ABSTRACT
For the rapid detection of carbapenemase-producing Enterobacteriaceae (CPE), immunochromatographic lateral flow tests (ICT) have recently been developed. The aim of this study was to assess the new multiplex ICT Resist-3 O.K.N. and to investigate if it can be performed directly from susceptibility testing plates. Additionally, the impact of the inoculum and carbapenem disks on sensitivity and specificity was evaluated. The new ICT was challenged using 63 carbapenem-resistant Enterobacteriaceae (CRE) isolates, including 51 carbapenemase producers. It was assessed under five different conditions directly from Mueller-Hinton agar (MHA): 1 μl or 10 μl of inoculum harvested in the absence of antibiotic pressure or 1 μl taken from the inhibition zone of either an ertapenem, imipenem, or meropenem disk. The sensitivity of the ICT was 100% for OXA-48-like and KPC carbapenemases and 94.4% for the NDM carbapenemase with the 1-μl inoculum. When harvested adjacent to a carbapenem disk, the sensitivity increased to 100%. Additionally, with zinc-supplemented MHA, both the sensitivity increased and the NDM band became visible faster (mean time, 8 ± 3.9 min for MHA compared to 1.9 ± 1.5 min for MHA plus zinc; P = 0.0016). The specificity of the ICT was 100%. The Resist-3 O.K.N. ICT is a sensitive and rapid test for the detection of three highly prevalent carbapenemases. However, false-negative results for NDM can occur. We recommend an inoculum of 1 μl that is harvested adjacent to an ertapenem or meropenem disk and the use of agars with sufficient zinc content to achieve the best performance.
KEYWORDS: carbapenemase, immunochromatographic assay, Mueller-Hinton agar, Enterobacteriaceae, New Delhi metallo-beta-lactamase, immunochromatographic assay, KPC, NDM, OXA-48, zinc
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae (CPE) are an important threat to global public health, which seriously compromise antibiotic treatment options in severe infections. OXA-48, KPC, VIM, and NDM are the most important carbapenemases worldwide, with great differences in prevalences in different regions (1). The phenotypic detection of carbapenemases can be difficult using standard methods, as there is a considerable variation in the MICs for carbapenems. The highest MICs are usually recorded for ertapenem, with meropenem and/or imipenem MICs that are lower and sometimes even in the susceptible range (≤1 mg/liter [CLSI] and ≤2 mg/liter [EUCAST]). Reliable phenotypic tests are available only for some carbapenemases and usually require 2 to 24 h (2). Molecular detection of carbapenemases by PCR or next-generation sequencing is considered to be the gold standard but is costly and not available in all laboratories. Highly sensitive and specific immunochromatographic lateral flow tests (ICT) have recently been developed, initially for the detection of OXA-48, and are now also available for KPC- and NDM-type carbapenemases (3, 4). These assays detect carbapenemase-specific epitopes, are very rapid (∼15 min), and have demonstrated excellent sensitivity and specificity across different carbapenemase variants and bacterial species (4–6). ICTs have the potential to considerably improve both quality and time of detection for the routine microbiology laboratory. Currently, there are little data on their sensitivity and specificity and no data on the impact of different culture media, inocula, or antibiotic disks on test performance.
In the present study, we evaluated a new multiplex ICT and assessed the influence of inoculum size, agar, and carbapenem disk on the sensitivity and specificity.
The Resist-3 O.K.N. assay for Enterobacteriaceae (Coris BioConcept, Gembloux, Belgium) is a new multiplex ICT based on monoclonal antibodies against carbapenemases of the OXA-48-like, KPC, and NDM types. According to the manufacturer's instructions, single colonies should be used, but no information is given on suitable media. Frequently, the ICT is performed as a confirmatory test after susceptibility testing has already been done. In these cases, the ICT is not performed from isolated colonies but directly from susceptibility testing plates, usually Mueller-Hinton agar (MHA). Currently, there are no data on the performance of the ICT from confluent growth on MHA, nor has the exact amount necessary for testing been systematically assessed. Additionally, it is unclear to what extent the antibiotic disks present on MHA have an influence on the results of the ICT. The aim of the present study was hence to investigate the performance of the new multiplex ICT directly from MHA and to assess if carbapenem disks, culture media, and inocula have an impact on sensitivity, specificity, and time to detection.
MATERIALS AND METHODS
Clinical isolates.
