Abstract
Understanding of antigen-presenting cell (APC) participation in tissue inflammation and metabolism has advanced through numerous studies using systems biology approaches. Previously unrecognized connections between these research areas have been elucidated in the context of inflammatory disease involving innate and adaptive immune responses. A new conceptual framework bridges APC biology, metabolism, and cytokines in the generation of effective T-cell responses. Exploring these connections is paramount to addressing the rising tide of multi-organ system diseases, particularly chronic diseases associated with metabolic syndrome, infection, and cancer. Focused research in these areas will aid the development of strategies to harness and manipulate innate immunology to improve vaccine development, anti-viral, anti-inflammatory, and anti-tumor therapies. This review highlights recent advances in APC “immunometabolism” specifically related to chronic viral and metabolic disease in humans. The goal of this review is to develop an abridged and consolidated outlook on recent thematic updates to APC immunometabolism in the areas of regulation and crosstalk between metabolic and inflammatory signaling and the integrated stress response and how these signals dictate APC function in providing T-cell activation Signal 3.
Keywords: macrophage, metabolism, ER stress, interferon, cholesterol
I. INTRODUCTION
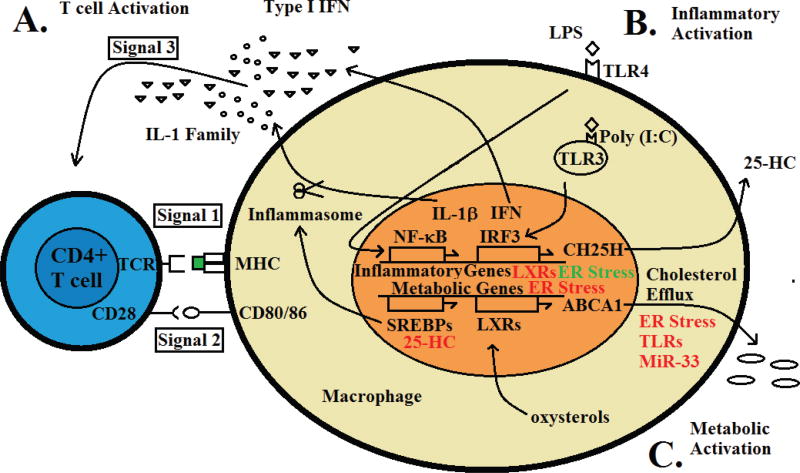
T-cell activation by pathogens proceeds in an ordered sequence; recognition of antigen through the T-cell receptor (TCR) in the context of “self” major histocompatability complex (MHC) (Signal 1); co-stimulation through TCR accessory molecules CD28, CD80/CD86, or intercellular adhesion molecule 1 (ICAM-1) (Signal 2); and a more enigmatic Signal 3 involving inflammatory cytokines provided by antigen-presenting cells (APCs). The requirement for Signal 3 in CD8+ T cells for acquisition of cytotoxic function has been well established such that interleukin-12 (IL-12) can provide Signal 3 and endow CD8+ cells with cytotoxic ability, opposing the development of tolerance that occurs in the absence of this cytokine. Subsequently, IL-1β or type I interferons (IFNs) have been shown to act as Signal 3 for CD4+ T cells. The involvement of IL-12, IL-1β, and IFN α/β strongly implicates upstream Toll-like receptor (TLR)-driven signaling in APCs as a critical component providing Signal 3 to T cells (Fig. 1A).
FIG. 1.
Immunometabolic signaling pathways contribute to regulation of T-cell responses by APCs. (A) Activation signaling for CD4+ T cells; Signal 1 TCR engagement by the MHC:peptide complex; Signal 2 co-stimulatory activation through CD28; and Signal 3-activating cytokine signal provided by IL-1 family members or type I IFN. (B) Inflammatory activation by PAMPS through TLR3 or TLR4; signaling through NF-κB, or IRF3; and induction of inflammatory gene expression. (C) Metabolic activation of transcription factors through oxysterols produced upon cholesterol accumulation (LXR) and induction of metabolic gene expression. Inhibitory interactions are presented in red and are described in the text. Activating interactions are presented in green and are also described in the text.
A critical component of APC activation involves signaling mediated by recognition of pathogen-associated molecular patterns (PAMPs) by cell-surface and endosomal pattern-recognition receptors (PRRs). Among the most well-characterized PRRs are the members of the TLR family. Ultimately, many inflammatory stimuli activate inflammasomes and type I IFN downstream of TLR signaling (Fig. 1B), Inflammation is only one function of APCs; many APCs, particularly those of myeloid origin, are highly metabolic. Increased metabolic activity in APCs occurs due to their role in phagocytosing and degrading dead and dying cells. Through their functions in the removal of apoptotic and necrotic cells, APCs encounter and metabolize bolus deliveries of lipids and cholesterol. APCs also produce and secrete a large amount of cytokines, which can induce endoplasmic reticulum (ER) stress. How APCs integrate this complex milieu of activating signals from multiple systems is not well understood.
Understanding of APC immune activation and metabolism have advanced independently, but major contemporary breakthroughs have demonstrated that these functions are related and are often co-regulated. Manipulation of either function to achieve reciprocal effects in the other pathway is a mechanism used by APCs and pathogens alike to maintain homeostasis or facilitate pathogen replication. The goal of this review is to begin to assimilate these conceptual advances into a systems-level understanding of APC activation, metabolism, and function and to incorporate insights provided through application of advanced molecular methods that have emerged recently among these disciplines into a unified concept of APC “immunometabolism.” Section II of this review examines the transcriptional control of crosstalk between macrophage metabolism and inflammation. Section III provides an overview of ER stress responses in APC function. Section IV highlights recent developments in the regulation of inflammation by specific bioactive metabolites and microRNAs (miRs). Section V briefly introduces several specific studies describing integration of metabolism, inflammation, and ER stress.
II. LIPID-ACTIVATED TRANSCRIPTION FACTORS AND MACROPHAGE PHEONTYPE
A. Transcriptional Control of Macrophage Metabolism and Inflammatory Responses
The nuclear receptors liver X receptors (LXRs) are ligand-activated transcription factors, the most well-described function of which is activation of metabolic gene expression in response to cholesterol metabolites.1 A role has emerged for TLRs and LXRs in a complex reciprocal crosstalk between the immune and metabolic systems at the level of APCs, particularly macrophages.
