Abstract
The cell cycle consists of four main phases: G1, S, G2, and M. Most cells undergo these cycles up to 40–60 times in their life. However, neurons remain in a nondividing, nonreplicating phase, G0. Neurons initiate but do not complete cell division, eventually entering apoptosis. Research has suggested that like cancer, Alzheimer’s disease (AD) involves dysfunction in neuronal cell cycle reentry, leading to the development of the two-hit hypothesis of AD. The first hit is abnormal cell cycle reentry, which typically results in neuronal apoptosis and prevention of AD. However, with the second hit of chronic oxidative damage preventing apoptosis, neurons gain “immortality” analogous to tumor cells. Once both of these hits are activated, AD can develop and produce senile plaques and neurofibrillary tangles throughout brain tissue. In this review, we propose a mechanism for neuronal cell cycle reentry and the development of AD.
23.1 Introduction
The cell cycle consists of four main phases that are necessary for division and replication: G1, S, G2, and M. Most cells undergo these cycles up to 40–60 times in their life. Neuronal precursors in developing brain and in the whole nervous system proliferate and undergo regular cell cycles and divisions (Kubiak and Smith 2010). Thereafter, in adults, this process ceases and the vast majority of neurons, save neuronal progenitor cells, never replicate. As such, most neurons are terminally differentiated, meaning that they remain in a nondividing, nonreplicating phase, G0, for most of their lives. If such neurons enter the cell cycle, recent evidence has shown that these neurons can initiate, but cannot complete, cell division and in consequence eventually enter an apoptotic-type neurodegeneration (Raina et al. 2001; Lee et al. 2009). Because of these properties, neurons are vulnerable to destructive neuropathies, such as Alzheimer’s disease (AD).
AD is a condition characterized by the destruction of neurons with two hallmarks: the senile plaque and the neurofibrillary tangle. Senile plaques are aggregations of amyloid-β (Aβ) protein that localize extracellularly (reviewed in Castellani et al. 2010). Senile plaques originate when the amyloid-β protein precursor (AβPP) is cleaved by α- and γ-secretases, resulting in Aβ aggregation and deposition in the brain (Korenberg et al. 1989). Currently, there is a debate about the role of amyloid aggregation and deposition. Some researchers suggest that Aβ is a toxic protein aggregate that causes the destruction of neurons (Robakis 2010), whereas others argue that Aβ has a protective effect, shielding neurons from oxidative damage (Moreira et al. 2008; Castellani et al. 2009). There may be some truth in both of these arguments such that while Aβ has known antioxidative properties (Hayashi et al. 2007; Nakamura et al. 2007), the large aggregates observed in AD would hinder such properties and lead to neuronal dysfunction and death (Zhu et al. 2007).
Neurofibrillary tangles, the other hallmarks of AD, are collections of protein found within neurons. They consist of hyperphosphorylated tau protein, which is typically associated with microtubules. Tau protein stabilizes microtubules and, at least in vitro, disassociates from microtubules when phosphorylated (Conde and Caceres 2009). As with Aβ, there is considerable debate over the role of tau phosphorylation in disease pathogenesis with some arguing that hyperphosphorylation of tau protein leads to microtubule destabilization and neuronal dysfunction (Iqbal et al. 1984; Grundke-Iqbal et al. 1986), whereas other investigators posit tau phosphorylation as the protective adaptation of neurons during stress (Smith et al. 2002; Lee et al. 2005).
In AD, senile plaques and neurofibrillary tangles are widespread throughout brain tissue and mirror other pathological changes. For example, in the past decade, research has shown that neuronal cell cycle reentry plays a fundamental role in the pathogenesis of AD. As such, AD can be considered as a disease of deregulation of cell cycle in neurons. Such an idea provided novel insights for the treatment of AD. However, before effective interventions can be implemented, a better understanding of cell cycle reentry involvement in AD must be achieved.
23.2 The Cell Cycle
To progress through the cell cycle, cells use proteins called cyclins and cyclin-dependent kinases (Cdks). In each cell stage, one set of cyclins are expressed while others are downregulated through controlled proteolysis (Udvardy 1996). To advance to the next stage, the current stage cyclins are downregulated, so that the next stage cyclins can be upregulated. Cyclin metabolism is largely dependent on the ubiquitin–proteasome pathway responsible for precisely regulated proteolysis. Anaphase-promoting complex/cyclosome is the major ubiquitin ligase involved in cell cycle regulation via cyclins recognition and targeting for destruction.
