ABSTRACT
Streptococcus pneumoniae colonizes the nasopharyngeal mucus in healthy individuals and can cause otitis media, pneumonia, and invasive pneumococcal diseases. In this study, we analyzed S. pneumoniae strains that caused 19 pneumonia episodes in long-term inpatients with severe underlying disease in a hospital during a period of 14 months (from January 2014 to February 2015). Serotyping and whole-genome sequencing analyses revealed that 18 of the 19 pneumonia cases were caused by S. pneumoniae strains belonging to 3 genetically distinct groups: clonal complex 9999 (CC9999), sequence type 282 (ST282), and ST166. The CC9999 and ST282 strains appeared to have emerged separately by a capsule switch from the pandemic PMEN 1 strain (Spain23F-ST81). After all the long-term inpatients were inoculated with the 23-valent pneumococcal polysaccharide vaccine, no other nosocomial pneumonia infections occurred until March 2016.
KEYWORDS: Streptococcus pneumoniae pneumonia, capsule switching, hospital-acquired infections, vaccination, whole-genome sequencing
INTRODUCTION
Streptococcus pneumoniae can asymptomatically colonize the nasopharynx for months in healthy humans (1) and cause pneumococcal diseases and deaths, especially in young children, the elderly, and persons with chronic illnesses or who are immunosuppressed (2–4). Pneumococcal colonization in the nasopharynx is a prerequisite for the onset of various types of pneumococcal diseases and for transmission from person to person through close contact (5, 6). Pneumonia is the third leading cause of death in Japan, and S. pneumoniae is the leading cause of community-acquired pneumonia in adults (7). Most of the pneumonia cases caused by S. pneumoniae are sporadic, but pneumonia outbreaks in closed spaces, such as hospitals and the Marine Corps, have been reported (8–10).
Some pneumococcal infections can be prevented by vaccination. Currently, the 10- and 13-valent pneumococcal conjugate vaccines (PCVs) and a 23-valent pneumococcal polysaccharide vaccine (PPSV23) are widely used. In Japan, PCV13 was introduced for children aged <5 years in 2013 and for adults in 2014. PPSV23 was introduced in 1988, and its routine immunization in adults aged ≥65 years was initiated in October 2014; however, the vaccination rate is still low (approximately 34% from April 2014 to March 2016 [http://www.e-stat.go.jp/SG1/estat/eStatTopPortal.do]). A report from Japan estimated that universal PPSV23 vaccination of individuals aged ≥65 years might prevent one-third of pneumococcal pneumonia cases (11).
The introduction of PCVs has dramatically reduced the incidence of invasive pneumococcal disease (IPD) among vaccinated young children (12, 13) and, as a result of herd immunity, has also decreased IPD among the elderly (14). However, the prevalence of non-PCV7-type IPD has recently increased among children and adults (6, 7). The phenomenon whereby the prevalence of nonvaccine serotypes increases while that of vaccine serotypes decreases is termed “serotype replacement” (15–17). Capsule switching from a vaccine type to a nonvaccine type is one cause of serotype replacement (18, 19). Several multidrug-resistant S. pneumoniae clones, including a global pandemic clone, Spain23F-ST81, were identified as serotypes caused by capsule switching (20–23).
In this study, we report that S. pneumoniae clones derived from Spain23F-ST81 that underwent capsule switching caused clusters of pneumococcal pneumonia infections among long-term inpatients with severe underlying diseases.
MATERIALS AND METHODS
Diagnosis of pneumonia and culture of S. pneumoniae.
Pneumonia was diagnosed based on positive X-ray findings and one or more clinical symptoms, including fever, rapid or difficult breathing, cough, and crackle in lung fields upon auscultation. Culturing of sputum samples and isolation of S. pneumoniae were performed as described previously by Tanaka et al. (24). Pathogens accounting for >50% of the colonies in the culture or presenting >1 × 107 CFU/ml of sputum were regarded as “dominant” (24).
Serotyping and antimicrobial susceptibility testing of S. pneumoniae strains.
All S. pneumoniae strains were plated onto Columbia agar with 5% sheep blood (Becton, Dickinson and Company Japan, Tokyo, Japan) overnight at 37°C with 5% CO2. Serotypes of the S. pneumoniae strains were determined by using the Quellung reaction with pneumococcal antisera (Statens Serum Institut, Copenhagen, Denmark). Because serotypes 11A and 11E could not be discriminated by the Quellung reaction, the 2 serotypes are indicated as serotype 11A/E. If the bacterial cells did not show a positive reaction with any antiserum, no obvious capsule was detected by India ink staining, and the bacterium was determined to be S. pneumoniae by the detection of lytA (25), the serotype of the strain was indicated as nontypeable (NT).
