ABSTRACT
In September 2016, 140 patients with primary symptoms of sore throat and fever were identified in a school dormitory in Osaka, Japan. Epidemiological and laboratory investigations determined that these symptomatic conditions were from a foodborne outbreak of group G streptococcus (GGS), with GGS being isolated from samples from patients, cooks, and foods. The strain of GGS was identified as Streptococcus dysgalactiae subsp. equisimilis of two emm types (stG652.0 and stC36.0). The causative food, a broccoli salad, was contaminated with the two types of S. dysgalactiae subsp. equisimilis, totaling 1.3 × 104 CFU/g. Pulsed-field gel electrophoresis (PFGE) of samples from patients, cooks, and foods produced similar band patterns among samples with the same emm type. This result suggested the possibility of exposure from the contaminated food. The average onset time was 44.9 h and the prevalence rate was 62%. This is the first report to identify the causative food of a foodborne outbreak by Streptococcus dysgalactiae subsp. equisimilis.
KEYWORDS: group G streptococcus, foodborne outbreak
INTRODUCTION
The genus Streptococcus consists of Gram-positive bacteria that form chains or pairs and are catalase negative. Beta-hemolytic streptococci are known causative bacteria of respiratory infections (https://web.archive.org/web/20071219224215/http://www.cdc.gov/ncidod/dbmd/diseaseinfo/groupastreptococcal_g.htm). Beta-hemolytic streptococci are classified according to the polysaccharide antigen in the cell wall (1). The main beta-hemolytic streptococci that cause pharyngitis are group A (GAS), C (GCS), and G (GGS). Numerous superficial antigenic factors have been described for GAS, GCS, and GGS. Among these, the M protein is type specific and is widely used as an epidemiological marker (2–4).
Streptococcus pyogenes (GAS) is a widely prevalent bacterial pathogen whose global distribution reflects the fact that humans constitute its primary biological host (5). In most cases, GAS infects shallow tissue sites, including the upper respiratory tract mucosal epithelium or the epidermal layer of the skin, and causes pharyngitis or impetigo. GAS is monitored at approximately 3,000 cooperating hospitals, and over 250,000 cases of GAS pharyngitis are reported annually in Japan (https://www.niid.go.jp/niid/en/aboutniid-2/865-iasr/5866-tpc426.html). S. pyogenes can also cause skin and soft tissue infections, septic arthritis, bacteremia, and endocarditis (6). Although GAS causes most streptococcal infections, GCS- and GGS-mediated infections have also been reported (7–9). On the other hand, the surveillance of beta-hemolytic streptococci excluding GAS is not sufficient.
Streptococci, including GAS and GGS, cause foodborne outbreaks (10–14). Although GAS foodborne outbreaks are rare, large-scale outbreaks exceeding 100 patients have been reported, requiring risk management. Similar to GAS, GGS also causes pharyngitis and streptococcal toxic shock syndrome (STSS) (15–19). GGS, which includes Streptococcus dysgalactiae subsp. equisimilis and S. dysgalactiae subsp. dysgalactiae, is a common component of the normal flora of human skin, pharynx, and gastrointestinal tract (20, 21). Foodborne outbreaks caused by GGS are also rare (13, 14) and have not been reported by isolation from causative foods.
Here, we provide the first report of a case of foodborne outbreak caused by GGS in Osaka, Japan, in September 2016 that was identified by isolating Streptococcus dysgalactiae subsp. equisimilis from the causative food.
MATERIALS AND METHODS
Epidemiological investigation.
The Osaka Institute of Public Health received a report from the Osaka Prefectural Affairs Division on 12 September 2016 that students of a vocational training school had developed symptoms of fever and pharyngitis. To determine whether these symptoms were due to an infectious disease or foodborne outbreak, we conducted an epidemiological investigation comprising interviews regarding patients' activities and a health survey to identify foods eaten, symptoms, and onset date for all students, teachers, and cooks. This school had a dormitory, and the investigation also included a survey about meals eaten by students in the dormitory (see Table S1 in the supplemental material).
