ABSTRACT
The current diagnostic marker of Lyme neuroborreliosis (LNB), the Borrelia burgdorferi sensu lato antibody index (AI) in the cerebrospinal fluid (CSF), has insufficient sensitivity in the early phase of LNB. We aimed to elucidate the diagnostic value of PCR for B. burgdorferi sensu lato in CSF from children with symptoms suggestive of LNB and to explore B. burgdorferi sensu lato genotypes associated with LNB in children. Children were prospectively included in predefined groups with a high or low likelihood of LNB based on diagnostic guidelines (LNB symptoms, CSF pleocytosis, and B. burgdorferi sensu lato antibodies) or the detection of other causative agents. CSF samples were analyzed by two B. burgdorferi sensu lato-specific real-time PCR assays and, if B. burgdorferi sensu lato DNA was detected, were further analyzed by five singleplex real-time PCR assays for genotype determination. For children diagnosed as LNB patients (58 confirmed and 18 probable) (n = 76) or non-LNB controls (n = 28), the sensitivity and specificity of PCR for B. burgdorferi sensu lato in CSF were 46% and 100%, respectively. B. burgdorferi sensu lato DNA was detected in 26/58 (45%) children with AI-positive LNB and in 7/12 (58%) children with AI-negative LNB and symptoms of short duration. Among 36 children with detectable B. burgdorferi sensu lato DNA, genotyping indicated Borrelia garinii (n = 27) and non-B. garinii (n = 1) genotypes, while 8 samples remained untyped. Children with LNB caused by B. garinii did not have a distinct clinical picture. The rate of detection of B. burgdorferi sensu lato DNA in the CSF of children with LNB was higher than that reported previously. PCR for B. burgdorferi sensu lato could be a useful supplemental diagnostic tool in unconfirmed LNB cases with symptoms of short duration. B. garinii was the predominant genotype in children with LNB.
KEYWORDS: Borrelia burgdorferi, children, Lyme disease, neuroborreliosis, cerebrospinal fluid, genotypic identification, PCR, polymerases
INTRODUCTION
Lyme borreliosis (LB) is caused by the spirochete Borrelia burgdorferi sensu lato, which is transmitted by Ixodes ticks. Several B. burgdorferi sensu lato genotypes can cause LB in humans. Borrelia afzelii, Borrelia garinii, and B. burgdorferi sensu stricto are the most common of these in Europe, whereas B. burgdorferi sensu stricto predominates in the United States. Different B. burgdorferi sensu lato genotypes seem to be differentially associated with the organ manifestation (skin, nervous system, joints) of LB (1–4).
In Europe, Lyme neuroborreliosis (LNB) is the most common disseminated manifestation of LB. Direct detection of B. burgdorferi sensu lato DNA by PCR has been useful for the diagnosis of LB in skin biopsy specimens and synovial fluid, but the sensitivity of this method in the cerebrospinal fluid (CSF) of LNB patients is considered to be low (10 to 30%) (5, 6). LNB is currently diagnosed indirectly by the combination of neurological symptoms, detection of CSF pleocytosis, and detection of intrathecally produced B. burgdorferi sensu lato antibodies (Ab), which are expressed as an antibody index (AI) (5, 7). In any infection, it takes time before the specific Ab production begins, and previous studies have shown that the AI has insufficient sensitivity in the early phase of LNB (8). Partly due to the frequent manifestation of acute facial nerve palsy (FNP), children assessed for LNB often have symptoms of short duration (8, 9), and no accurate diagnostic marker of LNB exists for this group. It has been suggested that PCR for B. burgdorferi sensu lato may be a useful supplemental diagnostic tool in the early phase of LNB and for immunocompromised LNB patients (5, 7, 10, 11). The diagnostic value of B. burgdorferi sensu lato PCR in CSF samples from children with LNB is uncertain, and B. burgdorferi sensu lato PCR has been tested only on small groups for which case definitions and laboratory methods differed significantly (12–16).
Approximately 70% of children with LNB present with FNP with or without symptoms of mild meningitis, and 20 to 30% have no cranial neuropathies but do have symptoms of mild meningitis, headache, and/or fatigue (9, 17, 18). It has been speculated that the variety of symptoms observed in children with LNB is related to the B. burgdorferi sensu lato genotype causing the disease (19). B. burgdorferi sensu lato genotypes have been determined in a few studies on LNB (2, 11, 13, 20–22). Only two of these studies, both conducted in adults, have reported clinical symptoms related to the B. burgdorferi sensu lato genotype (2, 21). The clinical symptoms of children with LNB differ from those of adults (9, 18), and the relationship between clinical symptoms and the B. burgdorferi sensu lato genotype in children with LNB has not been explored.
The aims of this study were (i) to evaluate the sensitivity and specificity of real-time PCR in the detection of B. burgdorferi sensu lato DNA in the CSF of children with symptoms suggestive of LNB, (ii) to elucidate the B. burgdorferi sensu lato genotypes associated with LNB in children, and (iii) to assess whether the clinical picture in children with LNB is related to the B. burgdorferi sensu lato genotype.
MATERIALS AND METHODS
Subjects.
The study area, southwest Norway, including Hordaland, Rogaland, and Vest- and Aust-Agder counties, is a region of LB endemicity. Approximately 290,000 children ≤18 years old live in this area (Statistics Norway, Table 07459 [http://www.ssb.no/en/statbank/table/07459/tableViewLayout1/?rxid=2a7fbd41-3bdb-4e19-ae2d-e9c236579f9e; accessed 11 July 2016]). In a multicenter prospective study of children with LNB, all children aged 3 months to 18 years with symptoms suggestive of LNB who were admitted to the pediatric departments of five hospitals from autumn 2011 to spring 2014 were invited to participate. Children who had been given antibiotics prior to admission were excluded. The inclusion criteria for the various diagnostic groups were defined prior to the start of the study, and the children were subsequently allocated to the groups based on laboratory findings upon admission, as shown in Table 1 and as described in more detail elsewhere (23). Children with probable LNB were considered likely to have LNB because LNB is the predominant cause of aseptic meningitis in children in this region (24), and it has been shown previously that this group has a clinical presentation similar to that of children with confirmed LNB in this area (9). In this study of PCR for B. burgdorferi sensu lato, all diagnostic groups listed in Table 1 were included, except for the nonmeningitis group, because the inclusion criteria for this group were wide, and we expected that this group would include a large number of children with an unclear likelihood of having LB and a low pretest probability of having positive PCR results. For the estimations of sensitivity and specificity, children with confirmed and probable LNB were included as LNB patients, whereas children with non-Lyme aseptic meningitis (NLAM) and negative controls were included as non-LNB controls. These decisions were made prior to the PCR analyses.
