SUMMARY
Background
Hypomethylating agents azacitidine and decitabine have shown efficacy in myelodysplastic syndromes and acute myeloid leukaemia (AML), but responses are limited and of short duration, possibly due to short half-lives and suboptimal bone marrow exposure. Guadecitabine (SGI-110), a dinucleotide of decitabine and deoxyguanosine, is resistant to degradation by cytidine deaminase thus achieving longer half-life and exposure of its active metabolite, decitabine. We aimed to assess the safety and clinical activity of subcutaneously given guadecitabine in treatment-naïve older patients with AML who were not candidates for intensive chemotherapy.
Methods
In this multicentre, open-label, phase 2 study, treatment-naïve older patients from 14 North American medical centres with AML who were not candidates for intensive chemotherapy were randomly assigned (1:1, using a computer algorithm) to subcutaneous guadecitabine at 60 or 90 mg/m2 on days 1–5 (5-day schedule) of a 28-day treatment cycle. Subsequently, patients were assigned sequentially to subcutaneous guadecitabine at 60 mg/m2 in a 10-day schedule (at least 2 cycles administered on days 1–5 and 8–12, with subsequent cycles given on a 5-day schedule). The objective was to evaluate and compare the activity and safety of two doses and schedules of guadecitabine, with a primary endpoint of composite complete response (including complete response [CR], CR with incomplete platelet recovery [CRp], and CR with incomplete neutrophil recovery regardless of platelets [CRi]). Response was evaluated in all patients (as-treated) as long as a patient received at least one guadecitabine treatment. Secondary endpoints included safety, survival, and pharmacodynamics. We present the final trial analyses, although at time of database lock, 15 patients were still being monitored for survival including six continuing treatment. This study is registered with ClinicalTrials.gov, number NCT01261312
Findings
Between Aug 24, 2012, and Sep 15, 2014, 103 patients received treatment: 51 on a 5-day schedule of guadecitabine (24 received 60 mg/m2, 27 received 90 mg/m2) and 52 on the 10-day schedule. Median age was 77 years. Poor prognostic features included ECOG status ≥2 (39 [38%]), poor-risk cytogenetics (43 [42%]), and secondary AML (37 [36%]). Characteristics were generally balanced across doses and schedules. CRc rates were 54% (13 patients), 59% (16 patients), and 50% (26 patients) for the 5-day 60, 5-day 90, and 10-day 60 mg/m2 regimens, respectively. The most common grade 3 or higher adverse events, regardless of relationship to treatment, for the 5- and 10-day schedules, respectively, included febrile neutropenia (31 [61%] and 36 [69%]), thrombocytopenia (25 [49%] and 22 [42%]), neutropenia (20 [39%] and 18 [35%]), pneumonia (15 [29%] and 19 [37%]), anaemia (15 [29%] and 12 [23%]), and sepsis (8 [16%] and 14 [27%]). The most common serious adverse events, regardless of relationship to treatment, for the 5- and 10-day schedules, respectively, were febrile neutropenia (27 [53%] and 25 [48%]), pneumonia (14 [27%] and 16 [31%]), and sepsis (8 [16%] and 14 [27%]).
Interpretation
In treatment-naïve older patients with AML not candidates for intensive chemotherapy, guadecitabine resulted in high CRc rates ≥50% with acceptable safety across the doses and schedules investigated. A phase 3 pivotal study in this patient population is ongoing to evaluate guadecitabine 60 mg/m2 in a 5-day schedule versus standard of care.
Funding
Astex Pharmaceuticals, Inc., Stand Up To Cancer
INTRODUCTION
Improved understanding of aberrant DNA methylation in cancer has resulted in investigations of hypomethylating agents (HMAs) for treating myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML) and other myeloid neoplasms (1,2). First generation epigenetically targeted HMAs include azacitidine and decitabine, both approved for treating MDS in the United States (3,4) and routinely used off-label for AML. Decitabine and azacitidine are also approved in Europe for treating AML in older patients who are not candidates for intensive chemotherapy, or for patients not eligible for haematopoietic stem cell transplant (HCT) (5,6). Despite routine adoption and clear evidence of activity, azacitidine and decitabine complete response (CR) rates in patients with high risk AML are relatively low (16%–20%).