Testing of the susceptibility of S. pneumoniae strains to 11 antibiotics was performed by using the broth microdilution method according to a Clinical and Laboratory Standards Institute (CLSI) protocol (26). The antibiotics evaluated were penicillin G (PCG), ampicillin, panipenem, meropenem, tebipenem, cefotaxime, cefditoren, tosufloxacin, erythromycin, clindamycin, and vancomycin. MIC breakpoints were defined according to CLSI criteria (26). MIC breakpoints of 4 antibiotics without criteria by the CLSI were determined as follows: MIC breakpoints for panipenem and tebipenem were the same as those defined for meropenem (susceptible [S], MIC of ≤0.25 μg/ml; intermediate [I], MIC of 0.5 μg/ml; resistant [R], MIC of ≥1 μg/ml); the MIC breakpoints of cefditoren were the same as those defined for cefpodoxime (S, MIC of ≤0.5 μg/ml; I, MIC of 1 μg/ml; R, MIC of ≥2 μg/ml); and MIC breakpoints for tosufloxacin were the same as those defined for levofloxacin (S, MIC of ≤2 μg/ml; I, MIC of 4 μg/ml; R, MIC of ≥8 μg/ml).
Whole-genome sequencing and phylogenetic analyses.
DNA libraries for whole-genome sequencing (WGS) were constructed by using the Nextera XT DNA sample prep kit (Illumina, San Diego, CA, USA) and then sequenced by using MiSeq (Illumina). To generate short-read mapping data for all S. pneumoniae strains compared with the reference genome sequence of serotype 23F strain ATCC 700669 (GenBank accession number FM211187), bwasw (27) and samtools (28) software were used, with default parameters. All single nucleotide polymorphisms (SNPs) were extracted with VarScan v.2.3.4 (29), using default parameters. The SNPs on the repetitive and recombinogenic region of ATCC 700669 identified by the MUMmer v.3.23 (30) and RecHMM (31) programs were excluded from the comparison. The genomic sequences of all the strains were concatenated to generate a pseudosequence for phylogenetic analyses. Maximum likelihood phylogenetic analyses were performed by using RAxML v8.2.0 (32), with 1,000 bootstrap iterations. The phylogenetic trees were visualized by using iTOL 3 (33). Sequence types (STs) of the S. pneumoniae strains were determined by the sequences of seven housekeeping genes (aroE, gdh, gki, recP, spi, xpt, and ddl) (34) obtained from the results of WGS. Allelic numbers and STs were assigned by using the pneumococcal Multilocus Sequence Typing (MLST) website (https://pubmlst.org/spneumoniae/). STs are shown as ST numbers (allelic numbers of the seven genes in order). Strains for which ≥5 of the 7 alleles were identical were classified as belonging to a clonal complex (CC).
Accession number(s).
Nucleotide sequence data obtained in this study have been submitted to the DNA Data Bank of Japan Sequenced Read Archive under accession numbers DRX114416 to DRX114435.
RESULTS
Pneumococcal pneumonia cluster infections and prevention by vaccination.
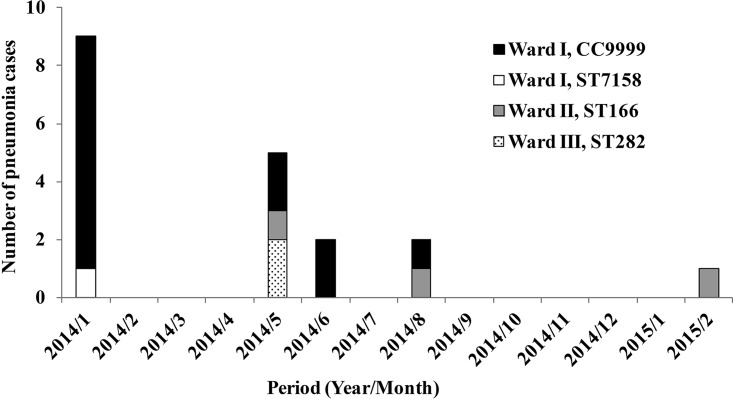
A cluster of pneumococcal pneumonia cases occurred among long-term inpatients with underlying diseases in 2014 at a hospital in western Japan. During a period of 14 months (from January 2014 to February 2015), totals of 14, 3, and 2 inpatients in three different wards (wards I, II, and III, respectively) were diagnosed with pneumonia (Fig. 1). S. pneumoniae was the predominant bacterium isolated from all 19 pneumonia patients and was therefore considered to be the causative pathogen. Prior to the cluster infection, neither the inpatients nor the personnel working in these wards had been vaccinated with pneumococcal vaccines. Because of the possibility that the pneumococcal cluster cases were hospital-acquired infections, all the long-term inpatients in the hospital were inoculated with the PPSV23 vaccine in February 2015. Up to March 2016, our surveillance deadline, no other nosocomial pneumonia infections had occurred in the three wards.
FIG 1.
Number and schedule of pneumonia episodes occurring in three wards and STs of the isolated S. pneumoniae strains. The horizontal axis shows the month of onset of the pneumonia cases, and the vertical axis shows the number of cases.
Serotypes and MICs of the S. pneumoniae strains.
One pneumococcal isolate from each of the 19 pneumonia cases was analyzed in this study. One strain isolated from an outpatient with pneumonia at the same time point (May 2014) was also analyzed for comparison. Serotyping and antimicrobial susceptibility testing of all 20 S. pneumoniae strains were performed (Table 1).
TABLE 1.