A total of 51 Enterobacteriaceae clinical isolates harboring different carbapenemases and 12 Enterobacteriaceae clinical isolates that were carbapenem resistant but carbapenemase negative were included in the study (Table 1). Carbapenemase-positive isolates expressed OXA-48-like (n = 23), KPC (n = 10), or NDM (n = 16) carbapenemase; two isolates were positive for OXA-48-like and NDM carbapenemases. Carbapenemase-negative isolates were resistant to ertapenem with MICs of ≥8 mg/liter, with varied MICs for imipenem and meropenem. The isolates had been previously characterized as part of other studies (6–8) or were obtained from the routine microbiology laboratory of the University Hospital Cologne and the University Hospital Frankfurt. CPE isolates expressed OXA-48-like (n = 23), KPC (n = 10), or NDM (n = 16) enzymes and belonged to six different species, with Klebsiella pneumoniae being the most frequent (n = 34), followed by Escherichia coli (n = 10) and Enterobacter cloacae (n = 5).
TABLE 1.
Isolates included in the study
| Carbapenemase production | No. of isolates |
MIC range (mg/liter) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae | E. coli | E. cloacae | E. aerogenes | P. mirabilis | R. ornithinolytica | All species | Ertapenem | Imipenem | Meropenem | |
| Carbapenemase positive | 34 | 10 | 5 | 1 | 1 | 51 | 0.12 to >32 | 0.5 to >32 | 0.12 to >32 | |
| OXA-48-like | 16 | 6 | 1 | 23 | 1 to >32 | 0.5 to >32 | 0.12 to >32 | |||
| OXA-48 | 12 | 1 | 1 | 14 | 1 to >32 | 0.5 to 8 | 0.12 to 16 | |||
| OXA-162 | 2 | 2 | 2 to >32 | 1 to 8 | 0.5 to >32 | |||||
| OXA-181 | 2 | 2 | 2 | 0.5 | 0.25 to 0.5 | |||||
| OXA-204 | 1 | 1 | >32 | 8 | >32 | |||||
| OXA-232 | 2 | 2 | 2 to 4 | 0.5 | 0.25 | |||||
| OXA-244 | 1 | 1 | 2 | 2 to 32 | 0.5 to 32 | 0.25 to 32 | ||||
| KPC | 10 | 10 | 16 to >32 | 32 to >32 | 32 to >32 | |||||
| KPC-2 | 9 | 9 | 16 to >32 | 32 to >32 | 32 to >32 | |||||
| KPC-3 | 1 | 1 | >32 | >32 | >32 | |||||
| NDM | 7 | 3 | 4 | 1 | 1 | 16 | 0.12 to >32 | 4 to >32 | 1 to >32 | |
| NDM-1 | 7 | 1 | 4 | 1 | 1 | 14 | 0.12 to >32 | 4 to >32 | 1 to >32 | |
| NDM-5 | 1 | 1 | >32 | >32 | >32 | |||||
| NDM-7 | 1 | 1 | >32 | >32 | >32 | |||||
| NDM-1/OXA-232 | 1 | 1 | >32 | >32 | >32 | |||||
| NDM-5/OXA-181 | 1 | 1 | 8 | >32 | >32 | |||||
| Carbapenemase negative | 4 | 3 | 3 | 2 | 12 | 8 to 32 | 0.5 to 16 | 1 to 4 | ||
| Total | 38 | 13 | 8 | 2 | 1 | 1 | 63 | |||
All strains were analyzed for the presence of the blaOXA-48-like, blaVIM, blaIMP, blaNDM, blaGIM, and blaKPC carbapenemase genes by PCR and subsequent DNA Sanger sequencing, as previously reported (6, 8–11). The MICs of ertapenem, meropenem, imipenem, and doripenem were determined using MIC test strips (Liofilchem, Roseto degli Abruzzi, Italy). For phenotypic identification of metallo-β-lactamases, KPC, or AmpC, a combination disk test was used with meropenem and one of the following β-lactamase inhibitors: EDTA, cloxacillin, or boronic acid (Liofilchem). The results were interpreted according to the manufacturer's recommendations.
Immunochromatographic test.
