Abstract
Background
Compound K (CK) is a ginsenoside, a metabolite of Panax ginseng. There is interest both in increasing skin health and antiaging using natural skin care products. In this study, we explored the possibility of using CK as a cosmetic ingredient.
Methods
To assess the antiaging effect of CK, RT-PCR was performed, and expression levels of matrix metalloproteinase-1, cyclooxygenase-2, and type I collagen were measured under UVB irradiation conditions. The skin hydrating effect of CK was tested by RT-PCR, and its regulation was explored through immunoblotting. Melanin content, melanin secretion, and tyrosinase activity assays were performed.
Results
CK treatment reduced the production of matrix metalloproteinase-1 and cyclooxygenase-2 in UVB irradiated NIH3T3 cells and recovered type I collagen expression level. Expression of skin hydrating factors—filaggrin, transglutaminase, and hyaluronic acid synthases-1 and -2—were augmented by CK and were modulated through the inhibitor of κBα, c-Jun N-terminal kinase, or extracellular signal-regulated kinases pathway. In the melanogenic response, CK did not regulate tyrosinase activity and melanin secretion, but increased melanin content in B16F10 cells was observed.
Conclusion
Our data showed that CK has antiaging and hydrating effects. We suggest that CK could be used in cosmetic products to protect the skin from UVB rays and increase skin moisture level.
Keywords: compound K, melanogenesis, Panax ginseng, skin protection, UVB irradiation
1. Introduction
Skin aging is divided into intrinsic and extrinsic causes and is affected by genetic and environmental causes, and leads to dryness, skin hyperpigmentation, wrinkles, and loss of elasticity [1], [2]. Intrinsic aging is associated with genetic factors, and extrinsic aging is mediated by UV light, pollution, repetitive muscle use, and gravity [2], [3]. UV irradiation of the skin is a main cause of aging. Photoaging alters dermal connective tissue, which suggests involvement of extracellular matrix proteins [4]. The amount of collagen related to skin elasticity and wrinkling is reduced by UV irradiation, and, in this process, proteolytic matrix metalloproteinases (MMPs) mediate and accelerate degradation of collagen [2], [5]. The barrier functions of the skin decline when skin loses water. Dry skin is itchy and has a scaly and flaky appearance [6], [7]. Hyaluronic acid (HA), a well-known component of the dermis, is a glycosaminoglycan and has water-retention properties [8], [9]. HA is synthesized by HA synthases (HAS-1, 2, and 3), which have been studied with respect to their effects on increased skin health [1], [8], [10]. Filaggrin (FLG) is an epidermal barrier protein that forms a protein-lipid matrix [11], which is important for maintaining skin moisture levels [12]. Transglutaminase (TGM) is an enzyme expressed constitutively in epidermal tissue. TGM catalyzes cornified epidermal cell envelope formation, which acts as a mechanical barrier to prevent water loss [13], [14]. Melanin plays a variety of roles in the skin, including protection from UV light, and is a physiological molecule involved in absorption of toxic molecules and in neural development [15]. Melanogenesis is the process by which the pigment melanin is synthesized by melanocytes [16]. Several reports have shown that melanin plays a critical role in skin protection, but more recent studies have focused on downregulation of melanogenesis for its whitening effects, as excessive melanin production may lead to the formation of age spots and freckles [17], [18].
Compound K (CK), 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, is a ginsenoside that was first isolated from soil bacteria [19], [20]. Akao et al [21] reported that CK was found in rat blood after oral administration of ginsenoside (G)-Rb1, a major saponin found in Panax ginseng [21], [22]. These results indicated that CK was a metabolite of P. ginseng, and many studies have been conducted to investigate the biological functions of CK. Recently, interest in CK has focused on its skin protective effects; CK has been reported to suppress MMP-1 in UVB- or tumor necrosis factor-α-stimulated dermal fibroblasts [23], [24]. The antiatopic dermatitis activity of CK was also reported using NC/Nga mice treated with Dermatophagoides farinae body extracts [25]. Although potential CK activities on skin health were found, a systemic and mechanistic understanding of its effects have not yet been fully elucidated. In this study, we focused on skin health care and potential molecular pathways involved in wrinkle formation, UVB protection, and skin hydration and evaluated the possibility of developing CK as a cosmetic ingredient.
2. Materials and methods
2.1. Materials
(3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) was obtained from AMRESCO (Solon, OH, USA). Compound K (purity: > 96%), L-3,4-dihydroxyphenylalanine (L-DOPA), α-melanocyte-stimulating hormone (α-MSH), arbutin, and mushroom tyrosinase were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM) were purchased from Gibco (Grand Island, NY, USA). Inhibitors (SB203583, SP600125, and U0126) were purchased from Millipore (Billerica, MA, USA). Total and phospho-specific antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Phospho- and total antibodies against p38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERK), inhibitor of κBα (IκBα), and β-actin were purchased from Cell Signaling (Beverly, MA, USA).
2.2. Cell culture
Mouse embryonic fibroblast NIH3T3 cells, mouse melanoma B16F10 cells, and human embryonic kidney HEK293T cells were cultured in DMEM supplemented with 5% FBS and 1% antibiotics (penicillin and streptomycin) under 5% CO2 at 37°C. Human keratinocyte HaCaT cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotics at 37°C under 5% CO2. For experiments, all cells were trypsinized.
