ABSTRACT
Modern advances in genomics provide an opportunity to reinterpret historical bacterial culture collections. In this study, genotypic antibiotic resistance profiles of Mycobacterium tuberculosis isolates from a historical 20-year-old multidrug-resistant tuberculosis (MDR-TB) culture collection in South Africa are described. DNA samples extracted from the phenotypically MDR-TB isolates (n = 240) were assayed by Hain line probe assay (LPA) for the confirmation of MDR-TB and by Illumina Miseq whole-genome sequencing (WGS) for the characterization of mutations in eight genes (rpoB, katG, inhA, rpsL, pncA, embB, gyrA, and rrs) that are known to code for resistance to commonly used anti-TB agents. LPA identified 71.3% of the TB isolates as MDR-TB, 18.3% as rifampin (RIF) monoresistant, 2% as isoniazid (INH) monoresistant, and 8.3% as susceptible to both RIF and INH (RIF+INH). In a subset of 42 randomly selected isolates designated as RIF+INH resistant by Löwenstein-Jensen (LJ) culture in 1993, LPA and WGS results confirmed MDR-TB. In all five INH-monoresistant isolates by LPA and in all but one (the wild type) of the 34 successfully sequenced RIF-monoresistant isolates, WGS revealed matching mutations. Only 26% of isolates designated as susceptible by LPA, however, were found to be wild type by WGS. Novel mutations were found in the rpoB (Thr480Ala, Gln253Arg, Val249Met, Val251Tyr, Val251Phe), katG (Trp477STOP, Gln88STOP, Trp198STOP, Trp412STOP), embB (Thr11Xaa, Gln59Pro), and pncA (Thr100Ile, Thr159Ala, Ala134Arg, Val163Ala, Thr153Ile, DelGpos7, Phe106Ser) genes. Three MDR-TB isolates showed mutations in both the gyrA and rrs genes, suggesting that extensively drug-resistant tuberculosis existed in South Africa well before its formal recognition in 2006.
KEYWORDS: historical isolates, molecular genetics, multidrug resistance, tuberculosis
INTRODUCTION
South Africa has one of the highest multidrug-resistant tuberculosis (MDR-TB) burdens globally, with an estimated total number of 10,000 MDR or rifampin-resistant (RR) TB cases in 2015 (1). Furthermore, extensively drug-resistant tuberculosis (XDR-TB) was first defined in 2006 by the World Health Organization (WHO) after an outbreak in Tugela Ferry, KwaZulu-Natal province (KZNP), South Africa. By the end of 2015, XDR-TB had been reported in 117 WHO member states, comprising 9.5% of the global 480,000 MDR cases (1, 2). However, detailed knowledge on drug resistance patterns and mutations associated with the MDR-TB epidemic in South Africa is limited, with only a few studies providing genomic data on MDR-TB strains in the country. These are mainly from the Western Cape and KwaZulu-Natal provinces (3, 4).
At the time of establishing the strain collection used in this study, the gold standard method for identification of MDR-TB strains was the agar proportion method in Löwenstein-Jensen (LJ) solid agar culture medium, a time-consuming process requiring several weeks to obtain adequate results. Currently, the liquid culture Bactec 960 MGIT system is widely recommended for the detection of Mycobacterium tuberculosis in clinical specimens and for drug sensitivity testing of first-line and certain second-line drugs. It is recommended as a reliable alternative with shorter turnaround times than those achieved by agar proportion methods (5, 6). Unfortunately, liquid culture methods are prone to higher rates of contamination, and consequently lost results, than solid agar methods. To overcome this problem, molecular assays are increasingly being employed for rapid detection of mutations associated with susceptibility in MDR-TB and XDR-TB.
In 2008 and 2016, the WHO endorsed the MTBDRplus and MTBDRsl line probe assays (Hain Lifescience, Germany), respectively, for detecting common mutations in regions of genes conferring drug resistance in M. tuberculosis. These molecular assays detect mutations in the “hot spot” region of genes that confer resistance to rifampin (RIF), isoniazid (INH), ethambutol (EMB), quinolones, and aminoglycosides (7).
With the recent development of next-generation sequencing (NGS) techniques, however, large-scale genome-sequencing projects have become possible. Furthermore, NGS is gradually entering the diagnostic laboratory for the detection of M. tuberculosis and its resistance to anti-TB drugs, promising decreased cost of sequencing and increased throughput (8).
Given the emerging molecular technologies that are becoming available recently, allowing for specific investigations into mutations associated with anti-TB drug resistance at the genomic level, we reanalyzed the drug resistance profiles of a historical South African MDR-TB strain collection dating from the period 1993 to 1995. The aim was to specifically describe the mutations associated with drug resistance in each isolate and to investigate whether any of these 20-year-old isolates harbored combinations of mutations that defined XDR-TB before this profile was formally reported in 2006. We also wanted to gain insights into the genetic diversity of MDR-TB strain types per geographical location, i.e., Western Cape Province (WCP) in the South and Gauteng Province (GP) in the North.
MATERIALS AND METHODS
Collection of samples.
A collection of 625 MDR-TB cultures, originally derived from sputum samples submitted to two routine TB laboratories (samples were collected from public hospital patients) in the Western Cape Province and Gauteng Province between 1993 and 1995, was centrally stored at the South African Medical Research Council (SAMRC) laboratories in Pretoria, South Africa, after a confirmation of their MDR-TB status. From this collection, 240 isolates (120 each from WCP and GP) were randomly selected in 2014 for DNA extraction and molecular analysis. Care was taken not to include more than one isolate from each patient represented in the collection. The demographic characteristics of patients represented by the 240 isolates were largely comparable. GP had slightly more males (77%) than females (23%) and a higher median age (38.1 years) than WCP, with 67% males, 33% females, and a median age of 33.3 years.
At the time of collection, sputum specimens were cultured on LJ medium and subjected to the proportion method as recommended by the WHO/International Union against Tuberculosis and Lung Disease (IUATLD) (9) for drug susceptibility testing of RIF and INH and routinely also for streptomycin (SM), EMB, and the second-line drugs kanamycin (KAN), capreomycin (CPM), cycloserine (CLS), and ofloxacin (OFX). Drug concentrations used were as follows: SM, 4 μg/ml; INH, 0.2 μg/ml; RIF, 4 μg/ml; EMB, 2 μg/ml; pyrazinamide (PZA), 100 μg/ml; KAN, 20 μg/ml; CPM, 2.5 μg/ml; CLS, 30 μg/ml; and OFX, 2 μg/ml.