To inhibit DNA methyltransferase and induce hypomethylation, HMAs incorporate into replicating DNA during S-phase of the cell cycle (2,7). Due to short half-lives of about 30 minutes, however, exposure of leukaemic cells to these drugs is limited (7,8), potentially abrogating their effectiveness. Epigenetically targeted agents with longer half-lives could theoretically improve response to therapy by enhancing incorporation of the active agent into dividing cells.
Guadecitabine (SGI-110) is a next-generation HMA that is not metabolised by cytidine deaminase, the enzyme that degrades decitabine. Guadecitabine is a dinucleotide of decitabine (active metabolite) and deoxyguanosine, which are linked by a phosphodiester bond. Gradual cleavage of this bond by phosphorylases and other enzymes results in slow release of decitabine, prolonging its in-vivo half-life and exposure to leukaemic cells during S-phase. The reduced peak plasma levels and prolonged half-life of subcutaneous guadecitabine may result in increased efficacy and reduced toxicity when compared to intravenously administered decitabine (9–11).
A phase 1 study of guadecitabine established 5 days of 60 mg/m2 as a biologically effective dose and schedule (12). In that study, promising clinical activity was reported in heavily pretreated MDS and AML patients. However, the phase 1 study did not investigate longer administration over 10 days, which was reported to result in a higher response rate for decitabine in single-centre studies (13,14). The objective of our phase 2 guadecitabine study was to evaluate and compare the activity and safety of two doses and schedules of guadecitabine in older treatment-naïve patients with AML who could not undergo intensive chemotherapy.
METHODS
Study design and participants
We describe here the phase 2 part of a phase 1/2 protocol (appendix page 1). The phase 2 part included different disease cohorts: treatment-naïve AML, relapsed or refractory AML, and MDS. We describe in this paper the treatment-naïve AML cohort of patients. Results from the relapsed or refractory AML and MDS phase 2 patient cohorts will be published separately. Treatment-naïve AML patients ≥65 years of age who were not candidates for intensive chemotherapy were randomly assigned to 5-day schedules (daily×5) of either 60 or 90 mg/m2 guadecitabine at 14 North American medical centres (appendix page 93). Subsequently, the protocol was expanded and additional patients were treated with guadecitabine 60 mg/m2 on a 10-day schedule (Days 1–5 and 8–12 of a 28-day cycle) to evaluate whether prolonged administration might improve efficacy without increased toxicity. The 10-day schedule study was not randomised but was conducted using the same study protocol and centres, after enrollment completed in the 5-day randomised study. Eligible patients had a confirmed diagnosis of treatment-naïve AML and were not candidates for intensive chemotherapy either due to age (≥75 years) or a combination of age (≥65 years) and at least one of these criteria: poor cytogenetics (defined as monosomies or partial deletions of chromosome 5 or 7 (del(5q), del(7q), −5, −7), abnormalities involving the long arm of chromosome 3 (q21;q26), t(6;9) (p23;q34), t(9;22) (q34;q11.2), or abnormalities including the long arm of chromosome 11 (11q23), or subjects with 3 or more unrelatedcytogenetic abnormalities of any kind); secondary AML following a prior diagnosis of MDS, prior chemotherapy or radiotherapy; Eastern Cooperative Oncology Group (ECOG) performance status of 2; or poor cardiopulmonary function (left ventricular ejection fraction [LVEF] <50%, or diffusing capacity of the lung for carbon monoxide [DLCO] or forced expiratory volume in the first second [FEV1] <50% of expected) unrelated to leukaemia. Patients with acute promyelocytic leukaemia were excluded. There was no upper limit to the total WBC count. Other eligibility criteria included ECOG performance status of 0 to 2 and adequate hepatorenal functions (creatinine ≤1·5 times the upper limit of normal [ULN], bilirubin ≤2 times the ULN, and hepatic transaminases ≤2·5 times the ULN). Patients with active central nervous system disease, other malignancies, active systemic infections, or uncontrolled non-AML life-threatening medical conditions were excluded. Hydroxyurea was allowed in cycle 1 only. Estimated life expectancy for eligible patients is 5–7 months at best (5,6) if untreated.