2.3. Treatment with compound K
A stock solution (20mM) of CK was prepared in 100% dimethyl sulfoxide (DMSO) and diluted with media to 5–10μM for the in vitro assay.
2.4. Cell viability assay
NIH3T3 cells and HaCaT cells were seeded onto 96-well plates at a density of 5.0 × 105 cells/mL in culture medium. After preincubation, CK (0–10μM) was added to cells, followed by incubation for 24 h. Cell viability was determined using a conventional MTT assay [26].
2.5. UVB irradiation
NIH3T3 cells (5.0 × 105 cells/mL) were seeded in six-well plates. NIH3T3 cells were pretreated with CK (0–10μM) for 30 min, and culture medium was replaced with 1 mL phosphate-buffered saline (PBS). NIH3T3 cells were irradiated using a UVB lamp (Bio-Link BLX-312; Vilber Lourmat, Collégien, France) with an emission wavelength peak of 312 nm. After removing the plate lid, cells were irradiated at 30 mJ/cm2. The PBS was removed, and cells were re-treated with CK at the appropriate concentration for 24 h [27].
2.6. Plasmid transfection and luciferase reporter gene assay
For the luciferase (Luc) reporter gene assay, HEK293T cells were transfected with 0.8 μg/mL of plasmids containing β-galactosidase and collagen (Col)1a1-Luc in the presence or absence of Flag-Smad3 using the polyethyleneimine (PEI) method in 24-well plates for 24 h. The cells were treated with CK for an additional 24 h. HEK293T cells were transfected with 0.8 μg/mL plasmid containing β-galactosidase, activator protein (AP)-1-Luc or cyclic adenosine monophosphate response element binding protein (CREB)-Luc for 24 h. Cells were treated with compound K for 24 h before termination. Luciferase assay was performed using the Luciferase Assay System (Promega, Madison, WI, USA), as previously reported [28], [29].
2.7. Analysis of mRNA levels by semi-quantitative RT-PCR
To quantify mRNA expression levels, NIH3T3 cells were pretreated with 0μM, 5μM, or 10μM CK for 30 min. UVB (30 mJ/cm2) irradiation was applied to cells, and cells were incubated for 24 h with CK. To evaluate hydration related mRNA expression levels, HaCaT cells were incubated with CK (0–10μM) for 24 h, and total RNA was isolated using the TRIzol reagent (Gibco) according to the manufacturer’s instructions. RT-PCR was performed as described previously [30].
2.8. Immunoblotting analysis
Preparation of total lysates of HaCaT cells was conducted according to previously reported methods [31]. Immunoblotting using the total lysates was carried out. The total and phospho-forms of JNK, ERK, p38, IκBα, and β-actin were visualized as described previously [32].
2.9. Melanin formation and secretion test
For the melanin formation assay, B16F10 cells (1.0 × 105 cells/well in 12-well plates) were treated with 100nM α-MSH, 30 μg/mL CK, or 1mM arbutin for 48 h. Melanin secretion was assessed by measuring the absorbance of the culture medium at 475 nm using a multidetection microplate reader. For melanin content analysis, cells were lysed with 20 μL cell lysis buffer (50mM Tris-HCl pH 7.5, 20mM NaF, 25mM β-glycerolphosphate pH 7.5, 120mM NaCl, and 2% NP-40 in distilled water). The lysed pellets were dissolved in 90 μL 1M NaOH containing 10% DMSO for 30 min at 55°C, after which the absorbance of the resulting solutions was measured at 405 nm [33].
2.10. Tyrosinase activity assay
To test tyrosinase activity, 50 μL of 2mM L-DOPA [dissolved in 50mM potassium phosphate buffer (pH 6.8)], 50 μL of DMSO [with or without CK at a final concentration of 10μM], and 900μM kojic acid (dissolved in potassium phosphate buffer) were mixed at room temperature for 15 min. Mushroom tyrosinase (100 units/mL) dissolved in potassium phosphate buffer was then added to the mixture. The absorbance of the mixture was then immediately measured at 475 nm using a multidetection microplate reader [34].
2.11. Statistical analyses
All data are presented as means ± standard deviations, and each experiment consisted of three or four replications. The Mann–Whitney U test was used to analyze statistical differences between groups. A p value < 0.05 was regarded as statistically significant. All statistical tests were performed using SPSS software (version 22.0, 2013; IBM Corp., Armonk, NY, USA).