Extraction of DNA from nonviable stored M. tuberculosis cultures.
Preparation of suspensions of M. tuberculosis colonies from the stored specimens depended on the state of preservation of the original cultures. For intact slopes, colonies were gently scraped off using an inoculation loop and further suspended (washed down) in 1 ml of sterile water in the original culture bottle. The suspension was then pipetted off and transferred into a 1.5-ml Eppendorf tube. For dried-out media and cultures, the dry substrate was crushed with an inoculation loop and a portion was transferred into a 1.5-ml Eppendorf tube. This was then gently crushed into finer fragments with a glass rod prior to adding 1 ml of sterile water and shaking. Eppendorf tubes containing suspensions obtained from either the intact or dried-out samples were centrifuged at 13,000 × g for 5 min, and the supernatant was discarded. DNA extraction was successfully achieved by applying the NucleoSpin Tissue kit (Macherey-Nagel, Germany) for extraction of genomic DNA as per the manufacturer's instructions. This procedure allowed for complete digestion of remnant protein from the growth medium, resulting in high-quality, pure DNA. DNA extraction was successful for all isolates included in this study.
Molecular drug resistance testing.
First-line drug susceptibility testing for RIF and INH was done using the GenoType version 2 MTBDRplus assay (Hain Lifescience, Germany). Amplification reactions were undertaken using a 35-μl primer nucleotide mix, 10 μl polymerase mix (Hain Lifescience, Germany), and 5 μl of genomic DNA. PCR and hybridization were performed and interpreted as per the manufacturer's instructions using GTBlot and GenoScan machines (Hain Lifescience, Germany). The PCR products were denatured in a special solution at room temperature, followed by hybridization with the provided buffer at 45°C for 30 min in a shaking water bath. After stringent washing of the PCR products, hybridization was detected by a colorimetric reaction to control for cross-contamination. Water, in lieu of the DNA template, was used as the negative control. Second-line drug susceptibility testing by methods similar to the ones described above was carried out using the GenoType MTBDRsl version 1 assay to detect resistance to fluoroquinolones (FQLs), amikacin (AMK), KAN, CPM, and EMB.
Library preparation.
Whole-genome sequencing (WGS) was carried out using the Illumina Miseq (Illumina Inc., USA) to detect mutations in eight genes that confer resistance to anti-TB drugs (rpoB, katG, inhA, embB, rpsL, pncA, gyrA, rrs). The DNA used for WGS was quantified using a Qubit (Invitrogen, Eugene, OR, USA). Concentrations of 1 to 5 ng/μl genomic DNA from 111 samples were prepared and used with the Nextera XT Sample Prep kit (Illumina, San Diego, CA, USA). Illumina WGS libraries were indexed into groups of 24 and pooled, as per the manufacturer's instructions (Illumina, San Diego, CA, USA). MiSeq NGS was performed using the MiSeq Reagent kit (V3) with 600 cycles. The sequencing was done as paired-end 150-base reads. The sequencing coverage was between 33× and 152×.
Bioinformatics analyses.
Sequence output quality control was achieved by using the trimmomatric software for the trimming of adaptors and artifacts to get the true DNA sequence of the genome. The same software was used to remove poor sequences with low base quality scores. Bioinformatics, including contig assembly, mutational analysis, and multiple sequence alignments, were performed using SeqMan NGen (V4) and LaserGene (V10) Core Suite software (DNAStar, Inc., Madison, WI, USA) with reference mapping.
Genotyping.
Genotyping was performed on all 240 MDR isolates using a commercial spoligotyping kit (Ocimum BioSolutions, India), via procedures previously described (10). Resulting spoligotypes were compared with the TB Drug Resistance Mutation Database (11) and with the updated version SITVIT 2 from the Pasteur Institute in Guadeloupe. Spoligotype clusters with more than 9 isolates were selected for characterization using the 24-locus mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) quadruplex typing kit (GenoScreen, France).
PCR amplification was done using a 96-well plate (LASEC, South Africa) in a total PCR volume of 10 μl consisting of 8 μl of MIRU-VNTR quadruplex mix and 2 μl of template DNA. The thermocycling profile included a 15-min denaturation step at 95°C, followed by 40 cycles of 1 min at 94°C, 1 min at 59°C, and 1.5 min at 72°C, with a final extension step at 72°C for 10 min. The 24-locus MIRU-VNTR typing method was subjected to electrophoresis using an ABI 3130 genetic analyzer (Applied Biosynthesis, USA). Sizing of PCR fragments and assignments of the alleles of the 24-loci were done using the MIRU-VNTR calibration kit (GenoScreen, France) and Gene Mapper software version 4.0 (Applied Biosystems, USA).
Dendrograms were constructed using the unweighted pair group method with arithmetic averages (UPGMA) algorithm for spoligotyping combined with 24-locus MIRU-VNTR typing, and the categorical coefficient was used to calculate the distance matrix.
RESULTS
Resistance profiles as determined by LPA.
Of the 240 MDR-TB isolates investigated by line probe assay (LPA), 171 (71%) were confirmed by MTBDRplus as MDR-TB (RIF and INH [RIF+INH] resistant); 98 were from WCP (n = 120) and 73 from GP (n = 120). Overall, 44/240 (18.0%) isolates were monoresistant to RIF (GP, 30; WCP, 14), 5/240 (2.1%) were monoresistant to INH (GP, 3; WCP, 2), and 20/240 (8.0%) showed no resistance to RIF or INH (GP, 14; WCP, 6) (Table 1).
TABLE 1.
Comparison of resistance profiles by original phenotypic drug susceptibility testing in 1993 to 1995 and by Genotype MTBDRplus or MTBDRsl on extracted DNA in 2014
| Resistance profile method and druga | No. (%) of resistant isolates |
|
|---|---|---|
| Gauteng Province (n = 120) | Western Cape Province (n = 120) | |
| Phenotypic MDR-TB profile (1993–1995) | ||
| RIF | 120 (100) | 120 (100) |
| INH | 120 (100) | 120 (100) |
| PZA | 42 (35) | 0 |
| EMB | 7 (6) | 35 (29) |
| ETM | 14 (12) | 45 (37) |
| SM | 17 (14) | 45 (37) |
| OFX | 1 (<1) | 1 (<1) |
| KAN | 1 (<1) | 0 |
| Genotypic resistance profile (Genotype MTBDRplus or MTBDRsl LPA) on extracted DNA (2014) | ||
| RIF+INH | 73 (61) | 98 (82) |
| RIF mono | 30 (25) | 14 (12) |
| INH mono | 3 (2) | 2 (2) |
| EMB | 11 (9) | 39 (34) |
| OFX+AMG | 1 (<1)b | 2 (2) |
| OFX mono | 1 (<1)b | 2 (2) |
| Susceptible | 14 (12) | 6 (5) |
Abbreviations: RIF, rifampin; INH, isoniazid; EMB, ethambutol; PZA, pyrazinamide; ETM, ethionamide; SM, streptomycin; OFX, ofloxacin; KAN, kanamycin; AMG, aminoglycoside; mono, monoresistant.
