ABSTRACT
Serology is the preferred method to confirm a Chagas disease diagnosis and to screen blood donors. A battery of assays is often required due to the limited accuracy of single assays. The Elecsys Chagas assay is a newly developed, double-antigen sandwich assay for use on the Elecsys and cobas e immunoassay analyzers, intended to identify individuals infected with Trypanosoma cruzi, for diagnosis and screening. The performance of the Elecsys Chagas assay was evaluated in comparison with those of other widely used T. cruzi antibody assays, at multiple sites (Europe/Latin America). Relative sensitivity and specificity were assessed by using samples from blood donors, pregnant women, and hospitalized patients from regions where Chagas disease is endemic and from regions of nonendemicity. The Elecsys Chagas assay had an overall relative sensitivity of 100% (n = 674). Overall relative specificities were 99.90% (n = 14,681), 100% (n = 313), and 100% (n = 517) for samples from blood donors, pregnant women, and hospitalized patients, respectively. The analytical specificity was 99.83% (n = 594). The Elecsys Chagas assay detected T. cruzi antibodies in two World Health Organization (WHO) standard T. cruzi reference panels (panels 09/188 and 09/186) at a 1:512 dilution, corresponding to a cutoff sensitivity of approximately 1 mIU/ml. The Elecsys Chagas assay demonstrated robust performance under routine conditions at multiple sites in Europe and Latin America. In contrast to other available Chagas assays, the Elecsys assay uses a reduced number of recombinant T. cruzi antigens, resulting in a significantly smaller number of cross-reactions and improved analytical specificity while being highly sensitive.
KEYWORDS: Chagas disease, electrochemiluminescence immunoassay, serology, Trypanosoma cruzi
INTRODUCTION
Chagas disease (American trypanosomiasis) is caused by the flagellate protozoan Trypanosoma cruzi and affects 6 million to 7 million people worldwide, mainly in Latin America (1, 2). Vector-borne transmission via insects of the subfamily Triatominae occurs in the Americas; however, infection can also be transmitted congenitally and via blood transfusion, organ transplantation, and the ingestion of food/beverages contaminated with parasites (3–6). Although it mainly affects individuals living in regions of endemicity, the disease has now spread to other regions and continents (7–10).
The infection is characterized by an acute, often asymptomatic stage lasting 8 to 12 weeks, when active parasitemia is evident. During this stage, diagnosis can be performed by direct microscopy of blood for circulating parasites or via PCR. The infection subsequently enters a chronic phase (1, 11, 12), and the majority of individuals remain asymptomatic. However, up to 30% of individuals will develop Chagas cardiomyopathy over decades, and up to 10% will develop gastrointestinal, neurological, or oligosymptomatic alterations that require treatment (1, 11, 12); these pathologies typically develop over years to decades. Generally, in the chronic phase of infection, the management of specific symptoms/conditions is necessary (13–15). The availability of curative treatment is a controversial topic; the WHO recommends treatment of adults with antiparasitic drugs to prevent disease progression and congenital transmission in pregnant women (1).
Importantly, chronically infected individuals represent a substantial population capable of transmitting the infection, particularly through blood or organ donation or from mother to child (4, 6). During the chronic phase, individuals exhibit low-level or no parasitemia, and thus, direct microscopy is inappropriate. PCR detects the parasite in 40 to 65% of patients with chronic disease (16, 17); consequently, diagnosis relies upon the detection of T. cruzi antibodies by serological methods (13, 17, 18).
The most commonly used serological methods are enzyme-linked immunosorbent assay (ELISA) and immunofluorescence indirect test (IFI) (13, 17, 19); a few automated systems based on chemiluminescence have been introduced (38, 40). The diagnostic approach to Chagas disease is heterogeneous, with guidelines varying according to location (i.e., laboratory, region, or country) and purpose (i.e., screening of blood/organ donors versus diagnosis of patients with symptomatic disease) (22–26). Conventional tests based on total antigens show cross-reactivity between T. cruzi and Leishmania spp. or Trypanosoma rangeli; therefore, confirmation of the presence of T. cruzi antibodies commonly requires the use of at least two tests that are based on different methods/antigens. Furthermore, the resolution algorithms (i.e., sequence, type, and number of tests used) also vary by region (22, 25, 27). However, with the availability of assays with improved sensitivity and specificity, it has been suggested that a single assay may now be adequate for screening and diagnosis (28).
The primary aim of this study was to assess the relative sensitivity and specificity of the fully automated Elecsys Chagas assay in comparison with those of other state-of-the-art T. cruzi antibody assays. Secondary aims were the evaluation of analytical specificity and analytical sensitivity at the cutoff (CO) in dilution series against WHO standards.
MATERIALS AND METHODS
Study design.
The analytical performance of the Elecsys Chagas assay was evaluated at five independent laboratories in Europe and Latin America and at the Roche Diagnostics assay development facility. The study was performed between August 2015 and July 2016. All samples were anonymized or pseudonymized residual fresh or frozen serum/plasma samples from either daily routine or blood donor testing (Table 1).
TABLE 1.
Sample cohorts and assays used for relative sensitivity and specificity evaluation
| Site | Cohort type | No. of samples | Source | Condition | Matrix | Comparator assay tested |
|---|---|---|---|---|---|---|
| Relative specificity | ||||||
| Hagen, Germany | Blood donors | 4,391 | Blood screening | Fresh | Serum | Abbott Prism Chagas |
| Pievesestina, Italy | Blood donors | 5,244 | Blood screening | Fresh | Serum | Ortho T. cruzi ELISA; DiaSorin Liaison XL Murex |
| Hospitalized patients | 500 | Daily routine | Frozen | Serum | DiaSorin Liaison XL Murex | |
| Pregnant women | 239 | Daily routine | Frozen | Serum | DiaSorin Liaison XL Murex | |
| Bucaramanga, Colombia | Blood donors | 2,707 | Blood screening | Fresh | Plasma | Abbott Architect Chagas |
| Buenos Aires, Argentina | Blood donors | 1,056 | Blood screening | Fresh | Serum | Abbott Architect Chagas |
| Blood donors | 1,283 | Blood screening | Frozen | Serum | Abbott Architect Chagas | |
| Hospitalized patients | 17 | Daily routine | Fresh | Serum | Abbott Architect Chagas | |
| Pregnant women | 74 | Daily routine | Fresh | Serum | Abbott Architect Chagas | |
| Relative sensitivity | ||||||
| Madrid, Spain | Chagas positive | 500 | Collection of stored samples | Frozen | Serum/plasma | Ortho T. cruzi ELISA |
| Buenos Aires, Argentina | Chagas positivea | 174 | Serotheque | Frozen | Serum | Abbott Architect Chagas |
Samples collected at the Universidad Nacional del Litoral, Santa Fe, Argentina.
Prior to the start of the study, ethical approval (or waiver) was obtained from the relevant local authorities. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation guidelines for good clinical practice. Where necessary, donors/participants provided written informed consent.
Elecsys Chagas and comparator assays.
The Elecsys Chagas assay is an automated electrochemiluminescence immunoassay for the qualitative determination of antibodies to T. cruzi for use on cobas e analyzers (clinical chemistry analyzer and immunochemistry analyzer) in equipped laboratory settings. The assay is based on a double-antigen sandwich principle, utilizing soluble forms of recombinant T. cruzi antigens derived from flagellar calcium binding protein, flagellar repetitive antigen, and cruzipain (the major cysteine proteinase of T. cruzi).
During the first incubation, 18 μl of a sample is added to a reaction mixture containing biotin-labeled and ruthenium-labeled T. cruzi antigens to form antibody-antigen immune complexes (with one antigen binding site of the patient's specific immunoglobulin G [IgG] binding the biotinylated antigen and the other paratope binding the ruthenium-labeled antigen). In a second incubation, the IgG–double-antigen sandwich complex is bound via biotin to streptavidin-coated beads and subsequently transferred to the measuring cell, where the microparticles are magnetically captured on the surface of the electrode. Chemiluminescent emission from the ruthenium label is induced by the application of voltage to the electrode and measured by using a photomultiplier. The results are automatically determined by software based on comparison of the electrochemiluminescence signal obtained from the reaction product with the cutoff value previously obtained during system calibration. The total assay time is 18 min.
