Abstract
Background
Panax ginseng is a well-known immune modulator, and there is concern that its immune-enhancing effects may negatively affect patients with rheumatoid arthritis (RA) by worsening symptoms or increasing the risk of adverse effects from other drugs. In this randomized, crossover clinical trial, we evaluated the impact of Korean Red Ginseng (KRG) on disease activity and safety in RA patients.
Methods
A total of 80 female RA patients were randomly assigned to either the KRG (2 g/d, n = 40) treatment or placebo (n = 40) groups for 8 wk, followed by crossover to the other treatment group for an additional 8 wk. The primary outcome was the disease flare rate, defined as worsening disease activity according to the disease activity score 28 joints-erythrocyte sedimentation rate (DAS28-ESR). The secondary outcomes were development of adverse events (AEs) and patient reported outcomes. Outcomes were evaluated at baseline and 8 wk and 16 wk. The outcomes were compared using the Chi-square test.
Results
Of the 80 patients, 70 completed the full study. Their mean age was 51.9 yr, and most exhibited low disease activity (mean DAS28-ESR 3.5 ± 1.0) at enrollment. After intervention, the flare rate was 3.7% in each group. During KRG treatment, 10 AEs were reported, while five AEs were developed with placebo; however, this difference was not statistically significant (p = 0.16). Gastrointestinal- and nervous system-related symptoms were frequent in the KRG group.
Conclusion
KRG is not significantly associated with either disease flare rate or the rate of AE development in RA patients.
Keywords: effect, Korean Red Ginseng, rheumatoid arthritis, safety
1. Introduction
Rheumatoid arthritis (RA) is a common chronic autoimmune disease with a worldwide prevalence rate of 0.5–1% [1]. It can be a debilitating and painful condition that affects multiple joints in individual patients and can lead to substantial loss of function and mobility [2]. The ultimate goal of RA treatment is to reduce pain by controlling inflammation, to prevent or delay joint damage, and to enhance patient quality of life [2], [3]. Some patients report that conventional RA medicine often fails to improve health, does not reliably alleviate pain, and can produce undesirable side effects [4]. Therefore, many RA patients have pursued complementary and alternative medicine (CAM) as part of their treatment [5]. CAM has become increasingly popular for RA patients, with an estimated 18–94% reporting some level of CAM use throughout the world, across different geographic and ethnic groups, and among people of different social and economic backgrounds [6], [7]. Previous reports have shown that a near-majority of Korean RA patients have had experiences with CAM use, and among patients who had never used CAM before their RA diagnosis, roughly 11% used CAM within 1 yr [8].
Panax ginseng Meyer is a herb that has been used as a component of CAM for hundreds of years in Korean traditional medicine [9]. The principal active components of P. ginseng are the ginsenosides (or triterpenoid saponins) of ginseng, as well as approximately 38 additional types of ginsenosides that have been identified [10]. Prior experimental work suggests that ginseng's antiinflammatory effect could provide a feasible way to treat RA symptoms [11], [12]; for example, by regulating tumor necrosis factor expression or Th17 cell activity [13], [14]. A significant concern, however, is that ginseng treatment could exacerbate this autoimmune disorder because it leads to immune enhancement. Furthermore, few health practitioners recommend additional health food supplements for acute patients that are prescribed a broad range of drugs, because this might increase the risk of adverse events (AEs) [7]. However up to now, there has been no evidence of an interaction between RA treatment and Korean Red Ginseng (KRG), and there has been no randomized clinical trial examining the effect of P. ginseng on RA patients. The aim of the present study was to evaluate the impact of KRG on disease activity and safety in RA patients.