TABLE 1.
Criteria for classification of children with signs or symptoms suggestive of LNB
| Diagnostic group | CSF pleocytosisa | Laboratory findings/physical examinationb |
|---|---|---|
| LNB patients | ||
| Confirmed LNB | Yes | 1. AI positive |
| Probable LNB | Yes | 1. AI negative |
| 2. Positive for B. burgdorferi sensu lato Ab in serum and/or CSF at admission or, if available, at follow-up, and/or EM in the past 10 wks | ||
| 3. No other agents for meningitis identified | ||
| Possible LNB conditions | ||
| Possible LNB | Yes | 1. AI negative |
| 2. CSF and serum negative for B. burgdorferi sensu lato Ab; no EM in the past 10 wks | ||
| 3. No other agents of meningitis identified | ||
| Possible peripheral LNB | No | 1. Facial nerve palsy in season (May–November) |
| 2. Serum positive for B. burgdorferi sensu lato Ab at admission and/or, if available, at follow-up, and/or EM in the past 10 wks | ||
| Non-LNB controls | ||
| Non-Lyme aseptic meningitis | Yes | 1. AI negative |
| 2. CSF negative for B. burgdorferi sensu lato Ab | ||
| 3. Other, more likely agents of meningitisc identified in CSF, serum, or feces | ||
| 4. No EM in the past 10 wks | ||
| Negative controls | No | 1. No facial nerve palsy |
| 2. Serum and CSF negative for B. burgdorferi sensu lato Ab | ||
| 3. No EM ever | ||
| 4. Duration of symptoms, >1 mo, or >1 mo between the start of symptoms and a negative result for B. burgdorferi sensu lato Ab in serum at available follow-up test | ||
| Excluded: nonmeningitis | No |
Defined as >5 × 106 white blood cells/liter in CSF.
AI, B. burgdorferi sensu lato antibody index (IgG and/or IgM); Ab, antibodies (IgG and/or IgM); EM, erythema migrans.
Children with CSF pleocytosis were diagnosed with enterovirus meningitis if (i) enterovirus RNA was detected by PCR in the CSF and B. burgdorferi sensu lato Ab were absent in the CSF or (ii) enterovirus was detected in the feces and B. burgdorferi sensu lato Ab were absent from both the serum and CSF. Children with CSF pleocytosis and a recent history of airway symptoms (coughing or dyspnea) were diagnosed with Mycoplasma-associated meningoencephalitis if both IgG and IgM were positive for Mycoplasma in the serum and B. burgdorferi sensu lato Ab were absent from the CSF and serum (isolated low-titer serum B. burgdorferi sensu lato IgM was allowed).
The study was approved by the Regional Committee for Medical Health and Research Ethics in Western Norway, and for each child, one of the parents provided written informed consent.
Data collection and routine laboratory tests.
On admission, all children included and/or their parents were interviewed with a standardized questionnaire, including questions regarding the type and duration of symptoms and a history of erythema migrans (EM). For each child, a blood sample was taken and a lumbar puncture was performed. The blood samples were analyzed for the total count of white blood cells (WBC), C-reactive protein, and Ab against B. burgdorferi sensu lato and Mycoplasma pneumoniae. The CSF samples were analyzed for the total count of WBC, the percentage of mononuclear cells, the protein level, and the presence of B. burgdorferi sensu lato Ab and enterovirus RNA (PCR). Additional tests for other infectious agents were ordered by the attending physician based on clinical findings. B. burgdorferi sensu lato Ab were detected in serum and CSF, and the AI was calculated, according to internal routines at the three hospitals providing laboratory services to the five participating hospitals. The tests applied were the Liaison Borrelia IgM and Borrelia IgG (DiaSorin, Saluggia, Italy), Enzygnost Lyme link VlsE/IgG, and Borreliosis/IgM (Siemens Healthineers, Erlangen, Germany) assays. The AI was determined according to the method of Reiber (25) or by the use of the IDEIA Lyme neuroborreliosis kit (Oxoid, Cheshire, UK).
Preparation of DNA.
CSF samples were stored frozen at −70°C at each hospital and were transported collectively on dry ice to the Department of Medical Microbiology, Hospital of Southern Norway Trust, Kristiansand, Norway, where all the DNA preparation and molecular analysis were performed in 2016. The laboratory conducting the analysis is the LB diagnostic reference laboratory in Norway, and these analyses have been performed there routinely since 2008. The PCR methods used are validated according to the standard of Norwegian Accreditation and are regularly evaluated by international external quality assessments (Quality Control for Molecular Diagnostics [QCMD]).
Samples that contained <200 μl CSF were excluded. Thawed CSF samples were centrifuged at 10,000 × g for 1 h. All the pelleted material was resuspended in 200 μl CSF and was incubated in ATL lysis buffer supplemented with proteinase K (1 h; 56°C) before completion of the DNA extraction protocol according to the manufacturer's instructions (QIAamp DNA minikit [Qiagen, Venlo, The Netherlands]). The elution volume of each sample was 200 μl. All DNA extracts were stored at −70°C for later analyses. Genotyping was performed on DNA extracts frozen and stored for 6 months after analysis for detection of B. burgdorferi sensu lato DNA.
B. burgdorferi sensu lato-specific PCR methods.
DNA samples were tested using two independent real-time assays targeting the ospA and 16S rRNA genes to detect B. burgdorferi sensu lato. The sequences and final concentrations of all primers and probes are given in Table 2. The assays were performed using 5 μl of DNA in a 15-μl reaction mixture consisting of 5 mM MgCl2, 0.5 U uracil DNA-glycosylase (Eurogentec S.A., Seraing, Belgium), and LightCycler FastStart DNA master mix (Roche), with primers and a probe. The following thermocycling parameters were used on a LightCycler 480 system: 2 min at 40°C followed by 10 min at 95°C and 47 cycles of 15 s at 95°C, 30 s at 60°C, and 20 s at 72°C. The ospA and 16S rRNA gene real-time assays used in this study have been validated against several panels of Borrelia strains in order to document the ability to detect strains relevant to human disease (26). The specificity of the assays has been challenged by testing DNA extracted from >130 CSF samples from adult non-LB controls (27) without generating any false-positive results.
TABLE 2.