Characteristics of pneumococcal pneumonia episodes and of the isolated Streptococcus pneumoniae strains
| Episode | Date of specimen collection (yr/mo/day) | Wardb | Age of patient (yr) | Sex of patiente | Diagnosis | Specimen type | S. pneumoniae strain | Serotypec | ST | MIC (μg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCG | ABPC | PAPM | MEPM | TBPM | CTX | CDTR | TFLX | EM | CLDM | VCM | ||||||||||
| 1 | 2014/1/20 | I | 25 | F | Pneumonia | Sputum | SP2637 | 10A | 9999 | 2 | 4 | 0.06 | 0.5 | 0.06 | 2 | 4 | 1 | 2 | ≤0.12 | 0.25 |
| 2 | 2014/1/20 | I | 23 | F | Pneumonia | Sputum | SP2638 | 6A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.12 | 2 | 4 | 2 | ≥8 | ≤0.12 | 0.5 |
| 3 | 2014/1/20 | I | 20 | F | Pneumonia | Sputum | SP2639 | 10A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.12 | 4 | 4 | 1 | 4 | ≤0.12 | 0.25 |
| 4 | 2014/1/20 | I | 27 | F | Pneumonia | Sputum | SP2640 | 6A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.12 | 4 | 4 | 2 | ≥8 | ≤0.12 | 0.5 |
| 5 | 2014/1/20 | I | 29 | M | Pneumonia | Sputum | SP2641 | 10A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.06 | 4 | 4 | 2 | 4 | ≤0.12 | 0.5 |
| 6 | 2014/1/20 | I | 34 | M | Pneumonia | Sputum | SP2642 | 10A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.06 | 4 | 4 | 1 | 4 | ≤0.12 | 0.25 |
| 7 | 2014/1/20 | I | 24 | F | Pneumonia | Sputum | SP2643 | 10A | 7158 | 1 | 1 | 0.03 | 0.12 | 0.015 | 0.5 | 0.5 | ≤0.12 | ≥8 | ≥8 | 0.25 |
| 8 | 2014/1/20 | I | 5 | M | Pneumonia | Sputum | SP2644 | 6A | 9999 | 2 | 4 | 0.12 | 1 | 0.12 | 2 | 4 | 2 | ≥8 | ≤0.12 | 0.5 |
| 9 | 2014/1/20 | I | 20 | M | Pneumonia | Sputum | SP2645 | 10A | 9999 | 1 | 2 | 0.12 | 0.5 | 0.06 | 2 | 2 | 2 | 4 | ≤0.12 | 0.25 |
| 10 | 2014/5/19 | I | 14 | F | Pneumonia | Sputum | SP2759 | 10A | 9999 | 2 | 4 | 0.12 | 0.5 | 0.12 | 4 | 2 | 2 | 4 | ≤0.12 | 0.25 |
| 11 | 2014/5/23 | I | 39 | M | Pneumonia | Sputum | SP2761 | 6A | 9999 | 4 | 8 | 0.25 | 0.5 | 0.12 | ≥8 | ≥8 | 2 | 0.25 | 0.25 | 0.5 |
| 12 | 2014/6/9 | I | 31 | M | Pneumonia | Sputum | SP2762 | 10A | 10024 | 4 | 4 | 0.25 | 1 | 0.12 | 4 | 2 | 1 | 4 | 0.25 | 0.5 |
| 13 | 2014/6/13 | I | 28 | F | Pneumonia | Sputum | SP2763 | 10A | 9999 | 2 | 2 | 0.06 | 0.25 | 0.06 | 2 | 2 | 1 | 2 | ≤0.12 | 0.25 |
| 14 | 2014/8/6 | I | 35 | M | Pneumonia | Sputum | SP3000 | 10A | 9999 | 1 | 2 | 0.12 | 0.5 | 0.06 | 2 | 2 | 1 | 2 | ≤0.12 | 0.25 |
| 15 | 2014/5/20 | II | 49 | M | Pneumonia | Sputum | SP2760 | 11A/Ed | 166 | 2 | 4 | 0.12 | 1 | 0.06 | 2 | 1 | ≥16 | ≥8 | ≥8 | 0.25 |
| 16 | 2014/8/21 | II | 23 | M | Pneumonia | Sputum | SP2998 | 11A/Ed | 166 | 2 | 4 | 0.12 | 0.5 | 0.06 | 2 | 1 | ≥16 | ≥8 | ≥8 | 0.25 |
| 17 | 2015/2/9 | II | 60 | M | Pneumonia | Sputum | SP2999 | 11A/Ed | 166 | 2 | 4 | 0.06 | 0.5 | 0.06 | 2 | 1 | ≥16 | ≥8 | ≥8 | 0.25 |
| 18 | 2014/5/12 | III | 30 | M | Pneumonia | Sputum | SP2756 | 6A | 282 | 2 | 4 | 0.12 | 0.5 | 0.06 | 1 | 1 | 0.25 | 2 | ≤0.12 | 0.25 |
| 19 | 2014/5/13 | III | 27 | F | Pneumonia | Nasal cavity | SP2757 | NT | 282 | 1 | 2 | 0.06 | 0.5 | 0.06 | 0.5 | 0.5 | ≤0.12 | 1 | ≤0.12 | 0.25 |
| 20 | 2014/5/19 | O | 3 | F | Pneumonia | Nasal cavity | SP2758 | 15A | 63 | 2 | 4 | 0.06 | 0.5 | 0.06 | 1 | 0.5 | 0.25 | ≥8 | ≥8 | 0.25 |
PCG, penicillin G; ABPC, ampicillin; PAPM, panipenem; MEPM, meropenem; TBPM, tebipenem; CTX, cefotaxime; CDTR, cefditoren; TFLX, tosufloxacin; EM, erythromycin; CLDM, clindamycin; VCM, vancomycin. MICs of intermediate resistance are indicated in boldface type, and those of resistance are indicated in boldface and italic type.