Of a total of 117 isolates instead of 120.
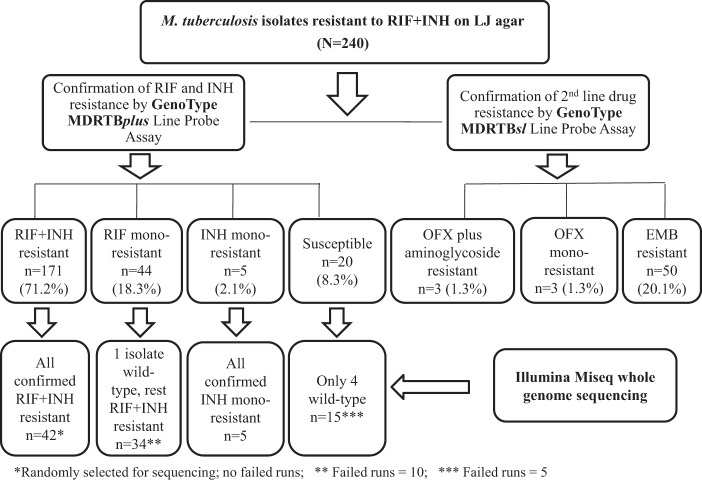
For three MDR-TB isolates from the GP (n = 120), no results were obtained for OFX or for the aminoglycosides. Of the remaining 237 isolates, 3 showed resistance to both OFX and an aminoglycoside by second-line LPA (GP, 1; WCP, 2). In three more isolates, one was phenotypically resistant to OFX but susceptible to the aminoglycosides (WCP), one was susceptible to OFX (GP), and the 3rd (WCP) had no phenotypic results recorded for OFX. A further three of the MDR-TB isolates (1.0%) were monoresistant to OFX (GP, 1; WCP, 2). None of the isolates showed monoresistance to AMK or KAN. Fifty isolates were resistant to EMB, of which 42 were originally also detected by LJ drug susceptibility testing (Table 1 and Fig. 1).
FIG 1.
Confirmation of drug resistance status by line probe assay and whole-genome sequencing of 240 Mycobacterium tuberculosis isolates originally designated as multidrug resistant in 1993 by proportion method drug susceptibility testing in Löwenstein-Jensen agar medium.
Illumina Miseq whole-genome sequencing.
Funding limitations prevented sequencing of all 240 isolates. Whole-genome sequencing was performed on 42 randomly selected isolates with RIF+INH resistance originally detected by the LJ proportion method and confirmed by LPA, as well as on all 69 isolates that showed discrepant results for RIF and INH between the original LJ phenotypic DST results and the LPA results (44 isolates monoresistant to RIF, 5 monoresistant to INH, and 20 susceptible to both RIF and INH). Fifteen sequencing runs (10 in the RIF-monoresistant group and 5 in the susceptible group) failed, however, leaving a total of 96 (86.5%) selected isolates having results interpretable by WGS. All 42 RIF+INH isolates confirmed by LPA were also confirmed by WGS. Of the 34 RIF-monoresistant isolates identified by LPA and successfully sequenced, all except one (the wild type [WT]) were confirmed by WGS as resistant to RIF only. All five of the INH monoresistant isolates by LPA were confirmed by WGS. WGS was successfully conducted for 15 of the 20 isolates susceptible to RIF and INH by LPA. Four were shown to be wild type by WGS, two were monoresistant to INH, one was monoresistant to RIF, and eight were resistant to RIF+INH. Eleven of the RIF-susceptible group of isolates had mutations compatible with RIF+INH resistance (Fig. 1 and Table 2).
TABLE 2.
MTBDRplus line-probe assay (LPA) results and associated mutations observed by Illumina MiSeq whole-genome sequencing (WGS) for confirmation of rifampin-isoniazid resistance as originally defined by LJ culture in 20-year-old M. tuberculosis isolates (n = 240)
| Resistance category results by LPA (no. tested/total no.; %) | No. of isolates selected for WGS (total n = 111) | Description of mutations by WGSa |
No. of isolates (total n = 96) with indicated mutation(s) | |
|---|---|---|---|---|
| rpoB | katG | |||
| RIF+INH resistant (171/240; 71.3%) | 42b | Ser531Leu | Ser315Thr | 15 |
| His526Try | Ser315Thr | 7 | ||
| Ser531Leu | Ser315Thr, Arg463Leu | 3 | ||
| Ser526Asp/TyrSTOP | Ser315Thr | 2 | ||
| Ser531Leu | Ser315Ile | 1 | ||
| Asp516Val | Ser315Thr/SerSTOP | 1 | ||
| Ser531Thr | Ser315Thr | 1 | ||
| Asp516Val | Ser315Asn | 1 | ||
| Thr525Pro | DelCpos526, Ser315Thr | 1 | ||
| Asp516Val | Ser315Ile | 1 | ||
| His526Asp | Ser315Asn | 1 | ||
| Leu533Pro | Ser315Asn | 1 | ||
| His526Leu | Trp91Arg, Arg463Leu | 1 | ||
| Thr480Ala, Ser531Leu | Ser315Thr | 1 | ||
| His526Tyr | Ser315Arg | 1 | ||
| Ser531Leu | Ser315Thr, Arg463Leu, Ala464Leu | 1 | ||
| His526Tyr | Trp91Arg, Ser140Gly, Arg463Leu | 1 | ||
| Gln409Arg, Ser450Leu | Arg463Leu | 1 | ||
| His 526 Tyr | Ile200Thr (inhA gene) | 1 | ||
| RIF monoresistant (44/240; 18.3%) | 44c | His531Leu | No mutations | 4 |
| His531Leu | Arg463Leu | 2 | ||
| His531Leu | Del2185G | 1 | ||
| His531Leu | Pro131Arg, Arg463Leu | 1 | ||
| His531Leu | Tyr98Cys | 1 | ||