Characteristics and sequences of primers and probes used for detection and genotype differentiation of B. burgdorferi sensu lato
| Target organism(s) | Target genea | Oligonucleotide sequence (5′–3′)b | Primer/probe concn (μM) | Reference |
|---|---|---|---|---|
| B. burgdorferi | ospA | F, ATATTTATTGGGAATAGGTCTAATAT | 0.5/0.4 | 33 |
| R, CTTTGTCTTTTTCTTTRCTTACAAG | ||||
| P, AAGCAAAATGTTAGCAGCCTTGA | ||||
| B. burgdorferi | 16S rRNA gene | F, GCTGTAAACGATGCACACTTGGT | 0.5/0.2 | 39 |
| R, GGCGGCACACTTAACACGTTAG | ||||
| P, TTCGGTACTAACTTTTAGTTAA-MGB | ||||
| B. afzelii and B. bissettiae | uvr-Ba | F, GCTCAGCGYATTAGGCTTGCTACT | 0.5/0.2 | Modified method based on reference 28c |
| R, GCTCAACAACAATTACMGTATTGCC | ||||
| P, AAGGCCAATGCTTGG (LNA) | ||||
| B. garinii and B. bavariensis | hbb-Bg | F, GGAAATTAGTTTATGTCTTTTTCAAG | 0.5/0.2 | Modified method based on reference 29c |
| R, TAAGCTCTTCAAAAAAAGCATCTA | ||||
| P, AGACCGAAGATTACTAAATCAGATATTGT | ||||
| B. burgdorferi sensu stricto, B. bissettiae, B. afzelii | hbb-Bb | F, GGAAATTAGTTTATGTCTTTTTCAAG | 0.5/0.2 | Modified method based on reference 29c |
| R, TAAGCTCTTCAAAAAAAGCATCTA | ||||
| P, AGACCAAAGGTTACTAAGTCAGACATTG | ||||
| B. garinii and B. spielmanii | nifS-Bg | F, ATAATTTATTTTGACAATGCAGCA | 0.5/0.5 | 28 |
| R, TTTTATGCTAGATTGAATTGCAAA | ||||
| P, CTAGCATTGAATATTATGAAAATTACAAC | ||||
| B. bavariensis | nifS-Bav | F, ATAATTTATTTTGACAATGCAGCA | 0.5/0.5 | 28 |
| R, TTTTATGCTAGATTGAATTGCAAA | ||||
| P, CTATCATTGAATATTATGAAAATTACAAC |
ospA, outer surface protein A; uvr, excinuclease ABC subunit A; hbb, protein H; nifS, aminotransferase.
F, forward primer; R, reverse primer; P, probe; MGB, minor groove binder; LNA, lock nucleic acids. LNA bases are indicated by boldface letters.
The probes used in the uvr and hbb assays are based on the references given and a redesign by Olfert Landt (TIB Molbiol, Berlin, Germany).
All samples were first analyzed in duplicate by both assays (ospA and 16S rRNA genes), and samples with inconsistent results were subsequently analyzed in triplicate, as shown in Fig. 1. Samples were considered positive for B. burgdorferi sensu lato DNA if both assays gave positive results after one or two runs, or if more than one replicate in one assay was positive after two runs. When both assays gave negative results for all replicates, either in the first run or when positive results in one assay could not be confirmed in the second run, the samples were considered to be negative for B. burgdorferi sensu lato DNA. All samples were tested for inhibition.
FIG 1.
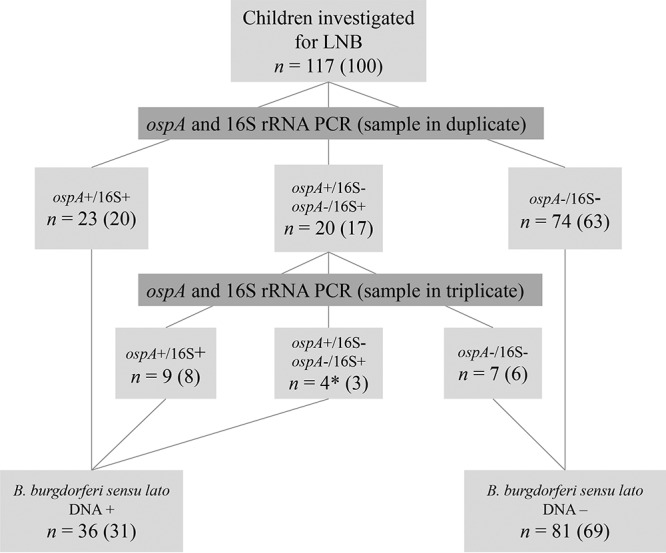
Flow chart of the PCR testing algorithm for the detection of Borrelia burgdorferi sensu lato DNA in CSF samples from children investigated for Lyme neuroborreliosis (LNB), with results. The two real-time PCR assays applied targeted the ospA and 16S rRNA genes. The total numbers and percentages (in parentheses) of children with various test outcomes are given in the boxes. *, all four children had >1 replicate positive for ospA, and no children had a positive result for the 16S rRNA gene only.
Differentiation of B. burgdorferi sensu lato genotypes by real-time PCR.
The samples identified as positive for B. burgdorferi sensu lato DNA were further analyzed with five singleplex real-time PCR assays for genotype determination (Table 2) (28, 29). The PCR thermocycling conditions were the same as those used for the ospA and 16S rRNA gene PCR assays. All assays were validated against a panel consisting of nine B. burgdorferi sensu lato genotypes (B. garinii, B. afzelii, B. burgdorferi sensu stricto, B. bissettiae, B. spielmanii, B. japonica, B. bavariensis, B. valaisiana, and B. lusitaniae) distributed by the ESCMID Study Group for Lyme Borreliosis (ESGBOR) and used in a Europe-wide study on the sensitivity and specificity of different PCR amplification protocols for the detection of B. burgdorferi sensu lato, conducted in 2016 (30). All samples were first screened by the B. afzelii uvr (uvr-Ba), B. garinii hbb (hbb-Bg), and B. burgdorferi sensu lato hbb (hbb-Bb) assays. Samples were tested in two to four replicates, depending on whether the result was conclusive or not after two runs and on the amount of DNA available. Subsequently, all samples were analyzed by the B. garinii nifS (nifS-Bg) and B. bavariensis nifS (nifS-Bav) assays (one or two replicates depending on the results and the amount of DNA), which were performed after the initial screening in an attempt to distinguish between B. garinii and B. bavariensis. Due to a high risk of false-negative results for the samples that contained small amounts of B. burgdorferi sensu lato DNA, genotype determination was based on positive PCR results only.
Statistical analysis.
Differences in clinical and laboratory characteristics between groups with different outcomes by the B. burgdorferi sensu lato PCR or by genotyping were analyzed by the Pearson chi-square and Fisher exact tests for categorical variables and by the Kruskal Wallis and Mann-Whitney U tests for continuous variables. Statistical analyses were performed using SPSS Statistics 23 (IBM, New York, NY, USA). The 95% confidence interval (CI) for sensitivity and specificity were calculated by VassarStats online clinical calculator 1 (www.vassarstats.net).