O, outpatient.
NT, nontypeable.
Serotype 11E could not be discriminated from serotype 11A by the Quellung reaction used in this study.
F, female; M, male.
Serotyping revealed that the 14 strains isolated from the patients in ward I were of serotypes 10A (10 strains) and 6A (4 strains), the 3 strains from ward II were of serotype 11A/E, the 2 strains from ward III were of serotype 6A and NT, and the strain from the outpatient was of serotype 15A.
MICs of 11 test antibiotics for the pneumococcal strains (except SP2643) isolated from the same ward were similar to each other. SP2643 had a different MIC profile compared to those of the other S. pneumoniae strains isolated from ward I. As expected, strain SP2758, isolated from the outpatient, had different MICs than those of the other strains (Table 1).
WGS analysis of the isolated strains.
To clarify the genetic relatedness among the S. pneumoniae strains, WGS analyses were performed. First, STs were determined to obtain a brief view of the overall relationship of all 20 S. pneumoniae strains. ST is represented by a combination of an ST number (e.g., ST9999) and a 7-digit allelic profile of the seven genes in order (e.g., 2, 4, 2, 4, 4, 1, 26). Twelve strains (8 of serotype 10A and 4 of serotype 6A) from ward I were of ST9999 (2, 4, 2, 4, 4, 1, 26). The remaining 2 strains of 10A from ward I, SP2762 and SP2643, were of ST10024 (2, 4, 2, 4, 4, 586, 26) and ST7158 (7, 12, 1, 1, 10, 1, 14), respectively. The ST9999 and ST10024 strains were different by 1 base in the xpt gene and were grouped into CC9999. Five of seven alleles of ST9999 strains were identical to those of ST81 (4, 4, 2, 4, 4, 1, 1), the global pandemic clone Spain23F-ST81 (23). Because ST7158 was different from ST9999 and ST81 in 6 of the 7 alleles, SP2643 was deemed to be unrelated to the CC9999 and ST81 strains. The 3 strains isolated from ward II were of ST166 (7, 11, 10, 1, 6, 1, 1), and the 2 strains from ward III were of ST282 (30, 4, 2, 4, 4, 1, 1). ST282 differed from ST81 by a single allele. The STs of the strains revealed that the S. pneumoniae strains belonging to CC9999 and ST282 were evolutionarily closely related to each other and ST81 in terms of their biology. The strain isolated from the outpatient was of ST63 and was unrelated to the other strains (Table 1).
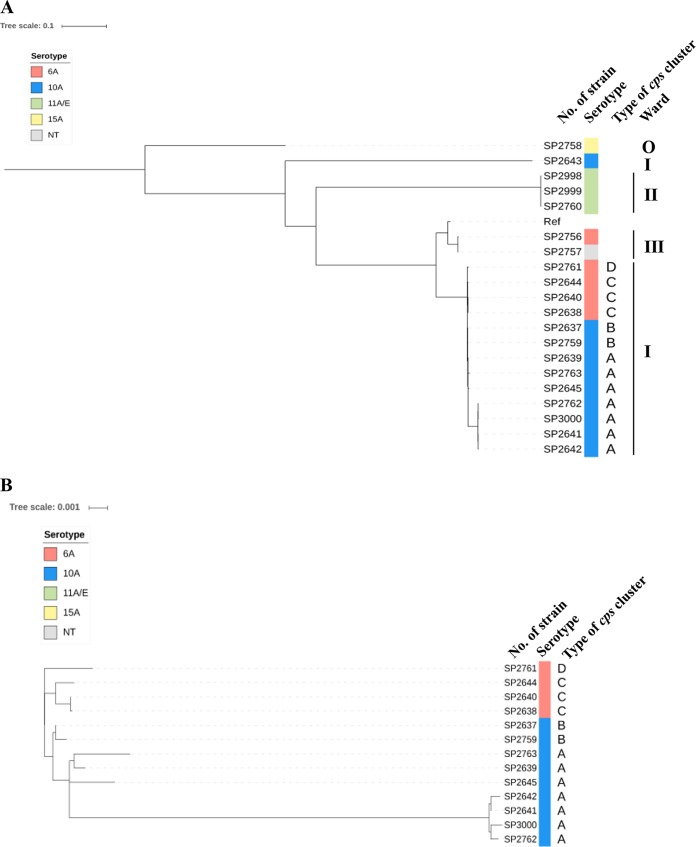
Subsequently, the whole-genome sequences of all 20 strains were compared to clarify whether the S. pneumoniae strains were of the same clone. The genomic sequence of strain ATCC 700669 was obtained from the NCBI database and used as a reference. Mutation sites were detected by mapping analyses of the 20 strains, and their genetic relatedness is indicated as a dendrogram in Fig. 2.
FIG 2.