1. LXRs and Macrophage Metabolism
The two receptors of the LXR family, α and β, are similar in sequence (77% amino acid similarity in DNA- and ligand-binding domains), but different in tissue distribution, with LXRβ expressed ubiquitously and LXRα being restricted to highly metabolically active sites including macrophages and the liver.1,2 The principle role of LXRs is to remove excess cholesterol at the cellular and organism levels through the process of “reverse cholesterol transport” involving trafficking peripheral cholesterol to the liver through high-density lipoproteins for excretion in the bile and feces.2 This is mediated through up-regulation of LXR target genes involved in cholesterol efflux (ATP-binding cassette transporters including ABCA1) and apolipoprotein cholesterol acceptors. In addition to cholesterol efflux, LXRs regulate fatty acid synthesis through up-regulation of sterol regulatory element-binding protein (SREBP)-1c3,4 and fatty acid synthase (FAS).5 Carbohydrate metabolism is regulated by LXRs through suppression of hepatic gluconeogenesis and induction of tissue uptake of glucose.2,6 LXRs also act as glucose sensors regulating signaling in response to glucose through binding glucose directly and induction of carbohydrate response element binding protein.7,8
2. LXRs and Macrophage Inflammatory Responses
a. LXR Crosstalk with TLRs
Numerous studies have demonstrated crosstalk between the LXRs and inflammatory signaling through TLRs.2 Under inflammatory conditions, TLR3 or TLR4 negatively affect cholesterol efflux through IFN regulatory factor 3 (IRF3)-mediated suppression of LXR-induced expression of cholesterol transporters.9 In this way, inflammation contributes directly to foam cell development and atherosclerosis. Intriguingly, this relationship appears to be, at least partially, bidirectional because activation of LXRs reduces inflammatory gene expression induced by TLR4, IL-1β, or tumor necrosis factor α (TNF-α) signaling.10,11 An interesting interpretation of these results has been presented recently suggesting that TLR activation decreases cholesterol efflux in a feedforward mechanism to potentiate further amplification of TLR signaling and the inflammatory response itself as an integrated response to pathogens.12 Ultimately, upon resolution of infection and diminution of TLR agonists, established cholesterol accumulation induces LXR activation as a natural “brake” to restore the system to homeostasis (Fig. 1C). A constant low-level barrage of TLR agonists or inflammasome triggers (theoretically provided by cholesterol crystal deposition or inflammatory modified lipoproteins) then could circumvent this brake, establishing chronic inflammatory diseases.12 To fully understand the impact of transcriptional crosstalk between inflammation and metabolism, it is necessary to examine proposed mechanisms of this crosstalk.
b. Mechanisms of Immune Modulation by LXRs: Direct and Indirect Effects
Mechanisms of transcriptional activation of LXRs are relatively well described. LXRs belong to the class of nuclear receptors that bind the promoters of target genes containing LXR response elements (LXREs) in heterodimeric association with retinoid X receptors.1,2 Nuclear receptors basally recruit co-repressors to these promoters, inhibiting gene expression, which is further reinforced by histone deacetylases (HDACs) and chromatin-modifying factors.13,14 Particular corepressors on inflammatory genes include silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) and nuclear receptor corepressor.13,15–20 Upon ligand binding, co-repressors are released and exchanged for co-activators to activate transcription of target genes.14,21–24
Despite being the focus of intense research, the mechanism of inhibition of TLR-signaling induced gene expression (of genes lacking LXREs and thus without direct binding by LXRs) by these receptors has remained somewhat nebulous. An elaborate mechanism known as “trans-repression” has been described for LXR control of inflammatory gene expression involving SUMOylation by SUMO2 or SUMO3 and involving E3 ligase HDAC4.11,19,25–27 In this model, under normal conditions, the promoters of inflammatory genes (including inducible nitric oxide synthase 2 [iNOS or NOS2]) are bound by repressors including HDACs, NCOR, or SMRT, preventing attachment of co-activators. Upon inflammatory activation of TLR4, a signaling cascade results in ubiquitination and degradation of the corepressor complex by the 19S proteasome. Concomitant activation of nuclear factor κB (NF-κB) binding to sequence-specific elements in inflammatory gene promoters and recruitment of co-activators induces target gene expression.1,2 Ligand-induced SUMOylation of LXR by SUMO2 or SUMO3 is proposed to allow LXR binding to the corepressor complex, preventing its degradation by the proteasome and thus maintaining repression of gene expression in the presence of inflammatory stimuli.1,2,18
A very recent study28 has presented a competing theory explaining the anti-inflammatory effects of LXR activation on TLR signaling. In this model, transcriptional activation of LXRs is linked directly to both cholesterol homeostasis and inflammatory repression through direct transcriptional activation of the LXR target cholesterol transporter ABCA1. This work showed that ABCA1 affects inflammatory repression by reduction of lipid raft cholesterol content reducing recruitment of myeloid differentiation primary response gene 88 (MYD88) and TNF receptor associated factor 6 (TRAF6), therefore inhibiting TLR activation-induced phosphorylation of mitogen-activated protein kinases and activation of NF-κB and ultimately leading to reduced expression of inflammatory target genes.28 The relevance of this model was demonstrated for activation of TLR2, TLR4, and TLR9, but not TLR3. Support for this model of LXR-mediated inflammatory repression has been provided by earlier studies demonstrating that ABCA1 and ABCG1 can negatively regulate TLR signaling by depleting lipid rafts where TLR signaling complexes are assembled.29–31 Several studies have been published recently identifying non-coding regulatory miRs (miR-33a and miR-33b) produced from parent genes of the SREBP family.31–34 The SREBPs regulate fatty acid (SREBP-1c) and cholesterol (SREBP-2) biogenesis and miR-33 appears to reinforce these effects to elevate cellular cholesterol by targeting and reducing ABCA1 expression.32–34 Further contemporary support for the model has been presented in a recent study reporting that miR-33 can enhance inflammatory gene expression by modulating lipid rafts and NF-κB activation induced by TLR4 agonists in a mechanism involving ABCA1 and ABCG1.31
Using technical advances in promoter-enrichment-quantitative mass spectrometry, studies have recently begun to identify LXR corepressors that are recruited specifically during the contraction phase of transcriptional activation induced by LXR agonists (8–16Hrs).14 This work demonstrated that nuclear receptor coactivator 5 (NCOA5), a corepressor that interacts with LXR, is only recruited to target gene (ABCA1) promoters upon LXR ligand stimulation during this contraction phase. Intriguingly, TLR3 (but not TLR4) agonists could enhance recruitment of NCOA5 and repression of gene expression at early time points only in the presence of LXR ligand, providing an additional layer of complexity to LXR/TLR crosstalk.
According to the corepressor–coactivator exchange and trans-repression mechanisms outlined above, LXRs can affect gene expression in either direct or indirect fashion. It is therefore not surprising that putative LXREs have been identified in a limited number of inflammatory genes themselves, potentially allowing for more direct control of inflammation by LXRs. Specifically, TNF-α and IFN-γ genes have been reported to contain LXREs and to respond to ligand stimulation.35,36 Although these reports are isolated, it will be interesting to see how direct regulation of inflammatory genes by LXRs is integrated contextually into the complex crosstalk that has been demonstrated to exist between LXRs and innate immunity. To establish thematic understanding of APC effects on T-cell activation and Signal 3, it is pertinent to emphasize direct effects of metabolic regulators on Signal 3 itself.
c. LXRs and Signal 3
The mechanisms and effects described thus far demonstrate how LXRs can affect inflammatory signaling. In the context of metabolic effects on APC function, specifically T-cell Signal 3 provided by inflammasomes and type I IFNs, it is noteworthy that several direct effects of LXRs have been described. Inflammasome activation proceeds in a two-signal cascade. An initial priming stimulus through TLR/ MYD88 activates NF-κB as a first signaling cascade. NF-κB activation induces expression of IL-1β, IL-18, and NOD-, LRR-, and pyrin domain-containing 3 (NLRP3). A multi-protein complex termed the inflammasome is assembled from NLRP3 and apoptosis-associated speck-like protein (ASC). A second signaling cascade activated via a variety of damage-associated molecular patterns (i.e., adenosine triphosphate, reactive oxygen species [ROS] or PAMPs, and involving cell surface receptors such as pannexins and potassium channels) activates NLRP3 and the cleavage function of the inflammasome. Once activated, the inflammasome cleaves Pro-caspase 1 to the mature Caspase-1, which subsequently cleaves pro-proteins of IL-1β and IL-18 to the mature forms that can be secreted and function in inflammation and T-cell activation. In an early study on the anti-inflammatory effects of LXRs, LXR activation was shown to reduce both lipopolysaccharide (LPS)-induced expression of IL-1β and IL-1β-induced expression of IL-6 and iNOS.10 A recent report indicates that LXRs can similarly regulate IL-18. In that study, LXR activation was shown to inhibit both LPS-induced gene and protein expression of IL-18 and also processing of Pro-IL-18 by regulating Pro-caspase-1 expression and activation. LXR ligand activation also induced inhibitory IL-18 binding protein (IL-18BP).37 Having reviewed transcriptional control of macrophage metabolism and inflammatory responses by LXRs, the next section highlights a specific example of feedback regulation by a bioactive cholesterol metabolite acting on another major metabolic transcription factor, SREBP-1.