All patients provided written informed consent. The protocol was approved by the institutional review board or an independent ethics committee at each centre.
Randomisation and masking
A computer algorithm (dynamic randomization) developed by Astex (Astex Pharmaceuticals, Pleasanton, CA, USA) was used to randomly assign (1:1) patients to 28-day cycles of subcutaneous guadecitabine at 60 or 90 mg/m2 daily×5. Once randomized, allocation was not concealed in this open-label trial, but the treating physician could not know the next assignment due to the multicentre nature of the study and dynamic randomization method. Study clinics, under direction of the physician, enrolled and treated patients according to their random assignment generated by Astex. After 51 patients were treated, an additional cohort was enrolled sequentially to receive guadecitabine 60 mg/m2 on the 10-day schedule without randomization.
Procedures
Planned doses of subcutaneous guadecitabine were 60 or 90 mg/m2 on 5-day schedules (days 1–5 of a 28-day cycle) or 60 mg/m2 on a 10-day schedule (days 1–5 and 8–12 of a 28-day cycle).
Patients on the 10-day schedule were to receive at least 2 cycles of 10-day treatment, after which they could receive treatment on a 5-day schedule (days 1–5). All patients were recommended to receive at least six cycles of therapy and to continue treatment until disease progression or unacceptable toxicity. Dose delays or reductions were allowed to manage toxicity. Physicians could extend cycles from 28 days up to 42 days to allow for marrow recovery in case of significant marrow hypocellularity, and additional delay or dose reduction may have been applied based on the physician’s judgment.
The haematological response (primary endpoint) to guadecitabine was monitored by analysis of blood and bone marrow aspirates. After the initial screening of bone marrow aspirate at baseline, the frequency of follow-up bone marrow aspirates was based on the results of peripheral blood assessments. Complete peripheral blood counts and differential were done each week to measure blast percentage, granulocyte and platelet numbers, and haemoglobin concentration. If a response was noted on blood counts, a bone marrow aspirate was done immediately to confirm and at two month intervals while responding. Response was not centrally reviewed.
Patient-reported and investigator-observed (through physical examinations, clinical haematology and laboratory tests, and electrocardiograms) adverse events were collected throughout the trial.
Whole blood samples for demethylation assays were collected weekly during the first cycle. Global DNA methylation was measured by the long interspersed nuclear element (LINE-1) methylation assay, and changes in methylation from baseline were assessed as previously described (15).
Outcomes
The primary objective was to measure the composite complete response (CRc) rate based on the International Working Group (IWG) response criteria for AML (16) including CR, CR with incomplete platelet recovery (CRp), and CR with incomplete neutrophil recovery regardless of platelets (CRi). Response was evaluated in all patients (as-treated) as long as a patient received at least one guadecitabine treatment. Secondary endpoints included response assessments of CR, CRp, CRi, and partial response (PR); time to response; duration of response; incidence of blood and platelet transfusions; overall survival; safety (including assessment of adverse events and abnormal laboratory values); and pharmacokinetics.
Response rates for CR, CRp, CRi, and PR were defined as the number of patients who had a best response of, for example, CR, divided by the total number of patients included in the analysis data set. Time to response was defined as the number of days from the day a subject received the first dose of guadecitabine (C1D1) to the first day of response. Duration of response was analyzed for CRc and CR only. Duration of CR (or CRc) was defined from the first time a complete response of CR (or CR, CRp, or CRi) was observed to time of relapse defined as the earliest time point whereby BM blasts or PB blasts became ≥5% and stayed at that level in subsequent visits while patients were still on study. Incidence of blood and platelet transfusions was analyzed, but these endpoints were primarily meant for the MDS part of the phase 2 trial, so they are not reported in this cohort. Survival was defined from the start of therapy until death or last follow-up. Adverse events were mapped to the appropriate system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA) version 14.0. Severity of adverse events was categorised according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). Pharmacokinetics data were collected from a small subset of patients in this study; these data will be combined with those from the other phase 2 parts of this study and published separately.
