Abstract
Background
Ginseng saponin has long been used as a traditional Asian medicine and is known to be effective in treating various kinds of pain. Ginsenoside Rf is one of the biologically active saponins found in ginseng. We evaluated ginsenoside Rf’s antinociceptive and anti-inflammatory effects, and its mechanism of action on adrenergic and serotonergic receptors, in an incisional pain model.
Methods
Mechanical hyperalgesia was induced via plantar incision in rats followed by intraperitoneal administration of increasing doses of ginsenoside Rf (vehicle, 0.5 mg/kg, 1 mg/kg, 1.5 mg/kg, and 2 mg/kg). The antinociceptive effect was also compared in a Positive Control Group that received a ketorolac (30 mg/kg) injection, and the Naïve Group, which did not undergo incision. To evaluate the mechanism of action, rats were treated with prazosin (1 mg/kg), yohimbine (2 mg/kg), or ketanserin (1 mg/kg) prior to receiving ginsenoside Rf (1.5 mg/kg). The mechanical withdrawal threshold was measured using von Frey filaments at various time points before and after ginsenoside Rf administration. To evaluate the anti-inflammatory effect, serum interleukin (IL)-1β, IL-6, and tumor necrotizing factor-α levels were measured.
Results
Ginsenoside Rf increased the mechanical withdrawal threshold significantly, with a curvilinear dose–response curve peaking at 1.5 mg/kg. IL-1β, IL-6, and tumor necrotizing factor-α levels significantly decreased after ginsenoside Rf treatment. Ginsenoside Rf’s antinociceptive effect was reduced by yohimbine, but potentiated by prazosin and ketanserin.
Conclusion
Intraperitoneal ginsenoside Rf has an antinociceptive effect peaking at a dose of 1.5 mg/kg. Anti-inflammatory effects were also detected.
Keywords: analgesics, antiinflammatory agents, ginsenosides, pain, postoperative
1. Introduction
Ginseng, the root of Panax ginseng Meyer, is a traditional Asian herbal medicine that has been used for more than thousands of years to reduce neuralgia, toothache, abdominal pain, and chest pain [1]. Ginseng saponins, also known as ginsenosides, have a steroid-like chemical structure consisting of four rings with sugar moieties attached. Ginsenosides have biological properties similar to those of histamines, opioids, adrenaline, and acetylcholine [2]. Several experimental studies have demonstrated the antinociceptive effects of ginseng extracts in various pain models including those of abdominal, neuropathic, chronic, and incisional pain [3], [4], [5], [6], [7]; the mechanisms of action that have been suggested to explain this effect include antagonism of adrenergic, cholinergic, gamma-aminobutyric acid, N-methyl-D-aspartate, and opioid receptors [3], [5], [8], [9], [10].
More than 20 different ginsenosides have been found in ginseng, making them major components of this herbal medicine [11]. Among those, ginsenoside Rf, a trace ginsenoside extract (3.48%), has been shown to have an antinociceptive effect [4], [5], [12]. Although the antinociceptive effect of ginseng extracts has been studied previously in an incisional pain model [3], [6], [9], the antinociceptive effects of isolated ginsenoside Rf have not been studied in a rat incisional mode, which induces mechanical hyperalgesia through surgical incision of the plantar surface of the hind paw and thus simulates human postoperative pain [13]. In addition, ginsenoside Rf’s antinociceptive mechanism of action is not known; it has been hypothesized to act upon adrenergic receptors, similar to other ginsenosides, or serotonin receptors, although this pathway has not been previously identified in this type of pain model.
Surgical incision and intraoperative injury induce postoperative pain by increasing central neuronal excitability leading to peripheral and central sensitization [14]. Proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrotizing factor (TNF)-α are known to exacerbate postoperative pain and mediate the pain mechanism and hyperalgesia [15], [16], [17], [18]. If sensitization is sustained, postoperative pain may progress to chronic pain, which can lead to physical, psychological, and social disability.
The aim of the present study was to evaluate the antinociceptive effect of ginsenoside Rf and its mechanism of action, including its effects on adrenergic and serotonergic receptors in a rat incisional pain model. Proinflammatory cytokine levels, including IL-1β, IL-6, and TNF-α, were also assessed to determine the anti-inflammatory effect of ginsenoside Rf.
2. Materials and methods
Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Chung-ang University (2016-00014). The present study was performed according to the guidelines established by the National Institutes of Health, the policies of the International Association for the Study of Pain for the use of laboratory animals, and the recommended guideline in the Animal Research Reporting In Vivo Experiments statement [19].
Ginsenoside Rf was obtained from Ambo institute (Daejon, Korea). The HPLC purity of Rf used in the present study was 99.01%. Prazosin, yohimbine, and ketanserin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ketorolac was purchased from Hanmi Pharmaceutical Corporation (Seoul, Korea).
2.1. Animal preparation and incisional pain model
Adult male Sprague–Dawley rats weighing 250–300 g (Coretec Laboratories, Seoul, Korea) were used. They were habituated in the colony room for 1 wk before experimentation. Each cage housed with two rats at 22 ± 0.5°C with a 12:12 h light–dark cycle. Food and water were available ad libitum.
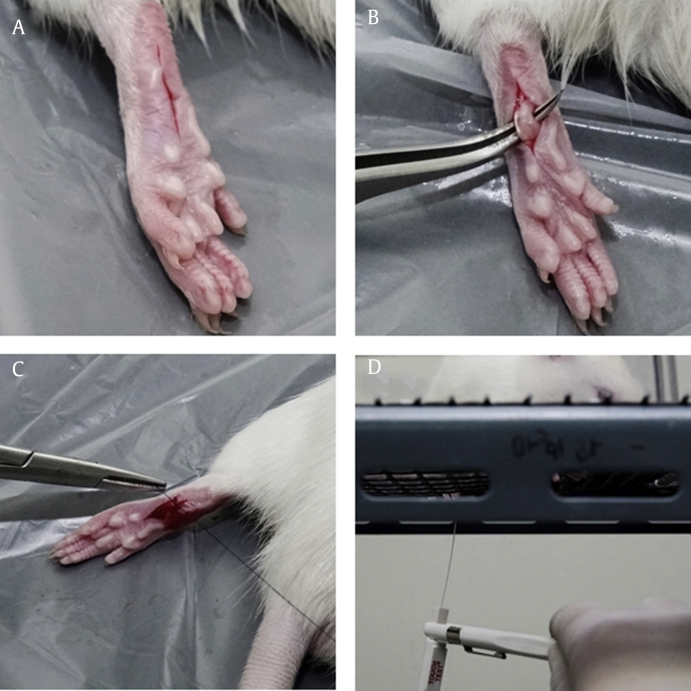
All the experiments were performed between 8:00 AM and 1:00 PM to avoid diurnal variation. One investigator prepared the incisional pain model as previously described [13]. Briefly, the animals were anesthetized with isoflurane (Aerane Solution; Ilsung-medicine, Seoul, Korea) using a chamber for induction and a nonrebreathing circuit system with mask delivery for maintenance. After subcutaneous injection of cefazolin (Cefazoline, 20 mg/kg; Chong Kun Dang Pharmaceutical Corporation, Seoul, Korea) and aseptic draping, a 1-cm skin incision was made in the area between 0.5 cm distal to the tibiotarsus and digits on the plantar area of the left hind paw. The plantaris muscle was then elevated, and an incision was made longitudinally, keeping the muscle origin and insertion site intact. The skin was sutured with 5-0 nylon, and prophylactic antibiotic salve (Mupirocin; Hanal Biopharma, Seoul, Korea) was applied (Fig. 1). Lesions were checked daily, and rats with suspected wound dehiscence or infection were excluded.
Fig. 1.