2. Methods
2.1. Study population
Patients with RA were screened according to the inclusion and exclusion criteria and enrolled prospectively between March 2015 and September 2015. Inclusion criteria were as follows: (1) female RA patients between the ages of 19 and 80 yr who satisfied either the 1987 American College of Rheumatology criteria or 2010 American College of Rheumatology/European League Against Rheumatism criteria; and (2) low disease activity [i.e., score less than 3.2 on the Disease Activity Score (DAS) 28-joint-erythrocyte sedimentation rate (ESR), DAS 28-ESR]. Exclusion criteria were as follows: (1) pregnant or breast-feeding; (2) any laboratory test abnormality; (3) use of ginseng extract within the last 2 mo; (4) known allergy to ginseng extract; (5) regular use of corticosteroids; and (6) moderate or high disease activity (≥ 3.2 on the DAS28-ESR).
All patients provided informed consent. This study was approved by the Institutional Review Board of Hanyang University Hospital (HYUH 2015-01-001). The study protocol was registered with the Clinical Research Information Service of the Republic of Korea (KCT0001516).
2.2. Study design
We undertook a crossover trial to allow each patient to serve as his or her own control. A total sample size of 69 provided a power of 0.8 to detect noninferiority using a one-sided t test when the relative margin of equivalence was 0.3 and the significance level was 0.05. We utilized a crossover design with an equal number of patients in each sequence. However, considering a withdrawal rate of 14–15%, we required 40 patients in each arm. Sample size was calculated using PASS 2008.
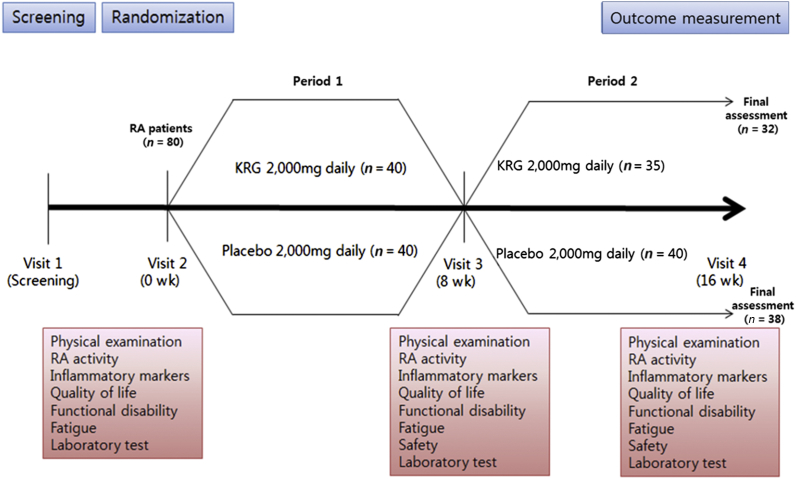
Patients were randomized to receive either 8 wk of treatment with KRG (Period 1) followed by 8 wk of placebo (Period 2) or 8 wk of placebo (Period 1) followed by 8 wk of treatment with KRG (Period 2). Treatment doses were 2 g of KRG using 500 mg tablets, which were manufactured by the Korea Ginseng Corporation (Seoul, Korea) and were composed of ginsenoside Rg1 + Rb1 + Rg3 > 5.5 mg/g and cellulose. Placebo tablets, identical in size, weight, color, and taste, were also provided from Korea Ginseng Corporation (Figure 1). The use of nonsteroidal antiinflammatory drugs or disease-modifying antirheumatic drugs for controlling the RA disease activity was not restricted during the study period. Randomization was performed by a third party using a computer-generated random sequence. Study investigators, participants, and their caregivers were blinded by ensuring both KRG and placebo medication was delivered in identical capsules and boxes, with neither the investigator providing the medication nor the participants being aware of the allocated treatment. All randomized participants underwent baseline assessment consisting of a physical examination, history, and laboratory testing. Outcomes were measured at baseline and wk 8 and 16.
Fig. 1.
Study design.