All isolates were tested with the ICT under five different test conditions. A bacterial suspension equivalent to 0.5 McFarland was inoculated on Mueller-Hinton II (Oxoid, Wesel, Germany) and ertapenem, imipenem, and meropenem disks (Oxoid) were placed on the agar. The plates were incubated for 18 h at 37°C. After incubation, bacteria were harvested from the middle of the plate using either a 10-μl loop or a full 1-μl loop (see Fig. S1 in the supplemental material). Additionally, to analyze the effect of antibiotics, the 1-μl inoculum was also tested when taken from the edge of the inhibition zones of all three carbapenem disks. The final results were read after 15 min. Additionally, the time for the test to become positive was documented in order to quantify the effect of media, antibiotic disks, and inocula.
Effect of zinc sulfate and of different MH agars on the detection of NDM carbapenemases.
Nine different NDM-producing isolates (NDM-1, n = 5; NDM-5, n = 1; NDM-7, n = 1, OXA-232 plus NDM-1, n = 1; and OXA-181 plus NDM-5, n = 1) were selected for further analysis with zinc-supplemented MHA. For this purpose, 100 μl of 10 mM zinc sulfate was spread on the surface of MHA using a Drigalski spatula, and plates were dried for 10 min. Afterwards, the agars were inoculated as described above.
Furthermore, all NDM-positive isolates were tested on Mueller-Hinton II agar from Oxoid and an additional four manufacturers (Mueller-Hinton II agar from Liofilchem, Roseto degli Abruzzi, Italy; Mueller-Hinton E agar from bioMérieux, Nürtingen, Germany; Mueller-Hinton agar from Mast Diagnostica, Reinfeld, Germany; and BD Mueller-Hinton II agar from Becton Dickinson, Heidelberg, Germany).
Statistical analysis.
The chi-square test with Yates correction or the two-sided Fisher's exact test was used where appropriate. The Wilcoxon matched-pairs signed-rank test was used to compare nonparametric data. P values of <0.05 were considered statistically significant.
RESULTS
The ICT and carbapenem-resistant clinical isolates.
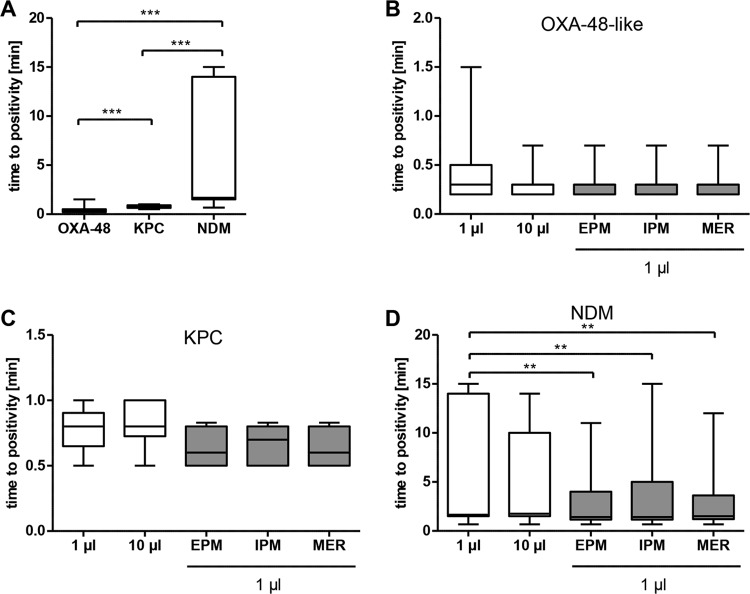
The performance of the ICT was evaluated on 63 Enterobacteriaceae clinical isolates (51 isolates producing a total of 53 carbapenemases and 12 carbapenem-resistant isolates without a carbapenemase). Isolates belonged to the species K. pneumoniae (n = 38), E. coli (n = 13), E. cloacae (n = 8), Enterobacter aerogenes (n = 2), Proteus mirabilis (n = 1), and Raoultella ornithinolytica (n = 1) (Table 1). All isolates were tested under five different conditions. Two different inocula were taken from the middle of MHA to assess the performance from MHA in the absence of antibiotic pressure using a full 1-μl inoculation loop (I) or a 10-μl loop (II). To investigate the impact of carbapenems on test results, three additional tests were done: harvesting 1 μl from the edge of the inhibition zone next to an ertapenem (III), meropenem (IV), or imipenem (V) disk.
Effect of the inoculum.