Outcomes from patients randomly assigned to the 60 and 90 mg/m2 daily×5 cohorts were compared for safety and efficacy. The combined results from daily×5 were then compared with the 10-day schedule results.
Statistical analysis
Initially for the entire phase 2 dose expansion study, a minimum of 30 subjects in each disease cohort (r/r AML; treatment-naïve AML, HMA treatment-naïve MDS, and r/r MDS) was planned for enrollment. The sample size of 30 subjects in each cohort was selected so that if no responses were observed, it could be concluded with 95% confidence that the response rate in that cohort was <10% and therefore not worthy of further development. The protocol allowed a Safety Review Committee to expand the number of subjects in one or more cohort to 50 subjects if justified by promising efficacy and safety data, for better assessment of efficacy in the cohort(s). Members of the SRC reviewed the efficacy and safety data, and the sample size was expanded to include up to 50 subjects in each cohort randomly assigned to 60 mg/m2 or 90 mg/m2 on a 5-day schedule. Subsequently, the 10-day treatment schedule was first introduced for up to 50 r/r AML subjects (Amendment 3), based on favorable emerging efficacy and safety published data from a decitabine 10-day regimen in AML (13, 14). Thereafter, the 10-day schedule was also introduced for up to 50 treatment-naïve AML subjects (Amendment 5) treated with 60 mg/m2 (not randomised), based on emerging favorable data from the 10-day schedule in r/r AML subjects (implemented from Amendment 3).
Data analyses are descriptive unless otherwise specified. Ad hoc significance tests are provided for assessing the relative importance of an effect. All patients who received guadecitabine were included in the analyses.
Differences in response rates between treatment groups were compared using Fisher’s exact test. The 95% confidence intervals (CIs) of response rates were based on binomial distributions. Time to response, duration of response, and survival were analysed using the Kaplan-Meier estimator and log-rank test. The 95% CIs of median time to response, median duration of response and median survival were provided based on Kaplan-Meier estimates with a Log-Log transformation. For responding subjects without a documented relapse, the duration of response was censored on the day of the last assessment. For subjects without a death date, the survival time was censored on the last day the subject was known alive. In addition, logistic regression and Cox regression models were used to explore influences of potential baseline predictors (ECOG status [0–1 vs 2–3], cytogenetics [poor risk vs other, based on NCCN Guidelines, V.2.2014], secondary AML [no vs yes], age [<75 vs ≥75 years], treatment schedule [10-day vs 5-day], PB blasts [below or above median baseline value, or <12% vs ≥12%]) on CRc and survival, respectively.
Treatment-emergent adverse events were summarised. These were defined as events that first occurred or worsened after the first dose of study treatment given on Cycle 1 Day 1 (C1D1) until 30 days after the last dose of study treatment or the start of an alternative anticancer treatment for AML, whichever occurred first, though events that occurred more than 30 days after the last dose of study treatment or start of an alternative anticancer treatment for AML were also considered treatment-emergent if the events were both serious and related to the study treatment. The number and percentage of patients experiencing treatment-emergent adverse events were summarised by MedDRA system organ class and preferred term, as well as by CTCAE grade. Related adverse events, serious adverse events, and related serious adverse events were summarized similarly. In summarizing adverse events, if a subject reported the occurrence of a particular adverse event more than once, the event was counted once with its worst CTCAE grade.
Analyses and statistical reporting used SAS version 9.3.
This study is registered with ClinicalTrials.gov, number NCT01261312.