RESULTS
In total, 217 children were assessed for symptoms suggestive of LNB. For 7 children, the laboratory data necessary for a diagnostic classification were missing; 91 children were diagnosed as not having meningitis; and samples from 2 children had an insufficient volume of CSF for the PCR analysis (see Materials and Methods regarding the inclusion and categorization of patients). The CSF samples of the remaining 117 children were analyzed by PCR. Of these, 76 were diagnosed as LNB patients (58 with confirmed LNB and 18 with probable LNB), 13 as having possible LNB conditions (6 with possible LNB and 7 with possible peripheral LNB), and 28 as non-LNB controls (12 with non-Lyme aseptic meningitis [NLAM] and 16 negative controls). For 6 of the 18 children with probable LNB, the amount of CSF was insufficient for AI analysis. The median CSF volume before centrifugation was lower for LNB patients (0.6 ml; interquartile range [IQR], 0.5 to 0.8 ml) than for non-LNB controls (0.90 ml; IQR, 0.75 to 1.0 ml) (P < 0.01).
Detection of B. burgdorferi sensu lato DNA in CSF by PCR.
The testing algorithm and the outcomes of PCRs targeting the B. burgdorferi sensu lato ospA and 16S rRNA genes in the CSF samples of the children are shown in Fig. 1. No samples were excluded due to inhibition.
The numbers of children in the various diagnostic groups who tested positive or negative for B. burgdorferi sensu lato DNA by PCR are shown in Table 3. All samples from the non-LNB controls were negative in duplicate in both assays. The rate of detection of B. burgdorferi sensu lato DNA did not differ between children with confirmed and probable LNB. The 12 children with probable LNB and a negative AI had symptoms of shorter duration (median, 3 days; IQR, 2 to 4.75 days) than those of all 58 children with a positive AI (confirmed LNB) (median duration of symptoms, 8 days; IQR, 4 to 17 days) (P = 0.025). B. burgdorferi sensu lato DNA was detected in CSF from 7 of the 12 children (58%) with probable LNB and a negative AI, compared to 26 of the 58 children (45%) with a positive AI (not significant).
TABLE 3.
Numbers of children positive and negative for B. burgdorferi sensu lato DNA by PCR in each diagnostic group
| PCR result | No. (%) of children in the following diagnostic group: |
|||||
|---|---|---|---|---|---|---|
| Confirmed LNB | Probable LNB | Possible LNB | Possible peripheral LNB | Non-Lyme aseptic meningitis | Negative control | |
| Positive | 26 (45) | 9 (50) | 0 (0) | 1 (14) | 0 (0) | 0 (0) |
| Negative | 32 (55) | 9 (50) | 6 (100) | 6 (86) | 12 (100) | 16 (100) |
| Total | 58 | 18 | 6 | 7 | 12 | 16 |
In children with symptoms suggestive of LNB, either diagnosed as LNB patients or categorized as non-LNB controls, the sensitivity of PCR for B. burgdorferi sensu lato in CSF was 46% (95% confidence interval [CI], 35 to 58%), and the specificity was 100% (95% CI, 85 to 100%).
B. burgdorferi sensu lato genotypes in children with suspected LNB.
Of the 36 samples in which B. burgdorferi sensu lato DNA was detected, 16 had DNA detected by the hbb-Bg and nifS-Bg PCR assays, and the organisms were identified as B. garinii (see Materials and Methods). For 11 samples, DNA was detected only by the hbb-Bg assay, indicating the presence of either B. garinii or B. bavariensis. Since the nifS-Bav assay failed to detect DNA in any sample, the presence of B. bavariensis was considered less likely. In one sample, DNA was detected only by the hbb-Bb assay, indicating the presence of either B. burgdorferi sensu stricto, B. afzelii, or B. bissettiae. For eight samples, no genotype could be determined. There was a tendency toward a higher genotype detection rate among children for whom all four replicates of the ospA and 16S rRNA gene assays (8/8) were positive than for children who had only one to three replicates positive (20/28 [71%]) (not significant).
Clinical variables and detection of B. burgdorferi sensu lato DNA and genotypes.
The clinical characteristics and laboratory data from the LNB patients tested by PCR for B. burgdorferi sensu lato are shown in Table 4. Children who tested positive for B. burgdorferi sensu lato by PCR had higher levels of white blood cells (WBC) in CSF and a tendency toward a higher age than those who tested negative for B. burgdorferi sensu lato by PCR. There was also a tendency for a higher number of children who were positive for B. burgdorferi sensu lato by PCR to have symptoms of short duration (<14 days). Otherwise, no differences were found between the groups.
TABLE 4.
Clinical characteristics and laboratory findings for LNB patients testing positive and negative for B. burgdorferi sensu lato by PCR
| Variablea | Value for group |
P valueb | |
|---|---|---|---|
| PCR positive (n = 35) | PCR negative (n = 41) | ||
| Clinical characteristics | |||
| Age (yr) | 7.5 (5–9) | 6 (4.5–8) | 0.072 |
| Male gender | 17/18 (49) | 21/20 (51) | 0.818 |
| Erythema migrans in the past 10 wks | 2/26 (7) | 4/32 (11) | 0.688c |
| Headache | 25/9 (74) | 25/13 (66) | 0.477 |
| Facial nerve palsy | 24/11 (69) | 27/14 (66) | 0.806 |
| Other cranial neuropathyd | 2/32 (6) | 3/37 (8) | 1.00c |
| Other neurological symptomse | 9/24 (27) | 10/29 (26) | 0.876 |
| CN without GIS | 2/29 (7) | 4/31 (11) | 0.676c |
| GIS with CN | 18/12 (60) | 18/17 (51) | 0.488 |
| GIS without CN | 10/24 (29) | 13/28 (32) | 0.830 |
| Duration of symptoms (days) | 7 (3–14) | 8 (3–20.5) | 0.418 |
| Duration of symptoms, <14 days | 25/10 (71) | 22/19 (54) | 0.112 |
| Laboratory findings in serum | |||
| White blood cells (109/liter) | 7.6 (6.8–8.3) | 8.2 (7.4–9) | 0.260 |
| Median CRP (mg/liter) | 1 (1–4) | 1 (1–4) | 0.675 |
| Borrelia IgG | 28/7 (80) | 37/4 (90) | 0.206 |
| Borrelia IgM | 18/17 (51) | 21/20 (51) | 0.985 |
| Laboratory findings from cerebrospinal fluid | |||
| Vol before centrifuging (ml) | 0.6 (0.5–1.0) | 0.6 (0.5–0.80) | 0.312 |
| White blood cells (106/liter) | 262 (60–335) | 88 (35–189) | 0.008 |
| % mononuclear cells | 95 (93–97) | 95 (89–99) | 0.657 |
| Protein concn (g/liter) | 0.54 (0.36–0.86) | 0.42 (0.29–0.68) | 0.278 |
| Borrelia IgG or IgM index (AI) | 26/7 (79) | 32/5 (87) | 0.394 |
Values for categorical variables are given as follows: number of patients with the characteristic/number of patients without the characteristic (percentage of patients with the characteristic). Values for continuous variables are medians (interquartile ranges). CN, cranial neuropathy; GIS, general inflammation symptoms; CRP, C-reactive protein.