Environmental and laboratory investigations. (i) Samples.
Specimens of blood and pharyngeal swabs from patients were collected. The detection of Streptococcus was conducted by evaluating pharyngeal swabs from 25 patients and 11 cooks, samples from 69 meals (supplied for 5 days, three meals a day) and drinking water, and 11 samples collected by swabbing various parts of the kitchen. All food samples that were stored frozen after cooking were collected and examined. In addition, blood specimens and pharyngeal swabs were examined for viruses.
(ii) Cultures.
Pharyngeal swabs were inoculated on blood agar and incubated at 35°C under microaerophilic conditions overnight. Each of the food samples (2 g) and swabs were incubated in 10 ml of Q medium at 35°C overnight (22). Q medium was used as the enrichment and selection medium. The medium, comprising 2.5% heat infusion broth (Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA), 0.5% tryptose (BD), 0.5% yeast extract (BD), 2.8% sodium chloride, 5.0 ml of a 0.1% aqueous sodium azide solution (per 100 ml), and 0.22 ml of an aqueous crystal violet solution (per 100 ml), was autoclaved at 121°C for 15 min. The pH was adjusted to 7.0 using a 10% sodium hydroxide solution. Defibrinated horse blood was added to make a final concentration of 5%. The cultures of hemolytic colonies that were confirmed to be catalase-negative were used for confirmation of the bacteria's Lancefield group using a Seroiden Strepto kit (Eiken Chemical Co., Ltd., Japan). Bacterial isolates were identified using a Rapid ID32 Strep system (bioMérieux, Marcy-l'Etoile, France). Trypticase soy agar with 5% sheep blood (BD) was used as the blood agar for the isolation of Streptococcus dysgalactiae subsp. equisimilis.
(iii) M protein gene typing.
For DNA extraction, colonies were picked from the blood agar, suspended in 100 μl of Tris-EDTA buffer in a 1.5-ml tube, and heated at 95°C for 10 min on a heat block. The suspension was centrifuged at 8,500 × g for 5 min, and the supernatant was used as the DNA template. emm typing was carried out according to the Centers for Disease Control and Prevention (CDC) method (https://www.cdc.gov/streplab/index.html).
(iv) Pulsed-field gel electrophoresis.
All isolates were characterized by pulsed-field gel electrophoresis (PFGE) after digesting the DNA with SmaI (Roche Diagnostics GmbH, Mannheim, Germany) using a CHEF-DR system (Bio-Rad, CA) (23). The DNA fragments were separated on 1% Seakem Gold agarose gel (Lonza, Basel, Switzerland) in 0.5× Tris-Borate-EDTA (TBE) buffer. The electrophoresis conditions were as follows: block 1 was run at 12°C and 6 V/cm for 11 h with time intervals between 5 and 15 s, and block 2 was run at 12°C and 6 V/cm for 8.5 h with time intervals between 5 and 45 s. The DNA of Salmonella enterica serovar Braenderup strain H9812 was digested with XbaI and used as the molecular marker for PFGE analysis. PFGE patterns were interpreted according to the criteria described by Tenover et al. (24).
(v) Determination of the amount of S. dysgalactiae subsp. equisimilis in causative food.
The number of S. dysgalactiae subsp. equisimilis organisms in food was calculated by direct plate colony counting and the most probable number (MPN) method. Broccoli salad and rice were examined using the three-tube and five-tube methods, respectively. In direct plate colony counting, 5 to 10 g of S. dysgalactiae subsp. equisimilis-positive samples was added to a 10-fold volume of heart infusion (HI) broth and stirred. A portion of this sample (1 ml) was used for direct plate colony counting. Mixtures were diluted 10- to 1,000-fold and applied to Columbia CNA with 5% sheep blood agar (CNA agar) (BD). After incubating at 35°C for 22 ± 2 h, colonies were counted to calculate the number of CFU per gram. For the MPN method, the remaining S. dysgalactiae subsp. equisimilis sample solution in HI broth was diluted in a series of decimal dilutions with HI broth and incubated at 35°C for 6 h for the recovery of S. dysgalactiae subsp. equisimilis. We previously prepared a modified Q medium containing reduced HI broth so that the required Q medium composition would be obtained upon the addition of the sample solution. The modified Q medium was added to each dilution solution and incubated at 35°C for 22 ± 2 h. The MPN was calculated with reference to the International Organization for Standardization (25).