The following comparison assays were performed according to the manufacturers' recommendations (including calibration and the respective control runs): whole-cell lysate Ortho T. cruzi ELISA (Ortho Clinical Diagnostics, Johnson & Johnson, High Wycombe, UK), Abbott Architect Chagas and Abbott Prism Chagas (Abbott Diagnostics, IL, USA), DiaSorin Liaison XL Murex Chagas (DiaSorin SpA, Saluggia, Italy), Wiener Lab Chagatest ELISA recombinante v.4.0 (Wiener Lab Group, Rosario, Argentina), NovaTech NovaLisa Chagas (NovaTech Immundiagnostica GmbH, Dietzenbach, Germany), and Biokit Bioelisa Chagas IgG (Biokit SA, Barcelona, Spain).
Relative sensitivity.
The relative sensitivity of the Elecsys Chagas assay was evaluated at sites in Europe (n = 1) and Latin America (n = 1) by using precharacterized Chagas disease-positive samples obtained from Chagas disease-infected patients in both regions. Comparison assays were the whole-cell-lysate Ortho T. cruzi ELISA and the Abbott Architect Chagas assay. Samples tested with the Ortho T. cruzi ELISA were previously characterized by using in-house assays (ELISA-Centro Nacional de Microbiología [CNM] and IFI-CNM) (29), Wiener Lab Chagatest ELISA recombinante v.4.0, and PCR (30), while samples tested with the Abbott Architect Chagas assay were previously characterized with at least two of the following serology assays: Wiener Lab Chagatest ELISA recombinante v. 4.0, Wiener Lab Chagatest hemagglutination indirect test (HAI), and in-house IFI (antigens and controls were provided by the National Institute of Parasitology Dr. Mario Fatala Chaben, Buenos Aires, Argentina). Tests for samples with nonreactive results in the comparison assay were repeated in triplicate.
Relative specificity.
The relative specificity of the Elecsys Chagas assay was evaluated at four sites in Europe (n = 2) and Latin America (n = 2), using samples from blood donors, pregnant women, and hospitalized patients obtained in both regions.
For the testing of samples from blood donors, comparison assays were the Ortho T. cruzi ELISA, the DiaSorin Liaison XL Murex Chagas assay, the Abbott Prism Chagas assay, and the Abbott Architect Chagas assay. For the testing of samples from pregnant women and hospitalized patients, comparison assays were the DiaSorin Liaison XL Murex Chagas and the Abbott Architect Chagas assays.
Initial determinations were carried out with single measurements. Samples with discrepant and concordant reactive results were repeated in duplicate for the respective assays and were considered to be repeatedly reactive (RR) if either of the retest results had a signal/cutoff ratio of ≥1.00.
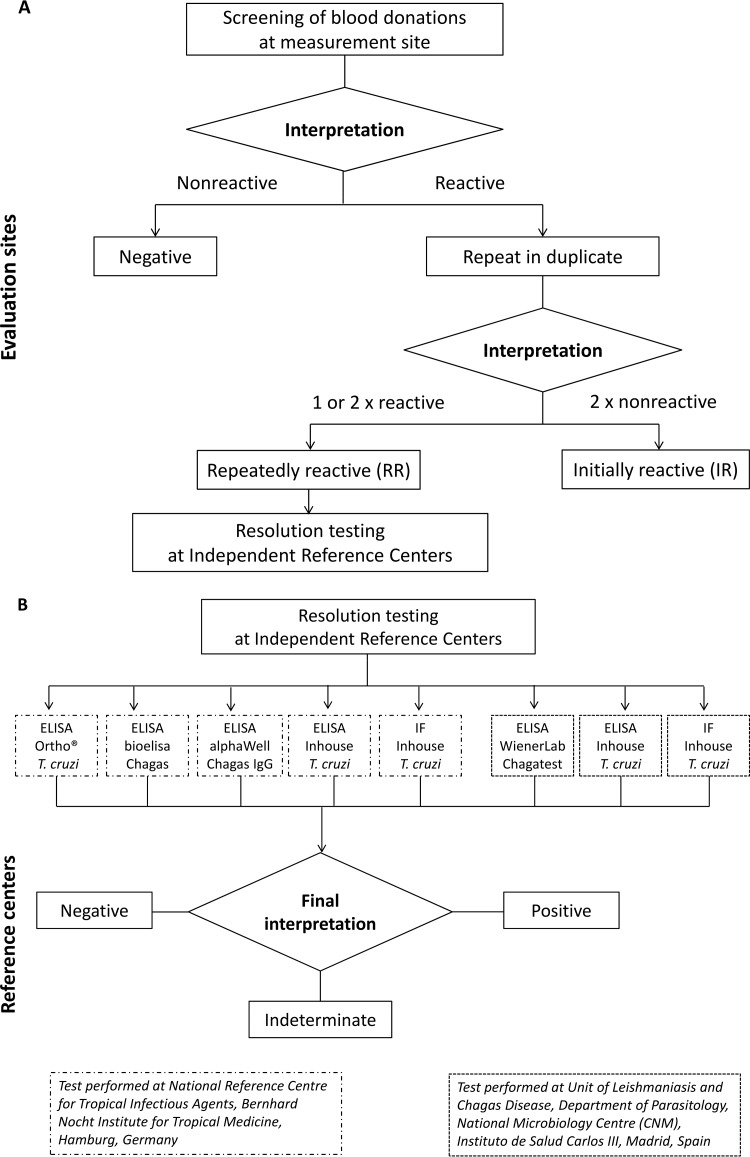
Initially reactive (IR) samples with incomplete retesting, or without retesting, were considered RR. IR and RR gray-zone samples for the Abbott Architect Chagas assay were considered reactive. Furthermore, an aliquot of discrepant and concordant reactive samples was stored for further resolution testing at two reference centers according to their local diagnostic algorithms (representing the surrogate “gold standard”) (see below and Fig. 1).
FIG 1.
(A) Sample workflow for evaluation of relative specificity. (B) Resolution algorithm for evaluation of samples found repeatedly reactive during specificity testing. Resolution testing was performed at two independent reference centers. ELISA, enzyme-linked immunosorbent assay; IF, immunofluorescence.
Analytical specificity.
The analytical specificity of the Elecsys Chagas assay was evaluated at two sites using potentially cross-reacting samples for other infectious diseases (see Table 4), e.g., leishmaniasis, malaria, Epstein-Barr virus, dengue virus, syphilis, toxoplasmosis, and human African trypanosomiasis.
TABLE 4.
Analytical specificity of the Elecsys Chagas assay with potentially cross-reactive samplesa
| Potentially cross-reacting condition or disease stateb | Total no. of samples | No. (%) of nonreactive samples | No. (%) of reactive samples |
|---|---|---|---|
| Epstein-Barr virus | 26 | 26 (100) | 0 |
| Dengue virus | 87 | 87 (100) | 0 |
| Leishmaniasis | 241 | 241 (100) | 0 |
| Malaria | 204 | 203 (99.5) | 1 (0.5) |
| Syphilis | 19 | 19 (100) | 0 |
| Toxoplasmosis | 15 | 15 (100) | 0 |
| Human African trypanosomiasis | 2 | 2 (100) | 0 |
| Total | 594 | 593 | 1 |
Samples were tested at Roche Diagnostics Centralized and Point of Care Solutions (Penzberg, Germany), unless stated otherwise.