By the Mann-Whitney U test for continuous variables and the Pearson chi-square for categorical variables, except where otherwise indicated. P values of <0.05 are shown in boldface.
By Fisher's exact test.
Trigeminal nerve affection (pain [n = 2], sensory loss [n = 1]), oculomotor nerve affection (n = 2).
Mainly neck stiffness.
LNB patients in whose samples B. garinii was detected (including children with either B. garinii or B. bavariensis) had higher levels of WBC in CSF, and more had symptoms of short duration (<14 days), than LNB patients who tested negative for B. burgdorferi sensu lato by PCR (Table 5). Based on these results, we performed an additional logistic regression analysis for children who were B. garinii positive or B. burgdorferi sensu lato negative by PCR. In the final analysis, a <14-day duration of symptoms (odds ratio [OR], 4.1; 95% CI, 1.2 to 13.6) (P = 0.021) and the log-transformed level of WBC in CSF (OR, 1.8; 95% CI, 1.1 to 3.0) (P = 0.029) were independently associated with the detection of B. garinii. There was no interaction between a <14-day duration of symptoms and the log-transformed level of WBC in CSF (P = 0.098). The other variables in Table 5 were tested with forward stepwise modeling, but none contributed to distinguishing between children who were B. garinii positive and those who were B. burgdorferi sensu lato negative by PCR. The presence of cranial neuropathies other than FNP was associated with cases of LNB positive for B. burgdorferi sensu lato by PCR in which no genotype was detected. No further differences in clinical symptoms or laboratory findings were found between children with LNB caused by B. garinii, those with LNB positive for B. burgdorferi sensu lato by PCR but with no genotype detected, and those with LNB negative for B. burgdorferi sensu lato by PCR. The child with LNB caused by a genotype other than B. garinii (either B. afzelii, B. burgdorferi sensu stricto, or B. bissettiae) had an isolated headache for 3 weeks but no cranial neuropathy or other symptoms.
TABLE 5.
Clinical characteristics and laboratory findings for LNB patients with different genotyping outcomes
| Variablea | Value for patients with the following genotyping outcome: |
P valuec |
|||||
|---|---|---|---|---|---|---|---|
| A (B. gariniib) (n = 27) | B (untyped) (n = 7) | C (negative for B. burgdorferi sensu lato by PCR) (n = 41) | Overall | A vs B | A vs C | B vs C | |
| Clinical characteristics | |||||||
| Age (yr) | 7 (5–9) | 7.5 (6–11) | 6 (4.5–8) | 0.200d | |||
| Male gender | 14/13 (52) | 5/2 (71) | 21/20 (51) | 0.655e | |||
| Erythema migrans in the past 10 wks | 1/22 (4) | 1/3 (25) | 4/32 (11) | 0.278e | |||
| Headache | 17/9 (65) | 7/0 (100) | 25/13 (66) | 0.182e | |||
| Facial nerve palsy | 19/8 (70) | 5/2 (71) | 27/14 (66) | 0.436e | |||
| Other cranial neuropathyf | 0/26 (0) | 2/5 (29) | 3/37 (8) | 0.034e | 0.040e | 0.272e | 0.154e |
| Other neurological symptomsg | 7/19 (27) | 2/4 (33) | 10/29 (26) | 0.924e | |||
| CN without GIS | 2/21 (9) | 0/7 (0) | 4/31 (11) | 1.00e | |||
| GIS with CN | 13/9 (59) | 5/2 (71) | 18/17 (51) | 0.585e | |||
| GIS without CN | 7/19 (27) | 2/5 (29) | 13/28 (32) | 0.929e | |||
| Duration of symptoms (days) | 7 (3–10) | 18 (7–30) | 8 (3–20.5) | 0.130d | |||
| Duration of symptoms, <14 days | 22/5 (82) | 3/4 (43) | 22/19 (54) | 0.031e | 0.061e | 0.019h | 0.696 |
| Laboratory data from cerebrospinal fluid | |||||||
| Vol before centrifuging (ml) | 0.60 (0.5–1.0) | 0.7 (0.6–1.0) | 0.6 (0.5–0.8) | 0.467d | |||
| White blood cells (106/liter) | 266 (60–350) | 81 (47–274) | 88 (35–189) | 0.025d | 0.241i | 0.007i | 0.520i |
| % mononuclear cells | 95 (94–97) | 96 (90–98) | 95 (89–99) | 0.862d | |||
| Protein concn (g/liter) | 0.53 (0.36–0.86) | 0.54 (0.4–0.84) | 0.42 (0.29–0.68) | 0.542d | |||
| Borrelia IgG or IgM index (AI) | 19/6 (76) | 6/1 (86) | 32/5 (87) | 0.596e | |||
| No. with all 4 replicates PCR positive/no. with 1–3 replicates positive (% with all 4 replicates positive) | 7/20 (26) | 0/7 (0) | 0.299e | ||||
Except where otherwise noted, values for categorical variables are given as follows: number of patients with the characteristic/number of patients without the characteristic (percentage of patients with the characteristic). Values for continuous variables are medians (interquartile ranges). CN, cranial neuropathy; GIS, general inflammation symptoms.
In 11 children, B. garinii could not be definitely distinguished from B. bavariensis.
P values of <0.05 are shown in boldface.
Determined by the Kruskal-Wallis test.
Determined by Fisher's exact test.
Trigeminal nerve affection (pain [n = 2], sensory loss [n = 1]), oculomotor nerve affection (n = 2).
Mainly neck stiffness.
Determined by the Pearson chi-square test.
Determined by the Mann-Whitney U-test.
DISCUSSION
In this study using two B. burgdorferi sensu lato-specific PCR assays, B. burgdorferi sensu lato DNA was detected in the CSF of 46% of children diagnosed with LNB, a rate higher than those reported in most previous studies of both children and adults (6, 10–15, 31). B. garinii was the predominant genotype associated with LNB in children in southwest Norway. Children with LNB caused by B. garinii did not have a distinct clinical picture.
Detection of B. burgdorferi sensu lato DNA for the diagnosis of LNB.