B. Regulation of Macrophage Inflammatory Pathways by Bioactive Lipid Metabolites
Through interaction between TLR signaling and transcription factors such as LXRs, inflammatory signaling in macrophages can affect macrophage metabolism. As described above, metabolic signaling can also affect reciprocal changes in inflammation. Although the LXRs couple cholesterol metabolism to the inflammatory response, another recently described mechanism couples lipogenesis to inflammation and, importantly, directly affects components of Signal 3 through the NLRP inflammasome and involves a signaling bioactive lipid mediator. A recent report demonstrated that SREBP-1a protected mice from lethal challenge with LPS and lethal bacterial-induced sepsis.38 The mechanism was shown to involve a direct interaction between SREBP-1a and inflammasome component NLRP-1a (Fig. 1C). SREBP-1a induction of NLRP-1a was shown to be important for Caspase-1 activation and secretion of IL-1β and IL-12. Surprisingly, SREBP-1a was shown to be a direct target of NF-κB, allowing LPS-induced inflammation to activate lipogenesis. A subsequent study identified an unexpected activity of an IFN-induced metabolic enzyme, cholesterol 25-hydroxylase (CH25H), producing a bioactive signaling lipid mediator, 25-hydroxycholesterol (25-HC), and suppressing IL-1β-mediated inflammation downstream of type I IFN. A series of studies had previously demonstrated that 25-HC inhibits maturation, translocation, and resultant activation of SREBPs, critical control nodes of cholesterol and fatty acid biosynthesis, through proteasomal degradation mediated by insulin-induced gene 1 (INSIG1).39–43 In this study, the absence of CH25H and resultant elimination of 25-HC was shown to allow exaggerated inflammasome activity with overproduction of IL-1 family members due to loss of regulation by SREBP.44 These studies identified 25-HC as a critical component of IFN-induced inhibition of inflammasome signaling with a dual role in regulation of lipogenesis through SREBPs.
III. ROLE OF STRESS RESPONSES IN APC FUNCTION OF MACROPHAGES AND DENDRITIC CELLS
A. Connection among Metabolic, ER, and Oxidative Stress in APC Functions
As discussed in the previous section, control of lipid metabolism is crucial for proper functioning of immune cells. The transcription factors SREBP1 and LXR are involved in macrophage and T-cell function and crosstalk through effects on metabolism and TLR and inflammasome signaling.45,46 Metabolic disturbance accompanied by immune changes can also exert a significant effect on cellular stress responses. There are a number of metabolic byproducts and lipids, which can trigger activation of the ER stress response. As with TLR signaling, there exists a bidirectional relationship between ER stress responses and lipid metabolism. Conversely, ER stress can trigger activation of different factors and pathways involved in lipid metabolism. Links between X-box-binding protein 1 (XBP1), a downstream target of the unfolded protein response (UPR) sensor inositol-requiring enzyme 1α (IRE1α), and metabolism have been studied extensively in the context of liver function. XBP1 regulates transcription of many genes involved in fatty acid synthesis, including SCD-1 (stearoyl-CoA desaturase-1), ACC2 (acetyl-CoA carboxylases 2), and DGAT2 (diacyl glycerol acyl transferase). Upon exposure to tunicamycin (which disrupts glycosylation of newly synthesized proteins, resulting in ER stress), liver tissue exhibited down-regulation of lipid metabolic pathways of many genes such as FAS, SREBP1, PGC-1α (peroxisome proliferator-activated receptor coactivator), CEBPα (CCAAT-enhancer-binding protein α), and PPARα (peroxisome-proliferator-activated receptor α).47 Acute ER stress can also modulate cholesterol metabolism in human hepatoma cells by causing ABCA1 redistribution to tubular perinuclear compartments.48 Considering these strong connections between metabolic signaling and ER stress, it is not surprising that ER stress signaling affects the antigen-presenting functions of macrophages and dendritic cells (DCs).
B. Understanding ER Stress Responses
The molecular signals released during infection or disease states are capable of eliciting stress responses within immune cells. Many studies have described different roles of specific stresses in aiding immune responses generated through APCs. The following section reviews the contribution and manipulation of oxidative stress and ER stress toward optimal or defective functioning of macrophages and DCs.
The ER is a large vesicular compartment that is actively involved in protein folding and protein trafficking within cells. It is also critical to the proper function of other organelles and multiple signaling cascades. An increase in secreted and membrane-embedded protein translation or a decrease in protein-folding capacity can result in a buildup of unfolded or misfolded proteins in the ER lumen, a condition known as ER stress. UPR is an adaptive intracellular signaling pathway that responds to ER stress by attenuating global protein translation and degrading unfolded proteins. Canonical UPR signaling is initiated by activation of three ER membrane-bound transducers: IRE1α, Activating Transcription Factor 6 (ATF6), and Double-Stranded RNA-Activated protein kinase-like ER kinase (PERK). Through transcriptional and translational reprogramming, the UPR is a cellular mechanism for stressed cells to adapt to and survive ER stress conditions. APCs, by virtue of their secretory demand, rely heavily on ER functioning and subsequent UPR signaling. Their reliance on ER makes them susceptible and sensitive to ER imbalance that compromises ER function. Understanding how the UPR affects specific functions at the cellular level and host-related factors that affect this are of great importance and this is discussed below.