Genetic relationships among the S. pneumoniae strains isolated from the cluster of pneumonia cases. (A) Single maximum parsimony tree reconstructed from the whole-genome sequences of all 20 S. pneumoniae strains analyzed in this study and reference strain ATCC 700669. (B) Enlarged tree including the 13 CC9999 strains isolated from ward I. Branch lengths represent the genetic distance, and the bars represent the percent difference. Serotypes of the strains are shown in the colors indicated at the top left. NT, nontypeable; Ref, reference strain ATCC 700669; O, outpatient strain. Based on sequences from the pbp2x to pbp1a genes, the CC9999 strains were further divided into types A, B, C, and D (see also Fig. 3B).
Strain SP2643 (serotype 10A, belonging to ST7158, isolated from ward I) and SP2758 (serotype 15A, isolated from the outpatient) were unrelated to the other strains tested. The 13 S. pneumoniae strains belonging to CC9999 isolated from ward I, the 3 serotype 11A/E strains from ward II, and the 2 ST282 strains from ward III were gathered in 3 separate clusters (Fig. 2A). The ST282 strains (SP2756 and SP2757) were closer to ATCC 700669 than the strains belonging to CC9999. The 3 serotype 11A/E strains were clustered together but were distant from ATCC 700669 and the CC9999 and ST282 strains. The results of all the phylogenetic analyses showed that 3 clusters of pneumonia infection (except for episode 7) occurred separately in each of the wards. Cases 7 and 20, caused by SP2643 and SP2758, respectively, were sporadic and unrelated to any other cases.
To clarify the relationship among the strains isolated from ward I, an enlarged view of the phylogenetic tree is shown in Fig. 2B. The 9 strains belonging to serotype 10A and the other 4 S. pneumoniae strains belonging to serotype 6A are clustered together. These results showed that the serotype 10A and 6A strains seemed to be independently derived from a single ancestor strain via capsule switching.
Recombination points for the capsule switching of S. pneumoniae ST282 and CC9999 strains.
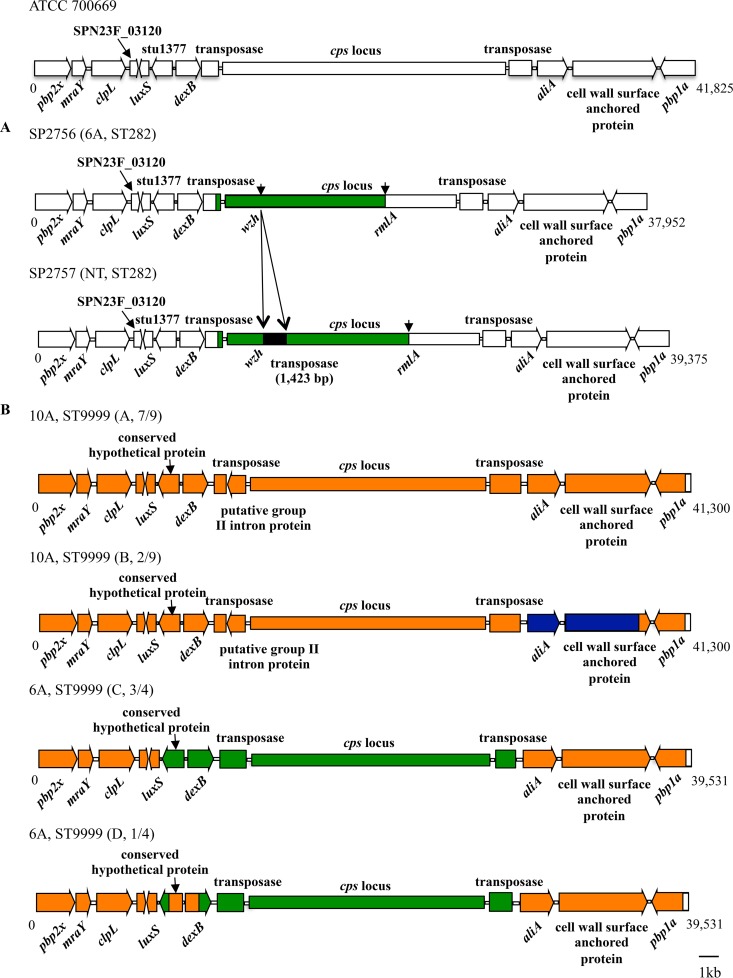
To elucidate the junction sites of capsule switching, DNA sequences around the capsule (cps) loci of the S. pneumoniae strains were compared. Because recombination points for capsule switching likely lay distal to the penicillin-binding protein 2x (pbp2x) and pbp1a genes (35), sequences between pbp2x and pbp1a of the ST282 and CC9999 strains were firstly assembled and compared with that of the corresponding region (bp 291868 to 333692) of ATCC 700669 (Fig. 3).
FIG 3.
Linear genetic arrangement between the pbp2b and pbp1a genes and the predicted recombination points of the ST282 (A) and CC9999 (B) S. pneumoniae strains. The sequence of ATCC 70069 (GenBank accession number FM211187) was obtained from the NCBI database and used as a reference. Regions with different sequences between the strains are displayed in different colors, whereas those corresponding to open reading frames with identical sequences are shown with the same colors.