| His531Leu | Ser315Thr | 1 | ||
| His531Leu | Asp381Glu | 1 | ||
| His531Leu | Pro232Arg | 1 | ||
| His531Leu | Asp1426Arg | 1 | ||
| His531Leu | del 1426G (Missense) | 1 | ||
| His531Leu | Pro29Ser, Met420Thr | 1 | ||
| His531Leu | Pro232Ala | 1 | ||
| His531Leu | Ser100Ala (inhA gene) | 1 | ||
| His526Tyr | Arg463Leu | 2 | ||
| His526Tyr | Ins19A | 1 | ||
| His526Tyr | Phe594Ser | 1 | ||
| His526Tyr | Gln88STOP | 1 | ||
| His526Asp | Gly699Glu | 1 | ||
| His526Asp | Leu378Arg | 1 | ||
| Ser531Trp | Ala122Gly/Ala | 1 | ||
| Ser531Trp | Trp477STOP | 1 | ||
| Ser522Leu | Trp412STOP | 1 | ||
| Asp516Val | Arg463Leu | 1 | ||
| Asp516Val | Ins576A | 1 | ||
| Gln253Arg, His256Arg | No mutations | 1 | ||
| His526Arg | Trp198STOP | 1 | ||
| Leu511Pro/LeuSTOP | Gly699GLU | 1 | ||
| DelC526 | Arg463Leu, Trp477STOP | 1 | ||
| Wild type | Arg463Leu, Del entire codon pos 480 | 1 | ||
| INH monoresistant (5/240; 2%) | 5 | Val251Phe | Thr380Ile | 2 |
| Ser522Leu | Ser315Thr, Arg463Leu | 1 | ||
| Val249Met, Val251Tyr | Ser315Thr, Arg463Leu | 1 | ||
| Ser531Leu | Ser315Thr | 1 | ||
| RIF + INH susceptible (20/240; 8.3%) | 15d | Wild type | Wild type | 4 |
| Ser531Leu | No mutations | 2 | ||
| No mutations | Ser315Thr, W198STOP | 1 | ||
| His526Leu/His (mixed) | Ser315Thr | 1 | ||
| Ser531Leu | Pro232Ala | 1 | ||
| Ser531Leu | Glu553Lys | 1 | ||
| His526Leu/Ala | Gly299Ser | 1 | ||
| Ile572Phe/IleSTOP | Arg104Trp | 1 | ||
| Asp516Val | DelGpos1284 | 1 | ||
| Ile572Phe/IleSTOP | Gly299Arg/GlySTOP | 1 | ||
| Ser531Leu/SerSTOP | Arg463Leu | 1 | ||
Novel mutations present by WGS are shown in bold type.
Randomly selected from the 171 isolates.
Sequencing failed in 10 isolates (DNA concentration was too low for WGS), leaving 34 isolates with WGS results.
Sequencing failed in 5 isolates (DNA concentration was too low for WGS), leaving 15 isolates with WGS results.
Description of mutations. (i) Resistance to rifampin.
The rpoB gene was successfully sequenced in 76 isolates designated as RIF resistant by LJ and LPA. Resistance was confirmed by WGS in all isolates, except one (WT) (Table 2). In 15 successfully sequenced RIF+INH isolates found to be resistant by LJ but susceptible by LPA, 10 isolates (66.7%) showed mutations in rpoB by WGS. Of the total of 86 isolates with rpoB gene mutations by WGS, amino acid positions 531 (n = 47; 54.7%), 526 (n = 24; 27.9%), and 516 (n = 6; 7.0%) were most commonly affected. WGS also detected five novel mutations (Thr480Ala, Gln253Arg, Val249Met, Val251Tyr, Val251Phe) and one deletion at position 526 (Table 2).
(ii) Resistance to isoniazid.
Eighty-four isolates had katG mutations (GP, 30; WCP, 54) by LPA. INH resistance was due mostly to mutations in the katG gene at position 315 (44; 52.4%). Four novel stop codon mutations in the katG gene were found in this study (Trp477*; Gln88*; Trp198*; and Trp412*) (Table 2). Three isolates had mixed mutations. Some isolates had double or triple mutations per gene. Six indels were observed in this study. There were 11 wild-type katG isolates. Only two mutations were found in the inhA gene, and both mutations were novel and contributed to INH resistance. Both of these mutations (Ser100Ala; Ile200Thr) were from the GP isolates. No mutations were found in the promoter region of the inhA gene.
(iii) Resistance to pyrazinamide.
Thirty-three of the 96 isolates with interpretable results had single mutations in the pncA gene (GP, 14; WCP, 21), and 7 had double mutations (GP, 3; WCP, 4). Three heteroresistant (mixed isolates with both resistant and susceptible strains) mutations were detected in the pncA gene (for GP, Gln10Xaa; for WCP, Thr168Xaa, His71Xaa). We also detected seven indels and seven novel pncA mutations (for WCP, Thr100Ile, Thr159Ala, and Ala134Arg; for GP, Val163Ala, Thr153Ile, Del G pos 7, and Phe106Ser). Only one isolate (from WCP) had a mutation in the promoter region of the pncA gene (−11A>R) (Table 3).
TABLE 3.