Incisional pain model preparation and mechanical withdrawal threshold measurement. (A) Skin incision was made on the plantar area of the hind paw. (B) The plantaris muscle was elevated, and an incision was made longitudinally, keeping the muscle origin and insertion site intact. (C) The incision was closed with two interrupted horizontal mattress sutures. (D) To measure the nociceptive threshold, von Frey filaments were applied vertically to the midplantar surface of the incisional hind paw.
2.2. Drug administration
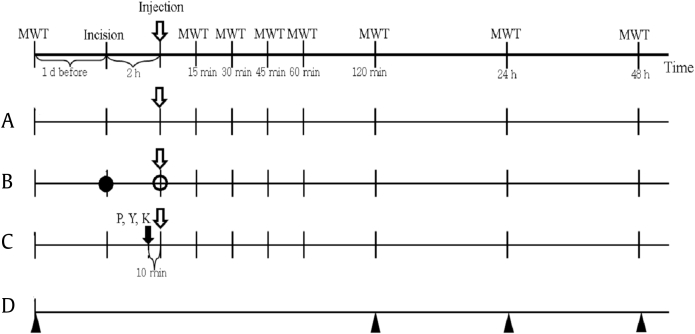
Dose response test Fifty rats were randomly divided into five groups to evaluate the antinociceptive effect of different doses of ginsenoside Rf. Two h after the incision, each rat in the four ginsenoside Rf dosing groups (Rf 0.5, Rf 1, Rf 1.5, and Rf 2) as well as Group C (the control) were injected with ginsenoside Rf (0.5 mg/kg, 1.0 mg/kg, 1.5 mg/kg, or 2.0 mg/kg) or 0.9% saline vehicle, respectively (Fig. 2). Ginsenoside Rf was dissolved in distilled water with an intraperitoneal (IP) injection volume of 10 mL/kg. Each solution was prepared in opaque syringes with sequential number according to a randomization list generated by an investigator who was not involved in any other stages of the study. Random sequence generation was performed using PASS 11 software (NCSS, Kaysville, UT, USA).
Fig. 2.
Experimental protocol. (A) Dose–response test. (B) Positive control and naïve groups. (C) Mechanism test. (D) Blood sampling. Vehicle (0.9% saline) or ginsenoside Rf was intraperitoneally injected 2 h after plantar incision (open arrow,  ). The mechanical withdrawal threshold (MWT) with the von Frey filaments was measured at each time point (vertical lines). The black circle (●) represents the time point when incision was made in the positive control group but not in Naïve group. At the time point indicated by the white circle (○), 30 mg/kg ketorolac was injected in the positive control group, whereas 0.9% saline was administered to the Naïve group. Black arrow (
). The mechanical withdrawal threshold (MWT) with the von Frey filaments was measured at each time point (vertical lines). The black circle (●) represents the time point when incision was made in the positive control group but not in Naïve group. At the time point indicated by the white circle (○), 30 mg/kg ketorolac was injected in the positive control group, whereas 0.9% saline was administered to the Naïve group. Black arrow ( ): time that prazosin (P), yohimbine (Y), or ketanserin (K) were intraperitoneally injected. Black triangle (▲): blood sampling time point.
): time that prazosin (P), yohimbine (Y), or ketanserin (K) were intraperitoneally injected. Black triangle (▲): blood sampling time point.
Positive control and naïve group To assess the validity of the present study, the antinociceptive effect in the dosage (1.5 mg/kg) of ginsenoside Rf was compared with that in a positive control group receiving an analgesic and in the Naïve Group. The Positive Control Group (Group Keto 30, n = 10) was administered the nonsteroidal anti-inflammatory analgesic ketorolac (30 mg/kg IP) [20]. The Naïve group (n = 10) were not incised and were administered 0.9 % saline IP.
Mechanism test for the antinociceptive effect To examine whether the observed effects of ginsenoside Rf on mechanical hyperalgesia induced by plantar incision were mediated by either adrenergic (α1, α2) or serotonergic (5-HT2A) receptors, studies with specific receptor antagonists were utilized. Prazosin (1 mg/kg, α1 adrenergic receptor antagonist, n = 10), yohimbine (2 mg/kg, α2 adrenergic receptor antagonist, n = 10), ketanserin (1 mg/kg 5-HT2A receptor antagonist, n = 10), or saline was administered by IP injection 110 min after a skin incision was made in the hind paw. After 10 min, ginsenoside Rf (1.5 mg/kg) was injected into the peritoneal cavity at a volume of 10 mL/kg (Fig. 2). The drug dosage levels were based on previous studies on antinociceptive mechanisms of action [21], [22], [23], [24].
2.3. Pain behavioral test
The nociceptive threshold was measured using von Frey filaments. The rats were placed individually in a clear Plexiglas cage (21 × 27 × 15 cm) with a plastic grid floor (8 × 8 mm). Mechanical hyperalgesia was assessed via bending force in ascending order (4mN, 9mN, 20mN, 59mN, 78mN, 98mN, 147mN, and 254mN). The von Frey filaments were applied vertically to the midplantar surface of the incisional hind paw for 5 s or until there was a positive response. A positive response was noted when the rats showed a rapid withdrawal or flexion of the stimulated hind paw. The mechanical withdrawal threshold (MWT) was determined via the lowest bending force, and confirmed by additionally applying higher and lower bending forces than the MWT. If a positive response was not observed at 254mN, the bending force was considered the MWT. The MWT measurement was conducted at the following time points after ginsenoside Rf administration: 1 d before the incision (BI); 2 h after plantar incision (AP); 15 min, 30 min, 45 min, 60 min, 80 min, and 120 min; 24 h and 48 h after ginsenoside Rf administration (Fig. 2). All behavioral assessments and animal experiments were carried out by an expert investigator blinded to the groups.
2.4. Measurement of IL-1β, IL-6, and TNF-α levels
Blood samples from the tails of the rats were collected 1 d BI, 120 min, and 24 h and 48 h after administration of ginsenoside Rf or vehicle control (Fig. 2). Blood samples were centrifuged at 1,000 g for 20 min, and the supernatants were stored at −80°C until analysis. IL-1β, IL-6, and TNF-α levels were measured using a commercially available enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA). Briefly, the 96-well plate was coated with each anticytokine capture antibody (Ab). If the related cytokine was present in the supernatant, it bound to the coated capture Ab to form a complex that was detected by the addition of a second detection Ab. Between each step, the plate was washed, and after the final washing, a tetramethylbenzidine substrate solution was added. Generation of a blue color indicated that the targeted cytokine was present in the sample. The optical density was measured at 450 nm using an enzyme-linked immunosorbent assay microplate reader (Molecular Devices, Sunnyvale, CA, USA). After generating a standard curve, the concentration of each cytokine in the sample was calculated in pg/mL.
2.5. Rotarod test
As the pain–behavioral test could be sensitively affected by drug-induced motor dysfunction, the rotarod test was used to exclude motor dysfunction or sedative effects. The rat rotarod treadmill test (Yusunglab, Seoul, Korea) was conducted 1 d BI, and 2 h and 24 h after ginsenoside Rf or saline administration. The rats were individually placed on a cylindrical platform rotating at 12 rev/min, and were allowed to adjust to this environment for 60 s for an accurate assessment. Each trial lasted for 120 s or until the rats fell, and then the rotarod test time was scored.