A total of 164 Leishmania-positive and 100 malaria-positive serum/plasma samples were tested in Madrid, Spain; samples (serotheque) were previously stored frozen. Samples used for the analytical specificity study were derived from commercial vendors (Acess Biologicals, USA; Slieagen LLC, USA, Cerba Specimens Services, France; Trina Bioreactives AG, Switzerland; BioClinical Partner Inc., USA; and DiaServe GmbH, Germany), routine laboratories, and institutions (Instituto de Salud Carlos III, Madrid, Spain). Characterization of the samples was done by either serological analysis, parasitological certificate, or clinical definition.
Analytical sensitivity at the cutoff.
Serial dilutions of two anti-T. cruzi antibody preparations from the National Institute for Biological Standards and Control (NIBSC) were measured in a single run (single determination per sample) by using the Elecsys Chagas assay and comparison assays. The WHO 1st International Standard for Chagas (TcI) antibody in human plasma (NIBSC panel 09/188) freeze-dried preparation contains anti-T. cruzi antibodies and consists of seropositive samples from autochthonous individuals living in Mexico, the region where T. cruzi genotype I is endemic. The WHO 1st International Standard for Chagas (T. cruzi genotype II [TcII]) antibody in human plasma (NIBSC panel 09/186) freeze-dried preparation contains anti-T. cruzi antibodies and is representative of seropositive samples from autochthonous individuals living in Brazil, the region where T. cruzi II is endemic.
Each standard was dissolved in deionized water to a final concentration of 0.5 IU/ml. Serial 1:2 pool dilutions were performed by using Chagas-negative serum and distributed to the laboratories for analysis (dilutions ranged from 1:2 to 1:8,192, corresponding to theoretical concentrations of 250 to 0.0610 mIU/ml of the respective antibody standards).
Comparison assays were the Abbott Prism Chagas assay, the Abbott Architect Chagas assay, the DiaSorin Liaison XL Murex Chagas assay, the Wiener Lab Chagatest, the Ortho T. cruzi ELISA for whole-cell lysates, the NovaTech NovaLisa Chagas assay, and the Biokit Bioelisa Chagas IgG assay.
Confirmatory testing: resolution testing.
Discrepant and concordant reactive results from relative specificity testing underwent resolution testing at two independent reference centers using Conformité Européenne-labeled or in-house methods representing state-of-the-art Chagas antibody assays (Fig. 1). The final interpretation of the result for each sample was used as the basis for the assessment of relative specificity.
Confirmatory testing: neutralization testing.
If sufficient sample volumes remained after resolution testing (Fig. 1), discrepant Elecsys Chagas results for reactive samples from the blood donor cohort were retested by using an in-house neutralization method similar to that reported previously (31). Briefly, the antigen extract (aqueous ultrasonic lysate supernatant) from native T. cruzi (CL Brener or DM28c) was added to the samples to a final concentration of 50 μg/ml T. cruzi antigen extract to generate a competitive situation between the recombinant antigens used in the Elecsys Chagas assay and the native T. cruzi antigen extract. After this pretreatment procedure was done to form stable antigen-antibody complexes, samples were subsequently rerun with the Elecsys Chagas assay. The obtained cutoff index (COI) values were compared with those derived by using the untreated sample, and the recovery of the neutralized sample was calculated. A recovery of ≤25% of the initial concentration was interpreted as reconfirmation of the presence of anti-T. cruzi antibodies.
Data analyses.
Measurements were captured by using WinCAEv software; statistical analyses were performed by using R-package VCA (version 1.2.1) and SAS (version 9.3; SAS Institute). Interpretation of assay results was performed according to the package inserts of the respective assays.
Relative sensitivity and specificity, and analytical sensitivity, are expressed as point estimates and two-sided 95% confidence intervals (CIs). For the Elecsys Chagas assay, samples with a signal/cutoff (CO) ratio of ≥1.0 were considered reactive (i.e., positive for antibodies to T. cruzi), and those with a signal/CO ratio of <1.0 were considered nonreactive (i.e., negative for antibodies to T. cruzi). Results of the comparator assays were interpreted as follows: reactive if values were higher than or equal to the CO and nonreactive if values were lower than the CO for the whole-cell-lysate Ortho T. cruzi ELISA; reactive if signal/CO ratios were ≥1.0, gray zone if signal/CO ratios were ≥0.8 to <1.0, and nonreactive if signal/CO ratios were <0.8 for Abbott Architect Chagas; reactive if signal/CO ratios were ≥1.0 and nonreactive if signal/CO ratios were <1.0 for Abbott Prism Chagas; reactive if signal/CO ratios were ≥1.0 and nonreactive if signal/CO ratios were <1.0 for DiaSorin Liaison XL Murex Chagas; reactive if values were higher than or equal to the CO and nonreactive if values were lower than the CO for Wiener Lab Chagatest; reactive if values were higher than or equal to the CO for absorbance plus 10%, gray zone if values were ±10% of the CO signal, and negative if values were lower than the CO signal minus 10% for NovaTech NovaLisa Chagas; and positive if signal/CO ratios were ≥1.0, equivocal if signal/CO ratios were ≥0.9 to <1.0, and negative if signal/CO ratios were <0.9 for Biokit Bioelisa Chagas IgG.
RESULTS
Relative sensitivity.
A total of 674 precharacterized positive frozen samples were tested (Table 1). Precharacterization was performed via serology and PCR (n = 158) or via serology alone (n = 516), with and without clinical staging (n = 135 and n = 381, respectively) (Table 2).
TABLE 2.
Characterization of the cohort used to determine relative sensitivity
| Cohort of Chagas disease-positive samples (n = 674) | Tests usedd |
|---|---|
| Regions of endemicity (n = 174)a | |
| Serological characterization (n = 174) | ELISA, HAI, antibodies induced by T. cruzi (anti-T. cruzi homogenate, anti-FRA, anti-p2β, anti-B13) |
| Serological characterization + clinical stage characterization (n = 135)b | ELISA, HAI, antibodies induced by T. cruzi (anti-T. cruzi homogenate, anti-FRA, anti-p2β, anti-B13), chest and abdominal X rays, electrocardiogram, echocardiogram |
| Regions of nonendemicity (n = 500)c | |
| Serological characterization (n = 342) | In-house CNM IFAT, in-house CNM ELISA, Wiener Lab Chagatest ELISA recombinante v.4.0 |
| PCR + serological characterization (n = 158) | T. cruzi kDNA PCR, in-house CNM IFAT, in-house CNM ELISA, Wiener Lab Chagatest ELISA recombinante v.4.0 |
Samples provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Chronic stage I (n = 66), stage II (n = 44), and stage III (n = 25) Chagas disease.
Samples provided by the Instituto de Salud Carlos III, Spain.
Anti-FRA, anti-flagellar repetitive antigen; IFAT, indirect fluorescent antibody technique; kDNA, kinetoplast DNA.
All Chagas disease-positive samples were correctly identified by the Elecsys Chagas assay (sensitivity of 100%; 95% CI, 99.45 to 100%). The Abbott Architect assay correctly identified all Chagas disease-positive samples (sensitivity, 100%; 95% CI, 97.90 to 100%; n = 174), while the Ortho T. cruzi assay detected positive samples with a sensitivity of 96% (95% CI, 93.89 to 97.54%; n = 500).
Relative specificity.