2.3. Outcome measures
2.3.1. Primary outcome
2.3.1.1. RA flare as defined by DAS28
The primary outcome measure was the flare rate during the KRG period compared with the flare rate during the placebo period. The disease flare was defined as an increase of more than 1.2 in the DAS 28-joint count ESR compared to baseline. The DAS28 is widely used to quantify disease activity and therapeutic response for RA patients. The DAS28 is a complicated formula with several disease elements as follows: DAS28 = 0.56 × √(tender28) + 0.28 × √(swollen28) + 0.70 × ln(ESR) + 0.014 × patient global health visual analogue scale (VAS) [15].
2.3.2. Secondary outcome
2.3.2.1. AEs
Safety was assessed based on the type and severity of AEs. AEs were classified using the System Organ Class of the Medical Dictionary for Regulatory Activities (MedDRA version 11.1). Changes from baseline in clinical laboratory parameters and treatment emergent AEs that were determined by the investigators to be related to the study drug were also evaluated.
2.3.2.2. Fatigue assessed by VAS and Functional Assessment of Chronic Illness Therapy-Fatigue
We assessed the improvement or worsening of fatigue by VAS and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), which is a technique that has been validated for use in assessing RA patients [16]. The FACIT-Fatigue Subscale consists of 13 questions scored on a 0–4 Likert scale; the instrument yields a summed total score ranging between 0 and 52 (52 = no fatigue).
2.3.2.3. Functional disability assessed with the health assessment questionnaire-disability index
The health assessment questionnaire-disability index (HAQ-DI) is a self-completed questionnaire used as a comprehensive measure of functional disability in patients with a wide variety of rheumatic diseases. We used the Korean version of the HAQ-DI, which has been validated for cultural authenticity of the translation [17]. The HAQ-DI contains 20 items distributed across eight components; standing up, walking, dressing, hygiene, eating, reaching, gripping, and performing specific activities, with scores from 0 (without any difficulty) to 3 (unable to complete the task). A score of 0 represents no disability and 3 represents severe disability with high dependency.
2.3.2.4. Quality of life assessed with the five dimensions of the EuroQol questionnaire
The five dimensions of the EuroQol questionnaire (EQ-5D) are mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension consists of one item, which is scored on a three-level scale; 1 denotes no difficulty and 3 denotes extreme difficulty. The 243 possible health states are then converted to an index score between 0 (death) and 1 (perfect health). We used the Korean translation of the EQ-5D, which had been validated for cultural authenticity [18].
2.4. Statistical analysis
Descriptive data for means and standard deviations (SD) were based on independent measurements and used to describe the baseline and outcome characteristics of the study groups. The primary outcome measure (i.e., flare rate) was compared between treatment and placebo groups using the Chi-square test with intention to treat analysis. Safety was assessed for the total patients enrolled. The impact of KRG was evaluated by comparing the means of the differences for each measure during the KRG treatment and during the placebo treatment by per protocol (PP) analysis. The differences were compared using a mixed effect model assuming no carryover effect. All analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline characteristics
A total of 80 patients were initially enrolled and randomly assigned to one of the two groups, first receiving either KRG or placebo treatment. Participants were aged 51.9 ± 9.35 yr, with a disease duration of 7.8 ± 5.4 yr. Most had low disease activity (DAS28 ESR: mean 3.5 ± 1.0), and only 12.5% of patients had received a prescription for glucocorticoid drugs, with instructions to take only as needed. All baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the participants