2.6. Statistical analysis
The primary outcome measure of present study was the MWT stimuli using von Frey filaments. To estimate the group size for the study assessing the antinociceptive activity of ginsenoside Rf (dose–response test), a pilot study was conducted for measuring MWT in six incisional pain model rats (Group C). As the MWT of the pilot study did not pass the Shapiro–Wilk test, the data were analyzed after natural log transformation. The averages of the natural log transformed MWT at BI, AP, postdrug administration time points 15 min, 30 min, 45 min, 60 min, 80 min, 100 min, and 120 min, and 24 h and 48 h were 4.41 ln(mN), 0.96 ln(mN), 0.08 ln(mN), 0.02 ln(mN), 0.02 ln(mN), 0.04 ln(mN), 0.06 ln(mN), 0.12 ln(mN), 0.34 ln(mN), 1.11 ln(mN), and 1.80 ln(mN), respectively. The standard deviations of the natural log transformed MWT ranged from 0.04 to 0.10, and the autocorrelation between adjacent measurements in the same individual was 0.8. For our power calculations, we assumed that a first-order autocorrelation adequately represented the autocorrelation pattern. Furthermore, we wished to detect a 10%, 20%, 30%, and 40% increase in the MWT in groups Rf 0.5, Rf 1.0, Rf 1.5, and Rf 2 compared with Group C. With an α of 0.05 and a power of 80%, it was determined that nine rats per group was required. Considering a 10% follow-up loss, 50 rats were used for the present study.
To explore the antinociceptive mechanism of ginsenoside Rf, a sample size estimation was performed using the results from the ginsenoside Rf antinociceptive effect experiments. The data from Group Rf 1.5 were used because maximum antinociceptive activity was found in this group. The averages of the natural log transformed MWT in Group Rf 1.5 at BI, AP, postdrug administration time points 15 min, 30 min, 45 min, 60 min, 80 min, 100 min, 120 min, and 24 h and 48 h were 4.48 ln(mN), 0.42 ln(mN), 1.91 ln(mN), 2.76 ln(mN), 2.79 ln(mN), 2.73 ln(mN), 2.44 ln(mN), 2.11 ln(mN), 1.53 ln(mN), 1.67 ln(mN), and 1.82 ln(mN) , respectively. The standard deviations of the natural log transformed MWT ranged from 0.02 to 0.07, and an autocorrelation between adjacent measurements in the same individual was 0.7. We wished to detect a 25% and 50% increase in the MWT when ketanserin and prazosin were coadministered, respectively, as well as a 25% decrease in the MWT when yohimbine was coadministered compared with Group Rf 1.5. With an α of 0.05 and a power of 80%, it was determined that nine rats per group were required. Considering a 10% follow-up loss, a total 10 rats per group were utilized. PASS 11 software (NCSS, Kaysville, UT, USA) was used to calculate the sample size.
The Shapiro–Wilk test was used to test for the normality of variables. As the MWT did not pass the Shapiro–Wilk test, a natural log-transformation of the MWT was performed, and the natural log transformed MWT passed the Shapiro–Wilk test. As the IL-1β, IL-6, and TNF-α did not pass the Shapiro–Wilk test, we additionally checked the q–q plot, which did not show marked deviation from linearity. Therefore, the normal assumptions were applied for the repeated measured analysis of variance (ANOVA).
As Mauchly’s sphericity test indicated that the assumption of sphericity had been violated in the dose–response test [χ2(54) = 271.95, p < 0.001, Mauchly’s W = 0.001], the positive control-naive test [χ2(54) = 299.15, p < 0.001, Mauchly’s W = 0.000], the mechanism test [χ2(54) = 169.44, p < 0.001, Mauchly’s W = 0.005], IL-1 β [χ2(5) = 54.68, p < 0.001, Mauchly’s W = 0.310], and IL-6 [χ2(5) = 176.80, p < 0.001, Mauchly’s W = 0.018], the Wilk's Lambda’s multivariate ANOVA (MANOVA) followed by univariate ANOVA with Bonferroni correction was used. Because TNF-α passed the sphericity test [χ2(5) = 2.631, p = 0.757, Mauchly’s W = 0.942], repeated measures ANOVA followed by the Tukey test was used.
Individual measurements were expressed as the mean ± standard error and analyzed with SPSS 23.0 (IBM Corp., Armonk, NY, USA). A p value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of the dose–response of the antinociceptive effect of ginsenoside Rf
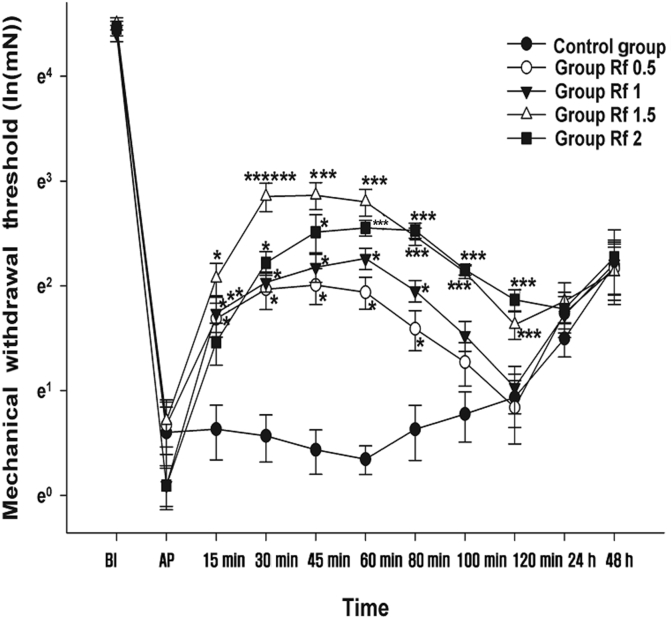
The changes in the MWT measured at BI, AP, and postdrug administration time points 15 min, 30 min, 45 min, 60 min, 80 min, 100 min, and 120 min, and 24 h and 48 h after the IP administration of ginsenoside Rf are shown in Fig. 3. The MANOVA results showed a statistically significant difference between the groups [F(55, 96.162) = 2.263, p < 0.001: Wilk’s lambda = 0.021].
Fig. 3.
Effects of intraperitoneal administration of ginsenoside Rf. Vehicle or ginsenoside Rf was intraperitoneally injected 2 h after plantar incision (AP). Graphs are presented as means (± standard error of the mean) of the mechanical withdrawal threshold with von Frey filaments. BI: 1 d before the plantar incision. AP: 2 h after plantar incision. Control group: 0.9% saline injection. Rf 0.5 group: 0.5 mg/kg injection of ginsenoside Rf. Rf 1 group: 1 mg/kg injection of ginsenoside Rf. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf. Rf 2 group: 2 mg/kg injection of ginsenoside Rf. * p < 0.05 when compared with the Control group.** p < 0.05 when the groups were compared with Rf 0.5 group. *** p < 0.05 when the groups were compared with Rf 1 group. 15–120 min; 15–120 min after injection of ginsenoside Rf. 24–48 h; 24–48 h after injection of ginsenoside Rf. BI, before incision; PI, plantar incision.
The MWT at BI and AP were not significantly different between groups. The MWT at 15 min, 30 min, 45 min, 60 min, and 80 min in Groups Rf 0.5 and Rf 1 significantly increased compared with that in Group C. In Groups Rf 1.5 and Rf 2, there were significant increases in the MWT at 15 min, 30 min, 45 min, 60 min, 80, and 120 min compared with Group C.
Compared with Group Rf 0.5, a significant increase in the MWT was observed at 15 min for Group Rf 1, from 30 min to 120 min for Group Rf 1.5, and from 60 min to 120 min for Group Rf 2. Only the MWT at 30 min for Group Rf 1.5 was significantly different from that of Group Rf 1.