A total of 14,681 samples from blood donors were tested (Fig. 1A); the relative specificities of the Elecsys Chagas assay (and comparison methods) after resolution testing (Fig. 1B) are summarized in Table 3. The Elecsys Chagas assay had overall relative specificities of 99.88% (for IR samples) (95% CI, 99.81 to 99.93%) and 99.90% (for RR samples) (95% CI, 99.83 to 99.94%). Relative specificities were 99.70% (for IR samples) (95% CI, 99.51 to 99.83%) and 99.74% (for RR samples) (95% CI, 99.56 to 99.86%) for the Latin American subgroup and 99.98% (for IR and RR samples) (95% CI, 99.93 to 100%) for the European subgroup. Overall, there were 26 qualitative discrepant results (n = 10 for Europe, and n = 16 for Latin America) versus the results of comparator assays, and 13 concordant reactive results (Latin America) were identified. The relative specificities (for RR samples) were 100% with the Ortho T. cruzi ELISA (95% CI, 99.93 to 100%) (n = 5,241), 99.96% with the DiaSorin Liaison assay (95% CI, 99.86 to 100%) (n = 5,244), 99.93% with the Abbott Prism assay (95% CI, 99.80 to 99.99%) (n = 4,391), and 99.78% with the Abbott Architect assay (95% CI, 99.61 to 99.89%) (n = 5,046).
TABLE 3.
Relative specificity of the Elecsys Chagas assay in blood donor samplesa
| Parameter | Value for test |
||||
|---|---|---|---|---|---|
| Elecsys Chagas | Ortho T. cruzi ELISA | DiaSorin Liaison XL Murex Chagas | Abbott Prism Chagas | Abbott Architect Chagas | |
| Overall cohort | |||||
| Total no. of samples | 14,681 | 5,241 | 5,244 | 4,391 | 5,046 |
| No. of confirmed positive samples | 8 | 0 | 0 | 0 | 8 |
| No. of negative samples | 14,673 | 5,241 | 5,244 | 4,391 | 5,038 |
| No. of IR samples | 25 | 0 | 2 | 6 | 15 |
| No. of RR samplesb | 23 | 0 | 2 | 3 | 14 |
| No. of RR samples confirmed positive/total no. of RR samples | 8/23 | 0/0 | 0/2 | 0/3 | 8/14 |
| Specificity (%) for IR samples (95% CI) | 99.88 (99.81–99.93) | 100 (99.93–100) | 99.96 (99.86–100) | 99.86 (99.70–99.95) | 99.78 (99.61–99.89) |
| Specificity (%) for RR samples (95% CI) | 99.90 (99.83–99.94) | 100 (99.93–100) | 99.96 (99.86–100) | 99.93 (99.80–99.99) | 99.78 (99.61–99.89) |
| European subgroup | |||||
| Total no. of samples | 9,635 | 5,241 | 5,244 | 4,391 | NA |
| No. of IR samples | 2 | 0 | 2 | 6 | NA |
| No. of RR samples | 2 | 0 | 2 | 3 | NA |
| No. of RR samples confirmed positive/total no. of RR samples | 0/2 | 0/0 | 0/2 | 0/3 | NA |
| Specificity (%) for IR samples (95% CI) | 99.98 (99.93–100) | 100 (99.93–100) | 99.96 (99.86–100) | 99.86 (99.70–99.95) | NA |
| Specificity (%) for RR samples (95% CI) | 99.98 (99.93–100) | 100 (99.93–100) | 99.96 (99.86–100) | 99.93 (99.80–99.99) | NA |
| Latin American subgroup | |||||
| Total no. of samples | 5,046 | NA | NA | NA | 5,046 |
| No. of IR samples | 23 | NA | NA | NA | 15 |
| No. of RR samplesb | 21 | NA | NA | NA | 14 |
| No. of RR samples confirmed positive/total no. of RR samples | 8/21 | NA | NA | NA | 8/14 |
| Specificity (%) for IR samples (95% CI) | 99.70 (99.51–99.83) | NA | NA | NA | 99.78 (99.61–99.89) |
| Specificity (%) for RR samples (95% CI) | 99.74 (99.56–99.86) | NA | NA | NA | 99.78 (99.61–99.89) |
CI, confidence interval; IR, initially reactive; NA, not applicable; RR, repeatedly reactive.
Thirteen reactive samples (in at least one assay) with incomplete retesting were considered RR for specificity calculations.
A total of 313 residual samples from pregnant women were tested. There were no discrepant or concordant reactive results. The Elecsys Chagas assay had an overall relative specificity of 100% (for IR and RR samples) (95% CI, 98.83 to 100%), which was generally consistent between the subgroups in the region of endemicity (Latin America) (100% for IR and RR samples [95% CI, 95.14 to 100%]) (n = 74 samples) and the region of nonendemicity (European) (100% for IR and RR samples [95% CI, 98.47 to 100%]) (n = 239 samples). Comparable results were obtained with the DiaSorin Liaison and Abbott Architect assays (data not shown).
A total of 517 residual samples from hospitalized patients were tested, and there were no discrepant or concordant reactive results. The Elecsys Chagas assay had an overall relative specificity of 100% (for IR and RR samples) (95% CI, 99.29 to 100%), which was generally consistent between the Latin American (100% for IR and RR samples [95% CI, 80.49 to 100%]) (n = 17 samples) and European (100% for IR and RR samples [95% CI, 99.26 to 100%]) (n = 500 samples) subgroups. Comparable results were obtained with the DiaSorin Liaison XL Murex Chagas and Abbott Architect assays (data not shown).
Analytical specificity.
A total of 594 potentially cross-reactive samples were tested with the Elecsys Chagas assay (Table 4), and the overall analytical specificity was 99.83% (95% CI, 99.07 to 100%).
A subgroup of precharacterized leishmaniasis-positive samples (n = 164) and malaria-positive samples (n = 100) from Spain were tested by both the Elecsys Chagas assay and the Ortho T. cruzi ELISA. In the leishmaniasis-positive cohort, all samples were nonreactive for T. cruzi antibodies with the Elecsys Chagas assay (analytical specificity of 100%; 95% CI, 97.78 to 100%), while 65 samples tested positive with the Ortho T. cruzi ELISA (analytical specificity of 60.37%; 95% CI, 52.44 to 67.91%). In the malaria-positive cohort, one sample was reactive with the Elecsys Chagas assay (low-level COI of 1.11; analytical specificity of 99.00%; 95% CI, 94.55 to 99.97%) and four samples tested positive with the Ortho T. cruzi ELISA (analytical specificity of 96.00%; 95% CI, 90.07 to 98.90%). Plasmodium-T. cruzi coinfection was ruled out. These samples were obtained from Spanish citizens and immigrant residents who had traveled to South Asia and/or Africa.
Six additional samples (n = 5 for dengue, and n = 1 for leishmaniasis) were excluded from the analysis because a coinfection could not be ruled out. These samples originated from a region where Chagas disease is endemic (Argentina) and were found to be reactive in the Elecsys Chagas assay (with COI values ranging from 13.3 to 206) as well as in at least one additional Chagas antibody assay. Four out of five dengue samples thereof were also found to be highly reactive in at least two comparator Chagas assays. Further resolution testing was not possible due to a lack of sample volume.
Analytical sensitivity at the cutoff.
The analytical sensitivities at the cutoff of the Elecsys Chagas assay and comparator assays (n = 3 automated assays, and n = 4 nonautomated ELISAs, reflecting local routine methods) were assessed by using two WHO standard NIBSC reference panels. The Elecsys Chagas assay detected T. cruzi antibodies at a 1:512 dilution for both reference panels (Table 5), corresponding to a cutoff sensitivity of approximately 1 mIU/ml. Comparison assays were found reactive at dilutions of 1:32 for panel 09/188 (T. cruzi I) and 1:16 for panel 09/186 (T. cruzi II), corresponding to cutoff sensitivities of 15.6 mIU/ml and 31.3 mIU/ml, respectively, or less sensitivity.
TABLE 5.