By comparing the sequences of ATCC 700669 and the ST282 strains (serotype 6A and NT), recombination sites were found in the region between the pbp2x and pbp1a genes (Fig. 3A). The recombination points for capsule switching from serotype 23F to serotype 6A of the 2 ST282 strains were present in the 3′-terminal region of the transposase gene located upstream of the cps locus and the rmlA genes, respectively. A 1,423-bp transposase gene was found in the wzh gene of SP2757, leading to a nonencapsulated mutant.
When the regions from pbp2x to pbp1a were compared between ATCC 700669 and the CC9999 strains, sequences in a region of about 300 bp at the 3′ terminus of the pbp1a gene were identical (Fig. 3B). Thus, it was presumed that the downstream recombination site was located at this region. However, sequences from pbp2x to the 5′-terminal region of pbp1a, excluding the cps locus, were different (97 to 98% identity) between ATCC 700669 and the CC9999 strains, although no obvious recombination point was found.
Based on sequences in the region from pbp2x to pbp1a, the serotype 10A CC9999 strains were further divided into types A and B, whereas the serotype 6A ST9999 strains were divided into types C and D, respectively (Fig. 2B and 3B). Type A included 7 of the serotype 10A strains, whereas type B included the remaining 2 strains. The type A and B strains appeared to be derived from further recombination in the region between aliA and the 5′-terminal region of the gene encoding the cell wall surface-anchored protein (Fig. 3B).
Sequences from pbp2x to luxS and those from aliA to pbp1a of the type C strains were identical to those of the serotype 10A type A strain, whereas those in the region related to capsular serotype were different (Fig. 3B). In the type D strain, the sequence from the 3′ terminus of the gene encoding the conserved hypothetical protein to the 5′ terminus of dexB was identical to the corresponding region of the serotype 10A CC9999 strains but different from those of the type C strains. These results suggest that the type D strain was derived from further recombination of the type C serotype 6A and 10A strains.
To identify the potential recombination site upstream of the cps locus of the CC9999 strains, we compared the ∼40-kb sequences upstream of the pbp2x gene of the 13 CC9999 strains and ATCC 700669. The sequences of the CC9999 strains were identical and shared 99% identity with that of ATCC 700669. Most of the differences in the sequences between the CC9999 strains and ATCC 700669 were located in regions not included in the open reading frames (data not shown). Potential recombination points also could not be found by sequence comparisons in this study.
DISCUSSION
This report describes a cluster of pneumonia infections caused by S. pneumoniae among long-term hospital inpatients. WGS analyses of the causative bacteria revealed that three different S. pneumoniae clones caused 18 pneumonia cases, with one clone being responsible for infection in each ward.
ST9999 and ST10024 are new STs, and only the S. pneumoniae strains isolated in the present study were registered in the MLST database (until January 2018). A total of 5,508 S. pneumoniae strains, including 417 serotype 10A and 6A strains, isolated in Japan were analyzed by serotyping and MLST analyses at our laboratory. The CC9999 strains were isolated only from the pneumococcal cases in this study (B. Chang, unpublished data). These results suggest that S. pneumoniae CC9999 is a unique clone that survived in ward I and/or the hospital. Therefore, the cluster infections caused by CC9999 in ward I are thought to be caused by in-hospital transmission via personnel or from patient to patient. However, 5 ST166 strains belonging to serotypes 9V, 23A, 15B, and 11A/E and 36 ST282 strains belonging to serotypes 6A, 6B, and 6D were also found in our collected S. pneumoniae isolates. These strains were isolated from different regions, including the city where this hospital is located in Japan (Chang, unpublished). Therefore, the possibility of multiple transmissions from family/friends/relatives to patients, in addition to in-hospital infection, should also be considered for the cause of cluster infections occurring in wards II and III. Unfortunately, because we could not investigate nasopharyngeal colonization by S. pneumoniae among inpatients without pneumonia and personnel working in the wards during the period of the infections, detailed infection routes are unclear in this study.
Nasopharyngeal colonization by S. pneumoniae is a prerequisite for pneumococcal infections. In our two previous surveys, approximately 25% and 33% of healthy Japanese children under 3 years old who lived in two different regions possessed S. pneumoniae in their nasopharynx (36, 37). To the best of our knowledge, systematic data on the rate of carriage of pneumococci in healthy adults are lacking. In our investigation, the rate of colonization in Japanese adults who do not suffer from pneumococcal infection is <1% (Chang, unpublished). Although the exact reason has not yet been clarified, it might be that more bacterial species colonize the nasopharyngeal mucosa of adults than children. In the nasopharynx, S. pneumoniae can acquire genes from other cocolonizing pneumococci and/or other bacteria by transformation, causing capsule switching and antibiotic resistance.
Capsule switching from a vaccine type to a nonvaccine type is one of the main reasons for serotype replacement in pneumococcal infections (18, 19). It was reported previously that a persistently carried S. pneumoniae strain may switch its capsule 1.5 × 10−3 times/week (4.6 × 10−5 to 4.8 × 10−3 times/week) (38). Surprisingly, a follow-up study of chronic pediatric otitis media reported by Hiller et al. showed that a total of ∼156 kb of genomic content containing the cps locus of the S. pneumoniae strain was replaced during a 7-month investigation period (39). Because the survey of the CC9999 strains in this study was short term, the research scope was limited, and the ancestor strain that first appeared in the hospital could not be confirmed. Therefore, the characteristics of the genetic transformation of the CC9999 strains are unknown. However, the serotype changed, and further recombination in the region around the cps locus was apparent in the strains isolated in this study, indicating the possibility that transformation among the CC9999 strains occurred at a high frequency (as for the Spain23F-ST81 clone). Therefore, although inpatients have been vaccinated with PPSV23, S. pneumoniae changes from a vaccine serotype to a nonvaccine serotype and causes further infections, which should be taken into account.