| Variable1) | RA patients (n = 80) |
|---|---|
| Age (yr) | 51.9 ± 9.35 |
| RA disease duration (yr) | 7.8 ± 5.4 |
| Female sex | 80 (100) |
| Rheumatoid factor positivity | 51 (63.8) |
| Anti-CCP positivity | 64 (80.0) |
| Body mass index (kg/m2) | 22.9 ± 3.1 |
| Comorbidity | 18 (22.6) |
| Hypertension | 16 (20.0) |
| Methotrexate | 66 (82.5) |
| Methotrexate dose (mg/wk) | 11.8 ± 2.7 |
| Hydroxychloroquine | 18 (22.5) |
| Sulfasalazine | 22 (27.5) |
| Leflunomide | 26 (32.5) |
| Biologic DMARDs | 10 (12.5) |
| Oral glucocorticoid use2) | 10 (12.5) |
| NSAIDs | 34 (42.5) |
| DAS28ESR | 3.5 ± 1.0 |
| DAS28CRP | 2.8 ± 0.7 |
| HAQ-DI | 0.39 ± 0.45 |
| EQ-5D | 0.85 ± 0.06 |
| General health VAS | 23.4± 16.8 |
| Fatigue VAS | 16.9 ± 18.4 |
| FACIT-F | 120.7 ± 17.5 |
anti-CCP, anti-cyclic citrullinated peptide antibody; CRP, c-reactive protein; DAS, disease activity score; DMARDs, disease-modifying anti-rheumatic drugs; EQ-5D, EuroQol-5 dimension; ESR, erythrocyte sedimentation rate; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, health assessment questionnaire-disability index; NSAIDs, non-steroidal anti-inflammatory drugs; RA, rheumatoid arthritis; VAS, visual analogue scale
Values are mean ± standard deviation, or number (percentage)
≤ 5 mg/d glucocorticoid was used, as needed
3.2. Patient withdrawal and follow up
Five patients withdrew during the first period; two due to AEs (1 due to nausea and 1 due to worsening of arthritis) and three due to withdrawal of consent. An additional five patients withdrew during the second period; four due to AEs (1 due to operation resulting from uterine myoma, 1 due to abnormalities in the liver function test, 1 due to development of fracture, and 1 due to headache) and one due to withdrawal of consent. In total, 70 patients successfully completed the study (Figure 1).
3.3. Impact of KRG on RA disease flare
We compared the RA flare rate during KRG treatment versus placebo treatment via intention to treat analysis. The flare rate was identical under each treatment, 3.7% of all patients. The mean disease activity score at baseline was 3.26 ± 1.16 before taking KRG and 3.26 ± 1.15 before taking placebo. After applying PP analysis, we found no statistically significant change after KRG treatment compared to the placebo treatment; KRG produced a mean 0.02 ± 0.75 decrease and placebo resulted in a decrease of 0.07 ± 0.87 (p = 0.77) (Table 2).
Table 2.
Change in rheumatoid arthritis (RA) flare rate after placebo and Korean Red Ginseng (KRG) treatments (total, n = 80)
| Change during placebo | Change during KRG | |
|---|---|---|
| Withdrawal or without flare | 77 (96.3) | 77 (96.3) |
| With flare | 3 (3.7) | 3 (3.7) |
Data are expressed as number (percentage)
Flare was defined as a change > 1.2 in DAS28ESR from baseline
3.4. Impact on KRG on fatigue
The mean change in fatigue after treatment is presented in Table 3, based on PP analysis. The mean baseline fatigue VAS was 16.43 ± 19.19 and 18.14 ± 20.17 before taking KRG and placebo, respectively. After treatment, the improvement in fatigue was better in the KRG period (2.14 ± 22.19) than in the placebo period (0.00 ± 22.46); however, this difference was not statistically significant (p = 0.57). The mean baseline fatigue scale was 42.87 ± 6.42 and 42.94 ± 5.64 before taking KRG and placebo, respectively. After treatment, the improvement according to the fatigue scale was greater in the KRG period (2.66 ± 6.37) compared to that of the placebo period (1.36 ± 6.14); again, however, this difference was not statistically significant (p = 0.25).
Table 3.