(vi) Growth of S. dysgalactiae subsp. equisimilis in food.
To confirm the growth of S. dysgalactiae subsp. equisimilis in food, a pseudopositive sample of broccoli salad was cooked using the same recipe as that of the causative food, and the isolates were inoculated at estimated concentrations of 100 to 1,000 CFU/g. Pseudopositive samples were frozen immediately after cooking, thawed, and used for analysis. The average temperature in Osaka at the time of the outbreak (September 2016) was 25.6°C. Given that the temperature was likely even higher in the kitchen during food preparation, we conducted our experiment at 30°C. Samples taken from these foods were incubated at 30°C, and cultured samples were picked after 2, 4, 8, 12, and 24 h in triplicates. The picked samples were diluted with sterile saline, applied to CNA blood agar, incubated at 35°C for 22 ± 2 h, and used to calculate the colony count.
(vii) Measure of ASO titers.
Anti-streptolysin O (ASO) antibody titers were measured using sera from 10 patients at onset and 1 month after the outbreak. ASO titers were measured using a latex agglutination turbidimetric immunoassay by LSI Medience Corporation (Tokyo, Japan). The collection of sera was approved by the ethics review committee of the Osaka Institute of Public Health. ASO titers at the time of the outbreak were compared with that collected 1 month after the outbreak in each of the 10 patients using a paired t test.
RESULTS
Epidemiological investigation.
The affected school was a vocational training school where students were in attendance for 6 months of the year. Students were 18 to 31 years old. All students stayed in the dormitory from Monday to Friday, and almost all students ate the same meals. Our interviews revealed that many patients had symptoms of sore throat and fever. The doctor who examined the patients also received reports of suspected streptococcal infection. The dormitory consisted of three floors, and four to six students lived in one room. Among 227 students and two teachers, 140 people developed mainly pharyngitis, fever, and headache from 9 to 13 September. The epidemiological investigation revealed no bias among dormitory rooms, floors, or groups. One hundred forty patients had clinical symptoms such as sore throat (89.3%), fever (84.3%), headache (46.4%), malaise (38.6%), arthralgia (36.4%), chills (27.9%), diarrhea (11.4%), nausea (6.4%), and stomachache (3.6%) (Table 1). The causative food was a broccoli salad with soy sauce (broccoli salad), which was served on 9 September during morning meals and eaten by most of the patients. The occurrence was unimodal (Fig. 1). The mean progression time was 44.9 h.
TABLE 1.
Clinical symptoms of patients falling ill
| Clinical symptom | No. of patients (n = 140) | % |
|---|---|---|
| Sore throat | 125 | 89.3 |
| Fever | 118 | 84.3 |
| Headache | 65 | 46.4 |
| Malaise | 54 | 38.6 |
| Arthralgia | 51 | 36.4 |
| Chills | 39 | 27.9 |
| Diarrhea | 16 | 11.4 |
| Nausea | 9 | 6.4 |
| Stomachache | 5 | 3.6 |
FIG 1.
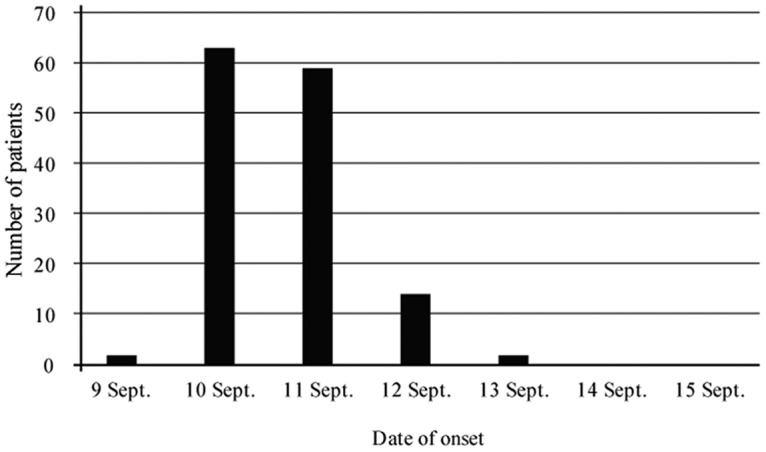
Number of patients between 9 and 15 September 2016.