23.3 Alzheimer’s Disease and the Cell Cycle Reentrant Neuron
Through this inducible progression, neurons will occasionally reenter the cell cycle from G0 to G1. Although this transition is regulated by the same cyclins/Cdks as normal mitosis, there are some key differences. In AD neurons, there are significantly elevated levels of cyclin D, Cdk4, and Ki67 (McShea et al. 1997; Zhu et al. 2007). The abundance of these markers signifies progression through the G1 phase and exit from G0. Interestingly, these markers are found in the cytoplasm of AD neurons rather than in the nucleus, their typical site of action (Vincent et al. 1997). Also, M-phase markers are found in AD neurons: increased MPM2 phosphoepitopes, Cdc25 A and B phosphatases, and binucleation, which may result of abortive mitotic karyokinesis (Vincent et al. 1998, 2001; Ding et al. 2000; Spremo-Potparevic et al. 2008; Zhu et al. 2008; Bajic et al. 2009). The ubiquitination system is also altered in AD (including ubiquitin-1 mutations; Tan et al. 2007; Tank and True 2009), which may influence both neuronal cell cycle regulation (Kubiak and Smith 2010) and, protein aggregation and accumulation (Haapasalo et al. 2010). Moreover, as some ubiquitin ligases, e.g., BRCA1, are overexpressed in AD neurons, the ubiquitination substrates, and thus, ubiquitination-dependent signaling, are most likely highly altered in AD (Evans et al. 2007).
Hernandez-Ortega et al. (2007) found that a hippocampal excitotoxic lesion would upregulate cell cycle markers in a progressive fashion in the entorhinal complex and dentate gyrus. They measured the levels of cyclin D1 and Cdk6 (G0/G1 transition), PCNA (late G1/early S transition), Cdk2 (G2/S transition), and cyclin B (G2 phase) and found that these markers elevate and decline in a sequential fashion in response to an AD-like stimulus. Levels of cyclin D1 and Cdk6 increased 1 day after kainic acid injection and remained elevated until day 15. PCNA rose for the first 7 days and then diminished in correlation with the rise of cyclin B. Cdk2 was detected over the first 15 days and then declined until day 30 (Hernandez-Ortega et al. 2007). Taking the progressive increase of cell cycle markers into account, these changes are representative of intentional reentry into the cell cycle rather than incidental increases in marker levels. The neurons seem to reenter the cell cycle with the intent of apoptosing or replicating (Fig. 23.1).
Fig. 23.1.
Neurons subject to loss of connections or other stressors exit G0 and reenter into the cell cycle that is abortive and leads to cell death
Further evidence for reentry into the cell cycle in AD neurons was demonstrated by Lopes et al. (2009). This study found that the pathway of Cdk5, a serine-threonine kinase involved in axonal guidance, cortical layering, and synaptic structure/plasticity, was overactivated and relocalized in AD and prion-induced pathologies. In cultured cortical neurons, levels of Cdk4, a downstream effector of Cdk5 and cell cycle marker of the G0/G1 transition, increased 13% by Aβ injection. Although the Cdk4 coactivator, cyclin D1, was not upregulated, it condensed into a nuclear/perinuclear pattern in response to Aβ. Levels of PCNA, a marker of S phase, increased 24% in response to Aβ. The number of apoptotic cells also increased by threefold in this study in response to Aβ. In contrast to findings in AD (Ogawa et al. 2003), levels of phosphorylated histone H3 (phH3), a marker of M phase, did not change, suggesting that these neurons did not undergo the G2/M transition. To prove that these changes in cell cycle markers were related, Lopes et al. (2009) treated the cells with roscovitine, a Cdk5 blocker. The increases in each case were inhibited with this treatment, suggesting that they were all mediated through the Cdk5 pathway. This study demonstrated that in response to Aβ, neurons will reenter and transit through the cell cycle up until M phase, and that this process is mediated by Cdk5 and its downstream cell cycle effectors.