Comparison of fatigue changes using VAS and FACIT after specified treatments
| Variable | Change during placebo | Change during KRG | p* |
|---|---|---|---|
| Fatigue VAS | 0.00 ± 22.46 | −2.14 ± 22.19 | 0.57 |
| FACIT-F | 1.88 ± 13.26 | 5.16 ± 13.79 | 0.18 |
| FACIT -Physical well being | 0.46 ± 2.66 | 0.84 ± 2.65 | 0.44 |
| FACIT -Social/family well-being | −0.43 ± 4.97 | 0.95 ± 4.63 | 0.12 |
| FACIT -Emotional well being | 0.56 ± 2.82 | 0.81 ± 2.40 | 0.55 |
| FACIT -Functional well being | −0.06 ± 4.57 | −0.10 ± 5.23 | 0.95 |
| FACIT -Fatigue scale | 1.36 ± 6.14 | 2.66 ± 6.37 | 0.25 |
Data are expressed as mean ± standard deviation
* For the difference between the changes during KRG versus placebo, using a mixed effect model
FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; KRG, Korean Red Ginseng; VAS, visual analogue scale
3.5. Impacts of KRG on functional disability and quality of life
The mean changes of functional disability and quality of life after beginning KRG or placebo treatment are summarized in Table 4. Using PP analysis, the mean baseline HAQ-DI was 0.35 ± 0.43 and 0.37 ± 0.47 before taking KRG and placebo, respectively. After beginning treatment, the improvement of HAQ-DI was greater with KRG (0.03 ± 0.24) compared to placebo (0.01 ± 0.34); however, this difference was not statistically significant (p = 0.74).
Table 4.
Comparison of changes in functional disability, quality of life, and general health VAS after specified treatments
| Variable | Change during placebo | Change during KRG | p* |
|---|---|---|---|
| HAQ-DI | −0.01± 0.34 | −0.03 ± 0.24 | 0.74 |
| EQ-5D | 0.00 ± 0.05 | −0.00 ± 0.07 | 0.78 |
Data are expressed as mean ± standard deviation
EQ-5D, EuroQol-5 dimension; HAQ-DI, health assessment questionnaire-disability index; KRG, Korean red ginseng
* For the difference between the changes during KRG compared to placebo, using a mixed effect model
The mean baseline EQ-5D was 0.85 ± 0.07 and 0.85 ± 0.05 before taking KRG and placebo, respectively. After intervention, the improvement of EQ-5D was not different between the two treatment groups (0.00 ± 0.07 in KRG and 0.00 ± 0.05 in placebo) (p = 0.78) (Table 4).
3.6. Safety
We evaluated the types and occurrences of AEs during both KRG treatment and placebo treatment. During the KRG period, 10 AEs were reported, while five AEs were reported during the placebo period; however, this difference was not statistically significant (p = 0.16). The most frequent AEs occurring during KRG treatment were gastrointestinal disorders (n = 5) and nervous system disorders (n = 3, 1 with headache, 1 with tingling in a finger, and 1 with a herniated disc). There were also three treatment emergent AEs in each period (Table 5).
Table 5.
Adverse events in each period in rheumatoid arthritis (RA) patients
| Variable | Placebo period n (TEAE) |
KRG period n (TEAE) |
p |
|---|---|---|---|
| Total | 5 (3) | 10 (3) | 0.16 |
| Hepatobiliary disorder | 2 (1) | 0 (0) | |
| Gastrointestinal disorder | 2 (2) | 3 (2) | |
| Skin and subcutaneous tissue disorder | 0 (0) | 2 (0) | |
| Injury, poisoning, or procedural complication | 1 (0) | 1 (0) | |
| Nervous system disorder | – | 31) (1) | |
| Genitourinary system | – | 1 (0) |
KRG, Korean red ginseng; TEAE, treatment emergent adverse events
headache, tingling of finger, herniated disc
4. Discussion
P. ginseng is a well-known immune modulator [19]. The immune system is comprised of several different types of cells that must maintain specialized functions; as such, each immune cell type is modulated differently by ginseng treatment [19]. Prior work has shown that ginseng promotes the generation of immunosuppressive regulatory T cells, and that it has a favorable effect on several autoimmune disease pathologies [20]. There is concern, however, that its immune enhancement could exacerbate symptoms in RA patients.