3. Results and discussion
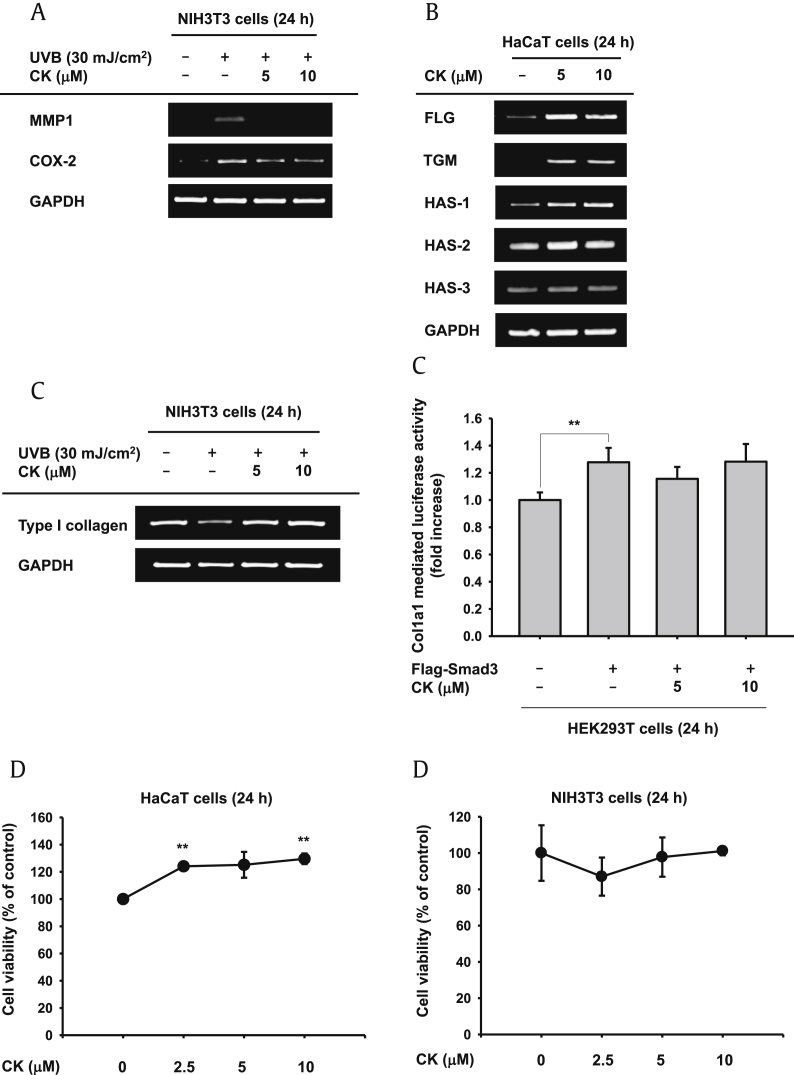
UVB irradiation is one cause of extrinsic skin aging (photoaging) and induces upregulation of various aging related factors including MMPs, inducible nitric oxide synthase or cyclooxygenase (COX)-2, and degradation of type I and III collagen [35], [36]. To investigate whether CK has protective effects on photoaging, we irradiated NIH3T3 cells with UVB (30 mJ/cm2) and conducted semiquantitative PCR to verify expression levels of aging biomarkers. Following UVB irradiation, the expression levels of MMP1 and COX-2 dramatically increased, and those were diminished by CK (Fig. 1A). Other MMPs—MMP2, MMP3, and MMP9—also mediate degradation of collagen and elastic fibers [35], [37]. We determined the mRNA levels of MMP2 and MMP9, but those factors were not changed by CK (data not shown). MMP1 is the prototype and main collagenase among the MMPs [37], [38]. Although CK only modulated MMP1, this result implies that CK has an antiaging effect. In addition, mRNA levels of type I collagen were tested. Type I collagen, which decreased in UVB irradiated cells, was recovered by treatment with CK (Fig. 1C, left panel). Smad3- and TGFβ-induced Col1a1 and Col1a2 gene expression, which encode type I collagen α1 and α2, respectively, which combine to make type I collagen [39], [40]. We cotransfected cells with the Flag-Smad3 construct and the Col1a1 promoter gene to confirm that increased levels of type I collagen were dependent on the Smad3 signaling pathway. The Col1a1 promoter was activated by Smad3, but Col1a1 promoter activity was not altered by treatment with CK (Fig. 1C, right panel), suggesting that the regulatory mechanism of CK is Smad3 independent. CK regulated expression of aging related factors (MMP1, COX-2, and type I collagen); thus, we concluded that CK had antiaging properties under UVB irradiation.
Fig. 1.
Effect of compound K (CK) on aging and skin hydration. (A) mRNA expression levels of aging factors [matrix metalloproteinase-1 (MMP1) and cyclooxygenase (COX)-2] were determined by RT-PCR in UVB (30 mJ/cm2) irradiated NIH3T3 cells. (B) HaCaT cells were treated for 24 h with CK. Increased mRNA expression levels of skin hydration factors [filaggrin (FLG), transglutaminase (TGM), hyaluronic acid synthases (HAS)-1, 2, and 3] were determined by RT-PCR. (C) mRNA expression levels of type I collagen were tested by RT-PCR in UVB irradiated NIH3T3 cells (left panel). Type I collagen promoter activity was tested in Smad3 induced HEK293T cells (right panel). (D) Cell cytotoxicity of HaCaT and NIH3T3 cells was tested using the MTT assay. * p < 0.05 and ** p < 0.01 compared to the normal or control groups.