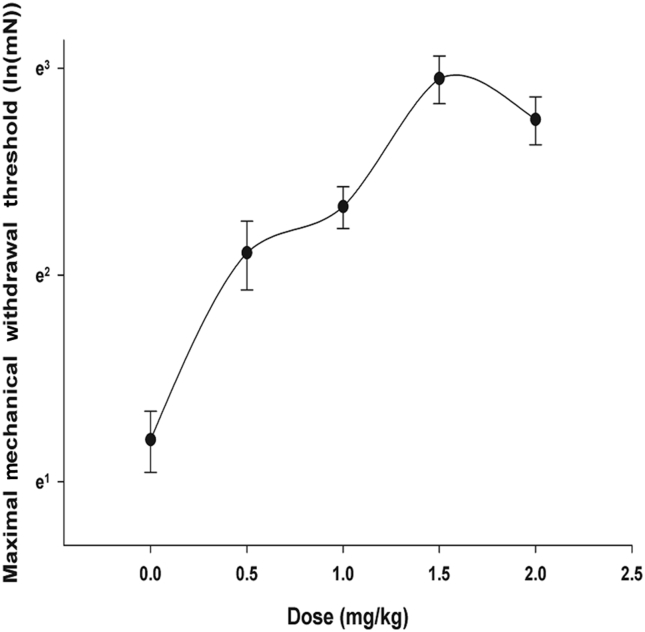
As shown in Fig. 4, the ginsenoside Rf-MWT dose–response curve is curvilinear, peaking at a ginsenoside Rf dosage of 1.5 mg/kg.
Fig. 4.
Dose–response curves in the incisional pain model for the effect of intraperitoneal injection of ginsenoside Rf on mechanical withdrawal threshold. Horizontal and vertical axes are dosage and the natural log transformation of maximum mechanical withdrawal threshold, respectively.
3.2. Comparison of the antinociceptive effect of ginsenoside Rf in positive control and naïve groups
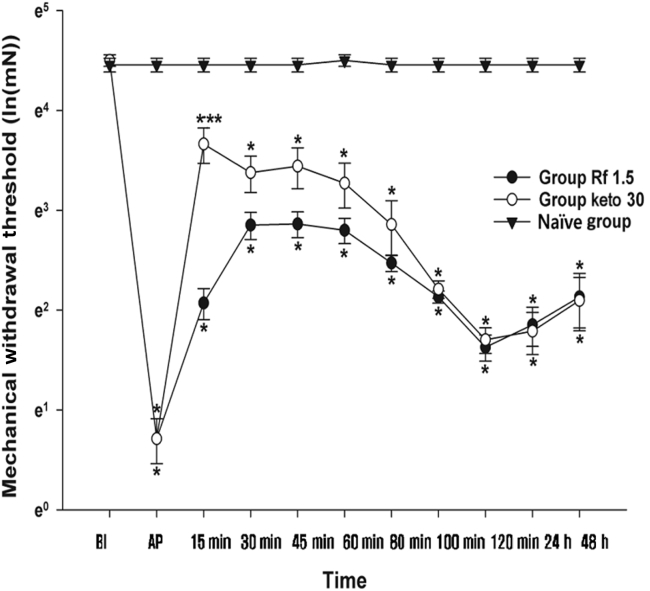
A comparison between the Rf 1.5, Positive Control, and Naïve Groups is shown in Fig. 5. The MWT at BI was not significantly different among the three groups. The MWT in the Positive Control Group, namely group Keto 30, showed a similar pattern to group Rf 1.5. The MWT in the Naïve group was relatively constant and similar to the BI value over the course of experimentation. Compared with the Naïve group, the Rf 1.5 and Keto 30 groups showed significantly different MWTs at all time points after incision. However, a statistically significant difference in the MWT was only observed at 15 min between the Keto 30 and Rf 1.5 groups.
Fig. 5.
Comparison of positive Control group (Group Keto 30) and Naïve group. Vehicle (0.9% saline), ketorolac, and ginsenoside Rf were intraperitoneally injected 2 h after plantar incision (AP) in the Naïve , Keto 30, and Rf 1.5 groups respectively. Graphs are presented as means (± standard error of the mean) of the MWT with von Frey filaments. BI: 1 d before the plantar incision. AP: 2 h after plantar incision. Naïve group: 0.9% saline injection. Keto group 30: 30 mg/kg injection of ketorolac. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf.* p < 0.05 when compared with Naïve group. ** p < 0.05 when the groups were compared with Rf 1.5 group. 15–120 min, 15–120 min after injection of ginsenoside Rf; 24–48 h, 24–48 h after injection; BI, before incision; MWT, mechanical withdrawal threshold; PI, plantar incision.
3.3. Effects of ginsenoside Rf on the rotarod test
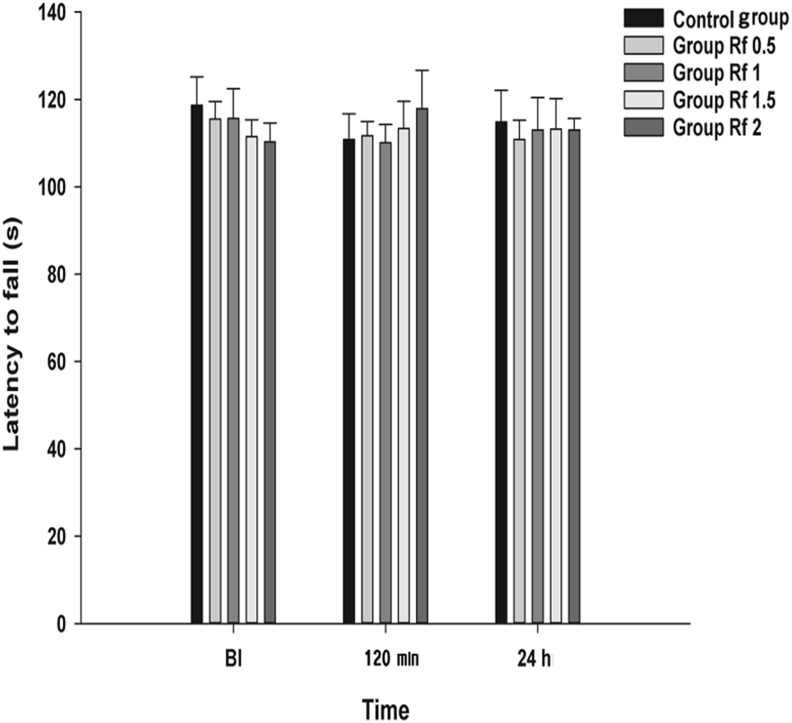
Rotarod performance between the groups was not significantly affected by the IP administration of ginsenoside Rf at BI, 120 min, and 24 h (p = 0.791, 0.893, and 0.994, respectively; Fig. 6).
Fig. 6.
Effects of intraperitoneal administration of ginsenoside Rf on rotarod test. Vehicle (0.9% saline) or ginsenoside Rf was intraperitoneally injected 2 h after plantar incision (AP). The graphs are presented as means (± standard error of the mean) of latency to fall. BI: 1 d before plantar incision. 120 min: 120 min after injection of ginsenoside Rf. 24 h: 24 h after injection of ginsenoside Rf. 48 h: 48 h after injection of ginsenoside Rf. Control group: 0.9% saline injection. Rf 0.5 group: 0.5 mg/kg injection of ginsenoside Rf. Rf 1 group: 1 mg/kg injection of ginsenoside Rf. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf. Rf 2 group: 2 mg/kg injection of ginsenoside Rf. BI, before incision.