Results from Illumina MiSeq whole-genome sequencing for detection of mutations in the emb B, pncA, gyrA, rrs, and rpsL genes of isolates originally defined in 1993 by LJ culture as resistant to isoniazid and rifampind
| Gene (antibiotic) | No. of isolates in which gene was found present by WGS/total no. tested (%) | Description of mutation(s) present by WGSb |
|
|---|---|---|---|
| Codon/nucleotide | No. of isolates | ||
| embB (EMB) | 34/96a (35.4) | Met306Ile | 14 |
| Met306Val | 7 | ||
| Gln497Argc | 3 | ||
| Thr11Xaac | 1 | ||
| Leu17Xaa, Gly25Xaa, Asp456Xaa, Met306Xaa | 1 | ||
| Met306Xaa | 1 | ||
| Gly406Aspc | 1 | ||
| Gln51Proc | 1 | ||
| Asp354Alac | 1 | ||
| Met306Xaa, Gly406Xaa | 1 | ||
| Glu378Alac | 1 | ||
| Tyr319Serc | 1 | ||
| Gln139Hisc | 1 | ||
| pncA (PZA) | 40/96 (41.7) | Trp68Gly | 1 |
| Asp8Asn | 2 | ||
| Trp68STOP | 1 | ||
| −11A>R | 1 | ||
| Phe13Leu | 1 | ||
| Trp68Arg | 1 | ||
| Cys72Try | 1 | ||
| Ins A pos 193 | 4 | ||
| Ala134Arg | 1 | ||
| Leu35Arg | 3 | ||
| Gln10Pro | 1 | ||
| Glu37STOP | 1 | ||
| Gly97Asp | 1 | ||
| Thr100Ile | 1 | ||
| Thr159Ala | 1 | ||
| Gly108Arg | 1 | ||
| Cys14Arg | 1 | ||
| His71Xaa | 1 | ||
| Thr168Xaa | 1 | ||
| Ile6Thr | 1 | ||
| Gln10Xaa | 1 | ||
| Leu35Arg, Val163Ala | 1 | ||
| Thr153Ile | 1 | ||
| Del G pos 7, Del G pos 391 | 1 | ||
| Thr76Pro | 2 | ||
| Phe106Ser | 1 | ||
| Leu4Ser | 1 | ||
| Ser164Leu | 1 | ||
| Ins G pos 392, Ins G pos 393 | 3 | ||
| His71Xaa, Thr168Xaa | 1 | ||
| Thr100Ile, Ile159Ala | 1 | ||
| gyrA (FQL) | 36/96 (37.5) | Ser91Pro | 1 |
| Ser95Thr, Gly668Asp | 12 | ||
| Gln613Glu | 10 | ||
| Ser95Thr, Gln21Glu, Gly668Asp | 1 | ||
| Cys254Arg | 1 | ||
| Gly247Ser | 4 | ||
| Ala90Xaa, Ser95Thr | 1 | ||
| Ser741Pro | 1 | ||
| Val384Ala | 1 | ||
| Leu252Arg | 1 | ||
| Glu21Gln, Ser95Thr, Gly668Asp | 1 | ||
| Asp89Xaa | 1 | ||
| Glu21Gln, Asp94Ala, Ser95Thr, Glt668Asp | 1 | ||
| rrs [16rRNA] (AMK, KAN) | 27/96 (11.3) | 1484G>T | 2 |
| 1403G>R, 1404T>Y | 1 | ||
| 492C>T | 15 | ||
| 517C>T | 4 | ||
| A514A>C | 2 | ||
| A1401A>R | 1 | ||
| 1260G>R, 1278A>W | 1 | ||
| 492C>T, 1260G>R, 1278A>W, 1445C>Y | 1 | ||
| rpsL (SM) | 11/96 (4.6) | Lys43Arg | 9 |
| Lys88Arg | 2 | ||
Includes all isolates successfully sequenced as per Table 2.
Novel mutations present by WGS are in bold type; bold type and underlining indicate that the mutation also conferred resistance phenotypically.
Discordant isolates (resistant by LJ and mutations present by WGS, but susceptible by LPA).
Abbreviations: LJ, Löwenstein-Jensen; LPA, line probe assay; WGS, whole-genome sequencing; PZA, pyrazinamide; EMB, ethambutol; FLQ, fluoroquinolone; AMK, amikacin; KAN, kanamycin; SM, streptomycin.
(iv) Resistance to ethambutol.
Of the 96 isolates sequenced (as per Table 2), a total of 34 isolates had EMB mutations (GP, 10; WCP, 24). EMB resistance was due to Met306Val and Met306Ile mutations in 21 isolates (GP, 4; WCP, 17). Two novel mutations were found in WCP isolates (Thr11Xaa and Gln51Pro) (Table 3).
(iv) Resistance to fluoroquinolones (ofloxacin) and injectable aminoglycosides (streptomycin, kanamycin, amikacin).
We observed three isolates with mutations in both the gyrA and rrs genes (in WCP, Ser91Pro with 1484G>T, Ala90Xaa with 1401A>G; in GP, Asp94Ala with 1484G>T), which would define XDR-TB in the clinical context. WCP had eight rpsL mutations (Lys43Arg), and GP had three (one Lys43Arg and two Lys88Arg). Isolates resistant to SM were not also resistant to AMK or KAN.
Spoligotyping results.
Spoligotyping results of all 240 isolates included in this study are provided in Table 4. Of these, 159 (GP, 81; WCP, 78) clustered into 28 shared international types (SIT) (GP, 11; WCP, 17), with each cluster consisting of 2 to 17 isolates. The remaining 40 isolates showed unique spoligotype SIT. Forty-one isolates were orphans (GP, 18; WCP, 23). Most of the isolates belonged to the following six lineages: T, LAM, Beijing, X, S, and Harlem. The other minor lineages were MANU2, U, EAI1_SOM, and H37Rv (Table 4).
TABLE 4.
Distribution of study isolates per the various shared types and corresponding lineages/sublineages as defined by spoligotyping for the Gauteng and Western Cape Provinces in South Africa
| Lineage | No. of lineages | Gauteng Province | Western Cape Province |
|---|---|---|---|
| T | 58 | 25 | 33 |
| LAM | 43 | 29 | 14 |
| Beijing | 34 | 17 | 17 |
| X | 28 | 9 | 19 |
| S | 19 | 11 | 8 |
| H | 8 | 3 | 5 |
| MANU2 | 3 | 0 | 3 |
| U | 4 | 0 | 4 |
| EAI1_SOM | 1 | 1 | 0 |
| H37Rv | 1 | 0 | 1 |
| Orphans | 41 | 18 | 23 |
| TOTAL | 240 | 113 | 127 |
The three largest groups were SIT1 (Beijing lineage), consisting of 34 isolates (GP, 17; WCP, 17), followed by SIT33 (LAM3) with 21 isolates (GP, 11; WCP, 10) and SIT53 (ill-defined T sublineage) consisting of 19 isolates (GP, 10; WCP, 9). Other minor groups were SIT34 (S family) (GP, 10; WCP, 4), SIT92 (X3 sublineage) (GP, 8; WCP, 4), SIT60 and SIT811 (LAM4 sublineage) (GP, 6; WCP, 1), SIT119 (X1 sublineage) (WCP, 4), and SIT44 (T5 sublineage) (WCP, 4). Other less frequent SITs were represented by three isolates and had a spoligotyping pattern resembling that of H37Rv. The 24-locus MIRU-VNTR typing was conducted on 78 isolates that had the largest SIT clusters of 9 to 17 isolates (ST1, ST33, ST53, and ST34), representing both GP and WCP. There were 64 unique and 7 clustered strains. The clusters of 16 isolates ranged between two and four isolates. Forty-three isolates were split as individual patterns in this study. When combining 24-locus MIRU-VNTR and spoligotyping, there were 26 number types detected with 64 unique isolates, and 14 isolates clustered into six clusters. The range of a cluster size was two to four isolates (Table 5).