In the absence of antibiotic pressure, the overall sensitivity of the ICT was 98.1% (52/53) with the 1-μl loop compared to 100% (53/53) with the 10-μl inoculum (P = 0.5). The sensitivity for both OXA-48-like and KPC carbapenemases was 100% compared to 94.4% to 100% for NDM, depending on the test conditions (Table 2). NDM was the most difficult result to read, because of a low intensity of the bands on the ICT, which can easily be missed. In the whole study, only one isolate of Proteus mirabilis with NDM-1 remained negative with the 1-μl loop despite repeated testing (n = 7), extended lysis, and reading time. However, when the 1-μl inoculum was harvested next to an antibiotic disk, the test became positive and sensitivity increased to 100% for NDM with ertapenem, imipenem, and meropenem (Table 2). Especially for NDM, the inoculum size was critical. When a smaller inoculum was used (<1 μl or incompletely filled 1-μl loop), the test remained negative in 4/5 tests for NDM-producing Enterobacteriaceae (Fig. S1).
TABLE 2.
Sensitivity of the ICT for the detection of different carbapenemases directly from Mueller-Hinton agar
| Inoculum (μl)a | Carbapenemase detectedb |
|||||
|---|---|---|---|---|---|---|
| OXA-48 like |
KPC |
NDM |
||||
| Sensitivity (% [no. detected/total no.]) | 95% CI (%) | Sensitivity (% [no. detected/total no.]) | 95% CI (%) | Sensitivity (% [no. detected/total no.]) | 95% CI (%) | |
| 1 (no AB) | 100 (25/25) | 86.3–100 | 100 (10/10) | 69.2–100 | 94.4 (17/18) | 72.7–99.9 |
| 10 (no AB) | 100 (25/25) | 86.3–100 | 100 (10/10) | 69.2–100 | 100 (18/18) | 81.5–100 |
| 1 EPM | 100 (25/25) | 86.3–100 | 100 (10/10) | 69.2–100 | 100 (18/18) | 81.5–100 |
| 1 IPM | 100 (25/25) | 86.3–100 | 100 (10/10) | 69.2–100 | 100 (18/18) | 81.5–100 |
| 1 MER | 100 (25/25) | 86.3–100 | 100 (10/10) | 69.2–100 | 100 (18/18) | 81.5–100 |
no AB, inoculum taken distant from antibiotic disk, with all others harvested next to an antibiotic disk; EPM, ertapenem; IPM, imipenem; MER, meropenem.
95% CI, 95% confidence interval.
To better quantify the effect of media and antibiotics on test performance, the time for the test to become positive was measured. The time to positivity differed between the three carbapenemases, with 10 to 90 s for OXA-48-like, 30 to 60 s for KPC, and 40 s to 15 min for NDM enzymes (P < 0.001) using a 1-μl loop in the absence of antibiotic pressure (Fig. 1A). When harvested next to ertapenem disks, the mean time to positivity decreased for all carbapenemases: for OXA-48-like from 18 s to 12 s (nonsignificant [n.s.]), for KPC from 46 s to 39 s (n.s.), and for NDM from 6.3 min to 3.1 min (P = 0.0025) (Fig. 1B to D). There was no correlation between MIC and time to positivity.
FIG 1.
Effect of the inoculum on time to positivity. Boxes indicate the quartiles and whiskers the range, and the median is marked as a black line. (A) Differences in time to positivity between OXA-48-like-, KPC-, and NDM-producing isolates for the 1-μl inoculum. (B to D) Impact of inoculum and antibiotics on time to positivity. The 1 μl and 10 μl designate inocula taken distant from the antibiotic disks. Gray boxes indicate 1-μl inoculum that was harvested next to an antibiotic disk. EPM, ertapenem; IPM, imipenem; MER, meropenem. **, P < 0.01; ***, P < 0.001.
The specificity of the ICT was 100% for OXA-48-like, KPC, and NDM carbapenemases when tested under all five test conditions and not compromised by antibiotic disks.
Effects of zinc and different media for the detection of NDM carbapenemases.
Of all carbapenemases, the results of OXA-48-like enzymes were the easiest to read, as the bands were distinct; in contrast, the detection of NDM was the most problematic, as the bands were faint (Fig. 2). When MHA was additionally supplemented with 100 μl of 10 mM zinc sulfate, the NDM band was more intense and became visible faster. The mean time to positivity was 8 min ± 3.9 min for MHA compared to 1.9 min ± 1.5 min for MHA plus zinc sulfate (P = 0.016, Fig. 2B). Additionally, the P. mirabilis isolate which tested false negative with the 1-μl inoculum tested positive when harvested from the zinc-supplemented plate.