Another interesting characteristic that separates the neuron from normal mitotic cells is the highly polarized state of its cytoskeleton (Nguyen et al. 2002). Because the neuron is constantly creating new synapses, neuronal microtubules are often in a state of flux, resulting in high levels of tau phosphorylation. This increased tau phosphorylation could cause problems for the neuron when it reenters the cell cycle, since mitosis requires microtubule remodeling for spindle assembly and transformation of its cytoskeleton (Conde and Caceres 2009). Under the circumstances of cell cycle reentry of this highly specialized neuron, mitotic-like hyperphosphorylation of tau could occur, producing neurofibrillary tangles and a disorganized mass of microtubule subunits (Bonda et al. 2010).
As a consequence, hyperphosphorylation of tau could undermine proper microtubule reorganization of cell replication and result in premature centromere division (PCD). PCD is a phenomenon where the centromeres prematurely divide in the G2 phase of the cell cycle, immediately after DNA replication in the S phase. Spremo-Potparevic et al. (2008) found that there was a three times higher incidence of PCD in the frontal lobe cortex of AD specimens than that of controls. This study suggests that since neurons underwent PCD, they must have reentered the cell cycle. Induction signals for PCD in these cases included loss of synaptic connections, cerebral hypoxia, Aβ, hormonal factors (estrogen), and mutations in presenilin 1 (Spremo-Potparevic et al. 2008). Upon review, these induction factors are associated with chromosomal damage and abortive mitogenesis, suggesting that premature division is the first step in neuronal apoptosis or dedifferentiation.
While there are differences between neuronal and normal mitotic cell cycle reentry, neuronal cell cycle reentry in control cases is in no way identical to neuronal reentry in AD. In 2001, Raina et al. discovered that AD does not activate the full set of caspases required for neuronal apoptosis. Instead, upstream caspases (caspase 8 and 9) were upregulated in AD, whereas downstream caspases (caspase 3, 6, and 7) stayed at control levels (Raina et al. 2001). This study suggests that AD neurons lacked effective apoptotic signal propagation to downstream caspase effectors, resulting in abortosis, a phenomenon consisting of apoptotic avoidance and neuronal survival (Raina et al. 2000, 2001).
To appreciate this unique process in AD, it is important to consider the differences between diseased and healthy neurons. For one, oxidative damage is highly associated with AD neurons and not with healthy neurons (Smith 2006). In 1998, Hampton et al. found that chronic oxidative stress inhibits the downstream propagation of caspase-mediated apoptotic signals (Hampton et al. 1998). If chronic oxidative stress induced apoptotic avoidance, or abortosis, in AD neurons, then this unique process would explain neuronal survival in the disease. Furthermore, recent evidence has shown Aβ to have antioxidant properties (Hayashi et al. 2007; Nakamura et al. 2007; Moreira et al. 2008). In response to the accumulation of oxidative damage in these resilient AD neurons, α- and γ-secretases could be induced to produce more Aβ for neutralization of future free radicals (Tamagno et al. 2002, 2005; Kim and Shen 2008).
23.4 Cell Cycle-Related Pathology of Alzheimer’s Disease
From the activation of cell cycle reentry induced by Aβ to the disruption of the microtubule network due to hyperphosphorylated tau protein, the cell cycle is intertwined with the pathology of AD. An important protein that initiates the pathology of AD is Aβ, which has numerous reported effects in the cell, ranging from the induction of apoptosis, promotion, or attenuation of cell survival, the activation of mitogen-activated protein kinase (MAPK), the promotion of tau phosphorylation, increases in oxidative stress and synapse loss, and the production of more Aβ.
One of the main pathways that mediate the effects of Aβ is the activation of the nerve growth factor (NGF) receptor, specifically the p75NTR isoform (Sakono and Zako 2010). Once activated, the NGF receptor can result in either a cell survival cascade or phosphorylation of JNK, a MAPK that is responsive to the accumulation of oxidative stress in the cell. Phosphorylated JNK negatively inhibits Bcl2, an antiapoptotic protein, resulting in upregulation of caspase 3 and apoptosis (Zhu et al. 2004b). What determines the path that the NGF receptor chooses must still be elucidated. Perhaps its choice for cell survival rather than apoptosis is a reflection of the circumstances that lead to AD. Other stimuli for the activation of JNK include macrophages and reactive oxygen species (ROS), which are both products of inflammation and another Aβ pathway (Zhu et al. 2001, 2003; Thakur et al. 2007).