TABLE 5.
Type, unique isolates, clustered isolates, cluster, and cluster range by spoligotyping and MIRU-VNTR analysis
| Method | No. of types | No. of unique isolates | No. of clustered isolates | No. of clusters | Cluster size range (no. of isolates) |
|---|---|---|---|---|---|
| Spoligotyping | 4 | 0 | 78 | 4 | 2 to 17 |
| 24-locus MIRU-VNTR | 26 | 62 | 16 | 7 | 2 to 4 |
| Spoligotyping + MIRU-VNTR | 26 | 64 | 14 | 6 | 2 to 4 |
DISCUSSION
The emergence of drug-resistant tuberculosis, in particular multidrug-resistant TB, has complicated M. tuberculosis eradication. This urgently calls for additional control measures such as characterization of resistant markers, new diagnostic methods, better drugs for treatment, and a more effective vaccine.
In this study, we reanalyzed 240 isolates collected between 1993 and 1995 from the Gauteng and Western Cape Provinces of South Africa that were phenotypically defined as multidrug resistant. Using current molecular methods in order to retrospectively assess the genotypic characteristics of the isolates, we aimed to confirm the original diagnosis and to specifically define any associated mutations.
Apart from confirming a large proportion of the earlier phenotypic results obtained from solid medium susceptibility testing in this study, LPA and NGS methods also revealed several misclassifications in the earlier data set compared with the WGS results in this study and additionally identified a few new putative resistance-conferring mutations that have not been reported elsewhere.
Mutations in the rpoB (3,519-bp) gene were most frequent at positions 531, 526, and 516. These results are in line with other studies in South Africa and other countries that reported the same trends (12). Mutation Ser531Leu, which is common in XDR-TB isolates (13), was also identified in two of the three XDR-TB isolates included in this study. Another mutation described as associated with XDR-TB and pre-XDR-TB (mutation 526), was seen in 24 MDR-TB isolates in our study, but not in the XDR-TB or pre-XDR-TB isolates. In this study, five novel mutations were identified in the rpoB gene (Thr480Ala, Gln253Arg, Val249Met, Val251Tyr, Val251Phe), all of which were outside the rifampin resistance-determining region (RRDR). Two of these mutations were responsible for RIF resistance, and two (Thr480Ala+Ser531Leu and Gln253Arg+His526Tyr) were paired with mutations within the RRDR of rpoB. It is not clear if these two mutations are involved in RIF resistance or if they represent compensatory mutations.
Resistance to INH was due mostly to mutations in the katG gene, particularly at position Ser315Thr. This correlated with published findings from South Africa and elsewhere (12, 14). In 96 isolates with phenotypic INH resistance in this study, 14 isolates had mutation Arg463Leu, and this mutation was found as a single mutation in the katG gene in seven of the isolates, suggesting an association with INH resistance. These seven observations applied to GP isolates only. In the WCP isolates, Arg463Leu mutations were also observed in seven isolates, all of which were also associated with Ser315Thr mutations in katG. Thirteen of the 14 isolates with an Arg463Leu mutation were of the Beijing strain type, and 1 was an X3 strain (GP). These findings seem to support observations from China that Arg463Leu mutations in katG are associated with INH resistance, particularly low-level resistance (15). If that is the case, then the Arg463Leu mutation might need to be taken into consideration as a target when developing rapid molecular diagnostic assays for INH resistance. However, it is possible that the resistance to isoniazid might have originated from the intergenic region between oxyR and ahpC or between Rv1482c and fabG1 (16).
We identified four novel stop codons, signifying an evolution in katG resistance genetics (Trp477*, Gln88*, Trp198S*, and Trp412*P). As for the Arg463Leu mutation, the stop codon mutations are not included in rapid genotyping assays; therefore, they might offer additional discriminatory power if included as targets.
Two novel mutations (Ser100Ala and Ile200Thr) were also identified in the inhA gene from INH-resistant GP isolates. The isolates lacked katG mutations, and therefore, these two mutations could be regarded as contributing to INH resistance. These mutations cannot be identified by the line probe assay since it detects only mutations on the promoter regions (−8, −15, and −16) of the inhA gene. No mutations were found in the promoter region of the inhA gene in any of the isolates in this study.
PZA mutations were scattered throughout the pncA gene, including its promoter region. We noted seven novel pncA mutations (in WCP, Thr100Ile, Thr159Ala, and Ala134Gly; in GP, Val163Ala, Thr153Ile, Del G pos 7, and Phe106Ser). Among these, only one mutation (FPhe106Ser) showed resistance to PZA phenotypically. More functional studies are needed to define the roles of the other six mutations in phenotypic PZA resistance. The promoter −11A>R mutation was from a WCP isolate and is known to contribute to phenotypic resistance to PZA. This mutation was first identified in the United States in 2007 (17) and has subsequently been reported in other parts of the world, including South Africa (18, 19). Mutation Leu35Arg, seen in four isolates in this study, was first reported in South Africa and later confirmed in America, where it reportedly contributes significantly to PZA resistance (17, 18, 20). Mutation Asp8Asn was found in one isolate in this study, albeit it has been previously identified in South Africa (18) and described as a significant contributor to PZA phenotypic resistance in this country.
Streptomycin mutations detected in this study in the rpsL gene were Lys43Arg (1 in GP, 8 in WCP) and Lys88Arg (two isolates). These mutations have previously been shown to be involved in SM resistance (21, 22).
Resistance to EMB was due mostly to the codon 306 of the embB gene (3 in GP, 17 in WCP). Two novel mutations were identified in codons 11 and 59. Isolates with a mutation in codon 11 contributed to EMB resistance phenotypically. GP had three isolates with Gln497Arg mutations. Mutations in codons 306, 497, and 406 are known to increase EMB's MICs in M. tuberculosis (23). One-third of the isolates did not carry mutations in codon 306 (used by rapid molecular tests to detect polymorphisms in the codon), but on codons 497 and 406, mutations are not detectable by rapid molecular methods. This explains the low sensitivity and specificity of the line probe assay in the detection of ethambutol resistance. These two mutations were confirmed as hot spot mutations in a study conducted in China (24). The inclusion of these two positions can improve the molecular diagnostic assays.