FIG 2.
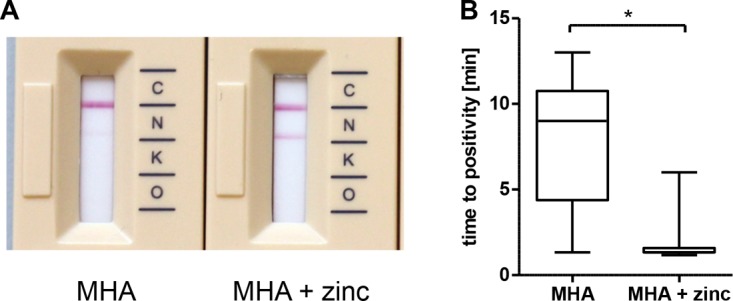
(A) Effect of zinc sulfate on the intensity of the NDM band. Inoculum harvested from MHA (left) with a very faint band compared to MHA supplemented with 100 μl of a 10 mM ZnSO4 solution (right). C, control band; N, NDM; K, KPC; O, OXA-48-like carbapenemases. (B) Impact of zinc sulfate on time to positivity of NDM-producing isolates. Boxes indicate the quartiles and whiskers the range. The median is marked as a black line; the asterisk corresponds to a P value of <0.05.
To investigate the impact of different MHA plates on NDM detection, the testing of NDM isolates was repeated with MHA from five different manufacturers. Even though the sensitivities were equal between all manufacturers, MHA from Mast Diagnostica showed the clearest positive test results regarding time and intensity, as observed by two investigators independently, followed by bioMérieux, BD, Liofilchem, and Oxoid. It is possible that the differences observed with media from different manufacturers were a result of different zinc concentrations used in these agars, which has also been previously shown to influence the performance of other tests for metallo-beta-lactamases, e.g., the modified Hodge test (12). Unfortunately, no information on the zinc content has been made available from the different companies.
DISCUSSION
This study demonstrates that the ICT is an excellent test for the detection of OXA-48-like, KPC, and NDM carbapenemases. However, in contrast to previous studies, we demonstrated that the performance depends on several factors when it is used with MHA: the right inoculum, antibiotic pressure, and the type and zinc content of MHA, at least for the detection of NDM. The overall sensitivity is equally good for KPC and OXA-48-like carbapenemases, as demonstrated in other studies (3–6, 13). In contrast, the sensitivity was lower than previously reported for NDM carbapenemases (4, 5), with a false-negative result observed for one NDM-1-producing P. mirabilis isolate. In both previous studies on this ICT, a sensitivity of 100% was reported also for NDM, but no NDM-producing P. mirabilis isolate was included in these evaluations. The P. mirabilis isolate from the present study additionally had low MICs for carbapenems (0.5 mg/liter for meropenem and 0.12 mg/liter for ertapenem), likely corresponding to a low expression level of NDM-1, which could explain the false-negative result observed with the 1-μl inoculum. Furthermore, Glupczynski et al. tested cultures from tryptic soy agar (TSA) with sheep blood, which probably contains a larger amount of zinc than MHA (5). The P. mirabilis isolate from the present study also tested positive when taken from TSA with sheep blood agar, confirming this hypothesis. In the present study, testing was done on MHA because the ICT is frequently used in clinical routine laboratories as a confirmatory test from this medium when regular susceptibility testing by disk tests or gradient tests has already been performed. One possibility to avoid the lower performance of the ICT for NDM is to do a subculture onto a blood-containing medium, but this would take another 10 to 24 h. However, this is not necessary, since a sensitivity of 100% can be achieved when a full 1-μl loop is taken adjacent to a carbapenem disk, likely because of the higher expression of carbapenemases in that area. Another possibility is to use a higher inoculum (10 μl) to increase sensitivity, but lysis of the higher inoculum was incomplete and migration of the suspension on the ICT was slowed; however, no false-negative or false-positive results were observed.
In this study, the effect of the inoculum, the type of MHA, and antibiotic disk used has been systematically assessed for the first time using molecularly characterized isolates with different carbapenemases. However, it has some limitations: since every isolate was tested under at least five different test conditions, the number of strains had to be limited. Furthermore, besides Enterobacteriaceae, no other Gram negatives were tested.