Aβ can also bind to the N-methyl d-aspartate (NMDA) receptor. Activation of this receptor results in abnormal Ca2+ homeostasis, perhaps mediated through dysregulation of Ca2+ ion channels and upregulated Ca2+ influx (Sakono and Zako 2010). Increased levels of Ca2+ can be disastrous for a cell, leading to increased oxidative stress, synapse loss, and activation of lipases and proteases that will destroy the cell and lead to apoptosis.
Another pathway of Aβ is the Frizzled (Fz) receptor. When Aβ is bound to Fz, Wnt signaling is inhibited. Wnt inhibits GSK-3β, which inhibits β-catenin. The net result is an upregulation of β-catenin and an increase in tau phosphorylation (Sakono and Zako 2010). The effects of Aβ are mediated through several pathways, which can determine the pathology of AD and the fate of the cell. To better understand this relationship, we postulate a mechanism for neuronal cell cycle reentry in neurons and how this might lead to AD.
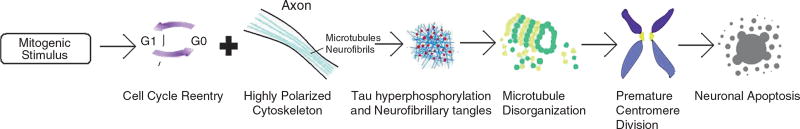
In the normal neuron, a mitotic stimulus (Aβ, estrogen, FGF, BMP, TGF-β, etc.) induces the cell to undergo cell cycle reentry. Because of its highly polarized cytoskeleton and the high activity of tau phosphorylation due to synapse creation, cell cycle reentry hyperphosphorylates tau and creates neurofibrillary tangles. These tangles aggregate with one another, disrupting the microtubule network and scattering microtubule-associated proteins across the cell. Despite this chaotic process, the neuron tends to reorganize its microtubule network in preparation for mitosis, but instead ends up in a cell crisis state. As a consequence of the disrupted microtubules, PCD is initiated, which activates the G2/M checkpoint. Activation of this checkpoint prevents the cell from progressing into mitosis and prolongs Cdk1 activity. Cdk1 is an interesting protein because it can act as a proapoptotic factor by phosphorylating Bcl2, an antiapoptotic protein, in addition to its role as a prerequisite of mitosis (Spremo-Potparevic et al. 2008). Thus, apoptosis is initiated, cellular proteins are degraded in a regulated fashion, and the cell dies before mitosis occurs (Fig. 23.2). This mechanism corresponds with findings showing that Cdk1 is expressed at higher levels in AD and localizes to the glia and neurofibrillary tangles (Vincent et al. 1997). Cdk1 promotes mitosis when localized in the nucleus and induces apoptosis in the cytoplasm.
Fig. 23.2.