Our environmental and epidemiological investigations indicated that the broccoli salad had been contaminated. The broccoli salad was prepared using 16.45 kg of frozen broccoli, 235 g of dried bonito shavings, and 0.7 liters of soy sauce and cooked as follows: frozen broccoli was placed in the refrigerator at noon on the previous day to thaw; the broccoli was boiled for 10 min at 5 a.m. and cooled under tap water, and the water was drained; the dried bonito shavings were toasted in a frying pan for 20 min; and the broccoli, dried bonito shavings, and soy sauce were then mixed in the kitchen at room temperature at 6 a.m. The broccoli salad was served for breakfast at 8 a.m. The examined broccoli salad was left at room temperature for 2 h or more before storing in a freezer.
Environmental and laboratory investigations. (i) Culture.
We inspected the patients' specimens for Legionella, Bordetella pertussis, hemolytic streptococcus, and viruses of respiratory disease. Because measles was prevalent in Osaka at the time of this case, we also conducted an examination for the measles virus. Real-time PCR was used to detect viruses of respiratory disease (parainfluenza viruses 1 to 4, respiratory syncytial virus, human metapneumovirus, enterovirus/rhinovirus, human bocavirus, human parechovirus, adenovirus, and human coronaviruses OC43, NL63, 229E, and HKU-1) and the measles virus (18, 19). DNA from Legionella and B. pertussis was extracted using a QIAamp DNA minikit (Qiagen, Hilden, Germany), and loop-mediated isothermal amplification (LAMP) was conducted using a Loopamp Legionella detection kit C and Loopamp Bordetella pertussis detection kit D (Eiken Chemical Co., Ltd., Tokyo, Japan). Viral genes of respiratory infection viruses were detected in some specimens but were not common pathogens in patients; bacteria of the genus Legionella and of B. pertussis and the measles virus were not detected in patients. We therefore concluded that these pathogens were not the cause of the outbreak. In addition, because the doctor's diagnosis strongly suggested streptococcal infection, patients' specimens were cultured on blood agar medium. The cultures of pharyngeal swab samples on blood agar medium revealed beta-hemolytic colonies. Further examination of the beta-hemolytic colonies led to the isolation of GGS from 19 of 25 patients, 1 of 11 cooks, and 2 of 69 foods. Drinking water and 11 swab samples from various parts of the kitchen were negative. GGS was isolated from the patients, a cook, and from the broccoli salad and cooked rice served for breakfast on 9 September. All isolated GGS samples were identified as S. dysgalactiae subsp. equisimilis.
(ii) Molecular typing.
Two kinds of S. dysgalactiae subsp. equisimilis with different emm types were isolated from foods, patients, and a cook (stC36.0 and stG652.0). S. dysgalactiae subsp. equisimilis of both emm types were isolated from the foods and one patient, while S. dysgalactiae subsp. equisimilis of one or the other emm type was found in 18 patients and the cook (Table 2).
TABLE 2.
S. dysgalactiae subsp. equisimilis emm types from specimens
| Isolated S. dysgalactiae subsp. equisimilis emm type | Source |
|---|---|
| stG652.0 and stC36.0 | Cooked rice |
| Broccoli salad | |
| 1 patient | |
| stG652.0 | 8 patients |
| 1 cook | |
| stC36.0 | 10 patients |
PFGE analysis requires digesting genomic DNA with a restriction enzyme, electrophoresing the fragmented DNA molecules, and comparing the band patterns. This analysis was used to estimate the differences in strains according to the DNA band patterns. PFGE produced unique band patterns among samples isolated from patients, the cook, and foods for each of the two emm types (stG652.0 and stC36.0). All DNA band patterns of S. dysgalactiae subsp. equisimilis stG652.0 isolated from foods, patients, and a cook were the same. Although we observed two DNA band patterns for stC36.0 isolated from foods and patients, these patterns differed by only one band. We therefore regarded these two patterns as belonging to the same strain (24).