C. ER Stress in APCs
1. ER Stress in Regulation of APC Maturation and Differentiation
High-mobility group box-1 (HMGB1) is a late inflammatory cytokine secreted by myeloid cells as well as NK cells. Studies have demonstrated the role of HMGB1 as an immunoregulatory molecule involved in DC maturation and differentiation. Recently, a study revealed that silencing of XBP1 in HMGB1-treated DCs decreased the expression of MHCII, CD80, and CD86 and resulted in a decrease in TNF-α production.49 Silencing of XBP1 also abrogated the APC function of DCs, leading to reduced levels of IFN-γ in T cells. Another target for ER stress is protein tyrosine phosphatase 1B (PTP1B); a tyrosine phosphatase involved in STAT3 dephosphorylation. Whereas ER stress activates PTP1B in skeletal muscle,50 loss of PTP1B causes a reduction in the DC maturation markers MHCII, CD80, and CD86 and leads to defective podosome formation in DC upon LPS stimulation.51 Differentiation of monocytes into macrophages is an important event in the initiation of immune responses. Induction of ER stress in monocytes leads to attenuation of macrophage differentiation capacity. THP1, a human monocytic cell line pretreated with an ER stress inducer, displayed no alteration of forward and side scatter and no increase of CD11b and CD68 expression level.52 Upon TLR signaling and immune activation, suppression of the C/EBP homologous protein pathway of UPR signaling is important for macrophage survival during the immune response.53
2. ER Stress in APC Antigen-Presenting Functions (Signal 1)
Potential effects of ER stress on antigen presentation are suggested from the observation that peptides derived from intracellular or extracellular pathogens are transported to the ER for association with MHC molecules. The translocation pathway of MHC peptide toward the cell surface is also initiated at the ER vesicular interface. Recently, a study described the role of HMG-CoA reductase degradation protein 1 (Hrd1), an ER-resident E3 ubiquitin ligase, in MHC-II expression on DCs,54 possibly by degrading transcription factor B lymphocyte-induced maturation protein 1 (BLIMP1). Although this study ruled out the potential role of ER stress in Hrd1-mediated MHC-II expression in DC, there might be a link between the ER stress pathway IRE1α-XBP1 and degradation of BLIMP1. Regarding MHC-I expression, ER-associated degradation (ERAD) is used by DCs to generate peptides for cross-presentation. Moreover, the IRE1α-XBP1 branch of the ER stress response has been implicated in cross presentation. In CD8a+ DCs, deletion of XBP1 leads to excessive endonuclease activity of IRE1α.55 Subsequently, activated IRE1α degrades mRNAs such as lysosomal-associated membrane protein 1 (LAMP1), TAP-binding protein (TAPBP), and ER-Golgi intermediate compartment protein 3 (ERGIC3), which are involved in the cross-presentation of antigen. Autophagy-mediated antigen processing is another important arm of MHC-I cross-presentation.56 Microtubule-associated protein 1A/1B-light chain 3 (LC3), a key component of the autophagy response, is activated upon ER stress. Phosphorylation of PERK induces an elevation in LC3 processing, thereby contributing to initiation of autophagy.57
3. ER Stress in Cytokine Production by APCs (Signal 3)
The role of ER stress responses in cytokine production has been widely studied in macrophages. ER stress has been shown to amplify cytokine production upon stimulation with TLR ligands. TLR2 and TLR4 ligands induce IRE1α activation in mouse J774 macrophages.58 Furthermore, activation of IRE1α in macrophages inhibited XBP1 splicing. IL-1β transcription was shown to be induced by IRE1α in a glycogen synthase kinase 3β (GSK3β)-dependent manner. GSK3, in turn, inhibits XBP1 splicing and thereby the transcriptional activity of XBP1s at inflammatory target genes, including TNF-α.59 Upon stimulation, IRE1α signaling is activated in a ubiquitination-dependent manner by TRAF6. This prevents the dephosphorylation by protein phosphatase 2A (PP2A) and subsequent inactivation.60 ATF4, one of the downstream targets of the PERK pathway of UPR signaling, binds directly to the IL-6 promoter.61 The ER stress response is also involved in the production of IFNs and IFN-stimulated genes (ISGs) through phosphorylation and nuclear translocation of IRF3 (Fig. 1B). Treatment with the ER stress inducer thapsigargin induces activation of stimulator of IFN genes (STING), an ER-resident protein. STING-induced IRF3 phosphorylation mediated by TANK-binding kinase 1 (TBK1) in turn induces transcription of ISGs. Retinoic acid-inducible gene I (RIG-I)-like receptor signaling also modulates IFN-β levels through a mechanism involving ER stress.62
In DCs, XBP1 can act as a double-edged sword. Under normal conditions, XBP1 enhances lipid metabolism in an ER-stress-dependent manner in response to inflammatory stimuli.63 This activity is necessary for optimum cytokine production by DCs by expanding the ER and Golgi compartment. Conversely, during tumor progression, DCs exposed to ROS reprogram XBP1 activity, thereby impairing lipid metabolism resulting in enhanced acquisition of immunosuppressive phenotype in DCs.63 Thymic stromal lymphopoietin (TSLP) produced by epithelial cells, as well as DCs themselves, acts on DCs to drive differentiation of T-Helper 2 (TH2) cells.64 Chemical induction of ER stress using tunicamycin (TM) or thapsigargin in conjunction with dectin-1 increased TSLP secretion from mDCs. This secretion of TSLP in mDCs is dependent on the IRE1α and PERK pathway of ER stress response because siRNA against these targets abrogated the production of TSLP by mDCs in a mechanism dependent on IL-1β production by the IRE1α branch of ER stress pathway.65 This demonstrates that specific control of different arms of the UPR is crucial for optimal functioning of antigen presentation in macrophages and DCs. Given transcriptional crosstalk and the role of ER stress in macrophage metabolism and inflammatory responses, studies elucidating a connection between bioactive lipids, miRs, and the IFN response would be crucial for the development of therapeutic agents.
IV. IFNS AND STEROL METABOLISM
In addition to transcriptional regulation of inflammation and metabolism by LXRs and the ER stress response, a contribution of bioactive metabolites to these processes has been described. Numerous microbial pathogens target host cell lipid metabolism to attain essential structural components required for replication. Cholesterol in particular, but also fatty acids and other metabolites, have been shown to be critical for replication of a number of human pathogens, viral, bacterial, and parasitic. It is attractive to speculate that host innate immune responses may have co-evolved to re-direct or override pathogen co-opted metabolic pathways to aide pathogen elimination. Compelling contemporary evidence for the existence of such pathways of specifically anti-viral immunity has been presented recently, elaborating a link between type I IFNs and cholesterol metabolism66 and independently for the putative IFN-stimulated cholesterol metabolism gene product CH25H and its enzymatic product 25-HC in the context of viral infection. New discoveries and the adaptation of new technologies in pursuit of understanding the intricate regulation of these pathways has prompted re-evaluation of this simplistic view of the host response as fundamentally reactionary and instead a concept emerges of an evolutionary “arms race” of actions and reactions by both host and pathogen in an ancient, yet undoubtedly still evolving, fight for survival.67
A. Lipid Signaling in Inflammatory Processes: Sterol Metabolism and Type I IFN
While evidence was accumulating steadily from multiple studies implicating TLRs and inflammatory signaling in transcription factor regulation of host metabolic pathways in APCs, a landmark study demonstrating this effect in anti-viral immunity was published in the journal PLOS Biology in 2011.66 Using a time-series analysis of microarray data in murine bone-marrow derived macrophages (BMDMs) either infected with virus or treated with IFN-γ, the investigators demonstrated selective, coordinated negative regulation of the complete sterol pathway.66 Gene and protein expression of all major sterol pathway nodes were reduced by viral infection. IFNs were shown to be sufficient for down-regulation of the sterol pathway, whereas other inflammatory cytokines including IL-1β, TNFα, and IL-6 could not mediate this effect. IFN-induced down-regulation of the sterol pathway resulted in reduced steadystate concentrations of free cholesterol and other major sterols in virus-infected or IFN-treated cells. Experiments confirmed that biochemically simulating the cellular IFN response to virus by inhibition of the sterol pathway is anti-viral both in vitro and in vivo and additional experiments implicated the proximal isoprenoid branch of sterol biosynthesis, particularly geranylgeranyl transferase type II, as the specific target of the cellular response against virus. Indeed, geranylgeraniol itself alleviated the anti-viral activity of IFN-β. The investigators demonstrated down-regulation of sterol biosynthesis for diverse enveloped and non-enveloped DNA and RNA viruses. Molecular mechanistic experiments demonstrated that type I IFN, type I IFN receptor (IFNAR), and tyrosine kinase 2 (Tyk2) receptor signaling are required for the reduction of sterol biosynthesis in a mechanism that involves transcriptional and translational down-regulation of sterol response element binding protein 2 (SREBP2). Inhibition of SREBP2 is proposed to be the terminal effector mechanism of the IFN response as a master switch allowing compounding of small negative changes in multiple intermediates to affect pathway inhibition of sterol synthesis. The investigators described a two-step model for anti-pathogenic inhibition of sterol biosynthesis in which a first signal is provided by pathogen activation of PRRs (including TLRs) inducing a type I IFN response. The second signal is then provided by type I IFN signaling through IFNAR impinging upon SREBP2 activation. Presciently at the time, the investigators noted that their notion of anti-viral activity of inhibiting SREBP2 implicated “negative feedback on SREBP-2 via oxysterol metabolites.” In short order, just such an interaction was indeed described in the context of multiple viruses for the oxysterol 25-HC.