This study assesses the safety of KRG treatment for RA patients. First, we examined the RA flare rate as our primary outcome. We found that KRG treatment was not associated with an increase in disease flare rate for RA patients. When we further investigated the impact of KRG treatment on disease flare rate or fatigue symptoms for patients with good compliance (pill count ≥ 80%), we found highly similar results compared to the placebo. In fact, we found a statistically significant improvement in terms of sleep disturbance during the KRG period (data not shown).
Fatigue is a dominant and burdensome symptom for RA patients. It has not generally received much attention in clinical care, however, and it is unclear whether and how fatigue can best be treated in RA patients. Our study suggests that fatigue tends to be improved through treatment with KRG, although the difference was not statistically significant. This lack of statistical significance might be due to an inadequate sample size, or it is possible that the participant group had an insufficient number of baseline fatigue scores to observe a statistical improvement after KRG treatment.
The occurrence of AEs was relatively frequent during KRG treatment compared to AE frequency during placebo treatment; however, this increase was not statistically significant. Of the various AEs we examined, the development of gastrointestinal and nervous system-related symptoms was more frequent than during placebo treatment. Some experimental studies have suggested that ginsenosides act on the central nervous system, particularly in neurodegenerative disorders [21]. Ginsenoside Rg3 has been reported to inhibit glutamate receptors (N-methyl-D-aspartate type and non-N-methyl-D-aspartate type), which contribute significantly to the occurrence of brain trauma and convulsions [22]. Several studies have also demonstrated the antinociceptive effects of P. ginseng in vitro and in vivo [23], [24]. We hypothesize that the nervous system-related AEs that we observed are related to the general effect of KRG on the nervous system.
Our study includes some important limitations. First, we did not consider a washout period, and all of our measurements were performed in a short KRG treatment period of 8 wk. Our main objective with this work, however, was to evaluate the safety rather than the effectiveness of KRG in RA patients, and our results demonstrate similar disease flare rate and incidence rate of AEs during KRG treatment and during placebo treatment. Therefore, we suggest that including a washout period would not significantly impact our results or conclusions. It is clear, however, that a study design that includes a washout period will be required for the proper evaluation of the effectiveness of KRG in alleviating RA symptoms. The second important limitation of our study is that it included only patients with low disease activity who were not taking corticosteroids. In clinical practice, many patients take KRG regardless of disease activity. Moreover, patients often experience greater fatigue under high disease activity status. Therefore, since our inclusion criteria restricted patients with low disease activity, it is difficult to generalize our results for all RA patients. Based on our study, however, patients with a broad range of disease activities can likely be enrolled in future studies that examine the effectiveness of KRG treatment.
This study was the first randomized clinical trial (RCT) with real-world RA patients and suggests that KRG treatment is a safe alternative for many RA patients. We believe this will be a key study that supports the development of future clinical trials involving KRG treatment. Further effort is required to effectively evaluate the effect of KRG for RA patients with moderate- to high-level fatigue. Various outcomes including fatigue reduction, corticosteroid sparing effects, and safety for long-term use should be considered.
In conclusion, we report that KRG is not associated with RA disease flare or the rate of AE development in RA patients. Further study with larger sample sizes will be required to adequately address the effectiveness of KRG in improving the fatigue symptoms of RA patients.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the 2014 grant from the Korean Society of Ginseng.
