of the Anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis. 2004;189:286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- 40.Clinical Trials. Gov. Ibalizumab plus optimized background regimen in patient with multi-drug resistant HIV. [Updated 2017 Feb 8]. Available from: 〈https://clinicaltrials.gov/ct2/show/NCT02475629〉.
- 41.Jacobson J.M., Kuritzkes D.R., Godofsky E., DeJesus E., Larson J.A., Weinheimer S.P. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an Anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin D.E., Salzwedel K., Allaway G.P. Bevirimat: a novel maturation inhibitor for the treatment of HIV-1 infection. Antivir Chem Chemother. 2008;19:107–113. doi: 10.1177/095632020801900301. [DOI] [PubMed] [Google Scholar]
- 43.Regueiro-Ren A., Liu Z., Chen Y., Sin N., Sit S.Y., Swidorski J.J. Discovery of BMS-955176, a second generation HIV-1 maturation inhibitor with broad spectrum antiviral activity. ACS Med Chem Lett. 2016;7:568–572. doi: 10.1021/acsmedchemlett.6b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedrich W.D., Hassan H.E., Wang H.B. Insights into CYP2B6-mediated drug–drug interactions. Acta Pharm Sin B. 2016;5:413–425. doi: 10.1016/j.apsb.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]