A fascinating update to this general theory has been elaborated recently in a study demonstrating that virus infection or type I IFN treatment of macrophages altered the balance of lipid metabolism to reduce de novo synthesis specifically and increase import of cholesterol and fatty acids in an IFNAR-dependent manner.68 Experimentally engineering an altered balance in the “set point” of lipid metabolism in mice by targeting SREBP cleavage-activating protein (SCAP) and thereby reducing lipid biogenesis relative to import phenocopied virus infection or type I IFN effects in reducing lipid synthesis and provided protection against viral infection. Remarkably, anti-viral activity was shown to be mediated by spontaneous and specific induction of a type I IFN response which was IFNAR-dependent in the absence of SCAP and ultimately discovered to be mediated by effects on SREBP2. In human and mouse studies, knockdown of SREBP2 spontaneously induced a type I IFN response that protected against multiple viruses in vitro. Finally, the mechanism of spontaneous induction of type I IFN was shown to involve cyclic GMP-AMP (cGAMP) synthase (cGAS), STING, TBK1, and IRF3.68 Importantly, in cells that were infected or exposed to IFN, compensatory increases in lipid import opposing decreased synthesis essentially maintained total lipid levels in cells. This prompted the investigators to propose a new theory that “acutely decreasing synthesized cholesterol appears to provide a novel “danger” signal that activates a type I IFN-mediated anti-viral response” and “reprogramming of lipid metabolism is to alter the balance between lipid synthesis and scavenging, rather than to decrease lipid pool sizes.” This suggests that lipids delivered to cells by exogenous uptake are less favorable to invading pathogens than de novo synthesized lipids, a provocative conclusion that suggests that there are still many unanswered questions in the interplay between host metabolism and anti-pathogen inflammatory responses. The unique interaction between sterols and IFN signaling suggested control by bioactive lipid mediators. The next section highlights a newly described example of metabolic regulation by the aforementioned 25-HC.
B. Regulation of Macrophage Anti-Viral Activity by Bioactive Lipid Metabolites
CH25H and its enzymatic product, 25-HC, comprise a unique example of a metabolic circuit with an emerging and seemingly independent role in immunity. The most historically well-defined role of 25-HC is inhibition of cholesterol biogenesis through inhibitory effects on HMG-CoA reductase (HMGCR), the rate-limiting enzyme of the mevalonate pathway.42,43,69 25-HC also regulates SREBPs through interactions involving SCAP and INSIG1.39–43
Unexpectedly, an exciting new role for CH25H and 25-HC in innate immunity has been described recently. In a series of studies, a molecular pathway was delineated in which murine macrophages significantly up-regulated CH25H in response to TLR stimulation, leading to production and secretion of 25-HC.70,71 Humans voluntarily injected with a TLR4 agonist also demonstrated increased serum 25-HC.70 Notably, studies in mice demonstrated the strongest TLR activation-induced effect on CH25H in tissues with substantial resident macrophage populations.71,72 This pathway was subsequently confirmed in DCs and shown to involve TLR3/4, TIR-domain-containing adapter-inducing IFN-β (TRIF), IRF3/NF-κB, IFN-β, IFNAR, and Janus kinase and signal transducer and activator of transcription 1 (JAK/STAT1), ultimately inducing CH25H responsible for converting cholesterol to 25-HC, which is then secreted73,74 (Fig. 1B). Another study has raised the possibility that IRF1 may also cooperate with STAT1 in the induction of CH25H.75
Before elaboration of the CH25H/25-HC molecular circuit, pioneering studies demonstrated anti-viral activity of this oxysterol against multiple viruses.76–80 After the description of this circuit, in rapid succession, evidence accumulated demonstrating anti-viral activity of 25-HC generalizable to a wide array of essentially unrelated viruses that may also be relevant to other side-chain-substituted derivatives of cholesterol.42,75,81–84 Most of these effects involve SREBP modulation by 25-HC, but an additional activity against oxysterol-binding proteins (OSBPs) and OSBP-related proteins (ORPs) involving phosphatidylinositol 4-kinase (PI4K) has also been described.83,84 An anti-viral effect of 27-hydroxycholesterol (27-HC) and 24-hydroxycholesterol (24-HC) has also been demonstrated.42,81 Notably, a number of studies have identified anti-viral activity of CH25H/25-HC against HCV potentially involving multiple mechanisms.43,69,78,79,85–87 Finally, an exciting study has been published recently examining the non-coding transcriptome in HCV-infected cells in the presence and absence of 25-HC treatment, demonstrating that 25-HC alters the miR environment of these cells. miR-185 and miR-130b were shown to be induced by 25-HC treatment in infected cells and to inhibit HCV through a mechanism involving regulation of hepatic lipid metabolism and virus-induced lipid microenvironments (miR-185 and miR-130b) and an effect on IFNs (miR-130b).88 Having reviewed the crosstalk among IFN signaling, cholesterol biosynthesis, and cholesterol metabolites in anti-viral immunity, the next section highlights an exciting new development in small, non-coding RNA regulation of these processes.
C. Prospective: Regulating the Regulators– Sterol Biogenesis and miRs
Concurrently with more thorough description of the interaction between host metabolism and immunity, the discovery of anti-viral pathways in invertebrates involving small RNA-mediated gene silencing and their correlates among the non-coding portion of the human genome, regulatory small RNAs, including miRs, have led to a paradigm shift in our understanding of the regulation of diverse cellular processes, including immune responses. This unique confluence of small RNAs, biochemical pathway regulation, and immune responses prompted studies investigating whether miRs might function to reinforce the IFN-mediated down-regulation of sterol biosynthesis elucidated in previous studies.66 Using systemic global analysis of RNA turnover via 4-thiouridine labeling, a unique regulatory mechanism involving cellular miR control of metabolic pathways that function in anti-viral immunity was uncovered.89 Upon IFN treatment of murine BMDMs, the investigators confirmed the previously described reduction in synthesis and abundance of sterol pathway transcripts including a major effect on SREBF2 (the gene encoding SREBP2). miR analysis identified increased synthesis and abundance of miR-155 and miR-342 in IFN-treated and mCMV-infected cells that was sensitive to type I IFN and IFNAR. miR-342 induction by IFN was shown to be regulated coordinately with the transcript from which the miR is derived, namely Enavasodilator stimulated phosphoprotein gene (EVL). miR-342 was shown to reduce the abundance of major transcripts of the sterol biosynthesis pathway through a regulatory interaction involving direct binding to the SREBF2 promoter with downstream reduction in immature and mature SREBP2 protein. miR-342 was also shown to reduce repressive function of the SREBF2-derived miR (miR-33), which was previously shown to target ABCA1 and ABCG1. Expectedly, miR-342 reduced levels of cholesterol metabolites in cells upon overexpression. Similar to the previous study, the miR-342-induced down-regulation of sterol metabolism resulted in broad-spectrum anti-viral activity.76,77 These studies from multiple groups42,68,89 have effectively combined insights from the integrated understanding of macrophage inflammatory pathways and metabolic signaling to make remarkable progress in advancing conceptual understanding of immunometabolism and present a compelling argument for describing innate immunity and metabolism as an integrated system.