Isolates resistant to fluoroquinolones (FQLs) had mutations at codons 90, 91, and 94 on the quinolone resistance-determining region (QRDR) of the gyrA gene, which is implicated in 70 to 90% of all FQL resistance mutations (25, 26). Fifteen isolates (WCP, 11; GP, 4) had Ser95Thr and Gly668Asp mutations. These mutations do not contribute to resistance but serve as evolutionary markers for classifying M. tuberculosis strains into the three principal genetic groups (PGGs) (27).
Resistance-conferring mutations in the rrs gene were due to 1484G>T and 1401A>R in this study. Mutations 1484G>T and 1401A>G cause high-level resistance to AMK, CPM, and KAN (28). Fifteen isolates had a C492T mutation (GP, 5; WCP, 10); however, this mutation does not confer resistance to AMK, CPM, or KAN (29). In this study, we also found two isolates from WCP with 514A>C and four isolates (2 in GP, 2 in WCP) with 517C>T mutations. These mutations have previously been reported in other studies, and they cause cross-resistance between AMK, CPM, and KAN (30). Other mutations identified in this study were at positions 1260, 1278 and 1445 but also did not seem to influence AMK, CPM, or KAN resistance.
Three of the MDR isolates were XDR-TB, suggesting that XDR-TB was already present in South Africa 10 years ahead of the first XDR-TB cases reported in Kwazulu-Natal province in 2006 (2). In the 1990s, however, there was no reason for screening strains routinely for resistance to second-line TB drugs as per the current-day XDR-TB definition, and if this screening had been pursued, it would have required culture-based methods to detect phenotypic resistance to the range of drugs available for treating MDR-TB. Such assays were at best available in specialized reference or research laboratories at the time. Two of these XDR-TB strains were Beijing, and one represented the X3 lineage. One Beijing XDR-TB strain harbored mutations in seven of eight genes tested in this study (rpoB, katG, embB, rpsL, pncA, gyrA, and rrs). This means that the strain was incurable and would not have responded to any antibiotics used to treat drug-resistant TB at the time.
Spoligotyping and 24 MIRU-VNTR were used to describe the diversity of M. tuberculosis strains that were circulating in the WCP and GP between the years 1993 to 1995. The T, LAM, and Beijing lineages were the largest genotypes that were circulating in both GP and WCP of South Africa and were the driving forces behind drug-resistant TB in the early 1990s. These genotypes were evenly distributed in both provinces.
The predominance of these genotypes in the two South African provinces reported here reflects the genotypes that are widely found in Africa and other parts of the world and concur with the current distribution of genotypes in South Africa. Generally, the even distribution of the Beijing family between GP and WCP in this study differs from reports suggesting that the genotype is more commonly found in WCP than in other provinces in South Africa.
Furthermore, the S and LAM4/F15/KZN genotypes were present in GP. These two genotypes are known to be dominant in the KZNP. Strain exchange between GP and KZNP is not unexpected, because of traditional migrant labor opportunities in GP that tended to draw labor from KZNP to GP.
MIRU-VNTR 24-locus analysis was specifically performed on a subgroup of strains representing the major spoligotype clusters. MIRU-VNTR further broke down the isolates into seven clusters of 16 isolates. The combination of spoligotyping and 24-locus MIRU-VNTR provided a better resolution of the large spoligotyping clusters, resulting in a final definition of six clusters of 14 isolates (Table 5).
In conclusion, Illumina Miseq WGS showed better concordance with the LJ phenotypic assay than with LPA. WGS detected mutations (13 rpoB, 32 katG, 1 inhA, and 10 embB mutations) that were missed by the LPA. The majority of these mutations were located outside the “hot spot” region normally detected by the LPA. Some of these mutations are novel and have not been reported elsewhere in the literature. This points to a need for confirming discordant results between phenotypic drug susceptibility testing (DST) and LPA by WGS. However, this study also showed high concordance between LPA and WGS in detecting common mutations, confirming that the LPA is a reliable screening assay for drug resistance.
Observations from this study also highlight the diagnostic advantages of WGS over rapid genotyping methods, as most conventional molecular assays are unable to detect mutations outside known “hot spot” regions. Our findings point to new opportunities for rapid molecular diagnostic test developers to improve on the current range of targets included in current assays.
Specifically, the R463L mutation on the katG gene is not currently included in rapid genotyping assays. Together with the novel mutations in the pncA gene, additional discriminatory power might be achieved if these targets were considered for inclusion in new rapid molecular tests for improved detection of INH- and PZA-resistant M. tuberculosis strains.
The limitations of the study include the following: WGS was not applied to all 240 isolates in the study to obtain a full picture of drug-resistant patterns in the two provinces because of the cost involved in employing the technology on such an extensive collection of isolates. Also, PZA and second-line drug susceptibility testing was not initially conducted on all the WCP isolates, as opposed to the GP isolates, raising the possibility that results from WCP might provide a biased picture of the mutation pattern. Finally, the cultures from which the genomic DNA was extracted for this study were nonviable, as they had been stored at a moderately low temperature (4°C) for decades and could not be recultured for repeat phenotypic DST.
Ethics.
Ethical approval was obtained from the University of Pretoria Ethics Committee (Ethics code 56/2013).
Study site.
The study was conducted at the laboratories of the South African Medical Research Council (MRC) in Pretoria, the Department of Medical Microbiology of the University of Pretoria, and for whole-genome sequencing, at the Research Laboratories of Longhorn Vaccines & Diagnostics, San Antonio, TX, USA. The MIRU-VNTR was conducted at the National Institute of Communicable Diseases, TB reference laboratory.
ACKNOWLEDGMENTS
N.E.M. received Ph.D. study bursaries from the University of Pretoria, the South African Medical Research Council, and the National Research Foundation of South Africa. She was also awarded a Global Infectious Diseases Research Training Fogarty Fellowship in 2013, which enabled access to next-generation sequencing laboratory facilities and training with Luke T. Daum (Longhorn Vaccines and Diagnostics, San Antonio, TX, USA) and James P. Chambers (University of Texas at San Antonio, San Antonio, TX, USA).