Cell cycle reentry in a normal neuron leads only to death
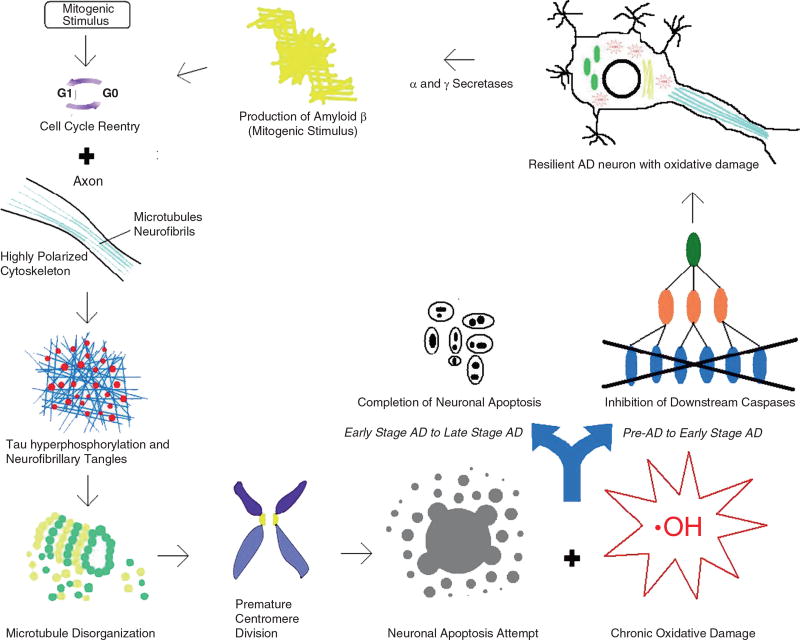
In the AD neuron, a mitotic stimulus (Aβ, estrogen, FGF, BMP, TGF-β, etc.) induces the cell to undergo reentry. Again, the high activity of tau phosphorylation in the neuron combines with the reorganization of the microtubule network to hyperphosphorylate tau protein. These proteins then aggregate with one another to create neurofibrillary tangles, disrupting the microtubule network. This disruption then initiates PCD and attempts to undergo apoptosis. However, the AD neuron contains significant amounts of oxidative damage-inducing activities, preventing the downstream caspases of apoptosis from immediately destroying the cell. Because of the neuronal accumulated oxidative damage, Aβ is upregulated to prevent future free radicals from further damaging the cell. Aβ can then begin a new cycle of reentry in other AD neurons. Eventually, after a few cycles of this process, the AD neuron is successful in its attempt to apoptose and dies (Fig. 23.3).
Fig. 23.3.
Cell cycle reentry in AD leads to a host of downstream sequelae including oxidative stress and death
23.5 Cell Cycle Dysregulation Commonalities for Alzheimer’s Disease and Cancer
Despite having different pathological results, cancer and AD do share similar etiologies. In cancer, abnormal cell cycle reentry instigates the uncontrolled proliferation and apoptotic avoidance resulting in tumor development and malignancy. In AD, abnormal reentry into the cell cycle initiates the pathway resulting in neurofibrillary tangles, apoptotic avoidance, and Aβ production. In 2000, Raina et al. described how even cell cycle control elements (Cdk4, p16, and p21) behave as oncoproteins in AD neurons (Raina et al. 2000).
In addition to cell cycle reentry, AD and cancer both require apoptotic avoidance to progress to a disease state. In AD, apoptotic avoidance allows the neuron to arrest in G2, accumulating oxidative damage and Aβ production. Oxidative damage may accumulate from the excessive amounts of mitochondria replicated in S phase (Sousa et al. 1997). In cancer, apoptotic avoidance is clearly necessary for the oncogenic cells to persist and proliferate indefinitely.
Since cancer and AD share similar etiologies, it is important to consider what result in their different conditions. On the most superficial level, AD is mediated through changes in the level of certain proteins, such as cell cycle regulators, Aβ, and regulators of tau phosphorylation, whereas cancer is mainly mediated through genetic mutations. However, this distinction becomes confusing because cell cycle reentry is involved in both processes. In AD, dysfunctional cell cycle reentry results in apoptosis/abortosis and delayed neuronal apoptosis. In cancer, dysfunctional cell cycle reentry leads to cell survival and the development of an immortal cell population.
Kim et al. suggests that these differences can be explained through the pathways of MAPK associated with each disease (Kim and Choi 2010). MAPKs are signaling cascades that involve a MAPK1, MAPK2, and MAPK3. MAPK3 phosphorylates MAPK2, which phosphorylates MAPK1, which then phosphorylates downstream effectors. AD is mostly associated with the MAPK1s, p38, and JNK. Oxidative stress activates the MAPK3, ASK1, which can then phosphorylate either MKK4/7 or MKK6. MKK4/7 phosphorylates JNK, which then activates caspase 3 and initiates apoptosis. MKK6 activates p38, which then leads to tau hyperphosphorylation.