Detection limits of the Elecsys Chagas and comparison assays using World Health Organization standard National Institute for Biological Standards and Control reference panels 09/188 (T. cruzi I) and 09/186 (T. cruzi II)a
| Dilution | Concn (mIU/ml) | Reactivity (signal/CO) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Elecsys Chagas | Abbott Prism Chagas | Abbott Architect Chagas | DiaSorin Liaison XL Murex Chagas | Wiener Lab Chagatest | Ortho T. cruzi ELISA | NovaTech NovaLisa Chagas | Biokit Bioelisa Chagas | ||
| Panel 09/188 | |||||||||
| 1:8,192 | 0.06 | 0.14 | 0.06 | 0.26 | 0.03 | 0.20 | 0.31 | 0.52 | |
| 1:4,096 | 0.12 | 0.21 | 0.08 | 0.13 | 0.03 | 0.14 | 0.23 | 0.61 | |
| 1:2,048 | 0.24 | 0.32 | 0.06 | 0.21 | 0.03 | 0.19 | 0.25 | 0.56 | 0.04 |
| 1:1,024 | 0.49 | 0.57 | 0.07 | 0.19 | 0.03 | 0.20 | 0.38 | 0.61 | 0.04 |
| 1:512 | 0.98 | 1.02 | 0.07 | 0.20 | 0.03 | 0.20 | 0.38 | 0.53 | 0.07 |
| 1:256 | 1.95 | 2.00 | 0.11 | 0.31 | 0.04 | 0.26 | 0.36 | 0.53 | 0.02 |
| 1:128 | 3.91 | 3.87 | 0.14 | 0.47 | 0.05 | 0.34 | 0.38 | 0.54 | 0.07 |
| 1:64 | 7.81 | 7.63 | 0.17 | 0.92 | 0.08 | 0.49 | 0.55 | 0.55 | 0.10 |
| 1:32 | 15.6 | 15.1 | 0.95 | 2.02 | 0.17 | 0.69 | 0.85 | 0.63 | 0.13 |
| 1:16 | 31.3 | 30.4 | 1.19 | 3.17 | 0.36 | 1.00 | 1.11 | 0.81 | 0.15 |
| 1:8 | 62.5 | 58.8 | 2.10 | 5.14 | 0.73 | 1.68 | 1.78 | 0.99 | 0.46 |
| 1:4 | 125 | 118 | 2.89 | 6.57 | 1.50 | 2.63 | 2.58 | 1.60 | 0.84 |
| 1:2 | 250 | 209 | 5.64 | 8.32 | 2.80 | 3.44 | 3.81 | 2.34 | 1.97 |
| Undiluted | 500 | 246 | 6.25 | 9.78 | 4.90 | 4.37 | 4.78 | 3.37 | 1.41 |
| Panel 09/186 | |||||||||
| 1:8,192 | 0.06 | 0.15 | 0.06 | 0.14 | 0.03 | 0.22 | 0.27 | 0.55 | |
| 1:4,096 | 0.12 | 0.21 | 0.05 | 0.14 | 0.03 | 0.21 | 0.32 | 0.64 | |
| 1:2,048 | 0.24 | 0.34 | 0.06 | 0.13 | 0.03 | 0.21 | 0.59 | 0.58 | 0.06 |
| 1:1,024 | 0.49 | 0.61 | 0.07 | 0.14 | 0.03 | 0.23 | 0.43 | 0.54 | 0.06 |
| 1:512 | 0.98 | 1.10 | 0.06 | 0.19 | 0.03 | 0.21 | 0.33 | 0.54 | 0.05 |
| 1:256 | 1.95 | 2.08 | 0.06 | 0.20 | 0.03 | 0.27 | 0.31 | 0.54 | 0.06 |
| 1:128 | 3.91 | 4.00 | 0.09 | 0.27 | 0.04 | 0.29 | 0.30 | 0.52 | 0.08 |
| 1:64 | 7.81 | 7.89 | 0.09 | 0.37 | 0.04 | 0.38 | 0.41 | 0.56 | 0.12 |
| 1:32 | 15.6 | 15.6 | 0.10 | 0.74 | 0.07 | 0.73 | 0.50 | 0.60 | 0.16 |
| 1:16 | 31.3 | 30.7 | 0.27 | 1.49 | 0.11 | 0.95 | 0.87 | 0.70 | 0.27 |
| 1:8 | 62.5 | 60.2 | 0.95 | 2.54 | 0.27 | 1.43 | 1.41 | 0.92 | 0.51 |
| 1:4 | 125 | 112 | 2.84 | 4.56 | 0.54 | 2.54 | 1.92 | 1.09 | 1.01 |
| 1:2 | 250 | 192 | 2.21 | 6.52 | 1.10 | 3.41 | 2.96 | 1.76 | 1.86 |
| Undiluted | 500 | 229 | 5.04 | 8.49 | 1.90 | 4.43 | 4.12 | 2.95 | 1.50 |
Data in boldface type represent reactive results; lightface type, nonreactive results. Data in italic type represent results within the “gray zone” for the NovaTech NovaLisa or the Abbott Architect assay.
Typical distribution of values.
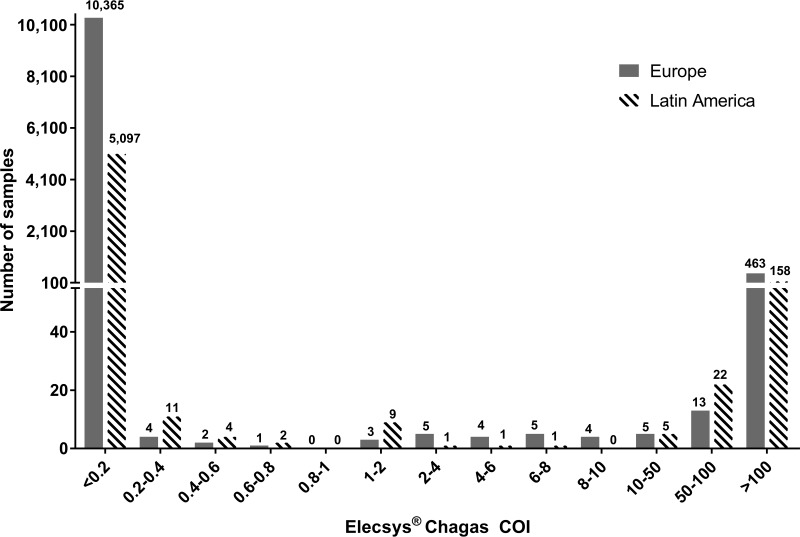
The distribution of COI values for 16,185 samples (reactive and nonreactive samples from blood donors, pregnant women, hospitalized patients, and confirmed Chagas disease-positive cases) is displayed in Fig. 2. The Elecsys Chagas assay revealed good discrimination between reactive and nonreactive samples. Only a small number of the samples were found to have low positive COI values in the Elecsys Chagas assay (n = 16,185; 12 samples with COI values ranging from 1 to 2, representing 0.07%) (Fig. 2).
FIG 2.
Distribution of COI values for reactive and nonreactive samples from blood donors, pregnant women, hospitalized patients, and Chagas-positive patients, measured with the Elecsys Chagas assay (n = 16,185) (suitable COI increments were chosen).
The lowest COI values observed for the precharacterized Chagas disease-positive cohort (n = 674) were 1.79 and 1.80, thus representing just 0.3% of the total positive-sample cohort. All other Chagas disease patient samples showed COI values ranging from 2.3 to >300.
Neutralization.
A total of 10 Elecsys assay-discrepant RR samples from the blood donor cohort underwent in-house neutralization testing to further assess the presence of antibodies to T. cruzi that might be undetectable by comparator methods with lower sensitivities. Five samples from regions of endemicity were successfully neutralized (≤25% recovery of the COI), and one sample from a region of endemicity showed a borderline tendency toward neutralization with the native antigen pretreatment procedure (26% recovery). Four samples could not be neutralized and revealed 28 to 98% COI recoveries (Table 6).
TABLE 6.