Role of the funding source
HMK, GJR, and representatives of the funding company (Astex), MA, YH, and EPR had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
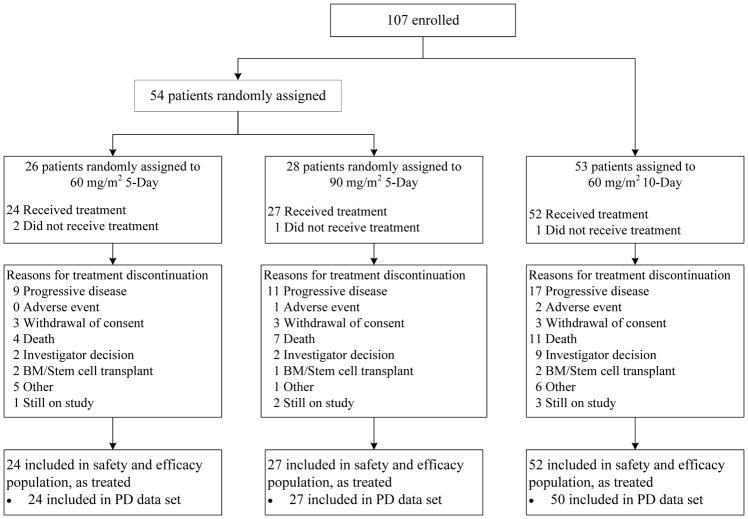
Between Aug 24, 2012, and Sep 15, 2014, we enrolled 107 patients: 54 were randomly assigned to 60 or 90 mg/m2 guadecitabine on the 5-day schedule (between Aug 24, 2012, and Sep 13, 2013) and 53 were assigned to 60 mg/m2 guadecitabine on the 10-day schedule (between Mar 24, 2014, and Sep 15, 2014; figure 1). Four patients (2 and 1 assigned to 60 and 90 mg/m2 guadecitabine on the 5-day schedule, respectively; and 1 assigned to the 10-day schedule) did not receive treatment and were not included in analyses (figure 1). The final analyses include all 103 treated patients: 51 on the 5-day schedule (24 and 27 who received 60 and 90 mg/m2, respectively) and 52 on the 10-day schedule.
Figure 1.
Study design
At the time of database lock on Aug 24, 2016, 15 patients were still being monitored for survival including six who were continuing treatment. The clinical data cut-off date (13 June 2016, date of the last patient observation included in the database) is the same for patients on the 5-day and 10-day schedules, so the length of follow-up is longer for patients on the 5-day schedule based on the enrollment dates above. Overall median follow-up was 953 days (31.8 months; IQR, 721–1040 days); median follow-up was 1017 days (33.9 months; IQR, 1013–1096 days) for patients on the 5-day schedule and 705 days (23.5 months; IQR 686–725 days) for patients on the 10-day schedule.
Overall median age was 77 years, and poor prognostic features included ECOG status ≥2 (39 [38%]), poor-risk cytogenetics (43 [42%]), and secondary AML (37 [36%]). Patient and disease characteristics were generally well-balanced between the different doses and schedules (table 1). Among patients receiving guadecitabine on the 5-day schedule, characteristics were similar between the randomised 60 and 90 mg/m2 dose groups, with exceptions of higher PB blast count (mean 29·7%) in the 90 mg/m2 group compared with the 60 mg/m2 group (mean 13·3%) and better ECOG performance status in the 90 mg/m2 group, with only 7 (26%) having ECOG of 2 (vs 11 [46%] in 60 mg/m2 group). There were no clinically significant differences in baseline patient characteristics between the 5-day and 10-day groups.
Table 1.