(iii) Estimation of the amount of S. dysgalactiae subsp. equisimilis in the causative foods.
Direct plate counting showed that the cooked rice contained <10 CFU/g, while the broccoli salad contained 1.3 × 104 CFU/g. The MPN method showed that the cooked rice had 3.3 MPN/g (95% confidence limits, 1.1 to 8.9 MPN/g), while the broccoli salad contained 4,600 MPN/g (95% confidence limits, 900 to 19,800 MPN/g).
(iv) Growth of S. dysgalactiae subsp. equisimilis in food.
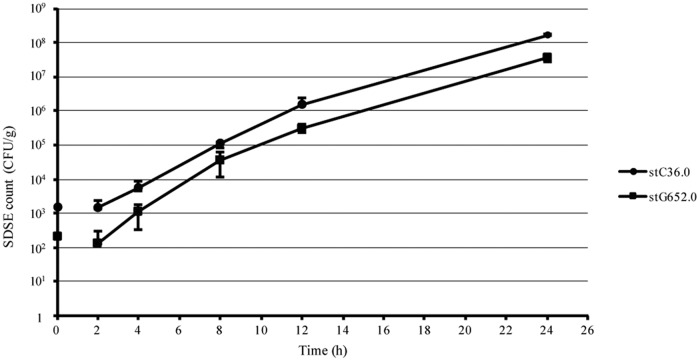
Additional experiments were conducted to confirm the growth of S. dysgalactiae subsp. equisimilis in the broccoli salad. The two S. dysgalactiae subsp. equisimilis emm types were added to the broccoli salad and the resulting growth of both S. dysgalactiae subsp. equisimilis types was observed. The results confirmed that S. dysgalactiae subsp. equisimilis proliferates even in vegetables (Fig. 2).
FIG 2.
Growth of Streptococcus dysgalactiae subsp. equisimilis (SDSE) in broccoli salad.
(v) Measure of ASO titers.
ASO is an antibody against streptolysin O, a representative extracellular product produced by beta-hemolytic streptococci of groups A, C, and G, and is used as an infection index. The ASO titers were measured in sera collected from S. dysgalactiae subsp. equisimilis-positive patients at the time of the outbreak and after 1 month. Analysis using the paired t test showed that the ASO titers were significantly higher 1 month after the outbreak than at the time of the outbreak (P < 0.01; data not shown).
DISCUSSION
We describe a foodborne outbreak of GGS from a school dormitory meal. Similar cases have been reported from an Israeli military base and an American college (13, 14) and were caused by meals served in dormitories or school cafeterias, as in the present case. The incidence rates of these previous reports were 46% and 31%, respectively. In contrast, the incidence rate in the present study was 62%, which is much higher than in previous reports. We speculate that this may be due to the large amount of bacteria in the contaminated food. The incubation period in the current case was 2 days, which is the same as that in the previous cases.
emm typing revealed that two types of S. dysgalactiae subsp. equisimilis (stG652.0 and stC36.0) were present in the food samples and one patient, while one or the other type was found in 18 patients and one cook (Table 2). Eight patients had stG652.0, while 10 patients had stC36.0. Patient symptoms were similar for the two S. dysgalactiae subsp. equisimilis types, suggesting that both types of S. dysgalactiae subsp. equisimilis were the causative bacteria. According to the PFGE results, the band patterns of stG652.0 and stC36.0 isolates from the patients, cook, and foods were similar in each S. dysgalactiae subsp. equisimilis type (data not shown). Consistent with the findings of Tenover et al. describing that PFGE patterns had differences of one to three bands at the time of outbreak (24), some stC36.0 isolates from patients indicated a one-band difference in this study. Therefore, we concluded that all isolates of stC36.0 were of the same strain. All isolates containing stG652.0 produced the same band pattern. S. dysgalactiae subsp. equisimilis is known to be part of the normal flora of the skin and oral cavity (26). Both stG652.0 and stC36.0 have been isolated from invasive or noninvasive GGS infections globally (18, 27–29) and are therefore not unique. Reports have indicated that multiple emm types cause GAS-mediated foodborne outbreaks (30, 31).