3.4. Effects of ginsenoside Rf on inflammatory responses
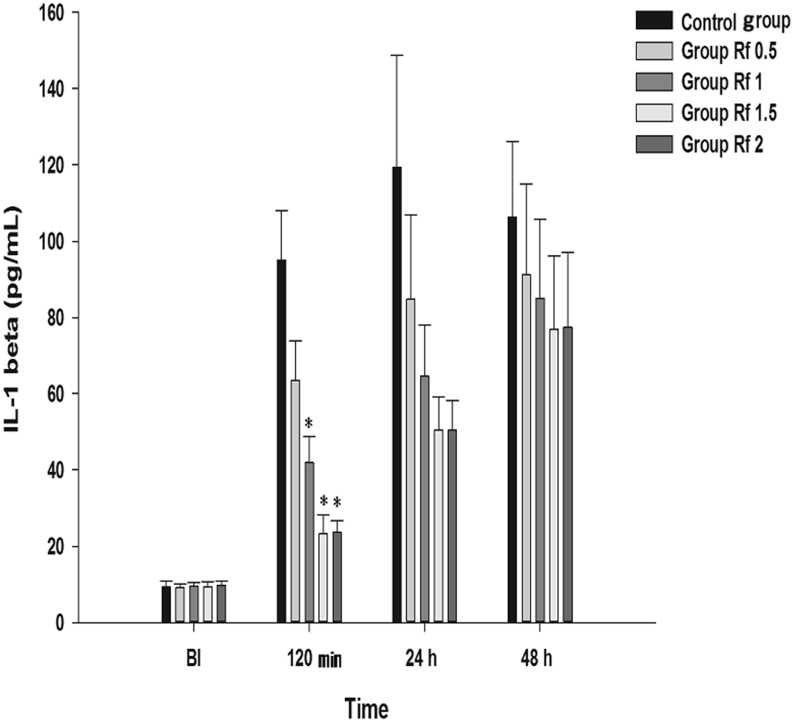
Effect on IL-1β The results of the MANOVA showed a statistically significant difference in the IL-1β levels between the groups [F(4, 45) = 14.051, p < 0.001: Wilk’s lambda = 0.445] (Fig. 7). In the Rf 1, Rf 1.5, and Rf 2 groups, significant decreases were observed at 120 min compared with that in Group C.
Fig. 7.
Effects of intraperitoneally injected ginsenoside Rf on IL-1β. Each bar is presented as the mean ± standard error of the mean. Graphs are presented as means (± standard error of the mean) of IL-1β. BI: 1 d before plantar incision. 120 min: 120 min after injection of ginsenoside Rf. 24 h: 24 h after injection of ginsenoside Rf. 48 h: 48 h after injection of ginsenoside Rf. Control group: 0.9% saline injection. Rf 0.5 group: 0.5 mg/kg injection of ginsenoside Rf. Rf 1 group: 1 mg/kg injection of ginsenoside Rf. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf. Rf 2 group: 2 mg/kg injection of ginsenoside Rf. * p < 0.05 when compared with Control group. BI, before incision; IL-1β, interleukin-1β.
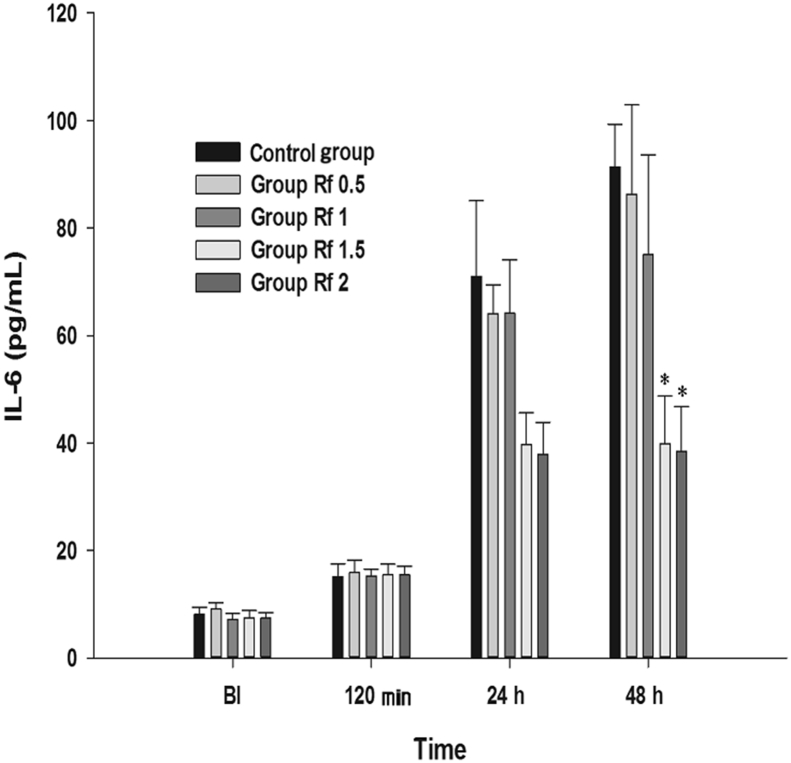
Effect on IL-6 The results of the MANOVA showed a statistically significant difference in the IL-6 levels between the groups [F(4, 45) = 6.615, p < 0.001: Wilk’s lambda = 0.630] (Fig. 8). In the Rf 1.5 and Rf 2 groups, significant decreases were observed at 48 h compared with that in group C.
Fig. 8.
Effects of intraperitoneally injected ginsenoside Rf on IL-6. Graphs are presented as means (± standard error of the mean) of IL-6. BI: 1 d before plantar incision. 120 m: 120 min after injection of ginsenoside Rf. 24 h: 24 h after injection of ginsenoside Rf. 48 h: 48 h after injection of ginsenoside Rf. Control group: 0.9% saline injection. Rf 0.5 group: 0.5 mg/kg injection of ginsenoside Rf. Rf 1 group: 1 mg/kg injection of ginsenoside Rf. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf. Rf 2 group: 2 mg/kg injection of ginsenoside Rf. * p < 0.05 when compared with Control group. BI, before incision; IL-6 = interleukin-6.
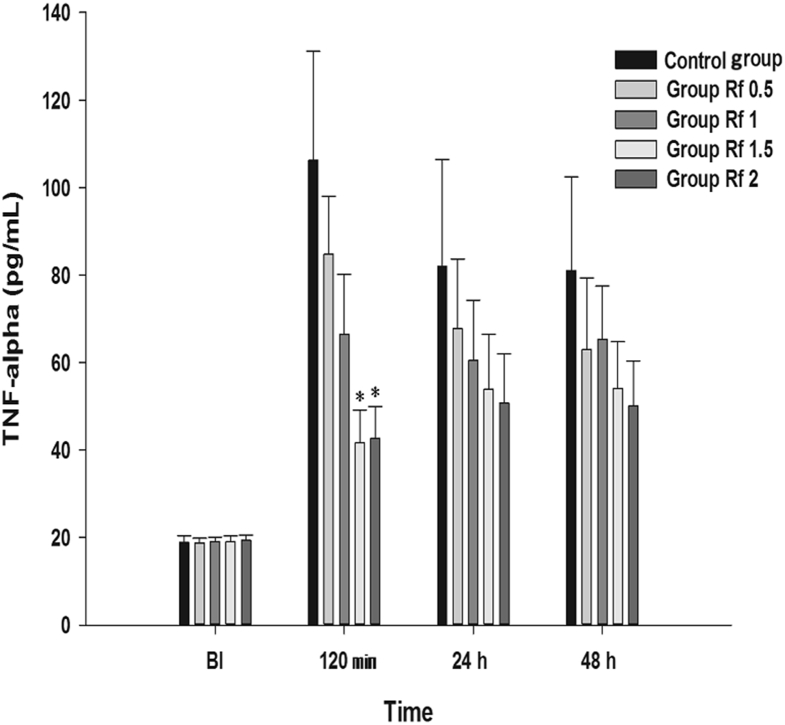
Effect on TNF-α In the Rf 1.5 and Rf 2 groups, significant decreases were observed in the TNF-α levels at 120 min compared with that in Group C (Fig. 9).
Fig. 9.
Effects of intraperitoneally injected ginsenoside Rf on TNF-α. Graphs are presented as means (± standard error of the mean) of TNF-α. BI: 1 d before plantar incision. 120 m: 120 min after injection of ginsenoside Rf. 24 h: 24 h after injection of ginsenoside Rf. 48 h: 48 h after injection of ginsenoside Rf. Control group: 0.9% saline injection. Rf 0.5 group: 0.5 mg/kg injection of ginsenoside Rf. Rf 1 group: 1 mg/kg injection of ginsenoside Rf. Rf 1.5 group: 1.5 mg/kg injection of ginsenoside Rf. Rf 2 group: 2 mg/kg injection of ginsenoside Rf. * p < 0.05 when compared with Control group. BI, before incision; TNF-α, tumor necrotizing factor-α.