Neutralization results for Elecsys Chagas assay-discrepant, repeatedly reactive samples from blood donors
| Study site | Comparator COI | Elecsys COIa | Elecsys COI after neutralization | Recoveryb (%) |
|---|---|---|---|---|
| Italy | 0.016 | 2.94 | 1.87 | 64 |
| Colombia | 0.023 | 1.16 | 0.298 | 26 |
| Colombia | 0.037 | 1.09 | 0.300 | 28 |
| Colombia | 0.554 | 1.47 | 0.515 | 35 |
| Argentina | 0.230 | 12.6 | 0.348 | 3 |
| Argentina | 0.99/0.97/1.14c | 40.8 | 2.04 | 5 |
| Argentina | 0.040 | 2.92 | 0.227 | 8 |
| Argentina | 0.080 | 1.60 | 0.132 | 8 |
| Argentina | 0.510 | 1.47 | 0.152 | 10 |
| Argentina | 0.030 | 1.03 | 1.01 | 98 |
COI as determined by Roche Diagnostics Centralized and Point of Care Solutions (Penzberg, Germany) prior to the neutralization procedure.
A recovery of ≤25% was assessed as successful neutralization (i.e., positive for anti-T. cruzi antibodies). Values in boldface type represent neutralizable/borderline and neutralizable samples.
The COI was repeatedly in the gray zone.
DISCUSSION
In the present study, the Elecsys Chagas assay demonstrated excellent analytical performance in a multicenter study in Europe and Latin America compared with established assays. Although the Elecsys Chagas assay uses a new combination of just three different recombinant T. cruzi antigens, the performance observed in the present study supports its use as a diagnostic and screening test. Since screening for blood products harboring T. cruzi is a critical component for blood safety, we also investigated the performance of the test on samples from blood donors from various regions of endemicity and nonendemicity. Importantly, a substantial number of serum samples from patients known to have Chagas disease from different regions were included in the study. Furthermore, the excellent analytical specificity was confirmed by using a large panel of potentially cross-reactive samples or samples from individuals with other infectious diseases. Finally, the performance of the Elecsys Chagas assay was also verified with samples from pregnant women and hospitalized patients, and the results were comparable, irrespective of whether the samples were sourced from regions of endemicity or from regions of nonendemicity.
A number of studies showed various T. cruzi antigens (either native or recombinant or as peptide or multiepitope antigen) to potentially be suitable for use as serodiagnostic tools (32–37). However, the numbers of samples used for evaluation varied, and the statistical power of the results may be limited. Studies including a significant number of blood donor screening samples, potentially cross-reacting samples, and proven reactive samples are found less frequently (20, 38–41). WHO-driven comparison activities were conducted over a decade ago (42), with specificity and sensitivity varying considerably among the 18 screening assays evaluated. A definitive resolution of the discrepant findings was difficult since “consensus positives” and “consensus negatives” always inherit a selection bias, while the true serological status cannot be revealed (42). It may now be timely to conduct comparative evaluations of the new assays that have become available since that WHO study was reported, to ascertain their relative efficacies.
The WHO seeks to promote the identification of novel diagnostic tests for Chagas disease (1). Although there are a variety of methods available to confirm the presence of T. cruzi infection (ELISA-based methods, immunofluorescence-based methods, immunoblotting, PCR, and microscopy), there is currently no gold standard, and testing of samples with multiple assays is often necessary, creating a combined gold standard as a surrogate (13, 17, 28). This leaves manufacturers of new, highly sensitive tests with a dilemma.
In the present study, the surrogate gold standard against which the Elecsys assay was compared included at least three serological assays or PCR (for the evaluation of relative sensitivity) and resolution testing at two independent reference centers using several commercial CE-labeled tests and well-evaluated in-house assays representing state-of-the-art methods (for the evaluation of relative specificity).
Our study results support the notion that existing methods worldwide may not be adequate to confirm the results for samples with low-level antibody concentrations, which may be best confirmed according to the epidemiological and clinical background. Equally, the results for samples with higher concentrations in this study could potentially be confirmed with any test. Moreover, our findings add to the evidence suggesting that single assays with improved sensitivity and specificity may be sufficient for screening and diagnosis purposes, respectively (28).
Since the Elecsys Chagas assay was developed without a gray zone, and a clear separation of reactive versus nonreactive samples was validated in this multicenter evaluation, the potential number of unclear and inconclusive results or “low-titer” samples following the initial analysis is expected to be reduced significantly compared with previously described assays (28) or resolution algorithms (27). A highly sensitive automated method to screen for Chagas disease, such as the Elecsys Chagas assay, could potentially increase the throughput of samples and will likely lead to improvements in diagnosis algorithms and, thus, in cost-effectiveness (28). Ultimately, the availability of improved assays for the detection of T. cruzi would be expected to better safeguard patients who require blood and organ donation and help to minimize misdiagnoses, which are major factors in delaying the appropriate health care response (21).
Strengths of this study are the inclusion of a significant proportion of samples from Latin America to evaluate the Elecsys Chagas assay under blood screening and diagnostic routine laboratory conditions in countries where the disease is endemic. This is important because geographical differences in the sensitivities of recombinant antigen-based rapid tests for T. cruzi infection have been demonstrated, possibly due to T. cruzi strain differences (43). Commercially available performance panels covering samples from an additional nine countries were all found to be reactive with the Elecsys assay (Roche internal data [not shown]), underlining the sensitivity for Chagas samples from South and Central America. The present study also included an analysis of reactive samples stored frozen for a period of years, demonstrating the general stability of the analyte (IgG per se). Moreover, in samples that showed a loss of reactivity with competitor assays during long-term storage, the Elecsys COI values ranged from 1.8 to 70.9, supporting the high sensitivity of this assay. Finally, the Elecsys Chagas assay was compared with several existing assays to ensure relevance to local protocols and thus to current benchmarks for performance. Evaluation with a commercially available seroconversion panel revealed a seroconversion sensitivity identical to those of competitors, thus reconfirming the sensitivity for samples derived from the early phase of infection (Table 7).
TABLE 7.
Evaluation of seroconversion sensitivity with a commercially available seroconversion panel, SeraCare Chagas (T. cruzi) AccuVert seroconversion panel 0615-0038
| Panel member | Bleed date (day.mo.yr) | No. of days since 1st bleed | Roche Diagnostics Elecsys Chagas signal/CO ratio | Interpretation of result |
|---|---|---|---|---|
| 1 | 31.07.2012 | 0 | 0.071 | Nonreactive |
| 2 | 10.09.2012 | 41 | 117 | Reactive |
| 3 | 17.09.2012 | 48 | 118 | Reactive |
| 4 | 24.09.2012 | 55 | 127 | Reactive |
| 5 | 01.10.2012 | 62 | 143 | Reactive |
| 6 | 08.10.2012 | 69 | 151 | Reactive |
| 7 | 15.10.2012 | 76 | 146 | Reactive |
| 8 | 29.10.2012 | 90 | 178 | Reactive |
| 9 | 12.11.2012 | 104 | 169 | Reactive |
| 10 | 26.11.2012 | 118 | 210 | Reactive |
Compared with the comparator tests, discrepant results were observed for 26 of 14,681 blood donor samples (0.17%) derived from regions of nonendemicity and endemicity, a significantly lower percentage than those observed with new-generation competitors (28). Since there is no established gold standard for the detection of anti-T. cruzi antibodies, we used a neutralization test to further investigate such discrepant reactive results obtained with the Elecsys test. The application of a neutralization test for the verification of discrepant reactive results in a highly sensitive assay to detect anti-T. gondii antibodies was described previously (31) and was successfully applied here for T. cruzi. The relative specificity (99.74%) observed in the present study for the subgroup of blood donors from Latin America may therefore be even higher due to the reconfirmed presence of specific antibodies in Elecsys-discrepant reactive samples. The in-house neutralization method to resolve discrepant reactive findings with state-of-the-art assays was used here for the first time within a multicenter study and was deemed to be an additional specific and valuable method. The use of the heterologous native T. cruzi antigen extract for supplemental neutralization testing avoids an inbreeding confirmatory situation for the recombinant antigens used by the Elecsys assay. However, we were unable to perform neutralization testing on all samples with qualitative discrepant results due to a lack of sufficient sample volumes in some cases. Due to the use of residual blood donor samples, there was also no possibility of serological donor follow-up to clarify questionable results.