The broccoli salad was contaminated with 1.3 × 104 CFU/g S. dysgalactiae subsp. equisimilis. GAS foodborne outbreak cases have mainly been reported in military bases and schools, in which the infected foods were salads containing eggs (13, 14, 32). This is similar to the present case, where the outbreak occurred at a vocational training school. In GGS- and GAS-mediated foodborne outbreaks, the cook or a patient is often reported as a carrier. Cases of causative “dressing” have also been reported (33), in which the cook was also a carrier of GAS. The one S. dysgalactiae subsp. equisimilis-positive cook in the present study ate but did not cook the contaminated foods, indicating that the cook was not the source of the contamination. Moreover, the cooked rice was contaminated with low concentrations of GGS in the present study. From our investigation of the cooking facility, cross-contamination between the cooked rice and the broccoli salad was unlikely to have occurred during the cooking process. However, the possibility of cross-contamination during storage remains, because the two foods were frozen and stored in the same box wrapped in cellophane for the postinspection of meals. Therefore, cross-contamination during storage is strongly suspected to be the cause of the spread of GGS to the rice.
We considered the broccoli salad, which contained a small amount of dried bonito shavings, to be a uniquely Japanese dish. Moreover, there are limited reports on the proliferation of S. dysgalactiae subsp. equisimilis in food, and the amount of S. dysgalactiae subsp. equisimilis required to cause a foodborne outbreak is unknown. Therefore, our growth examination confirmed that S. dysgalactiae subsp. equisimilis proliferates even in vegetables (Fig. 2). We estimated that the amount of S. dysgalactiae subsp. equisimilis in the contaminated food was 1,000 CFU/g and confirmed that it had reached more than 10,000 CFU/g in approximately 4 h. This high level of S. dysgalactiae subsp. equisimilis in the broccoli salad suggests that the food had been stored at room temperature after cooking. It also indicates that contaminated food requires only a short period of time to accumulate the amount of bacteria required to cause a foodborne outbreak. This result indicates that it is necessary to store cooked food at low temperatures to prevent a foodborne outbreak of S. dysgalactiae subsp. equisimilis.
The ASO titer is an indicator of streptococcal infection (34). ASO has been reported to continue to rise 3 to 5 weeks after infection. ASO titers were measured in sera collected from 10 patients at onset and 1 month after the outbreak. Values exceeding the reference titer would suggest that the patient was infected with hemolytic streptococci. All patient ASO titers were significantly elevated 1 month after the outbreak (data not shown), suggesting that the infections occurred from the same exposure.
This study is the first to report a case of food poisoning caused by GGS in Japan and revealed that S. dysgalactiae subsp. equisimilis is able to grow in broccoli salad. Our findings indicate that cooked foods need to be protected from contamination by S. dysgalactiae subsp. equisimilis. A foodborne outbreak caused by Streptococcus was considered unlikely because there were no gastrointestinal symptoms; rather, fever and sore throat were the main symptoms. From the symptoms, we considered that we could respond quickly by examining both general enteropathogenic bacteria and respiratory pathogenic bacteria. This case indicates the importance of investigating the possibility of a foodborne outbreak in cases of epidemic respiratory symptoms.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the Shijonawate Health Center and Division of Food Safety Promotion of Osaka Prefecture for help with the epidemiological investigation and sampling. We also thank the members of the Division of Microbiology, Osaka Institute of Public Health, for help with the laboratory investigation and K. Tomono in the Department of Infection and Control and Prevention, Osaka University Graduate School of Medicine, for clinical advice.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01884-17.