Next, we performed RT-PCR to study the skin hydrating effects of CK. HaCaT cells were treated with CK alone for 24 h, and total mRNA was isolated. FLG, TGM, and HAS-1 were significantly enhanced by CK treatment, the expression level of HAS-2 was also elevated slightly, and that of HAS-3 remained constant (Fig. 1B). Meanwhile, cytotoxicity by CK was not detected in HaCaT and NIH3T3 cells (Fig. 1D).
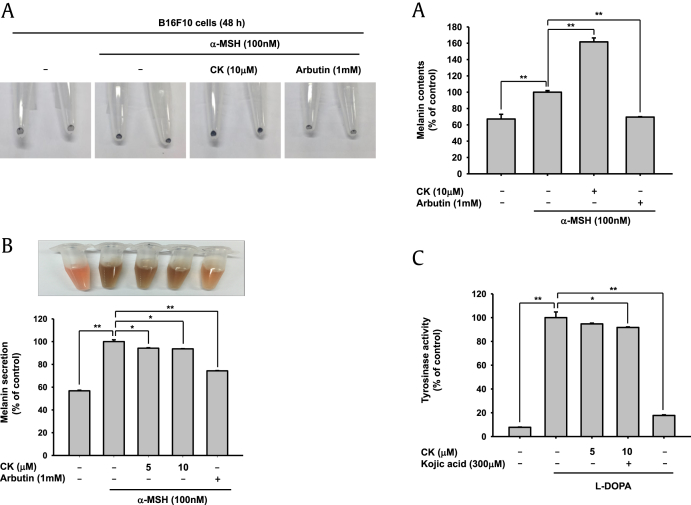
CK has the potential to improve skin conditions (Fig. 1). We further investigated whether CK had inhibitory action on melanogenesis. B16F10 cells were treated simultaneously with both CK and α-melanocyte-stimulating hormone (α-MSH) for 48 h. In this experiment, arbutin, a well-known whitening compound, was used as the positive control [15], [41]. We observed the pellet of harvested cells showing the melanogenic response. Following α-MSH treatment, melanin synthesis ability was greatly increased and, as expected, arbutin inhibited this response. By contrast, CK dramatically elevated melanin synthesis (Fig. 2A), unlike G-F1, which has been reported to increase intracellular melanin levels but inhibit secretion into the culture medium [33]. To confirm CK’s upregulation of melanin synthesis, the extracellular secretion level of melanin was measured with culture media. As Fig. 2B shows, the extracellular melanin level was not increased (Fig. 2B). Moreover, CK did not affect tyrosinase activity (Fig. 2C), implying that CK could not modulate melanin synthesis process as well as secretion pathway. Melanogenesis is a complex biological process to synthesize melanin, which protects the skin from UV irradiation [42], [43]. CK did not have any influence on tyrosinase activity or extracellular melanin secretion, but did increase intracellular melanin levels. The fact that CK stimulated melanogenesis led us to the hypothesis that CK does not have a whitening effect but might possess photoprotection activity at the cellular level. This UVB-protective effect suggests that CK can retard skin damage such as skin aging and wrinkle formation [5], [43]. It is possible that some biological activities of CK could be managed by melanin action [42].
Fig. 2.
Effect of compound K (CK) on melanin formation and secretion and tyrosinase activity. (A and B) B16F10 cells were treated with 100nM α-melanocyte-stimulating hormone (α-MSH) for 48 h. Melanin formation was tested by observing the color of harvesting cells, and the absorbance of B16F10 cell lysate was measured at 405 nm. Cell culture medium was collected and the absorbance of the medium was measured at 475 nm. Arbutin (1mM) was used as the positive control compound. (C) L-3,4-dihydroxyphenylalanine (L-DOPA), mushroom tyrosinase, and compound K (10μM) or kojic acid (300μM, as a positive control) were incubated with cells in 96 well plates. The absorbance at 475 nm was measured using a multi-detection microplate reader. * p < 0.05 and ** p < 0.01 compared to the normal or control groups.