The high analytical sensitivity of the Elecsys assay is reflected in comparisons of cutoff sensitivities based on the use of material accessible to the public, such as WHO reference material from the NIBSC. Such reference material may help to better benchmark the dilutional sensitivities and the individual cutoff settings of the respective assays. This approach is widely used to assess the performance of screening assays and is also an inherent part of the Common Technical Specifications (CTS) of European Commission directive 98/79 for screening assays (45). The recombinant assay format described here is highly sensitive, which contradicts the notion that only techniques that use whole parasites are sufficiently sensitive (44). Sensitivity assessment regarding different distinct typing units of T. cruzi was covered during the development of the assay by an analysis of 1,370 suspected Chagas disease-positive samples (all investigated samples were reactive in ≥3 assays) derived from 13 countries (Argentina, Bolivia, Chile, Spain, Ecuador, El Salvador, Honduras, Mexico, Nicaragua, Paraguay, Uruguay, the United States, and Venezuela), revealing 100% reactive results with the Elecsys Chagas assay (Roche internal data [not shown]) (the lowest observed signal/CO value for all samples was >2). Thus, the assay is suitable for blood donor management as well as for diagnostic use.
The development of highly sensitive and specific new assays for the detection of anti-T. cruzi antibodies thus helps to reduce expenses for additional second-line testing for the diagnosis of the disease and safeguards the sensitivity needed for blood screening purposes.
Conclusions.
The automated Elecsys Chagas assay demonstrated a robust and favorable performance under routine conditions at multiple sites in Europe and Latin America. In contrast to other available assays for Chagas disease, the Elecsys assay uses a reduced number of recombinant T. cruzi antigens, resulting in a significantly smaller number of cross-reactions, with improved analytical specificity, while still being highly sensitive, with high discriminatory power.
ACKNOWLEDGMENTS
This study was funded by Roche Diagnostics (Penzberg, Germany). Medical writing assistance was provided by David Evans and Emma McConnell (Gardiner-Caldwell Communications, Macclesfield, UK) and was funded by Roche Diagnostics (Penzberg, Germany).
We thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Miguel Vicco from the Universidad Nacional del Litoral (Santa Fe, Argentina) for the provision of Chagas-positive samples; DiaSorin SpA (Saluggia, Italy) for the free-of-charge provision of DiaSorin Liaison XL Murex Chagas for measurements performed at the Greater Romagna Hub Laboratory in Pievesestina, Italy; and Sigrid Reichhuber, Verena Rauchberger, Stefanie Hornauer, Michaela Eibl, and Christian Schuster for perfect technical assistance.
All authors fulfill International Committee of Medical Journal Editors authorship criteria.
Maria Delmans Flores-Chavez received funds from Roche Diagnostics to perform this study and received a speaker fee from Roche Diagnostics. Vittorio Sambri received research grants from Grifols, Abbott Iridica, Siemens Diagnostics, and DiaSorin; speaker fees from Abbott Diagnostics, DiaSorin, and Beckman Coulter; and funds from Roche Diagnostics to perform this study. Volkmar Schottstedt received funds from Roche Diagnostics to perform this study. Fernando Aparicio Higuera-Escalante received funds from Roche Diagnostics to perform this study. Dieter Roessler is employed by Roche Diagnostics. Monica Chaves is employed by Roche Diagnostics. Tina Laengin is employed by Roche Diagnostics. Alfredo Martinez received funds from Roche Diagnostics to perform this study and received a speaker fee from Roche Diagnostics. Bernhard Fleischer received funds from Roche Diagnostics to perform this study and received a speaker fee from Roche Diagnostics.
REFERENCES
- 1.WHO. 2017. Chagas disease (American trypanosomiasis) fact sheet. WHO, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs340/en/ Accessed 7 August 2017.
- 2.WHO. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–43. [PubMed] [Google Scholar]
- 3.Dias JP, Bastos C, Araújo E, Mascarenhas AV, Martins Netto E, Grassi F, Silva M, Tatto E, Mendonça J, Araújo RF, Shikanai-Yasuda MA, Aras R. 2008. Acute Chagas disease outbreak associated with oral transmission. Rev Soc Bras Med Trop 41:296–300. doi: 10.1590/S0037-86822008000300014. [DOI] [PubMed] [Google Scholar]
- 4.Neto VA, Doles J, Rassi A, Borges AP, de Rezende JM, de Oliveira Gomes MC. 1968. New case reports of Chagas' disease transmitted by blood transfusion. Rev Inst Med Trop Sao Paulo 10:46–51. (In Portuguese.) [PubMed] [Google Scholar]
- 5.Salas Clavijo NA, Postigo JR, Schneider D, Santalla JA, Brutus L, Chippaux JP. 2012. Prevalence of Chagas disease in pregnant women and incidence of congenital transmission in Santa Cruz de la Sierra, Bolivia. Acta Trop 124:87–91. doi: 10.1016/j.actatropica.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2006. Chagas disease after organ transplantation—Los Angeles, California, 2006. MMWR Morb Mortal Wkly Rep 55:798–800. [PubMed] [Google Scholar]
- 7.Piron M, Vergés M, Muñoz J, Casamitjana N, Sanz S, Maymó RM, Hernández JM, Puig L, Portús M, Gascón J, Sauleda S. 2008. Seroprevalence of Trypanosoma cruzi infection in at-risk blood donors in Catalonia (Spain). Transfusion 48:1862–1868. doi: 10.1111/j.1537-2995.2008.01789.x. [DOI] [PubMed] [Google Scholar]
- 8.Manne-Goehler J, Umeh CA, Montgomery SP, Wirtz VJ. 2016. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis 10:e0005033. doi: 10.1371/journal.pntd.0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ries J, Komarek A, Gottschalk J, Brand B, Amsler L, Jutzi M, Frey BM. 2016. A case of possible Chagas transmission by blood transfusion in Switzerland. Transfus Med Hemother 43:415–417. doi: 10.1159/000446264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores-Chavez MD, Merino FJ, Garcia-Bujalance S, Martin-Rabadan P, Merino P, Garcia-Bermejo I, Delgado A, Cuadros J, Working Group on Chagas Disease of Autonomous Community of Madrid. 2011. Surveillance of Chagas disease in pregnant women in Madrid, Spain, from 2008 to 2010. Euro Surveill 16(38):pii=19974. doi: 10.2807/ese.16.38.19974-en. [DOI] [PubMed] [Google Scholar]
- 11.Prata A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 12.Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL, Council on Chagas Disease of the Interamerican Society of Cardiology. 2013. Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol 62:767–776. doi: 10.1016/j.jacc.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 14.Martí-Carvajal AJ, Kwong JS. 2016. Pharmacological interventions for treating heart failure in patients with Chagas cardiomyopathy. Cochrane Database Syst Rev 7:CD009077. doi: 10.1002/14651858.CD009077.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rassi A Jr, Rassi A, Marcondes de Rezende J. 2012. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Hernández C, Cucunubá Z, Flórez C, Olivera M, Valencia C, Zambrano P, León C, Ramírez JD. 2016. Molecular diagnosis of Chagas disease in Colombia: parasitic loads and discrete typing units in patients from acute and chronic phases. PLoS Negl Trop Dis 10:e0004997. doi: 10.1371/journal.pntd.0004997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. 2002. Control of Chagas disease: a second report of the WHO Expert Committee. WHO, Geneva, Switzerland. [Google Scholar]