REFERENCES
- 1.Lancefield RC. 1933. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med 57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno AL. 1979. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun 26:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisno AL, Collins CM, Turner JC. 1996. M proteins of group C streptococci isolated from patients with acute pharyngitis. J Clin Microbiol 34:2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campo RE, Schultz DR, Bisno AL. 1995. M proteins of group G streptococci: mechanisms of resistance to phagocytosis. J Infect Dis 171:601–606. doi: 10.1093/infdis/171.3.601. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 6.Stevens DL. 1992. Invasive group A streptococcus infections. Clin Infect Dis 14:2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. 2002. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med 112:622–626. doi: 10.1016/S0002-9343(02)01117-8. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz IS, Keynan Y, Gilmour MW, Dufault B, Lagacé-Wiens P. 2014. Changing trends in β-hemolytic streptococcal bacteremia in Manitoba, Canada: 2007–2012. Int J Infect Dis 28:211–213. doi: 10.1016/j.ijid.2014.03.1376. [DOI] [PubMed] [Google Scholar]
- 9.Lother SA, Jassal DS, Lagacé-Wiens P, Keynan Y. 2017. Emerging group C and group G streptococcal endocarditis: a Canadian perspective. Int J Infect Dis 65:128–132. doi: 10.1016/j.ijid.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Tsakris A, Pournaras S, Hathi D, Douboyas J, Efstratiou A. 1999. Outbreak of rare serotype of group A streptococcus pharyngitis in a boarding college. Lancet 353:1585–1586. doi: 10.1016/S0140-6736(99)99028-1. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka D, Shima T, Isobe J, Watahiki M, Matsumoto M, Endoh M, Okuno R, Ogata K, Nagai Y. 2006. Epidemiology and molecular analysis of group A streptococci from patients involved in food-borne disease outbreaks in Japan between 1996 and 2003. Jpn J Infect Dis 59:202–203. [PubMed] [Google Scholar]
- 12.Okamoto F, Murakami K, Maeda E, Oishi A, Etoh Y, Kaida M, Makigusa M, Nakashima K, Jinnouchi Y, Takemoto H, Kakegawa H, Yamasaki C, Manabe S, Sasaki M, Ogata K, Ikebe T, Sera N. 2014. A foodborne outbreak of group A streptococcal infection in Fukuoka Prefecture, Japan. Jpn J Infect Dis 67:321–322. doi: 10.7883/yoken.67.321. [DOI] [PubMed] [Google Scholar]
- 13.Cohen D, Ferne M, Rouach T, Bergner-Rabinowitz S. 1987. Food-borne outbreak of group G streptococcal sore throat in an Israeli military base. Epidemiol Infect 99:249–255. doi: 10.1017/S0950268800067716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill HR, Caldwell GG, Wilson E, Hager D, Zimmerman RA. 1969. Epidemic of pharyngitis due to streptococci of Lancefield group G. Lancet ii:371–374. [DOI] [PubMed] [Google Scholar]
- 15.Zaoutis T, Attia M, Gross R, Klein J. 2004. The role of group C and group G streptococci in acute pharyngitis in children. Clin Microbiol Infect 10:37–40. doi: 10.1111/j.1469-0691.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.Lindbaek M, Høiby EA, Lermark G, Steinsholt IM, Hjortdahl P. 2005. Clinical symptoms and signs in sore throat patients with large colony variant beta-haemolytic streptococci groups C or G versus group A. Br J Gen Pract 55:615–619. [PMC free article] [PubMed] [Google Scholar]
- 17.Watsky KL, Kollisch N, Densen P. 1985. Group G streptococcal bacteremia. The clinical experience at Boston university medical center and a critical review of the literature. Arch Intern Med 145:58–61. doi: 10.1001/archinte.1985.00360010078011. [DOI] [PubMed] [Google Scholar]