In the first studies in which PCR was used to diagnose LNB in children, B. burgdorferi sensu lato DNA was detected in the CSF of 12 to 25% of the children with LNB (12–15). In these studies, the CSF volumes used were low (0.05 to 0.2 ml), the DNA extraction protocols applied may have led to low yields and impure DNA, and the target DNA was detected by conventional PCR. These factors may have reduced diagnostic sensitivity. Various approaches to the direct detection of B. burgdorferi sensu lato DNA in CSF may provide various results (32). In our study, the use of relatively large volumes of CSF (median, 0.6 ml for LNB patients), a B. burgdorferi sensu lato-concentrating step (centrifugation) prior to the isolation of DNA (33), high-quality isolated DNA, and replicate testing with two different PCR assays may have contributed to the high detection rate. In the first duplicate run of the ospA–16S rRNA gene screening, 23 samples were confirmed positive for B. burgdorferi sensu lato DNA and 20 samples were inconclusive (Fig. 1). Thirteen of these 20 samples were confirmed positive after the triplicate run. A less labor-intensive approach would be to rely on the results in the first duplicate run, regardless of whether samples were positive in one or two assays. This procedure would actually have increased the sensitivity of B. burgdorferi sensu lato PCR in this study. However, we chose to be conservative, based on the notion that positive results should be repeatable.
In three of the previous studies of children (12–14) and one study of adults (31) with LNB, nested PCR methods were conducted to increase assay sensitivity. This approach is likely to increase the risk of false-positive results, which may have been masked in studies without control groups (13, 14). In our study, diagnostic specificity was high: none of the non-LNB controls tested positive for B. burgdorferi sensu lato by PCR. All analyses were performed with enzymatic prevention of potential carryover contaminants and in a closed analysis system. All samples were analyzed by two independent PCR assays, and no sample was classified as positive for B. burgdorferi sensu lato DNA based on a single PCR. We consider the risk of false-positive results with these methods to be low, in accordance with the previous validation of these assays (26, 27).
Our results indicate a tendency toward a higher rate of detection of B. burgdorferi sensu lato DNA in children with LNB and symptoms of short duration (Table 4), in line with the work of Lebech (11). In areas of endemicity, LNB is the most common cause of acute peripheral FNP in children, a symptom that is recognized early (19). In the study region, we perform lumbar puncture routinely on all children with FNP, without waiting for any other symptoms or laboratory findings suggestive of LNB. This practice may have led to an overall short duration of symptoms in children diagnosed with LNB and therefore possibly a higher rate of detection of B. burgdorferi sensu lato DNA in CSF than that for adults (10, 11, 27, 34). Interestingly, in 7/12 children with probable LNB, symptoms of short duration, and a negative AI, B. burgdorferi sensu lato DNA could be detected. Furthermore, B. burgdorferi sensu lato DNA was detected in the CSF from one child categorized as having possible peripheral LNB who presented with isolated FNP for 2 days and no CSF pleocytosis (23). This may suggest that B. burgdorferi sensu lato PCR could be useful in the diagnosis of early LNB before a positive AI or even pleocytosis has developed.
Our estimates of the sensitivity and specificity of B. burgdorferi sensu lato PCR assume that the children in this study were categorized with correct diagnoses based on the current diagnostic criteria for LNB. These criteria have limited sensitivity in the early phase (5, 8).
B. burgdorferi sensu lato genotypes associated with LNB in children.
Previous European studies have shown that B. garinii is the most common genotype associated with LNB in adults (58 to 70%), followed by B. afzelii (14 to 35%) and B. burgdorferi sensu stricto (0 to 11%) (2, 11, 20–22). B. burgdorferi sensu lato genotypes detected in the CSF of children with LNB have been reported in one study identifying both B. garinii (n = 7) and B. afzelii (n = 4) in 12 children with PCR-positive LNB (13). Our study, the only report on B. burgdorferi sensu lato genotypes detected in CSF from Norwegian patients, suggests that B. garinii is the predominant genotype causing LNB in Norwegian children. However, we cannot definitely exclude the possibility that potentially present non-B. garinii genotypes cause LNB with lower loads in CSF than those detectable by the PCR assays used for the detection of B. burgdorferi sensu lato DNA in this study.
B. afzelii is the most common cause of erythema migrans, whereas B. garinii appears to preferentially disseminate in and affect the central nervous system (CNS) (20). Previous studies from southern Norway (35, 36) and neighboring Sweden (37) have shown that the major vector of LB, the nymphal Ixodes ricinus tick, is most frequently infected with B. afzelii. Our finding that B. garinii is the main cause of LNB in an environment presumably dominated by B. afzelii-infected ticks supports the theory that B. garinii is a more neurotropic bacterium, or exhibits a higher capacity to disseminate, than B. afzelii (20).
Some of the B. burgdorferi sensu lato DNA-positive CSF samples (8/36) in our study were not successfully genotyped. The most likely explanation supported by the laboratory findings is a concentration of B. burgdorferi sensu lato DNA beneath the detection limit of the PCR assays used for genotyping, although the presence of rare genotypes not targeted by the assays used cannot be excluded.
Clinical characteristics and B. burgdorferi sensu lato genotypes.
In this study, a high level of WBC in CSF and a <14-day duration of symptoms were independently associated with the detection of B. garinii. We do not know if these are clinical characteristics associated with B. garinii infection or if these factors only contribute to a higher rate of detection of B. burgdorferi sensu lato genotypes in general. It is possible that the concentration of B. burgdorferi sensu lato is higher in the early phase, before specific antibody-induced eradication occurs, and a high concentration of the organism could both increase the chance of detecting B. burgdorferi sensu lato genotypes and induce more inflammation. It is also possible that the B. garinii genotype may induce more CNS inflammation than other genotypes, but since a non-B. garinii genotype was detected in only one child, this conclusion cannot be drawn from our study.
Strle et al. found that LNB caused by B. garinii was associated with the acute symptoms of classical Bannwarth's syndrome in adults, whereas LNB caused by B. afzelii was associated with more-diffuse, long-lasting symptoms (2). However, Busch et al. managed to isolate all three of the most common B. burgdorferi sensu lato genotypes—B. garinii, B. afzelii, and B. burgdorferi sensu stricto—from adults with Bannwarth's syndrome (21). In our study, children in whom B. garinii was detected presented with various clinical pictures, similar to those described previously for children with LNB (9, 17, 18). Moreover, these symptoms did not differ from those of the group of children who tested positive for B. burgdorferi sensu lato by PCR with no genotype detected or the group testing negative for B. burgdorferi sensu lato by PCR. B. garinii may have been the predominant genotype in these two groups as well, and the only conclusion we can draw is that children with LNB caused by B. garinii do not have a uniform clinical picture.