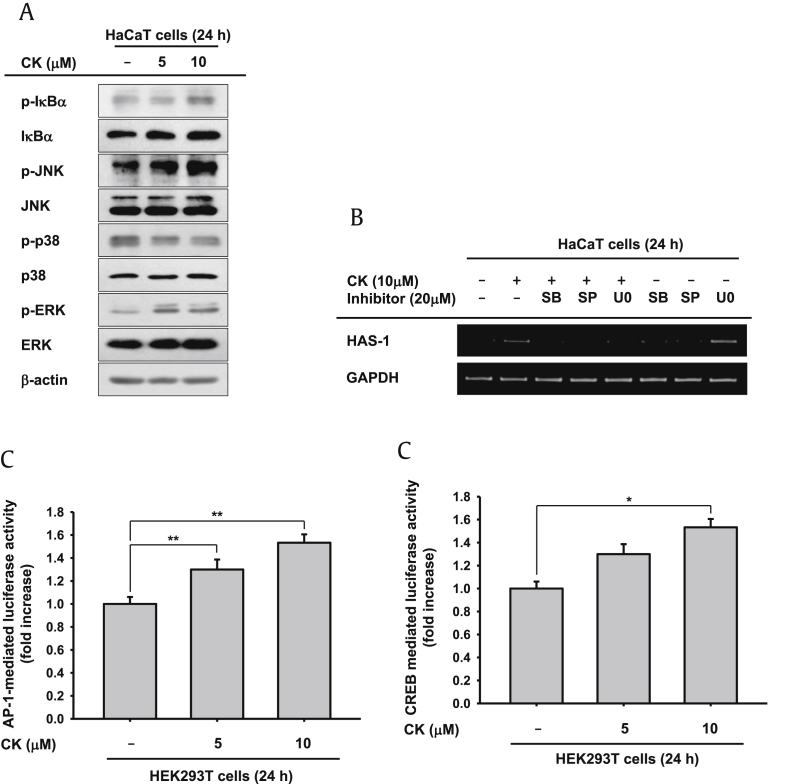
The mRNA levels of skin moisture increased in CK-treated HaCaT cells (Fig. 1B). We investigated how expression levels of skin hydrating factors were controlled. HaCaT cells were treated with CK for 24 h, and cells were prepared for immunoblotting. Previous reports demonstrated that mitogen-activated protein kinases (MAPKs: p38, ERK, and JNK) and Akt-signaling cascades played critical roles in HA synthesis [44], [45]. TGM expression was enhanced through MAPKs and the PI3K/nuclear factor (NF)-κB signal pathway [13], [46]. By contrast, Cyclopia intermedia extract augmented expression levels of FLG but attenuated phosphorylation of MAPKs under UVB irradiation conditions [47]. FLG was downregulated by STAT3 (signal transducer and activator of transcription 3) and ERK phosphorylation, as well [11]. Based on these results, MAPKs and IκBα phosphorylation levels were studied. Phosphorylation level of IκBα was slightly enhanced following treatment with CK at a concentration of 10μM. MAPKs, JNK, and ERK phosphorylation were all considerably augmented by CK (5–10μM; Fig. 3A). To confirm these results, we monitored changes in HAS-1 expression by RT-PCR when CK and MAPKs specific inhibitors (SB203580, SP600125, and U0126) were used to cotreat cells. The increased expression level of HAS-1 was abrogated by a JNK inhibitor (SP600125) in cotreated HaCaT cells (Fig. 1B). According to our RT-PCR result, HAS-1 expression was dependent on JNK activity. Here, we suggested that the factors involved in skin hydration were modulated through either the NF-κB or MAPKs pathways. To elucidate the regulatory mechanism of FLG, TGM, HAS-2, and HAS-3, additional experiments are needed. Furthermore, a promoter activity assay was conducted to demonstrate the regulatory mechanisms of skin hydration factors. We transfected AP-Luc or CREB-Luc and β-gal (as a control) constructs into HEK293T cells for 24 h and then exposed cells to CK for a further 24 h. Following CK treatment, both AP-1 and the CREB promoter were activated (Fig. 3C). NF-κB promoter activity was also examined, and its transcriptional activity was slightly increased (1.2-fold increase; data not shown), in accordance with phosphorylated IκBα (Fig. 3A).
Fig. 3.
Skin hydration effects of compound K (CK). (A) HaCaT cells were treated with CK for 24 h, and cells were harvested. Phosphorylation level of inhibitor of κBα and mitogen-activated protein kinases [c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38] were tested by immunoblotting. (B) HaCaT cells were pretreated with mitogen-activated protein kinases inhibitors (SB203580, SP600125, U0126) for 30 min and incubated in presence or absence of CK for 24 h. mRNA expression levels were determined by RT-PCR. (C) HEK293T cells were transfected with AP-1-Luc or cyclic adenosine monophosphate response element binding protein-Luc constructs and β-gal (as a control) for 24 h, and cells were treated with CK for an additional 24 h. Luciferase activity was measured using a luminometer. * p < 0.05 and ** p < 0.01 compared to the normal groups.
In this study, we evaluated the effects of CK on skin hydration, skin aging, wrinkle formation, and melanogenesis. CK prevented skin aging and degradation of collagen by regulation of MMP1 and COX-2 expression under UVB irradiation conditions. Our data showed that CK had skin hydrating effects by controlling FLG, TGM, HAS-1, and -2. CK-induced phosphorylated IκBα, JNK, and ERK affected expression levels of these molecules. Interestingly, CK increased melanin synthesis in B16F10 cells; this implied that CK cannot function as a whitening factor. However, our results (Fig. 1, Fig. 2) showed that CK played a pivotal role in skin protection. When skin was exposed to UV light, it caused skin aging, wrinkle formation, DNA damage and abnormal immune responses [42], [48], [49]. CK may possibly be used in natural UVB-blocking cosmetics because CK induced intracellular melanin synthesis. CK could be also served as an antiaging treatment because its photoprotective effects blocked collagen degradation and expression of aging factors (MMP1 and COX-2) [43]. Vitiligo is a depigmenting disease of the skin. This dermatologic disorder is caused by abnormal melanocytes. The exact cause of vitiligo is still unclear, but studies have suggested a wide range of causes from autoimmunity to autocytotoxicity to viral and neural causes [43], [50]. One vitiligo treatment is to expose the skin to UVB light to induce repigmentation [51]. However, exposure of skin to UVB light has potential harmful side effects [52]. It is possible that CK-induced melanin synthesis could be developed as a new method to treat or cure vitiligo.