- 18.Lopardo HA, Vidal P, Sparo M, Jeric P, Centron D, Facklam RR, Paganini H, Pagniez NG, Lovgren M, Beall B, Argentinian Streptococcus Study Group. 2005. Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J Clin Microbiol 43:802–807. doi: 10.1128/JCM.43.2.802-807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekelund K, Skinhøj P, Madsen J, Konradsen HB. 2005. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: epidemiological and clinical aspects. Clin Microbiol Infect 11:569–576. doi: 10.1111/j.1469-0691.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Poradosu R, Jaffe J, Lavi D, Grisariu-Greenzaid S, Nir-Paz R, Valinsky L, Dan-Goor M, Block C, Beall B, Moses AE. 2004. Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis 10:1455–1460. doi: 10.3201/eid1008.030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vartian C, Lerner PI, Shlaes DM, Gopalakrishna KV. 1985. Infections due to Lancefield group G streptococci. Medicine (Baltimore) 64:75–88. doi: 10.1097/00005792-198503000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Sato M. 1972. A new selective enrichment broth for detecting beta-hemolytic streptococci in throat cultures: quinoline derivate and three percent salt as an additional agent to Pike's inhibitors. Jpn J Microbiol 16:538–540. doi: 10.1111/j.1348-0421.1972.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen L, Levy D, Ferroni A, Gehanno P, Berche P. 1997. Molecular epidemiology of Streptococcus pyogenes in an area where acute pharyngotonsillitis is endemic. J Clin Microbiol 35:2111–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ISO. 1996. Microbiology of food and animal feeding stuffs–general requirements and guidance for microbiological examinations. 7128:1996 ISO, Geneva, Switzerland. [Google Scholar]
- 26.Facklam R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horii T. 2006. Acute peritonitis and salpingitis associated with streptococcal toxic shock syndrome caused by Lancefield group G-haemolytic Streptococcus dysgalactiae subsp. equisimilis. J Med Microbiol 55:953–956. doi: 10.1099/jmm.0.46507-0. [DOI] [PubMed] [Google Scholar]
- 28.Kittang BR, Skrede S, Langeland N, Haanshuus CG, Mylvaganam H. 2011. emm gene diversity, superantigen gene profiles and presence of SlaA among clinical isolates of group A, C and G streptococci from western Norway. Eur J Clin Microbiol Infect Dis 30:423–433. doi: 10.1007/s10096-010-1105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gherardi G, Imperi M, Palmieri C, Magi G, Facinelli B, Baldassarri L, Pataracchia M, Creti R. 2013. Genetic diversity and virulence properties of Streptococcus dysgalactiae subsp. equisimilis from different sources. J Med Microbiol 63:90–98. doi: 10.1099/jmm.0.062109-0. [DOI] [PubMed] [Google Scholar]
- 30.Gallo G, Berzero R, Cattai N, Recchia S, Orefici G. 1992. An outbreak of group A food-borne streptococcal pharyngitis. Eur J Epidemiol 8:292–297. doi: 10.1007/BF00144817. [DOI] [PubMed] [Google Scholar]
- 31.Falkenhorst G, Bagdonaite J, Lisby M, Madsen SB, Lambertsen L, Olsen KEP, Mølbak K. 2008. Outbreak of group A streptococcal throat infection: don't forget to ask about food. Epidemiol Infect 136:1165–1171. doi: 10.1017/S0950268807009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy M, Johnson CG, Kraa E. 2003. Tonsillopharyngitis caused by foodborne group A streptococcus: a prison-based outbreak. Clin Infect Dis 36:175–182. doi: 10.1086/345670. [DOI] [PubMed] [Google Scholar]
- 33.Decker MD, Lavely GB, Hutcheson RH, Schaffner W. 1985. Food-borne streptococcal pharyngitis in a hospital pediatrics clinic. JAMA 253:679–681. doi: 10.1001/jama.1985.03350290085031. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Huew BB. 1980. The sensitivity and specificity of an agglutination test for antibodies to streptococcal extracellular antigens: a quantitative analysis and comparison of the Streptozyme test with the anti-streptolysin O and anti-deoxyribonuclease B tests. J Pediatr 96:367–373. doi: 10.1016/S0022-3476(80)80674-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.