V. SYSTEMS-LEVEL INTEGRATION: LIPID METABOLISM AND ER STRESS
We have examined metabolism and ER stress and described how both biological systems impinge upon inflammation and immunity. However, metabolism, ER stress, and inflammation can also affect one another. Indeed, examples of integrative crosstalk have begun to emerge recently. The LXR target gene lysophosphatidylcholine acyltransferase 3 (Lpcat3) has been shown to regulate lipid-induced ER stress and inflammatory activation of proto-oncogene tyrosine-protein kinase Src (c-Src) through control of membrane composition and saturation.90 Macrophage fatty acid binding protein-4 (aP2) has been shown to act as a major regulator mitigating lipid-induced ER stress involving PERK and XBP-1 through actions on the LXR pathway and LXR targets including SCD-1 and FAS.91 In turn, ER stress has been shown to be a major contributor to hepatic steatosis through activation of SREBPs92 and ER stress can also inhibit cholesterol efflux and synthesis through LXR-independent effects on ABCA1 and effects on HMGCR.93 Therefore, it is appropriate and essential to view macrophages as major integrators of independent and dependent signals in the immune, metabolic, and ER stress responses.
VI. CONCLUDING REMARKS: OUTLOOK
According to the model developed by Tall and Yvan-Charvet,12 TLR- and IFN-mediated inflammation is initially a protective response to pathogens and feedforward inhibition of cholesterol efflux (Fig. 1B) and biogenesis and promotion of cholesterol uptake prolong and strengthen the TLR signal. Eventually, accumulation of cholesterol overrides the TLR effect and induces homeostatic activation of LXRs to remove cholesterol and restore balance in the system (Fig. 1C). Crosstalk between the systems is likely an evolutionary check on a futile cycle of simultaneous lipid biogenesis and catabolism, operating in response to conflicting signals in opposing pathways. According to this interpretation, prolonged low-level stimulation of TLRs (perhaps by cholesterol crystals, as suggested in Tall and Yvan-Charvet12) and possibly accentuated by ER stress (Fig. 1B) could produce a dangerous pathogenic cycle as has been described in atherosclerosis. Here, it has been observed that different areas of immunological foci, specifically atherosclerotic plaques, have distinctly different macrophage profiles with more inflammatory cells toward the interior of plaques yet, paradoxically, abundant anti-inflammatory macrophages toward the plaque periphery.94 This could be explained by temporal effects because the macrophages toward the plaque periphery could be those that have activated restorative metabolic activities such as LXRs, whereas those in the plaque core are subject to the inhibition of LXR induced by TLRs. The ultimate result of this crosstalk may prove pathogenic when the inflammatory activities in core macrophages are incapable of eliminating the offending stimulus and the anti-inflammatory macrophages (which have enhanced tissue-building and matrix-depositing capability) at the plaque periphery actively promote pathogen invasion or inflammatory activation and sustenance of the inflammatory TLR signal in the plaque core.
The emerging understanding of reciprocal interactions between inflammation and metabolism at the level of transcriptional control, ER stress, bioactive lipid mediators, and miRs are theoretically complex and involve an extensive network of checks and balances (Fig. 1). The multi-dimensional nature of this integrated-systems-level inter-relatedness has intriguing implications for the exquisite and intricate control of these pathways in homeostasis and underscores the involvement of major regulatory nodes such as LXRs, SREBPs, and IRE1α. Just as importantly, understanding how disturbances in the system are exploited acutely during pathogen infection can lend important insights into systems-level disturbance engendered during chronic inflammatory diseases. Indeed, many of the major breakthroughs discussed in this review involve astute targeted perturbations in the inflammatory network producing unexpected but highly informative corrections achieved through metabolic reprogramming. Although these discoveries advance our understanding of the system itself and its regulation, they have dubious implications against the backdrop of the steady march of chronic diseases. The outlook presented in Tall and Yvan-Charvet,12 specifically regarding atherosclerosis but likely applicable to a number of chronic diseases with an inflammatory component, is particularly inspiring. A new outlook of the systems-level integration of inflammatory and metabolic inputs, informed by the breakthroughs highlighted in this review, suggests a reason for optimism. Namely, increased understanding of these connections can empower researchers and physicians to make targeted interventions in these diseases that may achieve signal amplification and graded improvements in both measures, inflammation and metabolism, to ultimately improve health-related outcomes.
Empowering research to move forward by producing a consolidated theoretical model is important to encourage development of the theory, but must also acknowledge deficiency in current understanding. Although there is a reason for optimism, the outlook must be presented with caution. Many of the studies in this review focus on disease models and systemic perturbations in mouse systems. There are reported differences in regulation and expression of miR-33 in mouse and human.31 Similarly, regulation of inflammasome components by SREBPs has been suggested to be an artefact of back-crossing deficits in in-bred mouse strains.95 At least one report has challenged IFN sensitivity of CH25H in humans87 and, in particular, many aspects of CH25H and 25-HC expression and regulation are not understood in humans, where protein expression of CH25H is reported to be low.85 With these concerns in mind, acknowledgment must be made of studies that include a focused effort to realize applicability of findings across experimental models and consider translational impact. The excellent study by the Bensinger group achieves these goals laudably, melding studies in knock-out mice with in vitro experiments in primary human cells, including cells from patients with relevant human in-born errors of metabolism.68 It is the goal of the authors that careful application of the theoretical model developed in this review in studies designed from the outset with an understanding of application and translation to human disease may allow rapid advancement of these concepts to the clinical setting and allow physicians and researchers to continually improve and update our understanding of immunometabolism.
Acknowledgments
This work was supported by National Institutes of Health (Grants R01DK063222 and U19AI083024).