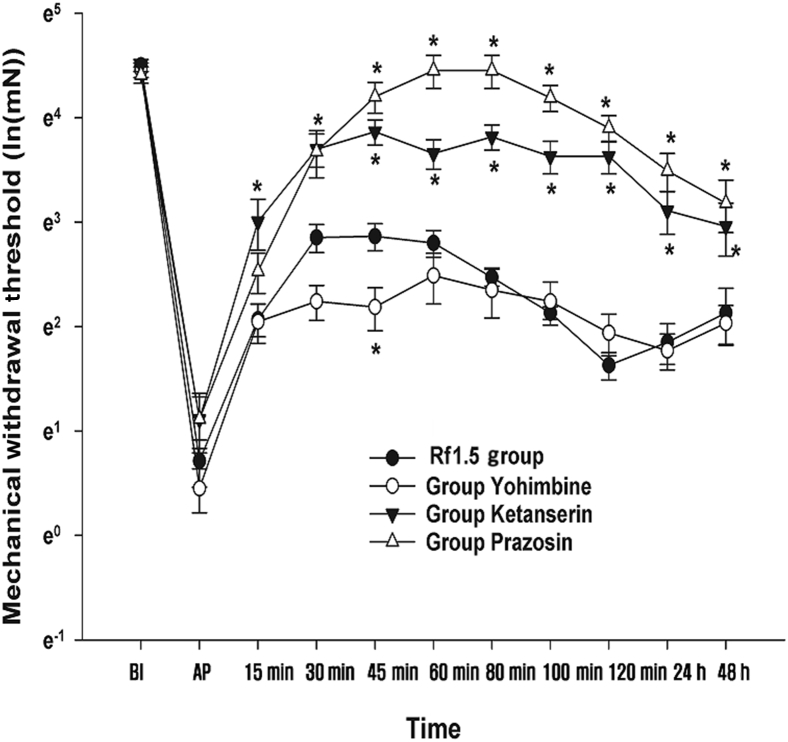
Effect of prazosin, yohimbine, and ketanserin on the antinociceptive effect of ginsenoside Rf The results of the MANOVA showed a statistically significant difference in the antagonism of the adrenergic and serotonergic receptors between the groups [F(66, 107.12) = 1.792, p < 0.001: Wilk’s lambda = 0.019] (Fig. 10). The MWT in the group Prazosin was significantly greater than the MWT in the group Rf 1.5, except at 15 min and 30 min postadministration of 1.5 mg/kg ginsenoside Rf IP. However, in the Yohimbine group, the MWT at 45 min postginsenoside Rf administration was significantly less than the MWT at the same time point in the group Rf 1.5. In the Ketanserin group, the MWTs at all time points after ginsenoside Rf administration were significantly higher than the MWT in the group Rf 1.5.
Fig. 10.
Effects of yohimbine, ketanserin, and prazosin on the antinociception of ginsenoside Rf. Ginsenoside Rf (1.5 mg/kg) was intraperitoneally administered 2 h after plantar incision (AP). Yohimbine, prazosin, and ketanserin were administered 10 min before the injection of ginsenoside Rf. The mechanical withdrawal threshold was measured at predetermined time points. The natural log transformation of the mechanical withdrawal threshold was plotted on the vertical axis. BI: 1 d before plantar incision. AP: 2 h after plantar incision. Rf 1.5 group : 1.5 mg/kg injection of ginsenoside Rf. Yohimbine group: 2 mg/kg injection of yohimbine. Ketanserin group: 1 mg/kg injection of ketanserin. Prazosin group: 1 mg/kg injection of prazosin. 15–120 m: 15–120 min after injection of ginsenoside Rf. 24–48 h: 24–48 h after injection of ginsenoside Rf. BI, before incision; PI, plantar incision.
4. Discussion
In the present study, the antinociceptive effect of ginsenoside Rf increased in a dose-dependent manner in the rat incisional pain model up to a dosage of 1.5 mg/kg, though the effect was not linear with dosage. With the exception of the 15-min time point, the ginsenoside Rf antinociceptive effect at 1.5 mg/kg was comparable to that of ketorolac, and its effect persisted 2 h after ginsenoside Rf administration, highlighting its antinociceptive properties.
Complications such as sedative effect or mortality were not observed during the behavioral test, the rotarod performance test, or during any other observational period.
Ginsenosides are a varied group of four-ring steroidal saponins concentrated mainly in the roots of ginseng plants. They are mainly divided into the panaxadiol (e.g., Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, and Rh1) and panaxatriol groups (e.g., Re, Rf, Rg1, Rg2, and Rh1) [25]. Previous studies have demonstrated the antinociceptive and anti-inflammatory effects of ginsenosides in various animal models. For example, ginsenosides Rc, Rd, and Re showed analgesic effects against pain induced by acetic acid and formalin [26]. Ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rf supraspinally or spinally injected were also found to exert an antinociceptive effect on substance P-induced pain [27]. Additionally, anti-inflammatory effects have been shown by ginsenosides Rb2, Rd, and Rg1 via inhibition of an increase in IL-6 [28], and by ginsenosides Re via reduction of TNF-α and IL-1β release [29].
Ginsenoside Rf belongs to the panaxadiol group of ginsenosides, and is especially abundant in Panax ginseng Meyer among all the Panax plants [25]. Although it is a trace extract component of ginseng, it has been shown to possess an antinociceptive effect [4], [5] in the substance P-induced pain model [27], the abdominal constriction test, and the tonic phase of the formalin test [4]. However, systemic administration of ginsenoside Rf failed to demonstrate an antinociceptive effect in the acute phase of the formalin test, the thermal tail-flick test, and the hot-plate test [4]. Furthermore, intracerebroventricular injection of ginsenoside Rf did not attenuate the nociceptive behavior induced by an intrathecal injection of substance P [27]. There has been a great deal of controversy surrounding the antinociceptive effect of ginsenoside Rf based on the results of various pain models. To date, no study has investigated its effect on the rat incisional pain model. Thus, we aimed to investigate whether systemic ginsenoside Rf administration has an antinociceptive effect on postoperative pain by using the rat incisional pain model.
In the present study, the peak antinociceptive effect of ginsenoside Rf was observed between 30 min and 60 min after administration, with the effect lasting for 2 h. These results are consistent with a previous study that showed the peak concentration of ginsenosides was achieved within 2 h [30]. Although the duration of antinociceptive effect is not >2 h, systemic ginsenoside Rf administration could play an important role in acute postoperative pain management, because mechanical hyperalgesia is usually most intense immediately after surgery [13], and preemptive analgesia could reduce hyperalgesia and allodynia after surgery [31].
We also found in this study that ginsenoside Rf’s antinociceptive effect was comparable to that of the positive control group (group Keto 30) except at the 15-min time point after incision, although ginsenoside Rf did not return the MWT to that of the Naïve Group. Therefore, we propose that ginsenoside Rf could be an alternative to other currently used analgesic agents.
Ginseng total saponin showed dose-dependent, incremental antihyperalgesic effects in the incisional pain model [3]. However, in the present study, ginsenoside Rf-MWT had a curvilinear-shaped dose–response relationship, similar to pregabalin, buprenorphine, and haloperidol, the effects of which were more pronounced at medium rather than high dosages [32], [33], [34]. The peak effective dose of ginsenoside Rf in the present study was 1.5 mg/kg, and achieved a significant antinociceptive effect.
There are several possible explanations as to why ginsenoside Rf had antagonistic effects in dosages >1.5 mg/kg. A metabolite of ginsenoside Rf may induce an antihyperalgesic effect at higher dosages. Alternatively, ginsenoside Rf may have a negative interaction with its metabolite. To investigate these mechanisms, future studies should examine ginsenoside Rf metabolites and investigate the effects of these metabolites on ginsenoside Rf. Comparison of the effects of different components and their interactions may also be needed.