References
- 1.Gabriel S.E. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 3.Whalley D., McKenna S.P., de Jong Z., van der Heijde D. Quality of life in rheumatoid arthritis. Br J Rheumatol. 1997;36:884–888. doi: 10.1093/rheumatology/36.8.884. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Hernández E., César Casasola-Vargas J., Lino-Pérez L., Burgos-Vargas R., Vázquez-Mellado J. Complementary and department for the first time. Analysis of 800 patients. Reumatol Clin. 2006;2:183–189. doi: 10.1016/S1699-258X(06)73044-3. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 5.Setty A.R., Sigal L.H. Herbal medications commonly used in the practice of rheumatology: mechanisms of action, efficacy, and side effects. Semin Arthritis Rheum. 2005;34:773–784. doi: 10.1016/j.semarthrit.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Efthimiou P., Kukar M. Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities. Rheumatol Int. 2010;30:571–586. doi: 10.1007/s00296-009-1206-y. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Remus C., Raut A. Complementary and alternative practices in rheumatology. Best Pract Res Clin Rheumatol. 2008;22:741–757. doi: 10.1016/j.berh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Han M., Sung Y.K., Cho S.K., Kim D., Won S., Choi C.B., Bang S.Y., Cha H.S., Choe J.Y., Chung W.T. Factors associated with the use of complementary and alternative medicine for Korean patients with rheumatoid arthritis. J Rheumatol. 2015;42:2075–2081. doi: 10.3899/jrheum.141447. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer D., Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 10.Choi S., Kim T., Shin Y., Lee C., Park M., Lee H., Song J. Effects of a polyacetylene from Panax ginseng on Na(+) currents in rat dorsal root ganglion neurons. Brain Res. 2008;29:75–83. doi: 10.1016/j.brainres.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Na J.Y., Kim S., Song K., Lim K.H., Shin G.W., Kim J.H., Kim B., Kwon Y.B., Kwon J. Anti-apoptotic activity of ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes: stabilization of mitochondria and the inhibition of caspase-3. J Ginseng Res. 2012;36:242–247. doi: 10.5142/jgr.2012.36.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y.S., Kang E.H., Lee E.Y., Gong H.S., Kang H.S., Shin K., Lee E.B., Song Y.W., Lee Y.J. Joint-protective effects of compound K, a major ginsenoside metabolite, in rheumatoid arthritis: in vitro evidence. Rheumatol Int. 2013;33:1981–1990. doi: 10.1007/s00296-013-2664-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.A., Kim S., Chang S.H., Hwang H.J., Choi Y.N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–1291. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Jhun J., Lee J., Byun J.K., Kim E.K., Woo J.W., Lee J.H., Kwok S.K., Ju J.H., Park K.S., Kim H.Y. Red ginseng extract ameliorates autoimmune arthritis via regulation of STAT3 pathway, Th17/Treg balance, and osteoclastogenesis in mice and human. Mediators Inflamm. 2014;2014:351856. doi: 10.1155/2014/351856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevoo M.L., van Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 16.Cella D., Yount S., Sorensen M., Chartast E., Sengupta N., Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32:811–819. [PubMed] [Google Scholar]
- 17.Bae S.C., Cook E.F., Kim S.Y. Psychometric evaluation of a Korean Health Assessment Questionnaire for clinical research. J Rheumatol. 1998;25:1975–1979. [PubMed] [Google Scholar]
- 18.Kim M.H., Cho Y.S., Uhm W.S., Kim S., Bae S.C. Cross cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res. 2005;14:1401–1406. doi: 10.1007/s11136-004-5681-z. [DOI] [PubMed] [Google Scholar]
- 19.Kang S., Min H. Ginseng, the 'Immunity Boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang I., Ahn G., Park E., Ha D., Song J.Y., Jee Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett. 2011;138:169–178. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y., Kim S., Markelonis G., Oh T. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. Neurosci Res. 1998;54:123. doi: 10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Radad K., Gille G., Liu L., Rausch W. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 23.Shin Y., Jung O., Nah J., Nam K., Kim C., Nah S. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999;32:653–659. doi: 10.1016/s0306-3623(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 24.Rhim H., Kim H., Lee D., Oh T., Nah S. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur J Pharmacol. 2002;436:151–158. doi: 10.1016/s0014-2999(01)01613-2. [DOI] [PubMed] [Google Scholar]