To date, more than 90 serotypes have been reported for S. pneumoniae (40). Serotyping is clearly essential for both the analysis of pneumococcal infections and the evaluation of the effect of pneumococcal vaccines. However, we found that serotype analysis alone was insufficient for the detection of pneumococcal transmission: (i) SP2643 was a serotype 10A strain, but it did not belong to the cluster infections occurring in ward I; (ii) two different serotypes, serotypes 6A and 10A, belonged to one longer ongoing outbreak; and (iii) serotype 6A and NT strains isolated from cases in ward III were closely related. Minimally, the combined use of serotyping and molecular typing is required for the study of pneumococcal infection. MLST and WGS analyses are currently the most commonly used methods for the molecular typing of S. pneumoniae. MLST is a simple and low-cost method, and many strains can be analyzed concurrently over a short time. The ST obtained by MLST can be used to probe aspects of the population and evolutionary biology of the organism. WGS analysis can determine whether the bacterial strains are of the same clone and whether the infection is an outbreak. However, WGS analysis is time-consuming and costly, and specific tools are required for genotyping. Thus, it is necessary to selectively use WGS and ST analyses properly when pneumococcal infections are being investigated.
Although PPSV23 was introduced to Japan in 1998, vaccine coverage has remained low. Prior to this outbreak, such cluster pneumonia cases had occurred among long-term inpatients in these wards. Usually, only antibiotic administration and symptomatic treatments were carried out, although the cause of pneumonia was unknown. Naturally, countermeasures against hospital infections, including vaccination with PPSV23, for inpatients with severe physical and mental disorders have not been performed for a long time. Although there are some difficulties, such as a lack of medical staff in the hospital and the difficulty in hygiene management for long-term inpatients, vaccination with PCV13 and/or PPSV23, at least for the prevention of pneumonia, is considered effective and indispensable. In summary, we recommend pneumococcal vaccination for subjects with an increased risk for pneumonia based on the findings of this study.
ACKNOWLEDGMENTS
Support for this project was awarded by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (16K09953) and the Japan Agency for Medical Research and Development (AMED). We have not been paid by a pharmaceutical company or other agency to write this report.
REFERENCES
- 1.Gray BM, Converse GM III, Dillon HC Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 2.Ishiwada N, Kurosaki T, Terashima I, Kohno Y. 2008. The incidence of pediatric invasive pneumococcal disease in Chiba prefecture, Japan (2003-2005). J Infect 57:455–458. doi: 10.1016/j.jinf.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 4.Pallarés R, Liñares J, Vadillo M, Cabellos C, Manresa F, Viladrich PF, Martin R, Gudiol F. 1995. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med 333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 6.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O'Brien KL, Pneumococcal Carriage Group. 2012. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Respiratory Society. 2017. The JRS guidelines for the management of community-acquired pneumonia in adults. Japanese Respiratory Society, Tokyo, Japan. [DOI] [PubMed] [Google Scholar]
- 8.Crum NF, Wallace MR, Lamb CR, Conlin AM, Amundson DE, Olson PE, Ryan MA, Robinson TJ, Gray GC, Earhart KC. 2003. Halting a pneumococcal pneumonia outbreak among United States Marine Corps trainees. Am J Prev Med 25:107–111. doi: 10.1016/S0749-3797(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 9.Kuroki T, Ishida M, Suzuki M, Furukawa I, Ohya H, Watanabe Y, Konnai M, Aihara Y, Chang B, Ariyoshi K, Oishi K, Ohnishi M, Morimoto K. 2014. Outbreak of Streptococcus pneumoniae serotype 3 pneumonia in extremely elderly people in a nursing home unit in Kanagawa, Japan, 2013. J Am Geriatr Soc 62:1197–1198. doi: 10.1111/jgs.12863. [DOI] [PubMed] [Google Scholar]
- 10.Millar MR, Brown NM, Tobin GW, Murphy PJ, Windsor AC, Speller DC. 1994. Outbreak of infection with penicillin-resistant Streptococcus pneumoniae in a hospital for the elderly. J Hosp Infect 27:99–104. doi: 10.1016/0195-6701(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, Ishida M, Hamaguchi S, Aoshima M, Ariyoshi K, Morimoto K, Adult Pneumonia Study Group-Japan. 2017. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis 17:313–321. doi: 10.1016/S1473-3099(17)30049-X. [DOI] [PubMed] [Google Scholar]
- 12.Weiss S, Falkenhorst G, van der Linden M, Imohl M, von Kries R. 2015. Impact of 10- and 13-valent pneumococcal conjugate vaccines on incidence of invasive pneumococcal disease in children aged under 16 years in Germany, 2009 to 2012. Euro Surveill 20(10):21057. doi: 10.2807/1560-7917.ES2015.20.10.21057. [DOI] [PubMed] [Google Scholar]