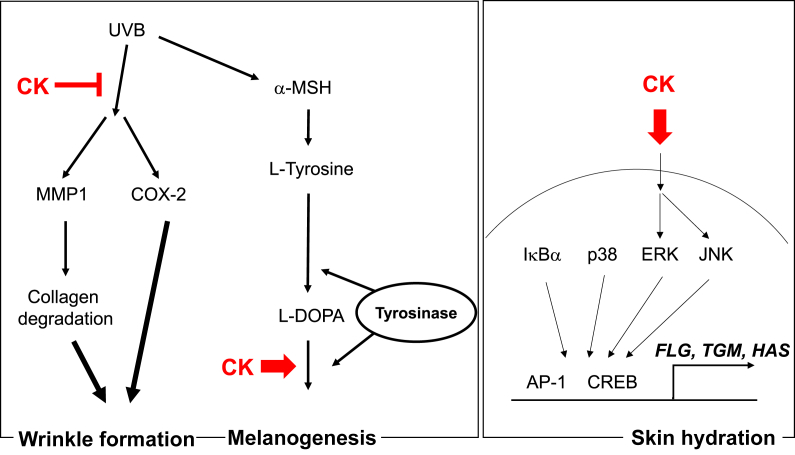
Taken together, our data conclusively demonstrated that CK had ability to increase skin moisture levels and melanin synthesis as well as to protect against UVB-induced photoaging by regulating the p38/AP-1/CREB pathway (Fig. 4). Our results indicated that CK could be used as an ingredient in cosmetics, for instance, in UVB-blocking, antiwrinkle, or skin hydrating cosmetics. Furthermore, we also suggest that CK has the potential to be developed as a treatment for vitiligo.
Fig. 4.
Action of compound K (CK) in photoaging, skin hydration, and melanogenesis. CK was found to suppress UVB-induced MMP1 and COX-2 expression and modulate L-DOPA-induced melnogenesis. Finally, it was revealed that CK is able to stimulate ERK and JNK activities linked to AP-1 and CREB activity in skin hydration process. AP-1, activator protein-1; COX-2, cyclooxygenase-2; CREB, cyclic adenosine monophosphate response element binding protein; ERK, extracellular signal–regulated kinases; IκBα, inhibitor of κBα; JNK, c-Jun N-terminal kinase; L-DOPA, L-3,4-dihydroxyphenylalanine; MMP1, matrix metalloproteinase-1.
Conflicts of interest
The authors have no conflicts of interest to declare.
Contributor Information
Jong-Hoon Kim, Email: jhkim1@chonbuk.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
Junseong Park, Email: superbody@amorepacific.com.
References
- 1.Kanlayavattanakul M., Lourith N., Chaikul P. Jasmine rice panicle: a safe and efficient natural ingredient for skin aging treatments. J Ethnopharmacol. 2016;193:607–616. doi: 10.1016/j.jep.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.M., Jung H.J., Choi J.S., Nam T.J. Anti-wrinkle effects of a tuna heart H2O fraction on Hs27 human fibroblasts. Int J Mol Med. 2016;37:92–98. doi: 10.3892/ijmm.2015.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farage M., Miller K., Elsner P., Maibach H. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 4.Quan T., Qin Z., Xia W., Shao Y., Voorhees J.J., Fisher G.J. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher G.J., Wang Z., Datta S.C., Varani J., Kang S., Voorhees J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1429. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J., Zhong A., Friedrich E.E., Jia S., Xie P., Galiano R.D., Mustoe T.A., Hong S.J. S100A12 induced in the epidermis by reduced hydration activates dermal fibroblasts and causes dermal fibrosis. J Invest Dermatol. 2017;137:650–659. doi: 10.1016/j.jid.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Kottner J., Kanti V., Dobos G., Hahnel E., Lichterfeld-Kottner A., Richter C., Hillmann K., Vogt A., Blume-Peytavi U. The effectiveness of using a bath oil to reduce signs of dry skin: a randomized controlled pragmatic study. Int J Nurs Stud. 2017;65:17–24. doi: 10.1016/j.ijnurstu.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Barnes L., Carraux P., Saurat J.H., Kaya G. Increased expression of CD44 and hyaluronate synthase 3 is associated with accumulation of hyaluronate in spongiotic epidermis. J Invest Dermatol. 2012;132:736–738. doi: 10.1038/jid.2011.384. [DOI] [PubMed] [Google Scholar]
- 9.Verdier-Sévrain S., Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6:75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Lauer M.E., Anand S., Mack J.A., Maytin E.V. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem. 2014;289:32253–32265. doi: 10.1074/jbc.M114.578377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.H., Bae H.C., Ko N.Y., Lee S.H., Jeong S.H., Lee H., Ryu W.I., Kye Y.C., Son S.W. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J Allergy Clin Immunol. 2015;136 doi: 10.1016/j.jaci.2015.04.026. 205–8. e209. [DOI] [PubMed] [Google Scholar]
- 12.Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3–11. doi: 10.1016/j.jdermsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Terazawa S., Mori S., Nakajima H., Yasuda M., Imokawa G. The UVB-stimulated expression of transglutaminase 1 is mediated predominantly via the NFκB signaling pathway: new evidence of its significant attenuation through the specific interruption of the p38/MSK1/NFκBp65 Ser276 axis. PLoS One. 2015;10:e0136311. doi: 10.1371/journal.pone.0136311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candi E., Schmidt R., Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 15.Lim Y.J., Lee E.H., Kang T.H., Ha S.K., Oh M.S., Kim S.M., Yoon T.J., Kang C., Park J.H., Kim S.Y. Inhibitory effects of arbutin on melanin biosynthesis of α-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch Pharm Res. 2009;32:367–373. doi: 10.1007/s12272-009-1309-8. [DOI] [PubMed] [Google Scholar]