Demographic and baseline characteristics, by schedule
| 5-day | 10-day | |||||||
|---|---|---|---|---|---|---|---|---|
| 60 mg/m2 (N=24) | 90 mg/m2 (N=27) | Total (N=51) | 60 mg/m2 (N=52) | |||||
| Age, y | ||||||||
| Mean (SD) | 77 | (7·5) | 78 | (6·3) | 78 | (6·8) | 77 | (6·0) |
| Median (range) | 78 | (62–92) | 77 | (66–92) | 78 | (62–92) | 77 | (66–92) |
| Sex, n (%) | ||||||||
| Male | 14 | (58) | 16 | (59) | 30 | (59) | 34 | (65) |
| Female | 10 | (42) | 11 | (41) | 21 | (41) | 18 | (35) |
| Race, n (%) | ||||||||
| White | 23 | (96) | 24 | (89) | 47 | (92) | 50 | (96) |
| Asian | 1 | (4) | 2 | (7) | 3 | (6) | 1 | (2) |
| Other | 0 | 0 | 1 | (2) | 1 | (2) | ||
| ECOG status, n (%) | ||||||||
| 0 | 3 | (13) | 8 | (30) | 11 | (22) | 5 | (10) |
| 1 | 10 | (42) | 12 | (44) | 22 | (43) | 26 | (50) |
| ≥2a | 11 | (46) | 7 | (26) | 18 | (35) | 21 | (40) |
| Cytogenetics, n (%) | ||||||||
| Intermediate risk | 13 | (54) | 14 | (52) | 27 | (53) | 28 | (54) |
| Poor risk | 11 | (46) | 12 | (44) | 23 | (45) | 20 | (38) |
| Not evaluated | 0 | 1 | (4) | 1 | (2) | 4 | (8) | |
| Secondary AMLb, n (%) | 10 | (42) | 13 | (48) | 23 | (45) | 14 | (27) |
| Bone marrow blast (%) | ||||||||
| Mean (SD) | 44·8 | (18·8) | 48·4 | (24·8) | 46·7 | (22·1) | 53·0 | (23·8) |
| Median (range) | 39·5 | (21–90) | 46·0 | (13–94) | 40·0 | (13–94) | 49·5 | (16–98) |
| Peripheral blood blast (%) | ||||||||
| Mean (SD) | 13·3 | (20·5) | 29·7 | (33·2) | 22·0 | (28·9) | 27·4 | (27·7) |
| Median (range) | 5·0 | (0–71) | 18·0 | (0–91) | 9·2 | (0–91) | 22·5 | (0–90) |
| White blood cell count (×109 cells/L) | ||||||||
| Mean (SD) | 10·6 | (15·0) | 9·1 | (12·3) | 9·8 | (13·5) | 9·7 | (14·1) |
| Median (range) | 2·5 | (0·7–50·0) | 2·9 | (0·9–51·4) | 2·8 | (0·7–51·4) | 4·0 | (0·5–87·7) |
ECOG=Eastern Cooperative Oncology Group scale, SD=standard deviation.
One patient in the 10-day cohort had an ECOG status of 3.
Secondary AML: following a prior diagnosis of MDS, prior chemotherapy or radiotherapy.
The median number of treatment cycles received was 4·0 (range 1–31) in the 60 mg/m2 5-day group, 5·0 (range 1–41) in the 90 mg/m2 5-day group, and 3·0 (range 1–26) in the 10-day group. In the 10-day cohort, 14 patients (27%) received only one cycle of 10-day treatment, while 28 (54%) received 2 cycles, and 10 (19%) received more than 2 cycles. Subsequent cycles were given on the 5-day schedule. Most patients received the planned dose. Of the patients who received treatment in cycles 2 and 3, no patient on the 5-day schedule had a dose reduction (defined as 20% or more lower than the planned cycle 1 dose), and only two patients (of 43 treated, 5%) and three patients (of 33 treated, 9%) had cycle 2 and 3 dose reductions, respectively, on the 10-day schedule. The proportion of dose reductions increased with subsequent cycles; in cycle 6, five patients (of 22 treated, 23%) on the 5-day schedule and four patients (of 18 treated, 22%) assigned to the 10-day schedule had a dose reduction. The protocol did not have any upper limit exclusion on total WBCs, so hydroxuyurea was allowed only in Cycle 1 to lower the counts in proliferative patients as judged by the investigator. Three (13%), 6 (22%), and 10 (19%) patients in the 60 mg/m2 5-day, 90 mg/m2 5-day, and 10-day cohorts, respectively, received hydroxyurea during the study.