Cancer is more associated with the MAPK pathway involving ERK 1/2. This pathway is mediated through activation of a GTPase, ras, which then goes downstream to activate K-ras, MEK, and then ERK. MEK 1/2 and phosphorylated ERK upregulate matrix metalloproteinases (MMP) and protect cancer cells. MMPs are critical for cancer progression because they degrade the extracellular matrix to allow for cancer cell migration. ERK 1/2 also downregulates proapoptotic BIM and upregulates antiapoptotic MCL-1 by phosphorylating FOXO3a and MCL-1. Phosphorylation of FOXO3a degrades the transcription factor, which is necessary for the production of BIM. Phosphorylation of MCL-1 stabilizes the protein (Kim and Choi 2010). The net effect of ERK is to inhibit apoptosis and promote cancer cell survival. Because AD and cancer use different MAPK pathways, the final results of their pathologies are different. In addition, ERK 1/2 has a negative feedback on β-secretase, an enzyme that cleaves AβPP to produce Aβ, whereas JNK and p38 have a positive feedback on the enzyme (Tamagno et al. 2005, 2008). This difference in MAPK regulation explains the excessive production of Aβ in AD and its absence in cancer.
These events in cancer have led to the establishment of the two-hit hypothesis (Knudson 1971). This theory suggests that there are two requirements for a cell to become cancerous. The first requirement is that the cell must have an upregulating mutation in an oncogene or a gene that promotes proliferation of the cell. An activated oncogene would result in abnormal cell cycle reentry and unlimited replication. The second requirement is that the cell must have an inactivating mutation in a tumor suppressor gene or a gene that inhibits cell proliferation. A tumor suppressor gene could produce a protein that promotes cell cycle arrest or induces apoptosis.
From the two-hit hypothesis of cancer, Zhu et al. (2004a, 2007) proposed the two-hit hypothesis of AD. This theory states that oxidative damage and cell cycle reentry are both necessary for a healthy neuron to become an AD neuron. The first hit in this theory also originates from abnormal cell cycle reentry. The process of AD is initiated when a mitogenic stimulus pushes the neuron to reenter the cell cycle. Typically, neurons that experience this “hit” undergo apoptosis and never progress to a disease state. However, when a neuron accumulates the second hit of chronic oxidative damage, apoptosis can be avoided, resulting in an “unlimited proliferation” of mitochondrial free radical production and Aβ deposition.
23.6 Conclusion
The dysregulation of cell cycle control is an integral part of both AD and cancer. Abnormal cell cycle reentry in a normal neuron leads to apoptosis. In aged subjects with AD, on the other hand, abnormal reentry triggers a cycle of oxidative damage and mitogen production with neurofibrillary tangles and Aβ deposition as a result of the condition. Cell cycle reentry is also a requirement of carcinogenesis, during which initiated cells must undergo dysregulation of the cell cycle to proliferate indefinitely and create tumors.
The similarities in these disease processes have led to the development of the two-hit hypothesis of AD. AD neurons must undergo the first hit of abnormal cell cycle reentry to develop the condition. With this event, neurons typically die, preventing the development of AD. However, with the second hit of chronic oxidative damage preventing apoptosis of the cell, neurons gain “immortality” analogous to tumor cells. Once both of these hits are activated, AD can develop and produce the pathophysiology commonly seen in this condition. Most cancers, as well as AD, are age-related diseases reflecting problems arising at the end of the human developmental process. Thus, the cell cycle control seems to escape fine regulation at those final steps, resulting in pathologies abbreviating our lives. It remains unclear how far this slippage is imprinted to our developmental program.
Abnormal cell cycle reentry raises the possibility of a new target for therapeutic intervention. Cell cycle inhibitors could be a possible solution to the progression of AD. In combination with current drug therapies for AD, millions of people could improve their AD and delay progression for a substantial number of years.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG031364 and AG028679 to MAS). JZK was supported by grants from ARC and LCC.
Contributor Information
Calvin Moh, Department of Pathology, Case Western Reserve University, 2103 Cornell Road, Cleveland, OH 44106, USA.
Jacek Z. Kubiak, CNRS UMR 6061, Institute of Genetics and Development, Cell Cycle Group, University of Rennes 1, IFR 140 GFAS, Rennes, France
Vladan P. Bajic, Institute of Biomedical Research, Galenika a.d, Belgrade, Serbia
Xiongwei Zhu, Department of Pathology, Case Western Reserve University, 2103 Cornell Road, Cleveland, OH 44106, USA.