- 18.Brasil PE, De Castro L, Hasslocher-Moreno AM, Sangenis LH, Braga JU. 2010. ELISA versus PCR for diagnosis of chronic Chagas disease: systematic review and meta-analysis. BMC Infect Dis 10:337. doi: 10.1186/1471-2334-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. 1991. Control of Chagas disease. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 811:1–95. [PubMed] [Google Scholar]
- 20.De Marchi CR, Di Noia JM, Frasch AC, Amato Neto V, Almeida IC, Buscaglia CA. 2011. Evaluation of a recombinant Trypanosoma cruzi mucin-like antigen for serodiagnosis of Chagas' disease. Clin Vaccine Immunol 18:1850–1855. doi: 10.1128/CVI.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet I, Dinardi G, Canevari C, Torabi N. 2016. Health care seeking behavior of persons with acute Chagas disease in rural Argentina: a qualitative view. J Trop Med 2016:4561951. doi: 10.1155/2016/4561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrade JP, Marin Neto JA, Paola AA, Vilas-Boas F, Oliveira GM, Bacal F, Bocchi EA, Almeida DR, Fragata Filho AA, Moreira MC, Xavier SS, Oliveira WA Jr, Dias JC. 2011. Latin American guidelines for the diagnosis and treatment of Chagas' heart disease: executive summary. Arq Bras Cardiol 96:434–442. doi: 10.1590/S0066-782X2011000600002. [DOI] [PubMed] [Google Scholar]
- 23.WHO. 2010. Screening donated blood for transfusion-transmissible infections: recommendations. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 24.Chin-Hong PV, Schwartz BS, Bern C, Montgomery SP, Kontak S, Kubak B, Morris MI, Nowicki M, Wright C, Ison MG. 2011. Screening and treatment of Chagas disease in organ transplant recipients in the United States: recommendations from the Chagas in Transplant Working Group. Am J Transplant 11:672–680. doi: 10.1111/j.1600-6143.2011.03444.x. [DOI] [PubMed] [Google Scholar]
- 25.Basile L, Oliveira I, Ciruela P, Plasencia A, Working Group for Developing the Catalonian Screening Programme for Congenital Transmission of Chagas Disease. 2011. The current screening programme for congenital transmission of Chagas disease in Catalonia, Spain. Euro Surveill 16(38):pii=19972. doi: 10.2807/ese.16.38.19972-en. [DOI] [PubMed] [Google Scholar]
- 26.Ministerio de Salud de la Nación. 2012. Guías para la atención al paciente infectado con Trypanosoma cruzi. Enfermedad de Chagas, Buenos Aires, Argentina. [Google Scholar]
- 27.Lapa JS, Saraiva RM, Hasslocher-Moreno AM, Georg I, Souza AS, Xavier SS, do Brasil PE. 2012. Dealing with initial inconclusive serological results for chronic Chagas disease in clinical practice. Eur J Clin Microbiol Infect Dis 31:965–974. doi: 10.1007/s10096-011-1393-9. [DOI] [PubMed] [Google Scholar]
- 28.Abras A, Gállego M, Llovet T, Tebar S, Herrero M, Berenguer P, Ballart C, Martí C, Muñoz C. 2016. Serological diagnosis of chronic Chagas disease: is it time for a change? J Clin Microbiol 54:1566–1572. doi: 10.1128/JCM.00142-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores-Chávez M, Cruz I, Rodríguez M, Nieto J, Franco E, Gárate T, Cañavate C. 2010. Comparison of conventional and non-conventional serological tests for the diagnosis of imported Chagas disease in Spain. Enferm Infecc Microbiol Clin 28:284–293. (In Spanish.) doi: 10.1016/j.eimc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Norman FF, Pérez-Ayala A, Pérez-Molina JA, Flores-Chavez M, Cañavate C, López-Vélez R. 2011. Lack of association between blood-based detection of Trypanosoma cruzi DNA and cardiac involvement in a non-endemic area. Ann Trop Med Parasitol 105:425–430. doi: 10.1179/1364859411Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köhler S, Rössler D, Hornauer S, Upmeier B, Franck J, Liesenfeld O. 2010. Neutralization assay to resolve discrepancies between positive results in new highly sensitive anti-Toxoplasma gondii IgG assays and negative results in reference tests. Eur J Clin Microbiol Infect Dis 29:359–363. doi: 10.1007/s10096-009-0864-8. [DOI] [PubMed] [Google Scholar]
- 32.Umezawa ES, Bastos SF, Camargo ME, Yamauchi LM, Santos MR, Gonzalez A, Zingales B, Levin MJ, Sousa O, Rangel-Aldao R, da Silveira JF. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J Clin Microbiol 37:1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silveira JF, Umezawa ES, Luquetti AO. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol 17:286–291. doi: 10.1016/S1471-4922(01)01897-9. [DOI] [PubMed] [Google Scholar]
- 34.Hernández P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, Beck E. 2010. Highly effective serodiagnosis for Chagas' disease. Clin Vaccine Immunol 17:1598–1604. doi: 10.1128/CVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izquierdo L, Marques AF, Gállego M, Sanz S, Tebar S, Riera C, Quintó L, Aldasoro E, Almeida IC, Gascon J. 2013. Evaluation of a chemiluminescent enzyme-linked immunosorbent assay for the diagnosis of Trypanosoma cruzi infection in a nonendemic setting. Mem Inst Oswaldo Cruz 108:928–931. doi: 10.1590/0074-0276130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brito CR, McKay CS, Azevedo MA, Santos LC, Venuto AP, Nunes DF, D'Ávila DA, Rodrigues da Cunha GM, Almeida IC, Gazzinelli RT, Galvão LM, Chiari E, Sanhueza CA, Finn MG, Marques AF. 2016. Virus-like particle display of the α-Gal epitope for the diagnostic assessment of Chagas disease. ACS Infect Dis 2:917–922. doi: 10.1021/acsinfecdis.6b00114. [DOI] [PubMed] [Google Scholar]
- 37.Longhi SA, Brandariz SB, Lafon SO, Niborski LL, Luquetti AO, Schijman AG, Levin MJ, Gómez KA. 2012. Evaluation of in-house ELISA using Trypanosoma cruzi lysate and recombinant antigens for diagnosis of Chagas disease and discrimination of its clinical forms. Am J Trop Med Hyg 87:267–271. doi: 10.4269/ajtmh.2012.11-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CD, Cheng KY, Jiang LX, Salbilla VA, Haller AS, Yem AW, Bryant JD, Kirchhoff LV, Leiby DA, Schochetman G, Shah DO. 2006. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion 46:1737–1744. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 39.Santos FL, de Souza WV, Barros MS, Nakazawa M, Krieger MA, Gomes YM. 2016. Chronic Chagas disease diagnosis: a comparative performance of commercial enzyme immunoassay tests. Am J Trop Med Hyg 94:1034–1039. doi: 10.4269/ajtmh.15-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Praast G, Herzogenrath J, Bernhardt S, Christ H, Sickinger E. 2011. Evaluation of the Abbott ARCHITECT Chagas prototype assay. Diagn Microbiol Infect Dis 69:74–81. doi: 10.1016/j.diagmicrobio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Leiby DA, Herron RM Jr, Read EJ, Lenes BA, Stumpf RJ. 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 42.Otani MM, Vinelli E, Kirchhoff LV, del Pozo A, Sands A, Vercauteren G, Sabino EC. 2009. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion 49:1076–1082. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 43.Verani JR, Seitz A, Gilman RH, LaFuente C, Galdos-Cardenas G, Kawai V, de LaFuente E, Ferrufino L, Bowman NM, Pinedo-Cancino V, Levy MZ, Steurer F, Todd CW, Kirchhoff LV, Cabrera L, Verastegui M, Bern C. 2009. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg 80:410–415. [PubMed] [Google Scholar]
- 44.Balouz V, Agüero F, Buscaglia CA. 2017. Chagas disease diagnostic applications: present knowledge and future steps. Adv Parasitol 97:1–45. doi: 10.1016/bs.apar.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Commission. 7 December 1998. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. OJ L 331 European Commission, Brussels, Belgium: https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en. [Google Scholar]