The peak antinociceptive dosage of ginsenoside Rf (1.5 mg/kg) observed in the present study was much lower than the dosage causing hemolysis (1,340 mg/kg IP) [2]. The therapeutic index for antinociception in the abdominal constriction test was also shown to be >20 [4]. Taken together, these results indicate that the use of ginsenoside Rf at a dosage with an antinociceptive effect may be well within the safety window of the compound.
Proinflammatory cytokines such as IL-1β, IL-6, and TNF-α were increased in the postoperative period [35], and by themselves induced more intense pain and vice versa [36]. IL-1β is known to induce upregulation of cyclo-oxygenase-2 [37] and increase substance P [38], which contributes to postoperative hyperalgesia. IL-1β, and IL-6 are also associated with the development of allodynia and hyperalgesia in the mono-neuropathic pain model [18]. TNF-α can induce neuromodulation in the pain signal pathway and promote the development of neuropathic pain in the chronic constriction pain injury model [16].
In the present study, IP injection of ginsenoside Rf significantly suppressed the production of IL-6, IL-1β, and TNF-α in a dose-dependent manner. These results demonstrated that ginsenoside Rf shows an anti-inflammatory effect by reducing the production of proinflammatory cytokines. Furthermore, it has been recently reported that ginsenoside Rf shows anti-inflammatory effect through suppression of the nuclear factor-kappa B pathway, which has a critical role in the induction of proinflammatory cytokines [39], [40]. Of note, intracerebroventricular-injected ginsenosides attenuate nociceptive behavior induced by IL-1β and TNF-α [41]; thus, we can deduce that the anti-inflammatory effect of ginsenoside Rf contributed to its antinociceptive effect, and that ginsenoside Rf could be used as an adjuvant analgesic.
A lower dosage of ginsenoside Rf was not found to significantly decrease the inflammatory cytokines in the present study. These results are consistent with those of a previous study in which IP injection of ginsenoside Rf (0.1–1 mg/kg) was ineffective in an immobilization stress model in reducing plasma IL-6 levels [28]. It is possible that the lower dosage of ginsenoside Rf did not show an anti-inflammatory effect because of its inability to achieve sufficient plasma concentrations of ginsenoside Rf.
The adrenergic receptor is located on Aδ- and C-fibers [42], which are involved in inducing hyperalgesia after an incision [43], [44], [45]. Therefore, we investigated the antinociceptive mechanism of ginsenoside Rf by using an α-1 adrenergic receptor antagonist (prazosin) and an α-2 adrenergic receptor antagonist (yohimbine). The results in the present study showed different outcomes for the two adrenergic receptor antagonists. The antinociceptive effect of ginsenoside Rf was significantly potentiated by prazosin, while yohimbine seemed to inhibit its effect. By contrast, several other studies did not show potentiation by prazosin, whereas some studies were in agreement with our yohimbine results.
Intrathecal injection of α-1 and -2 adrenergic receptor antagonists reversed the antinociceptive effect of intrathecal administration of total ginsenosides in the formalin test [10] and in the incisional pain model [9]. In the formalin test, the α-2 A, B, and C adrenergic receptor antagonists attenuated the antinociception of intrathecal total ginsenosides [46]. The type of ginsenosides, administration route, dosage, and type of pain model might play a role in this discrepancy.
5-HT is known to be involved in modulating nociceptive transmission via both inhibitory and facilitatory signaling pathways. One study revealed that 5-HT2 receptors mediated membrane depolarization and pronociceptive effects [47]. However, in the present study, ketanserin, a 5-HT2 receptor antagonist, potentiated the antinociceptive effect of ginsenoside Rf. Ketanserin may have an antinociceptive effect itself or potentiate analgesia by inhibiting the 5-HT2A receptor [48]. However, we did not evaluate ketanserin treatment alone in the present study. Consequently, it remains an open question whether the result was due to an additive or synergistic effect between ketanserin and ginsenoside Rf.
The present study has several limitations: (1) only mechanical hyperalgesia was assessed in the investigation of the antinociceptive effect of ginsenoside Rf; (2) the behavioral assessment was measured frequently, such that full recovery from an earlier test may not be achieved prior to a follow-up test. Although there was at least a 15-min interval between each MWT assessment, an earlier MWT measurement could still affect the outcome of a subsequent MWT measurement. However, the time between von Frey filament tests was > 10 min and was enough for rats to adapt to the environment; and (3) specific comparative groups using prazosin, yohimbine, and ketanserin alone were not included in the present study. Therefore, the mechanism of action behind ginsenoside Rf’s antinociceptive effect cannot be conclusively determined. Nevertheless, the present study indicates that the use of ginsenoside Rf in combination with other drugs such as prazosin and ketanserin may be more effective in achieving antinociception.
Ginsenoside Rf showed an antinociceptive effect in a rat incisional pain model. There was a curvilinear dose–response relationship for ginsenoside Rf and MWT, peaking at a dose of 1.5 mg/kg. Ginsenoside Rf also showed an anti-inflammatory effect.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2015R1A2A2A01005153).
References
- 1.Liu C.X., Xiao P.G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 2.Kaku T., Miyata T., Uruno T., Sako I., Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975;25:539–547. [PubMed] [Google Scholar]
- 3.Kim W.J., Kang H., Choi G.J., Shin H.Y., Baek C.W., Jung Y.H., Woo Y.C., Kim J.Y., Yon J.H. Antihyperalgesic effects of ginseng total saponins in a rat model of incisional pain. J Surg Res. 2014;187:169–175. doi: 10.1016/j.jss.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Mogil J.S., Shin Y.H., McCleskey E.W., Kim S.C., Nah S.Y. Ginsenoside Rf, a trace component of ginseng root, produces antinociception in mice. Brain Res. 1998;792:218–228. doi: 10.1016/s0006-8993(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 5.Nemmani K.V., Ramarao P. Ginsenoside Rf potentiates U-50,488H-induced analgesia and inhibits tolerance to its analgesia in mice. Life Sci. 2003;72:759–768. doi: 10.1016/s0024-3205(02)02333-0. [DOI] [PubMed] [Google Scholar]
- 6.Shin D.J., Yoon M.H., Lee H.G., Kim W.M., Park B.Y., Kim Y.O., Huang L.J., Cui J.H. The effect of treatment with intrathecal ginsenosides in a rat model of postoperative pain. Korean J Pain. 2007;20:100–105. [Google Scholar]
- 7.Kim W.J., Kang H., Kim J.E., Choi G.J., Shin H.Y., Baek C.W., Jung Y.H., Woo Y.C., Kim S.H., Lee J.H. Effect of intraperitoneal administered ginseng total saponins on hyperalgesia induced by repeated intramuscular injection of acidic saline in rats. J Med Food. 2014;17:657–662. doi: 10.1089/jmf.2013.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Ju N., Seok C., Yoon Hee K., Seok Chang K., Ki Yeul N., Jong Keun K., Seung Yeol N. Effect of spinally administered ginseng total saponin on capsaicin-induced pain and excitatory amino acid-induced nocicpetive responses. J Ginseng Res. 1999;23:38–43. [Google Scholar]
- 9.Kim I.J., Park C.H., Lee S.H., Yoon M.H. The role of spinal adrenergic receptors on the antinociception of ginsenosides in a rat postoperative pain model. Korean J Anesthesiol. 2013;65:55–60. doi: 10.4097/kjae.2013.65.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S.Y., Yoon M.H., Lee H.G., Kim W.M., Lee J.D., Kim Y.O., Huang L.J., Cui J.H. The role of adrenergic and cholinergic receptors on the antinociception of Korean Red Ginseng in the spinal cord of rats. Korean J Pain. 2008;21:27–32. [Google Scholar]