Mark A. Smith, Department of Pathology, Case Western Reserve University, 2103 Cornell Road, Cleveland, OH 44106, USA
Hyoung-gon Lee, Department of Pathology, Case Western Reserve University, 2103 Cornell Road, Cleveland, OH 44106, USA.
References
- Bajic VP, Spremo-Potparevic B, Zivkovic L, Bonda DJ, Siedlak SL, Casadesus G, Lee HG, Smith MA. The X-chromosome instability phenotype in Alzheimer’s disease: a clinical sign of accelerating aging? Med Hypotheses. 2009;73:917–920. doi: 10.1016/j.mehy.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Bajic VP, Spremo-Potparevic B, Casadesus G, Zhu X, Smith MA, Lee HG. Cell cycle aberrations and neurodegeneration: a review. Neuropathol Appl Neurobiol. 2010;36:157–163. doi: 10.1111/j.1365-2990.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, Zhu X, Perry G, Smith MA. Reexamining Alzheimer’s disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis. 2009;18:447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Ding XL, Husseman J, Tomashevski A, Nochlin D, Jin LW, Vincent I. The cell cycle Cdc25A tyrosine phosphatase is activated in degenerating postmitotic neurons in Alzheimer’s disease. Am J Pathol. 2000;157:1983–1990. doi: 10.1016/S0002-9440(10)64837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Raina AK, Delacourte A, Aprelikova O, Lee HG, Zhu X, Perry G, Smith MA. BRCA1 may modulate neuronal cell cycle re-entry in Alzheimer disease. Int J Med Sci. 2007;4:140–145. doi: 10.7150/ijms.4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo A, Viswanathan J, Bertram L, Soininen H, Tanzi RE, Hiltunen M. Emerging role of Alzheimer’s disease-associated ubiquilin-1 in protein aggregation. Biochem Soc Trans. 2010;38:150–155. doi: 10.1042/BST0380150. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Fadeel B, Orrenius S. Redox regulation of the caspases during apoptosis. Ann N Y Acad Sci. 1998;854:328–335. doi: 10.1111/j.1749-6632.1998.tb09913.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Shishido N, Nakayama K, Nunomura A, Smith MA, Perry G, Nakamura M. Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-beta peptide. Free Radic Biol Med. 2007;43:1552–1559. doi: 10.1016/j.freeradbiomed.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ortega K, Ferrera P, Arias C. Sequential expression of cell-cycle regulators and Alzheimer’s disease-related proteins in entorhinal cortex after hippocampal excitotoxic damage. J Neurosci Res. 2007;85:1744–1751. doi: 10.1002/jnr.21301. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Zaidi T, Thompson CH, Merz PA, Wisniewski HM. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62:167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg JR, Pulst SM, Neve RL, West R. The Alzheimer amyloid precursor protein maps to human chromosome 21 bands q21.105-q21.05. Genomics. 1989;5:124–127. doi: 10.1016/0888-7543(89)90095-5. [DOI] [PubMed] [Google Scholar]
- Kubiak J, Smith MA. Ubiquitin/proteasome system in mitotic and mitotic-like regulation during brain development and pathology. In: Di Napoli M, Wojcik C, editors. The ubiquitin proteasome system in the central nervous system: from physiology to pathology – 2008 update. Nova Science; Hauppauge, NY: 2010. pp. 113–130. [Google Scholar]
- Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer’s disease: pathogen or protector? Trends Mol Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Nunomura A, Zhu X, Castellani RJ, Richardson SL, Perry G, Felsher DW, Petersen RB, Smith MA. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol. 2009;174:891–897. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JP, Oliveira CR, Agostinho P. Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-beta and prion peptides. Cell Cycle. 2009;8:97–104. doi: 10.4161/cc.8.1.7506. [DOI] [PubMed] [Google Scholar]
- McShea A, Harris PL, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am J Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7:3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Shishido N, Nunomura A, Smith MA, Perry G, Hayashi Y, Nakayama K, Hayashi T. Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry. 2007;46:12737–12743. doi: 10.1021/bi701079z. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP. Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 2002;9:1294–1306. doi: 10.1038/sj.cdd.4401108. [DOI] [PubMed] [Google Scholar]