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 3.Cohen KA, Abeel T, McGuire AM, Desjardins CA, Munsamy V, Shea TP, Walker JB, Bantubani N, Almeida DV, Alvarado L, Chapman SB, Mvelase NR, Duffy EY. 2015. Evolution of extensively drug-resistant tuberculosis over four decades: whole genome sequencing and dating analysis of Mycobacterium tuberculosis isolates from KwaZulu-Natal. PLoS Med 12 (9):e1001880. doi: 10.1371/journal.pmed.1001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loerger TR, Koo S, No E-G, Chen X, Larsen MH, Jacobs WR Jr, Pillay M, Sturm AW, Sacchettini JC. 2009. Genome analysis of multi- and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 4:e7778. doi: 10.1371/journal.pone.0007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambau E, Viveiros M, Machado D, Raskine L, Ritter C, Tortoli E, Matthys V, Hoffner S, Richter E, Perez Del Molino ML, Cirillo DM. 2014. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother 70:686–696. doi: 10.1093/jac/dku438. [DOI] [PubMed] [Google Scholar]
- 6.Gomathi NS, Kumar V. 2014. Reliability of Mycobacterial Growth Indicator Tube (MGIT) 960 for the detection of isoniazid resistance in a tuberculosis endemic setting. Indian J Med Res 139:471–473. [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. 2008. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med 177:787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 8.Metzker ML. 2010. Sequencing technologies—the next generation. Nat Rev Genet 11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 9.WHO/International Union Against Tuberculosis and Lung Diseases (IUATLD) 2001. Guidelines for drug susceptibility testing. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. 2009. Tuberculosis drug resistance mutation database. PLoS Med 6:e2. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans J, Stead MC, Nicol MP, Segal H. 2009. Rapid genotypic assays to identify drug-resistant Mycobacterium tuberculosis in South Africa. J Antimicrob Chemother 63:11–16. doi: 10.1093/jac/dkn433. [DOI] [PubMed] [Google Scholar]
- 13.Dymova MA, Cherednichenko AG, Alkhovik OI, Khrapov EA, Petrenko TI, Filipenko ML. 2014. Characterization of extensively drug-resistant Mycobacterium tuberculosis isolates circulating in Siberia. BMC Infect Dis 14:478. doi: 10.1186/1471-2334-14-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daum LT, Rodriguez JD, Worthy SA, Ismail NA, Omar SV, Dreyer AW, Fourie PB, Hoosen AA, Chambers JP, Fischer GW. 2012. Next-generation Ion Torrent sequencing of drug resistance mutations in Mycobacterium tuberculosis strains. J Clin Microbiol 50:3831–3837. doi: 10.1128/JCM.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Yue J, Yang YP, Zhang HM, Lei JQ, Jin RL, Zhang XL, Wang HH. 2005. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J Clin Microbiol 43:5477–5482. doi: 10.1128/JCM.43.11.5477-5482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, Liu Z, Li C, Galwey N, Deng J, Zhou Y. 2013. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet 45:1255–1260. doi: 10.1038/ng.2735. [DOI] [PubMed] [Google Scholar]
- 17.Somoskovi A, Dormandy J, Parsons LM, Kaswa M, Goh Rastogi KS N, Salfinger M. 2007. Sequencing of the pncA gene in members of the Mycobacterium tuberculosis complex has important diagnostic applications: identification of a species-specific pncA mutation in “Mycobacterium canettii” and the reliable and rapid predictor of pyrazinamide resistance. J Clin Microbiol 45:595–599. doi: 10.1128/JCM.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsåker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol 46:3459–3464. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maningi NE, Daum LT, Rodriguez JD, Mphahlele M, Peters RPH, Fischer GW, Chambers JP, Fourie PB. 2015. Improved detection by next-generation sequencing of pyrazinamide resistance in Mycobacterium tuberculosis isolates. J Clin Microbiol 53:3779–3783. doi: 10.1128/JCM.01179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louw GE, Warren RM, Donald PR, Murray MB, Bosman M, van Helden PD, Young DB, Victor TC. 2006. Frequency and implications of pyrazinamide resistance in managing previously treated tuberculosis patients. Int J Tuberc Lung Dis 10:802–807. [PubMed] [Google Scholar]
- 21.Nair J, Rouse DA, Bai GH, Morris SL. 1993. The rpsL gene and streptomycin resistance in single and multiple drug-resistant strains of Mycobacterium tuberculosis. Mol Microbiol 10:521–527. doi: 10.1111/j.1365-2958.1993.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 22.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol 48:1683–1689. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safi H, Fleischmann RD, Peterson SN, Jones MB, Jarrahi B, Alland D. 2010. Allelic exchange and mutant selection demonstrate that common clinical embCAB gene mutations only modestly increase resistance to ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother 54:103–108. doi: 10.1128/AAC.01288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Zhang X, Jiang X, Yuan H, Lee JC, Barry CE III, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonova OV, Gryadunov DA, Lapa SA, Kuz'min AV, Larionova EE, Smirnova TG, Nosova EY, Skotnikova OI, Chernousova LN, Moroz AM, Zasedatelev AS. 2008. Detection of mutations in Mycobacterium tuberculosis genome determining resistance to fluoroquinolones by hybridization on biological microchips. Bull Exp Biol Med 145:108–113. doi: 10.1007/s10517-008-0034-5. [DOI] [PubMed] [Google Scholar]
- 26.Hoshide M, Qian L, Rodrigues C, Warren R, Victor T, Evasco HB II, Tupasi T, Crudu V, Douglas JT. 2014. Geographical differences associated with single-nucleotide polymorphisms (SNPs) in nine gene targets among resistant clinical isolates of Mycobacterium tuberculosis. J Clin Microbiol 52:1322–1329. doi: 10.1128/JCM.00857-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci U S A 94:9869–9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Q, Dai G, Long Q, Yu X, Dong L, Huang H, Xie J. 2013. Mycobacterium tuberculosis rrs A1401G mutation correlates with high-level resistance to kanamycin, amikacin, and capreomycin in clinical isolates from mainland China. Diagn Microb Inf Dis 77:138–142. doi: 10.1016/j.diagmicrobio.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 29.Engström A, Perskvist N, Werngren J, Hoffner SE, Jureen P. 2011. Comparison of clinical isolates and in vitro selected mutants reveals that tlyA is not a sensitive genetic marker for capreomycin resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 66:1247–1254. doi: 10.1093/jac/dkr109. [DOI] [PubMed] [Google Scholar]
- 30.Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L. 2009. High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother 53:5064–5068. doi: 10.1128/AAC.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]