Consequently, differences in clinical pictures may be related to factors other than the B. burgdorferi sensu lato genotype. Previous studies have found great heterogeneity in large restriction fragment patterns (LRFP), plasmid profiles, and surface proteins in B. garinii strains isolated from CSF (20, 21, 38). These factors may play a role in the host immune response to infection and may thereby influence the clinical presentation of LNB caused by B. garinii in children.
This is currently the largest study on B. burgdorferi sensu lato PCR in LNB diagnostics that includes only children. Other strengths of this study are the prospective inclusion of well-defined cases and clinically relevant control groups, the large CSF sample sizes, the methods used to control for contamination, and the use of replicate testing by two individual assays to detect B. burgdorferi sensu lato DNA. Potential limitations include the lack of a diagnostic confirmatory follow-up lumbar puncture for children with probable or possible LNB. There may be minor differences in the sensitivities of the three different AI assays used, but it is unlikely that this influenced our results, since both children with confirmed LNB and those with probable LNB (AI negative) were included in the estimates of the sensitivities of the PCR assays. The number of children in the control group could have been larger, and consequently, the 95% CI for specificity could have been smaller.
In conclusion, in this study of children with symptoms suggestive of LNB, the rate of detection of B. burgdorferi sensu lato DNA in CSF by two real-time PCR assays was higher than those reported previously. This study supports the use of B. burgdorferi sensu lato PCR as a supplemental diagnostic tool for children with suspected LNB, particularly in the early phase, when the AI is negative and the diagnosis cannot be confirmed. PCR-based genotyping suggests that B. garinii is the predominant genotype associated with LNB in children in Norway. Children with LNB caused by B. garinii did not have a uniform clinical picture, suggesting that factors other than the B. burgdorferi sensu lato genotype determine the clinical presentation of LNB. Future studies correlating the phenotypic and genotypic characteristics of B. burgdorferi sensu lato strains in CSF with markers of host immune response and clinical reports may identify these factors.
ACKNOWLEDGMENTS
This research received grants from Stavanger University Hospital and the EU-Interreg ÖKS project ScandTick Innovation for laboratory analyses, as well as a grant from the Western Norway Health Authority for a scholarship (to B.B.). The funders had no role in the study design, data collection and interpretation, and the decision to submit the work for publication.
We have no conflicts of interest or funding to disclose.
We thank the staff at the pediatric wards of the participating hospitals for the recruitment of patients. Finally, we thank the participating children and their parents.
REFERENCES
- 1.Logar M, Ružić-Sabljić E, Maraspin V, Lotric-Furlan S, Cimperman J, Jurca T, Strle F. 2004. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection 32:15–19. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 2.Strle F, Ružić-Sabljić E, Cimperman J, Lotric-Furlan S, Maraspin V. 2006. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis 43:704–710. doi: 10.1086/506936. [DOI] [PubMed] [Google Scholar]
- 3.Tijsse-Klasen E, Pandak N, Hengeveld P, Takumi K, Koopmans MP, Sprong H. 2013. Ability to cause erythema migrans differs between Borrelia burgdorferi sensu lato isolates. Parasit Vectors 6:23. doi: 10.1186/1756-3305-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanek G, Reiter M. 2011. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin Microbiol Infect 17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 5.Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I. 2010. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17:8–16. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 6.Ružić-Sabljić E, Cerar T. 2017. Progress in the molecular diagnosis of Lyme disease. Expert Rev Mol Diagn 17:19–30. doi: 10.1080/14737159.2016.1246959. [DOI] [PubMed] [Google Scholar]
- 7.Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, Kristoferitsch W, O'Connell S, Ornstein K, Strle F, Gray J. 2011. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect 17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 8.Ljøstad U, Skarpaas T, Mygland A. 2007. Clinical usefulness of intrathecal antibody testing in acute Lyme neuroborreliosis. Eur J Neurol 14:873–876. doi: 10.1111/j.1468-1331.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 9.Øymar K, Tveitnes D. 2009. Clinical characteristics of childhood Lyme neuroborreliosis in an endemic area of northern Europe. Scand J Infect Dis 41:88–94. doi: 10.1080/00365540802593453. [DOI] [PubMed] [Google Scholar]
- 10.Dunaj J, Moniuszko A, Zajkowska J, Pancewicz S. 2013. The role of PCR in diagnostics of Lyme borreliosis. Przegl Epidemiol 67:35–39, 119–123. [PubMed] [Google Scholar]
- 11.Lebech AM. 2002. Polymerase chain reaction in diagnosis of Borrelia burgdorferi infections and studies on taxonomic classification. APMIS Suppl 2002(105):1–40. [PubMed] [Google Scholar]
- 12.Christen HJ, Eiffert H, Ohlenbusch A, Hanefeld F. 1995. Evaluation of the polymerase chain reaction for the detection of Borrelia burgdorferi in cerebrospinal fluid of children with acute peripheral facial palsy. Eur J Pediatr 154:374–377. doi: 10.1007/BF02072106. [DOI] [PubMed] [Google Scholar]
- 13.Eiffert H, Ohlenbusch A, Christen HJ, Thomssen R, Spielman A, Matuschka FR. 1995. Nondifferentiation between Lyme disease spirochetes from vector ticks and human cerebrospinal fluid. J Infect Dis 171:476–479. doi: 10.1093/infdis/171.2.476. [DOI] [PubMed] [Google Scholar]
- 14.Huppertz HI, Schmidt H, Karch H. 1993. Detection of Borrelia burgdorferi by nested polymerase chain reaction in cerebrospinal fluid and urine of children with neuroborreliosis. Eur J Pediatr 152:414–417. doi: 10.1007/BF01955900. [DOI] [PubMed] [Google Scholar]
- 15.Zbinden R, Goldenberger D, Lucchini GM, Altwegg M. 1994. Comparison of two methods for detecting intrathecal synthesis of Borrelia burgdorferi-specific antibodies and PCR for diagnosis of Lyme neuroborreliosis. J Clin Microbiol 32:1795–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issakainen J, Gnehm HE, Lucchini GM, Zbinden R. 1996. Value of clinical symptoms, intrathecal specific antibody production and PCR in CSF in the diagnosis of childhood Lyme neuroborreliosis. Klin Padiatr 208:106–109. doi: 10.1055/s-2008-1046456. [DOI] [PubMed] [Google Scholar]