- 13.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A, Active Bacterial Core Surveillance of the Emerging Infections Program Network. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 14.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, Harrison LH, Schaffner W, Reingold A, Bennett NM, Hadler J, Cieslak PR, Whitney CG, Active Bacterial Core Surveillance Team. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 15.Frazão N, Hiller NL, Powell E, Earl JP, Ahmed A, Sá-Leão R, de Lencastre H, Ehrlich GD, Tomasz A. 2013. Virulence potential and genome-wide characterization of drug resistant Streptococcus pneumoniae clones selected in vivo by the 7-valent pneumococcal conjugate vaccine. PLoS One 8:e74867. doi: 10.1371/journal.pone.0074867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. 2011. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 17.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. 2013. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011. Emerg Infect Dis 19:1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai DR, Shahinas D, Buzina A, Pollock RA, Lau R, Khairnar K, Wong A, Farrell DJ, Green K, McGeer A, Low DE. 2009. Genome-wide dissection of globally emergent multi-drug resistant serotype 19A Streptococcus pneumoniae. BMC Genomics 10:642. doi: 10.1186/1471-2164-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. 2014. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med 2:387–394. doi: 10.1016/S2213-2600(14)70032-3. [DOI] [PubMed] [Google Scholar]
- 20.Domenech A, Ardanuy C, Grau I, Calatayud L, Pallares R, Fenoll A, Brueggemann AB, Liñares J. 2014. Evolution and genetic diversity of the Spain23F-ST81 clone causing adult invasive pneumococcal disease in Barcelona (1990-2012). J Antimicrob Chemother 69:924–931. doi: 10.1093/jac/dkt473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu FZ, Eutsey R, Ahmed A, Frazao N, Powell E, Hiller NL, Hillman T, Buchinsky FJ, Boissy R, Janto B, Bennett J, Longwell M, Ezzo S, Post JC, Tomasz A, Ehrlich GD. 2012. Capsular switch between two highly related Streptococcus pneumoniae strains only partially recreates the more virulent phenotype. PLoS One 7:e47983. doi: 10.1371/journal.pone.0047983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu EY, Chang JC, Lin JC, Chang FY, Fung CP. 2016. Important mutations contributing to high-level penicillin resistance in Taiwan19F-14, Taiwan23F-15, and Spain23F-1 of Streptococcus pneumoniae isolated from Taiwan. Microb Drug Resist 22:646–654. doi: 10.1089/mdr.2015.0261. [DOI] [PubMed] [Google Scholar]
- 23.Nesin M, Ramirez M, Tomasz A. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis 177:707–713. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka J, Ishiwada N, Wada A, Chang B, Hishiki H, Kurosaki T, Kohno Y. 2012. Incidence of childhood pneumonia and serotype and sequence-type distribution in Streptococcus pneumoniae isolates in Japan. Epidemiol Infect 140:1111–1121. doi: 10.1017/S0950268811001592. [DOI] [PubMed] [Google Scholar]
- 25.Llull D, López R, García E. 2006. Characteristic signatures of the lytA gene provide a basis for rapid and reliable diagnosis of Streptococcus pneumoniae infections. J Clin Microbiol 44:1250–1256. doi: 10.1128/JCM.44.4.1250-1256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CLSI. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. CLSI, Wayne, PA. [Google Scholar]
- 27.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H. 2011. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, McCann A, Weill FX, Blin C, Nair S, Wain J, Dougan G, Achtman M. 2014. Transient Darwinian selection in Salmonella enterica serovar Paratyphi A during 450 years of global spread of enteric fever. Proc Natl Acad Sci U S A 111:12199–12204. doi: 10.1073/pnas.1411012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 33.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enright MC, Spratt BG. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 35.Brueggemann AB, Pai R, Crook DW, Beall B. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 3:e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akeda H, Chang B, Nakamura Y, Hamabata H, Ameku K, Toma T, Tamanaha E, Ohnishi M. 2015. Impact of seven valent pneumococcal conjugate vaccine on nasopharyngeal carriage in young children in Okinawa, Japan. World J Vaccines 5:88–95. doi: 10.4236/wjv.2015.52011. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka T, Chang B, Shirai T, Iwaya A, Wada A, Yamanaka N, Okazaki M, SADO-Study Working Group. 2013. Individual risk factors associated with nasopharyngeal colonization with Streptococcus pneumoniae and Haemophilus influenzae: a Japanese birth cohort study. Pediatr Infect Dis J 32:709–714. doi: 10.1097/INF.0b013e31828701ea. [DOI] [PubMed] [Google Scholar]
- 38.Temime L, Boelle PY, Opatowski L, Guillemot D. 2008. Impact of capsular switch on invasive pneumococcal disease incidence in a vaccinated population. PLoS One 3:e3244. doi: 10.1371/journal.pone.0003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiller NL, Ahmed A, Powell E, Martin DP, Eutsey R, Earl J, Janto B, Boissy RJ, Hogg J, Barbadora K, Sampath R, Lonergan S, Post JC, Hu FZ, Ehrlich GD. 2010. Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection. PLoS Pathog 6:e1001108. doi: 10.1371/journal.ppat.1001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. 2015. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]