ABBREVIATIONS
- 25-HC
25-hydroxycholesterol
- ABC
ATP-binding cassette
- APC
antigen-presenting cell
- CD
cluster of differentiation
- CH25H
cholesterol 25-hydroxylase
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- IFNAR
type I IFN receptor
- IRE1α
inositol-requiring enzyme 1α
- IRF
interferon regulatory factor
- LXR
liver X receptor
- MHC
major histocompatability complex
- miR
microRNA
- NF-κB
nuclear factor κB
- NLRP
NOD-, LRR-, and pyrin-domain-containing protein
- SREBP
sterol regulatory element-binding protein
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- UPR
unfolded protein response
- XBP1
X-box-binding protein 1
References
- 1.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 2.Fessler MB. Liver X receptor: crosstalk node for the signaling of lipid metabolism, carbohydrate metabolism, and innate immunity. Curr Signal Transduct Ther. 2008;3(2):75–81. doi: 10.2174/157436208784223170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JA, Shimomura I, Shan B, Brown MS, goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–30. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14(22):2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277(13):11019–25. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 6.Dalen KT, Ulven SM, Bamberg K, Gustafsson J, Nebb HI. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J Biol Chem. 2003;278(48):48283–91. doi: 10.1074/jbc.M302287200. [DOI] [PubMed] [Google Scholar]
- 7.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445(7124):219–23. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 8.Cha J, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis: the carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282(1):743–51. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 9.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2016;12(4):805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 10.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala R, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122(5):707–21. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–16. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, Schulman IG, Glass CK. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol. 2003;23(16):5780–9. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie MA, Gold ES, Ramsey SA, Podolsky I, Aderem A, Ranish JA. An LXR-NCOA5 gene regulatory complex directs inflammatory crosstalk-dependent repression of macrophage cholesterol efflux. EMBO J. 2015;34(9):1244–58. doi: 10.15252/embj.201489819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB Kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16(2):245–55. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose DW, Johnson RS, Rosenfeld MG, Glass CK. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101(40):14461–6. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116(4):511–26. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 18.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Wilson TM, Rosenfeld MG, Glass CK. A SUMOylation dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin M, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol Cell. 2007;25(1):57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23(6):681–93. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huuskonen J, Fielding PE, Fielding CJ. Role of p160 coactivator complex in the activation of liver X receptor. arteriosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2004;24(4):703–8. doi: 10.1161/01.ATV.0000121202.72593.da. [DOI] [PubMed] [Google Scholar]
- 22.Huuskonen J, Vishnu M, Fielding PE, Fielding CJ. Activation of ATP-binding cassette transporter A1 transcription by chromatin remodeling complex. arteriosclerosis, thrombosis, and vascular biology. Arterioscler Thromb Vasc Biol. 2005;25(6):1180–5. doi: 10.1161/01.ATV.0000163186.58462.c5. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Lee J, Lee S, Lee JW. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol. 2008;22(6):1312–9. doi: 10.1210/me.2008-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakobsson T, Venteclef N, Toresson G, Damdimopoulos AE, Ehrlund A, Lou X, Sanyal S, Steffensen KR, Gustafsson JA, Treuter E. GPS2 is required for cholesterol efflux by triggering histone demethylation, LXR recruitment, and coregulator assembly at the ABCG1 locus. Mol Cell. 2009;34(4):510–8. doi: 10.1016/j.molcel.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Ghisletti S, Saijo K, Gandhi M, Aouadi M, Tesz GJ, Zhang DX, Yao J, Czech MP, Goode BL, Rosenfeld MG, Glass CK. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature. 2011;470(7334):414–8. doi: 10.1038/nature09703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe EH, Jou I. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol Cell. 2009;35(6):806–17. doi: 10.1016/j.molcel.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Jänne OA, Gustafsson JA, Steffensen KR, Treuter E. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24(4):381–95. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51(11):3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atheroscloerotic lesions. Circulation. 2008;118(18):1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai L, Azzam KM, Lin W, Rai P, Lowe JM, Gabor KA, Madenspacher JH, Aloor JJ, Parks JS, Näär AM, Fessler MB. MicroRNA-33 regulates the innate immune response via ATP binding cassette transporter-mediated remodeling of membrane microdomains. J Biol Chem. 2016;291(37):19651–60. doi: 10.1074/jbc.M116.723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. miR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–3. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328(5985):1566–9. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquart TJ, Allen RM, Ory DS, Baldán Á. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107(27):12228–32. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landis MS, Patel HV, Capone JP. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J Biol Chem. 2002;277(7):4713–21. doi: 10.1074/jbc.M108807200. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Ma X, Chen Y, Zhang L, Jiang M, Li X, Xiang R, Miao R, Hajjar DP, Duan Y, Han J. Identification of interferon-γ as a new molecular target of liver X receptor. Biochem J. 2014;459(2):345–54. doi: 10.1042/BJ20131442. [DOI] [PubMed] [Google Scholar]
- 37.Pourcet B, Gage MC, León TE, Waddington KE, Pello OM, Steffensen KR, Castrillo A, Valledor AF, Pineda-Torra I. The nuclear receptor LXR modulates interleukin-18 levels in macrophages through multiple mechanisms. Sci Rep. 2016;6:25481. doi: 10.1038/srep25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13(5):540–9. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279(50):52772–80. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Lee JN, Lee PCW, Goldstein JL, Brown MS, Ye J. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 2016;3(1):15–24. doi: 10.1016/j.cmet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci U S A. 2007;104(16):6511–8. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanc M, Hsieh W, Robertson K, Kropp K, Forster T, Shui G, Lacaze P, Watterson S, Griffiths SJ, Spann NJ, Meljon A, Talbot S, Krishnan K, Covey DF, Wenk MR, Craigon M, Ruzsics Z, Haas J, Angulo A, Griffiths WJ, Glass CK, Wang Y, Ghazal P. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38(1):106–18. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singaravelu R, Srinivasan P, Pezacki JP. Armand-Frappier Outstanding Student Award: the emerging role of 25-hydroxycholesterol in innate immunity. Can J Microbiol. 2015;61(8):521–30. doi: 10.1139/cjm-2015-0292. [DOI] [PubMed] [Google Scholar]
- 44.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345(6197):679–84. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129(1):33–6. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Fazilleau N, Mark L, McHeyzer-Williams L, McHeyzer-Williams M. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee A, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Röhrl C, Eigner K, Winter K, Korbelius M, Obrowsky S, Kratky D, Kovacs WJ, Stangl H. Endoplasmic reticulum stress impairs cholesterol efflux and synthesis in hepatic cells. J Lipid Res. 2014;55(1):94–103. doi: 10.1194/jlr.M043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu X, Yao F, Yao Y, Dong N, Yu Y, Sheng Z. Endoplasmic reticulum stress and its regulator XBP-1 contributes to dendritic cell maturation and activation induced by high mobility group box-1 protein. Int J Biochem Cell Biol. 2012;44(7):1097–1105. doi: 10.1016/j.biocel.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Panzhinskiy E, Hua Y, Culver B, Ren J, Nair S. Endoplasmic reticulum stress upregulates protein tyrosine phosphatase 1B and impairs glucose uptake in cultured myotubes. Diabetologia. 2013;56(3):598–607. doi: 10.1007/s00125-012-2782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Granados C, Prescott AR, Le Sommer S, Klaska IP, Yu T, Muckersie E, Giuraniuc CV, Grant L, Delibegovic M, Forrester JV. A key role for PTP1B in dendritic cell maturation, migration, and T cell activation. J Mol Cell Biol. 2015;7(6):517–28. doi: 10.1093/jmcb/mjv032. [DOI] [PubMed] [Google Scholar]
- 52.Komura T, Sakai Y, Honda M, Takamura T, Wada T, Kaneko S. ER stress induced impaired TLR signaling and macrophage differentiation of human monocytes. Cell Immunol. 2013;282(1):44–52. doi: 10.1016/j.cellimm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2015;47(2):146–7. doi: 10.1093/abbs/gmu128. [DOI] [PubMed] [Google Scholar]