- 17.Broekhuijsen-van Henten DM, Braun KP, Wolfs TF. 2010. Clinical presentation of childhood neuroborreliosis; neurological examination may be normal. Arch Dis Child 95:910–914. doi: 10.1136/adc.2009.176529. [DOI] [PubMed] [Google Scholar]
- 18.Skogman BH, Croner S, Nordwall M, Eknefelt M, Ernerudh J, Forsberg P. 2008. Lyme neuroborreliosis in children: a prospective study of clinical features, prognosis, and outcome. Pediatr Infect Dis J 27:1089–1094. doi: 10.1097/INF.0b013e31817fd423. [DOI] [PubMed] [Google Scholar]
- 19.Tveitnes D, Øymar K, Natås O. 2007. Acute facial nerve palsy in children: how often is it Lyme borreliosis? Scand J Infect Dis 39:425–431. doi: 10.1080/00365540601105764. [DOI] [PubMed] [Google Scholar]
- 20.Ružić-Sabljić E, Lotric-Furlan S, Maraspin V, Cimperman J, Pleterski-Rigler D, Strle F. 2001. Analysis of Borrelia burgdorferi sensu lato isolated from cerebrospinal fluid. APMIS 109:707–713. doi: 10.1034/j.1600-0463.2001.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 21.Busch U, Hizo-Teufel C, Boehmer R, Fingerle V, Nitschko H, Wilske B, Preac-Mursic V. 1996. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulsed-field gel electrophoresis and PCR. J Clin Microbiol 34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornstein K, Berglund J, Bergstrom S, Norrby R, Barbour AG. 2002. Three major Lyme Borrelia genospecies (Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii) identified by PCR in cerebrospinal fluid from patients with neuroborreliosis in Sweden. Scand J Infect Dis 34:341–346. doi: 10.1080/00365540110080313. [DOI] [PubMed] [Google Scholar]
- 23.Barstad B, Tveitnes D, Noraas S, Ask IS, Saeed M, Bosse F, Vigemyr G, Huber I, Øymar K. 2017. Cerebrospinal fluid B-lymphocyte chemoattractant CXCL13 in the diagnosis of acute Lyme neuroborreliosis in children. Pediatr Infect Dis J 36:e286–e292. doi: 10.1097/INF.0000000000001669. [DOI] [PubMed] [Google Scholar]
- 24.Tveitnes D, Natås OB, Skadberg Ø, Øymar K. 2012. Lyme meningitis, the major cause of childhood meningitis in an endemic area: a population based study. Arch Dis Child 97:215–220. doi: 10.1136/archdischild-2011-300526. [DOI] [PubMed] [Google Scholar]
- 25.Reiber H, Lange P. 1991. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 37:1153–1160. [PubMed] [Google Scholar]
- 26.Lager M, Faller M, Wilhelmsson P, Kjelland V, Andreassen A, Dargis R, Quarsten H, Dessau R, Fingerle V, Margos G, Noraas S, Ornstein K, Petersson AC, Matussek A, Lindgren PE, Henningsson AJ. 2017. Molecular detection of Borrelia burgdorferi sensu lato—an analytical comparison of real-time PCR protocols from five different Scandinavian laboratories. PLoS One 12:e0185434. doi: 10.1371/journal.pone.0185434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forselv KJN, Lorentzen AR, Ljostad U, Mygland A, Eikeland R, Kjelland V, Noraas S, Quarsten H. 2017. Does more favourable handling of the cerebrospinal fluid increase the diagnostic sensitivity of Borrelia burgdorferi sensu lato-specific PCR in Lyme neuroborreliosis? Infect Dis (Lond) doi: 10.1080/23744235.2017.1399315. [DOI] [PubMed] [Google Scholar]
- 28.Mukhacheva TA, Kovalev SY. 2014. Borrelia spirochetes in Russia: genospecies differentiation by real-time PCR. Ticks Tick Borne Dis 5:722–726. doi: 10.1016/j.ttbdis.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Portnoï D, Sertour N, Ferquel E, Garnier M, Baranton G, Postic D. 2006. A single-run, real-time PCR for detection and identification of Borrelia burgdorferi sensu lato species, based on the hbb gene sequence. FEMS Microbiol Lett 259:35–40. doi: 10.1111/j.1574-6968.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 30.Faller M, Hiergeist A, Margos G, Reischl U, Sing A, Fingerle V. 2016. Sensitivity and specificity of different amplification protocols for detection of Borrelia burgdorferi sensu lato: an EU-wide external quality assessment study, poster EV0540. 26th Eur Cong Clin Microbiol Infect Dis (ECCMID 2016), Amsterdam, The Netherlands, 9 to 12 April 2016. [Google Scholar]
- 31.Pícha D, Moravcová L, Vanousová D, Hercogová J, Blechová Z. 2014. DNA persistence after treatment of Lyme borreliosis. Folia Microbiol (Praha) 59:115–125. doi: 10.1007/s12223-013-0272-4. [DOI] [PubMed] [Google Scholar]
- 32.Cerar T, Ogrinc K, Cimperman J, Lotric-Furlan S, Strle F, Ružić-Sabljić E. 2008. Validation of cultivation and PCR methods for diagnosis of Lyme neuroborreliosis. J Clin Microbiol 46:3375–3379. doi: 10.1128/JCM.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooskens J, Templeton KE, Claas EC, van Dam AP. 2006. Evaluation of an internally controlled real-time PCR targeting the ospA gene for detection of Borrelia burgdorferi sensu lato DNA in cerebrospinal fluid. Clin Microbiol Infect 12:894–900. doi: 10.1111/j.1469-0691.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 34.de Leeuw BH, Maraha B, Hollemans L, Sprong H, Brandenburg AH, Westenend PJ, Kusters JG. 2014. Evaluation of Borrelia real time PCR DNA targeting OspA, FlaB and 5S–23S IGS and Borrelia 16S rRNA RT-qPCR. J Microbiol Methods 107:41–46. doi: 10.1016/j.mimet.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Kjelland V, Stuen S, Skarpaas T, Slettan A. 2010. Prevalence and genotypes of Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in southern Norway. Scand J Infect Dis 42:579–585. doi: 10.3109/00365541003716526. [DOI] [PubMed] [Google Scholar]
- 36.Quarsten H, Skarpaas T, Fajs L, Noraas S, Kjelland V. 2015. Tick-borne bacteria in Ixodes ricinus collected in southern Norway evaluated by a commercial kit and established real-time PCR protocols. Ticks Tick Borne Dis 6:538–544. doi: 10.1016/j.ttbdis.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelmsson P, Lindblom P, Fryland L, Ernerudh J, Forsberg P, Lindgren PE. 2013. Prevalence, diversity, and load of Borrelia species in ticks that have fed on humans in regions of Sweden and Aland Islands, Finland with different Lyme borreliosis incidences. PLoS One 8:e81433. doi: 10.1371/journal.pone.0081433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ružić-Sabljić E, Maraspin V, Lotric-Furlan S, Jurca T, Logar M, Pikelj-Pecnik A, Strle F. 2002. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr 114:544–550. [PubMed] [Google Scholar]
- 39.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A 101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]