- 16.Gillbro J., Olsson M. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int J Cosmet Sci. 2011;33:210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 17.Ko H.H., Chiang Y.C., Tsai M.H., Liang C.J., Hsu L.F., Li S.Y., Wang M.C., Yen F.L., Lee C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: role of MAPK and Akt pathways. J Ethnopharmacol. 2014;151:386–393. doi: 10.1016/j.jep.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Choi M.H., Shin H.J. Anti-melanogenesis effect of quercetin. Cosmetics. 2016;3:18. [Google Scholar]
- 19.Shin K.C., Choi H.Y., Seo M.J., Oh D.K. Compound K production from red ginseng extract by β-glycosidase from Sulfolobus solfataricus supplemented with α-L-Arabinofuranosidase from Caldicellulosiruptor saccharolyticus. PLoS One. 2015;10:e0145876. doi: 10.1371/journal.pone.0145876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh J., Kim J.S. Compound K derived from ginseng: neuroprotection and cognitive improvement. Food Funct. 2016;7:4506–4515. doi: 10.1039/c6fo01077f. [DOI] [PubMed] [Google Scholar]
- 21.Akao T., Kida H., Kanaoka M., Hattori M., Kobashi K. Drug metabolism: intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 22.Upadhyaya J., Kim M.J., Kim Y.H., Ko S.R., Park H.W., Kim M.K. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea. J Ginseng Res. 2016;40:105–112. doi: 10.1016/j.jgr.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C.S., Bae I.H., Han J., Choi G.Y., Hwang K.H., Kim D.H., Yeom M.H., Park Y.H., Park M. Compound K inhibits MMP-1 expression through suppression of c-Src-dependent ERK activation in TNF-alpha-stimulated dermal fibroblast. Exp Dermatol. 2014;23:819–824. doi: 10.1111/exd.12536. [DOI] [PubMed] [Google Scholar]
- 24.Shin D.J., Kim J.E., Lim T.G., Jeong E.H., Park G., Kang N.J., Park J.S., Yeom M.H., Oh D.K., Bode A.M. 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol suppresses UV-Induced MMP-1 expression through AMPK-mediated mTOR inhibition as a downstream of the PKA-LKB1 pathway. J Cell Biochem. 2014;115:1702–1711. doi: 10.1002/jcb.24833. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.R., Choi J., Kim J., Kim H., Kang H., Kim E.H., Chang J.H., Kim Y.E., Choi Y.J., Lee K.W. 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365–371. doi: 10.1016/j.jep.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 26.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung K.W., Choi Y.J., Park M.H., Jang E.J., Kim D.H., Park B.H., Yu B.P., Chung H.Y. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J Gerontol Series A Biol Sci Med Sci. 2015;70:959–968. doi: 10.1093/gerona/glu137. [DOI] [PubMed] [Google Scholar]
- 28.Baek K.S., Hong Y.D., Kim Y., Sung N.Y., Yang S., Lee K.M., Park J.Y., Park J.S., Rho H.S., Shin S.S. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015;39:155–161. doi: 10.1016/j.jgr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S., Kim Y., Jeong D., Kim J.H., Kim S., Son Y.J., Yoo B.C., Jeong E.J., Kim T.W., Lee I.H. Pyrrole-derivative of chalcone, (E)-3-phenyl-1-(2-pyrrolyl)-2-propenone, inhibits inflammatory responses via inhibition of Src, Syk, and TAK1 kinase activities. Biomol Ther (Seoul) 2016;24:595–603. doi: 10.4062/biomolther.2016.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh W., Nam G., Yang W.S., Sung G.H., Shim S.H., Cho J.Y. Chemical constituents identified from fruit body of Cordyceps bassiana and their anti-inflammatory activity. Biomol Ther (Seoul) 2017;25:165–170. doi: 10.4062/biomolther.2016.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Lee J., Rhee M.H., Yu T., Baek K.S., Sung N.Y., Kim Y., Yoon K., Kim J.H., Kwak Y.S. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015;39:61–68. doi: 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J.G., Kang W.S., Park K.T., Park D.J., Aravinthan A., Kim J.H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40:304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.H., Baek E.J., Lee E.J., Yeom M.H., Park J.S., Lee K.W., Kang N.J. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp Dermatol. 2015;24:150–152. doi: 10.1111/exd.12586. [DOI] [PubMed] [Google Scholar]