The present study demonstrates that the Resist-3 O.K.N. ICT is a sensitive and rapid test for the detection of three frequent carbapenemases. It is well suited for clinical microbiology laboratories because of its simplicity and speed. However, it has to be taken into account that false-negative results can occur if it is performed from confluent growth from MHA, especially with media with low zinc content. Before using the ICT under routine conditions for clinical isolates, we recommend testing several isolates (especially NDM producing) with the MHA used in the laboratory and to standardize the inoculum used for this assay. Additionally, we recommend using a full 1-μl inoculum that is harvested adjacent to an ertapenem or meropenem disk and from agars containing enough zinc to increase sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Faculty of Medicine, University Hospital Cologne. The ICT was supplied for evaluation free of charge by Coris BioConcept. MHA agar was provided by bioMérieux, Liofilchem, Mast, and BD.
We declare no conflicts of interest and have no association with Coris.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00050-18.
REFERENCES
- 1.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giske CG, Martinez-Martinez L, Canton R, Stefani S, Skov R, Glupczynski Y, Nordmann P, Wootton M, Miriagou V, Skov Simonsen G, Zemlickova H, Cohen-Stuart J, Gniadkowski M. 2017. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 2.0 European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden. [Google Scholar]
- 3.Wareham DW, Shah R, Betts JW, Phee LM, Momin MH. 2016. Evaluation of an immunochromatographic lateral flow assay (OXA-48 K-SeT) for rapid detection of OXA-48-like carbapenemases in Enterobacteriaceae. J Clin Microbiol 54:471–473. doi: 10.1128/JCM.02900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wareham DW, Abdul Momin MHF. 2017. Rapid detection of carbapenemases in Enterobacteriaceae: evaluation of the Resist-3 O.K.N. (OXA-48, KPC, NDM) lateral flow multiplexed assay. J Clin Microbiol 55:1223–1225. doi: 10.1128/JCM.02471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glupczynski Y, Jousset A, Evrard S, Bonnin RA, Huang TD, Dortet L, Bogaerts P, Naas T. 2017. Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J Antimicrob Chemother 72:1955–1960. doi: 10.1093/jac/dkx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koroska F, Göttig S, Kaase M, Steinmann J, Gatermann S, Sommer J, Wille T, Plum G, Hamprecht A. 2017. Comparison of phenotypic tests and an immunochromatographic assay and development of a new algorithm for detection of OXA-48-like carbapenemases. J Clin Microbiol 55:877–883. doi: 10.1128/JCM.01929-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göttig S, Hamprecht AG, Christ S, Kempf VA, Wichelhaus TA. 2013. Detection of NDM-7 in Germany, a new variant of the New Delhi metallo-beta-lactamase with increased carbapenemase activity. J Antimicrob Chemother 68:1737–1740. doi: 10.1093/jac/dkt088. [DOI] [PubMed] [Google Scholar]
- 8.Hamprecht A, Rohde AM, Behnke M, Feihl S, Gastmeier P, Gebhardt F, Kern WV, Knobloch JK, Mischnik A, Obermann B, Querbach C, Peter S, Schneider C, Schroder W, Schwab F, Tacconelli E, Wiese-Posselt M, Wille T, Willmann M, Seifert H, Zweigner J, DZIF-ATHOS Study Group. 2016. Colonization with third-generation cephalosporin-resistant Enterobacteriaceae on hospital admission: prevalence and risk factors. J Antimicrob Chemother 71:2957–2963. doi: 10.1093/jac/dkw216. [DOI] [PubMed] [Google Scholar]
- 9.Jazmati N, Hein R, Hamprecht A. 2016. Use of an enrichment broth improves detection of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in clinical stool samples. J Clin Microbiol 54:467–470. doi: 10.1128/JCM.02926-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamprecht A, Poirel L, Göttig S, Seifert H, Kaase M, Nordmann P. 2013. Detection of the carbapenemase GIM-1 in Enterobacter cloacae in Germany. J Antimicrob Chemother 68:558–561. doi: 10.1093/jac/dks447. [DOI] [PubMed] [Google Scholar]
- 11.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 12.Girlich D, Poirel L, Nordmann P. 2012. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 50:477–479. doi: 10.1128/JCM.05247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meunier D, Vickers A, Pike R, Hill RL, Woodford N, Hopkins KL. 2016. Evaluation of the K-SeT R.E.S.I.S.T. immunochromatographic assay for the rapid detection of KPC and OXA-48-like carbapenemases. J Antimicrob Chemother 71:2357–2359. doi: 10.1093/jac/dkw113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.