- 54.Melo-Cardenas J, Kong S, Fang D. A Hrd way for MHC-II expression. Oncotarget. 2015;6(26):21767–8. doi: 10.18632/oncotarget.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osorio F, Tavernier SJ, Hoffmann E, Saeys Y, Martens L, Vetters J, Delrue I, De Rycke R, Parthoens E, Pouliot P, Iwawaki T, Janssens S, Lambrecht BN. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α+ dendritic cells. Nat Immunol. 2014;15(3):248–57. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- 56.Gannage M, da Silva RB, Münz C. Antigen processing for MHC presentation via macroautophagy. Methods Mol Biol. 2013;960:473–88. doi: 10.1007/978-1-62703-218-6_35. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina M, Vargas-Castrillón J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntané J, Boscá L, Garcia-Monzón C, Martin-Sanz P, Valverde AM. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5:e1179. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinon F, Chen X, Lee A, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411–8. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S, Joe Y, Kim HJ, Kim Y, Jeong SO, Pae H, Ryter SW, Surh YJ, Chung HT. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J Immunol. 2015;194(9):4498–4506. doi: 10.4049/jimmunol.1401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu Q, Zheng Z, Chang L, Zhao Y, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, Zhang T, Kong Q, Li H, Chen S, Chen A, Kaufman RJ, Yang WL, Lin HK, Zhang D, Perlman H, Thorp E, Zhang K, Gang D. Toll-like receptor-mediated IRE1α activation as a therapeutic target for inflammatory arthritis. EMBO J. 2013;32(18):2477–90. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwasaki Y, Suganami T, Hachiya R, Shirakawa I, Kim-Saijo M, Tanaka M, Hamaguchi M, Takai-Igarashi T, Nakai M, Miyamoto Y, Ogawa Y. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes. 2013;63(1):152–61. doi: 10.2337/db13-0757. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Zeng L, Tian A, Bomkamp A, Rivera D, Gutman D, Barber GN, Olson JK, Smith JA. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol. 2012;189(9):4630–9. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cubillos-Ruiz J, Silberman P, Rutkowski M, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–38. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H, Ziegler SF. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91(6):877–86. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elder MJ, Webster SJ, Williams DL, Gaston JSH, Goodall JC. TSLP production by dendritic cells is modulated by IL-1β and components of the endoplasmic reticulum stress response. Eur J Immunol. 2016;46(2):455–63. doi: 10.1002/eji.201545537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanc M, Hsieh WY, Robertson KA, Watterson S, Shui G, Lacaze P, Khondoker M, Dickinson P, Sing G, Rodriguez-Martin S, Phelan P, Forster T, Strobl B, Müller M, Riemersma R, Osborne T, Wenk MR, Angulo A, Ghazal P. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 2011;9(3):e1000598. doi: 10.1371/journal.pbio.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thibault PA, Wilson JA. Virology: MicroRNA-lipid one-upmanship. Nat Chem Biol. 2015;11(12):905–6. doi: 10.1038/nchembio.1953. [DOI] [PubMed] [Google Scholar]
- 68.York A, Williams K, Argus J, Zhou Q, Brar G, Vergnes L, Gray EE, Zhen A, Wu NC, Yamada DH, Cunningham CR, Tarling EJ, Wilks MQ, Casero D, Gray DH, Yu AK, Wang ES, Brooks DG, Sun Rm, Kitchen SG, Wu TT, Reue K, Stetson DB, Bensinger SJ. Limiting cholesterol biosyn-thetic flux spontaneously engages type I IFN signaling. Cell. 2015;163(7):1716–29. doi: 10.1016/j.cell.2015.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pezacki JP, Sagan SM, Tonary AM, Rouleau Y, Bélanger S, Supekova L, Su AI. Transcriptional profiling of the effects of 25-hydroxycholesterol on human hepatocyte metabolism and the antiviral state it conveys against the hepatitis C virus. BMC Chem Biol. 2009;9:2. doi: 10.1186/1472-6769-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diczfalusy U, Olofsson KE, Carlsson A, Gong M, Golenbock DT, Rooyackers O, Fläring U, Björkbacka H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J Lipid Res. 2009;50(11):2258–64. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci U S A. 2009;106(39):16764–9. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsumiya T, Imaizumi T. How are STAT1 and cholesterol metabolism associated in antiviral responses? JAK-STAT. 2013;2(3):e24189. doi: 10.4161/jkst.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park K, Scott AL. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J Leukoc Biol. 2010;88(6):1081–7. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonald JG, Russell DW. Editorial: 25-hydroxycholesterol: a new life in immunology. J Leukoc Biol. 2010;88(6):1071–2. doi: 10.1189/jlb.0710418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mboko WP, Mounce BC, Emmer J, Darrah E, Patel SB, Tarakanova VL. Interferon regulatory factor 1 restricts gammaherpesvirus replication in primary immune cells. J Virol. 2014;88(12):6993–7004. doi: 10.1128/JVI.00638-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, Blaas D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci U S A. 1994;91(5):1839–42. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moog C, Aubertin A, Kirn A, Luu B. Oxysterols, but not cholesterol, inhibit human immunodeficiency virus replication in vitro. Antivir Chem Chemother. 1998;9(6):491–6. doi: 10.1177/095632029800900605. [DOI] [PubMed] [Google Scholar]
- 78.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99(24):15669–74. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sagan SM, Rouleau Y, Leggiadro C, Supekova L, Schultz PG, Su AI, Pezacki JP. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochem Cell Biol. 2006;84(1):67–79. doi: 10.1139/o05-149. [DOI] [PubMed] [Google Scholar]
- 80.Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by West Nile Virus perturbs the cellular immune response. Cell Host Microbe. 2007;2(4):229–39. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Liu S, Aliyari R, Chikere K, Li G, Marsden M, Smith J, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, Cheng G. Interferon-inducible cholesterol-25-Hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2016;38(1):92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Civra A, Cagno V, Donalisio M, Biasi F, Leonarduzzi G, Poli G, Lembo D. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Sci Rep. 2014;4:7487. doi: 10.1038/srep07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arita M, Kojima H, Nagano T, Okabe T, Wakita T, Shimizu H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J Virol. 2013;87(8):4252–60. doi: 10.1128/JVI.03546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roulin RS, Lotzerich M, Torta F, Tanner L, van Kuppeveld FJ, Wenk MR, Greber UF. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2016;16(5):677–90. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Wang S, Yi Z, Tian H, Aliyari R, Li Y, Chen G, Liu P, Zhong J, Chen X, Du P, Su L, Qin FX, Deng H, Cheng G. Interferon-inducible cholesterol-25-hydroxylase inhibits hepatitis C virus replication via distinct mechanisms. Sci Rep. 2014;4:7242. doi: 10.1038/srep07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anggakusuma, Romero-Brey I, Berger C, Colpitts CC, Boldanova T, Engelmann M, Todt D, Perin PM, Behrendt P, Vondran FW, Xu S, Goffinet C, Schang LM, Heim MH, Bartenschlager R, Pietschmann T, Steinmann E. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology. 2015;62(3):702–14. doi: 10.1002/hep.27913. [DOI] [PubMed] [Google Scholar]
- 87.Xiang Y, Tang J, Tao W, Cao X, Song B, Zhong J. Identification of cholesterol 25-hydroxylase as a novel host restriction factor and a part of the primary innate immune responses against hepatitis C virus infection. J Virol. 2015;89(13):6805–16. doi: 10.1128/JVI.00587-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singaravelu R, O’Hara S, Jones DM, Chen R, Taylor NG, Srinivasan P, Quan C, Roy DG, Steenbergen RH, Kumar A, Lyn RK, Özcelik D, Rouleau Y, Nguyen MA, Rayner KJ, Hobman TC, Tyrrell DL, Russell RS, Pezacki JP. MicroRNAs regulate the immunometabolic response to viral infection in the liver. Nat Chem Biol. 2015;11(12):988–93. doi: 10.1038/nchembio.1940. [DOI] [PubMed] [Google Scholar]
- 89.Robertson KA, Hsieh WY, Forster T, Blanc M, Lu H, Crick PJ, Yutuc E, Watterson S, Martin K, Griffiths SJ, Enright AJ, Yamamoto M, Pradeepa MM, Lennox KA, Behlke MA, Talbot S, Haas J, Dolken L, Griffiths WJ, Wang Y, Angulo A, Ghazal P. An interferon regulated MicroRNA provides broad cell-intrinsic antiviral immunity through multihit host-directed targeting of the sterol pathway. PLoS Biol. 2016;14(3):e1002364. doi: 10.1371/journal.pbio.1002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, Jung ME, Ford DA, Tontonoz P. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18(5):685–97. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15(12):1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rohrl C, Eigner K, Winter K, Korbelius M, Obrowsky S, Kratky D, Kovacs WJ, Stangl H. Endoplasmic reticulum stress impairs cholesterol efflux and synthesis in hepatic cells. J Lipid Res. 2014;55(1):94–103. doi: 10.1194/jlr.M043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22(5):365–72. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerlic M, Croker B, Cengia L, Moayeri M, Kile B, Masters S. NLRP1a expression in Srebp-1a–deficient mice. Cell Metab. 2016;19(3):345–46. doi: 10.1016/j.cmet.2014.02.002. [DOI] [PubMed] [Google Scholar]