- Ogawa O, Zhu X, Lee HG, Raina A, Obrenovich ME, Bowser R, Ghanbari HA, Castellani RJ, Perry G, Smith MA. Ectopic localization of phosphorylated histone H3 in Alzheimer’s disease: a mitotic catastrophe? Acta Neuropathol. 2003;105:524–528. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J Neurosci Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA. Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol. 2001;101:305–310. doi: 10.1007/s004010100378. [DOI] [PubMed] [Google Scholar]
- Robakis NK. Are Abeta and its derivatives causative agents or innocent bystanders in AD? Neurodegener Dis. 2010;7:32–37. doi: 10.1159/000266476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-beta and tau serve antioxidant functions in the aging and Alzheimer brain. Free Radic Biol Med. 2002;33:1194–1199. doi: 10.1016/s0891-5849(02)01021-3. [DOI] [PubMed] [Google Scholar]
- Smith MA. Oxidative stress and iron imbalance in Alzheimer disease: how rust became the fuss! J Alzheimers Dis. 2006;9:305–308. doi: 10.3233/jad-2006-9s334. [DOI] [PubMed] [Google Scholar]
- Sousa M, Barros A, Silva J, Tesarik J. Developmental changes in calcium content of ultrastructurally distinct subcellular compartments of preimplantation human embryos. Mol Hum Reprod. 1997;3:83–90. doi: 10.1093/molehr/3.2.83. [DOI] [PubMed] [Google Scholar]
- Spremo-Potparevic B, Zivkovic L, Djelic N, Plecas-Solarovic B, Smith MA, Bajic V. Premature centromere division of the X chromosome in neurons in Alzheimer’s disease. J Neurochem. 2008;106:2218–2223. doi: 10.1111/j.1471-4159.2008.05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Sun X, Hou FS, Oh HW, Hilgenberg LG, Hol EM, van Leeuwen FW, Smith MA, O’Dowd DK, Schreiber SS. Mutant ubiquitin found in Alzheimer’s disease causes neuritic beading of mitochondria in association with neuronal degeneration. Cell Death Differ. 2007;14:1721–1732. doi: 10.1038/sj.cdd.4402180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank EM, True HL. Disease-associated mutant ubiquitin causes proteasomal impairment and enhances the toxicity of protein aggregates. PLoS Genet. 2009;5:e1000382. doi: 10.1371/journal.pgen.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Wang X, Siedlak SL, Perry G, Smith MA, Zhu X. c-Jun phosphorylation in Alzheimer disease. J Neurosci Res. 2007;85:1668–1673. doi: 10.1002/jnr.21298. [DOI] [PubMed] [Google Scholar]
- Udvardy A. The role of controlled proteolysis in cell-cycle regulation. Eur J Biochem. 1996;240:307–313. doi: 10.1111/j.1432-1033.1996.0307h.x. [DOI] [PubMed] [Google Scholar]
- Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J Neurosci. 1997;17:3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging. 1998;19:287–296. doi: 10.1016/s0197-4580(98)00071-2. [DOI] [PubMed] [Google Scholar]
- Vincent I, Bu B, Hudson K, Husseman J, Nochlin D, Jin L. Constitutive Cdc25B tyrosine phosphatase activity in adult brain neurons with M phase-type alterations in Alzheimer’s disease. Neuroscience. 2001;105:639–650. doi: 10.1016/s0306-4522(01)00219-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ogawa O, Wang Y, Perry G, Smith MA. JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer’s disease. J Neurochem. 2003;85:87–93. doi: 10.1046/j.1471-4159.2003.01645.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004a;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang Y, Ogawa O, Lee HG, Raina AK, Siedlak SL, Harris PL, Fujioka H, Shimohama S, Tabaton M, Atwood CS, Petersen RB, Perry G, Smith MA. Neuroprotective properties of Bcl-w in Alzheimer disease. J Neurochem. 2004b;89:1233–1240. doi: 10.1111/j.1471-4159.2004.02416.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zhu X, Siedlak SL, Wang Y, Perry G, Castellani RJ, Cohen ML, Smith MA. Neuronal binucleation in Alzheimer disease hippocampus. Neuropathol Appl Neurobiol. 2008;34:457–465. doi: 10.1111/j.1365-2990.2007.00908.x. [DOI] [PubMed] [Google Scholar]