- 11.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 12.Nah S.Y., Park H.J., McCleskey E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc Natl Acad Sci U S A. 1995;92 doi: 10.1073/pnas.92.19.8739. 8,739–8,743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan T.J., Vandermeulen E.P., Gebhart G.F. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 14.Woolf C.J., Chong M.S. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi M., Martucci C., Ferrario P., Franchi S., Sacerdote P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugs. Anesth Analg. 2007;104:949–954. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- 16.Covey W.C., Ignatowski T.A., Renauld A.E., Knight P.R., Nader N.D., Spengler R.N. Expression of neuron-associated tumor necrosis factor alpha in the brain is increased during persistent pain. Reg Anesth Pain Med. 2002;27:357–366. doi: 10.1053/rapm.2002.31930. [DOI] [PubMed] [Google Scholar]
- 17.Beilin B., Shavit Y., Trabekin E., Mordashev B., Mayburd E., Zeidel A., Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822–827. doi: 10.1213/01.ANE.0000078586.82810.3B. [DOI] [PubMed] [Google Scholar]
- 18.Cui J.G., Holmin S., Mathiesen T., Meyerson B.A., Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 19.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1:94–99. doi: 10.4103/0976-500X.72351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim T.K., Kim Y.S., Yoon J.R., Han I.S., Kim J.S., Lee C.W. The effect of an intraperitoneal injection of ketamine and ketorolac on mechanical allodynia in rats with spinal nerve ligation. Korean J Anesthesiol. 2004;46:719–723. [Google Scholar]
- 21.Micov A., Tomic M., Popovic B., Stepanovic-Petrovic R. The antihyperalgesic effect of levetiracetam in an inflammatory model of pain in rats: mechanism of action. Br J Pharmacol. 2010;161:384–392. doi: 10.1111/j.1476-5381.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinardi G., Sierralta F., Miranda H.F. Adrenergic mechanisms in antinociceptive effects of non steroidal antiinflammatory drugs in acute thermal nociception in mice. Inflamm Res. 2002;51:219–222. doi: 10.1007/pl00000296. [DOI] [PubMed] [Google Scholar]
- 23.Reboucas E.C., Segato E.N., Kishi R., Freitas R.L., Savoldi M., Morato S., Coimbra N.C. Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology (Berl) 2005;179:349–355. doi: 10.1007/s00213-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 24.Yokogawa F., Kiuchi Y., Ishikawa Y., Otsuka N., Masuda Y., Oguchi K., Hosoyamada A. An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg. 2002;95:163–168. doi: 10.1097/00000539-200207000-00029. [DOI] [PubMed] [Google Scholar]
- 25.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin Y.H., Jung O.M., Nah J.J., Nam K.Y., Kim C.Y., Nah S.Y. Ginsenosides that produce differential antinociception in mice. Gen Pharmacol. 1999;32:653–659. doi: 10.1016/s0306-3623(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.-S., Han E.-J., Han K.-J., Lee H.-K., Suh H.-W. Antinociceptive effects of ginsenosides injected intracerebroventricularly or intrathecally in Substance P-Induced pain model. Planta Med. 2003;69 doi: 10.1055/s-2003-45145. 1,001–4. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.H., Moon Y.S., Lee T.H., Jung J.S., Suh H.W., Song D.K. The inhibitory effect of ginseng saponins on the stress-induced plasma interleukin-6 level in mice. Neurosci Lett. 2003;353:13–16. doi: 10.1016/j.neulet.2003.08.070. [DOI] [PubMed] [Google Scholar]
- 29.Paul S., Shin H.S., Kang S.C. Inhibition of inflammations and macrophage activation by ginsenoside-Re isolated from Korean ginseng (Panax ginseng C.A. Meyer) Food Chem Toxicol. 2012;50 doi: 10.1016/j.fct.2012.02.035. 1,354–61. [DOI] [PubMed] [Google Scholar]
- 30.Li L., Sheng Y.X., Zhang J.L., Wang S.S., Guo D.A. High-performance liquid chromatographic assay for the active saponins from Panax notoginseng in rat tissues. Biomed Chromatogr. 2006;20:327–335. doi: 10.1002/bmc.567. [DOI] [PubMed] [Google Scholar]
- 31.Wilder-Smith O.H. Preemptive analgesia and surgical pain. Prog Brain Res. 2000;129:505–524. doi: 10.1016/S0079-6123(00)29037-7. [DOI] [PubMed] [Google Scholar]
- 32.Boschen M.J. Pregabalin: dose–response relationship in generalized anxiety disorder. Pharmacopsychiatry. 2012;45:51–56. doi: 10.1055/s-0031-1291176. [DOI] [PubMed] [Google Scholar]
- 33.Cowan A., Lewis J.W., Macfarlane I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palao D.J., Arauxo A., Brunet M., Bernardo M., Haro J.M., Ferrer J., Gonzalez-Monclus E. Haloperidol: therapeutic window in schizophrenia. J Clin Psychopharmacol. 1994;14:303–310. [PubMed] [Google Scholar]
- 35.Bryan D., Walker K.B., Ferguson M., Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Salo M. Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol Scand. 1992;36:201–220. doi: 10.1111/j.1399-6576.1992.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 37.Samad T.A., Moore K.A., Sapirstein A., Billet S., Allchorne A., Poole S., Bonventre J.V., Woolf C.J. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 38.Perretti M., Ahluwalia A., Flower R.J., Manzini S. Endogenous tachykinins play a role in IL-1-induced neutrophil accumulation: involvement of NK-1 receptors. Immunology. 1993;80:73–77. [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn S., Siddiqi M.H., Aceituno V.C., Simu S.Y., Yang D.C. Suppression of MAPKs/NF-kappaB activation induces intestinal antiinflammatory action of ginsenoside Rf in HT-29 and RAW264.7 cells. Immunol Invest. 2016;45:439–449. doi: 10.3109/08820139.2016.1168830. [DOI] [PubMed] [Google Scholar]
- 40.Li P., Lv B., Jiang X., Wang T., Ma X., Chang N., Wang X., Gao X. Identification of NF-kappaB inhibitors following Shenfu injection and bioactivity-integrated UPLC/Q-TOF-MS and screening for related antiinflammatory targets in vitro and in silico. J Ethnopharmacol. 2016;194:658–667. doi: 10.1016/j.jep.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 41.Seo Y.J., Kwon M.S., Choi H.W., Jang J.E., Lee J.K., Sun Y., Jung J.S., Park S.H., Suh H.W. Intracerebroventricular ginsenosides are antinociceptive in proinflammatory cytokine-induced pain behaviors of mice. Arch Pharm Res. 2008;31:364–369. doi: 10.1007/s12272-001-1165-x. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki Y., Kumamoto E., Furue H., Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Pogatzki E.M., Gebhart G.F., Brennan T.J. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw 1 d after an incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 44.Vandermeulen E.P., Brennan T.J. Alterations in ascending dorsal horn neurons by a surgical incision in the rat foot. Anesthesiology. 2000;93 doi: 10.1097/00000542-200011000-00024. 1,294–1,302; discussion 6A. [DOI] [PubMed] [Google Scholar]
- 45.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Yoon M.H., Huang L.J., Choi J.I., Lee H.G., Kim W.M., Kim C.M. Antinociceptive effect of intrathecal ginsenosides through alpha-2 adrenoceptors in the formalin test of rats. Br J Anaesth. 2011;106:371–379. doi: 10.1093/bja/aeq367. [DOI] [PubMed] [Google Scholar]
- 47.Millan M.J. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 48.Huang J., Cai Q., Chen Y., Hong Y. Treatment with ketanserin produces opioid-mediated hypoalgesia in the late phase of carrageenan-induced inflammatory hyperalgesia in rats. Brain Res. 2009;1303:39–47. doi: 10.1016/j.brainres.2009.09.072. [DOI] [PubMed] [Google Scholar]