- 34.Kubo I., Nitoda T., Nihei K. Effects of quercetin on mushroom tyrosinase and B16-F10 melanoma cells. Molecules. 2007;12:1045–1056. doi: 10.3390/12051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichihashi M., Ando H., Yoshida M., Niki Y., Matsui M. Photoaging of the skin. Antiaging Med. 2009;6:46–59. [Google Scholar]
- 36.Zhang J.A., Yin Z., Ma L.W., Yin Z.Q., Hu Y.Y., Xu Y., Wu D., Permatasari F., Luo D., Zhou B.R. The protective effect of baicalin against UVB irradiation induced photoaging: an in vitro and in vivo study. PLoS One. 2014;9:e99703. doi: 10.1371/journal.pone.0099703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon H.J., Lee S.R., Shim S.N., Jeong S.H., Stonik V.A., Rasskazov V.A., Zvyagintseva T., Lee Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol Pharma Bull. 2008;31:284–289. doi: 10.1248/bpb.31.284. [DOI] [PubMed] [Google Scholar]
- 38.Wenk J., Schüller J., Hinrichs C., Syrovets T., Azoitei N., Podda M., Wlaschek M., Brenneisen P., Schneider L.A., Sabiwalsky A. Overexpression of phospholipid-hydroperoxide glutathione peroxidase in human dermal fibroblasts abrogates UVA irradiation-induced expression of interstitial collagenase/matrix metalloproteinase-1 by suppression of phosphatidylcholine hydroperoxide-mediated NFκB activation and interleukin-6 release. J Biol Chem. 2004;279:45634–45642. doi: 10.1074/jbc.M408893200. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh A.K., Yuan W., Mori Y., Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- 40.Verrecchia F., Chu M.L., Mauviel A. Identification of novel TGF-β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty A.K., Funasaka Y., Komoto M., Ichihashi M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res. 1998;11:206–212. doi: 10.1111/j.1600-0749.1998.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 42.ElObeid A.S., Kamal-Eldin A., Abdelhalim M.A.K., Haseeb A.M. Pharmacological properties of melanin and its function in health. Basic Clin Pharmacol Toxicol. 2016 doi: 10.1111/bcpt.12748. [DOI] [PubMed] [Google Scholar]
- 43.Yao C., Jin C.L., Oh I.G., Park C.H., Chung J.H. Melia azedarach extract stimulates melanogenesis through increase of tyrosinase-related protein 1 expression in B16F10 mouse melanoma cells. Int J Mol Med. 2015;35:1761–1766. doi: 10.3892/ijmm.2015.2182. [DOI] [PubMed] [Google Scholar]
- 44.Stuhlmeier K.M., Pollaschek C. Differential effect of transforming growth factor β (TGF-β) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-β-induced hyaluronan synthase 1 activation. J Biol Chem. 2004;279:8753–8760. doi: 10.1074/jbc.M303945200. [DOI] [PubMed] [Google Scholar]
- 45.Lim T.G., Jeon A.J., Yoon J.H., Song D., Kim J.E., Kwon J.Y., Kim J.R., Kang N.J., Park J.S., Yeom M.H. 20-O-β-D-glucopyranosyl-20 (S)-protopanaxadiol, a metabolite of ginsenoside Rb1, enhances the production of hyaluronic acid through the activation of ERK and Akt mediated by Src tyrosin kinase in human keratinocytes. Int J Mol Med. 2015;35:1388–1394. doi: 10.3892/ijmm.2015.2121. [DOI] [PubMed] [Google Scholar]
- 46.Bayardo M, Punzi F, Bondar C, Chopita N, Chirdo F. Transglutaminase 2 expression is enhanced synergistically by interferon-γ and tumour necrosis factor-α and tumour necrosis factor, Chopita N, Chirdo F. Trans–104. [DOI] [PMC free article] [PubMed]
- 47.Im A.R., Yeon S.H., Lee J.S., Um K.A., Ahn Y.J., Chae S. Protective effect of fermented Cyclopia intermedia against UVB-induced damage in HaCaT human keratinocytes. BMC Complement Altern Med. 2016;16:261. doi: 10.1186/s12906-016-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha R.P., Häder D.P. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 49.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 50.de Menezes A.F., Oliveira de Carvalho F., Barreto R.S., Santana Silva B., Shanmugam S., Gurgel R.Q., Souza Araújo A.A. Pharmacologic treatment of vitiligo in children and adolescents: a systematic review. Pediatr Dermatol. 2017;34:13–24. doi: 10.1111/pde.13024. [DOI] [PubMed] [Google Scholar]
- 51.Scherschun L., Kim J.J., Lim H.W. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J American Acad Dermatol. 2001;44:999–1003. doi: 10.1067/mjd.2001.114752. [DOI] [PubMed] [Google Scholar]
- 52.Ogilvy V., Preziosi R.F. Can carotenoids mediate the potentially harmful effects of ultraviolet light in Silurana (Xenopus) tropicalis larvae? J Anim Physiol Anim Nutr (Berl) 2012;96:693–699. doi: 10.1111/j.1439-0396.2011.01197.x. [DOI] [PubMed] [Google Scholar]