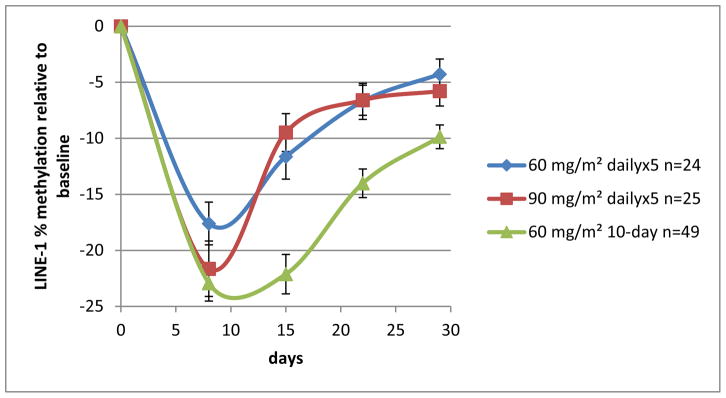
Guadecitabine administration on the 10-day schedule resulted in deeper, more prolonged DNA demethylation than on the 5-day schedule, as measured by LINE-1 during the first cycle (figure 2).
Figure 2.
Guadecitabine-LINE-1 demethylation in cycle 1 with the 5-day and 10-day schedules
The CRc rates did not differ between doses and schedules, ranging from 50% (26 of 52 subjects on the 10-day schedule) to 59% (16 of 27 subjects on the 90 mg/m2 dose 5-day schedule; table 2). All treated patients were evaluable for response analysis. For patients who responded, median time to best response was shorter for the 10-day cohort than the 5-day cohort (69 days [95% CI: 57–86] vs 89 days [95% CI: 57–103], respectively). Median response duration was similar for the 5-day and 10-day cohorts (186 days [95% CI: 95–362; 25 relapsed patients] and 269·5 days [95% CI: 176–421; 21 relapsed patients], respectively). Within the limitation of the sample size, multiple logistic regression analysis did not reveal a statistically significant (p<0.05) predictor of response (table 3).
Table 2.
Response, by regimen
| Response Rate, n (%) [95% Confidence Interval (CI)] a | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5-Day | 10-Day | |||||||
| Response Categoryb | 60 mg/m2 (N=24) | 90 mg/m2 (N=27) | Total (N=51) | 60 mg/m2 (N=52) | ||||
| Complete response (CR) | 9 | (37·5) | 11 | (40·7) | 20 | (39·2) | 17 | (32·7) |
| CR with incomplete blood count recovery (CRi) | 4 | (16·7) | 3 | (11·1) | 7 | (13·7) | 4 | (7·7) |
| CR with incomplete platelet recovery (CRp) | 0 | 2 | (7·4) | 2 | (3·9) | 5 | (9·6) | |
| Partial response (PR) | 1 | (4·2) | 1 | (3·7) | 2 | (3·9) | 1 | (1·9) |
| Nonresponder (NR) | 10 | (41·7) | 10 | (37·0) | 20 | (39·2) | 25 | (48·1) |
| Not evaluable | 0 | 0 | 0 | 0 | ||||
| CRc (CR+CRi+CRp) | 13 | (54·2) | 16 | (59·3) | 29 | (56·9) | 26 | (50·0) |
| [32·8 – 74·4] | [38·8 – 77·6] | [42·2 – 70·7] | [35·8 – 64·2] | |||||
AML=acute myeloid leukaemia; CRc=composite complete response.
Differences were not statistically significant.
Modified IWG 2003 AML Response Criteria (16).
Source: Table 14·3·1·1
Table 3.
Univariate and multiple logistic regression analysis of composite complete response (CRc)
| Effect | Multiple Logistic Regression Model | Univariate Logistic Model | ||||
|---|---|---|---|---|---|---|
| Odds Ratio Estimates | 95% Wald Confidence Limits | p value | Odds Ratio Estimates | p value | ||
| ECOG (0–1 vs 2–3) | 2.243 | 0.937 | 5.369 | 0.07 | 2.242 | 0.05 |
| Cytogenetics (others vs poor risk) a | 0.924 | 0.400 | 2.139 | 0.85 | 0.994 | 1.0 |
| Secondary AML (no vs yes) | 0.806 | 0.335 | 1.941 | 0.63 | 0.682 | 0.36 |
| Age (<75 vs ≥75 years) | 0.377 | 0.138 | 1.029 | 0.06 | 0.489 | 0.13 |
| Treatment (10-day vs 5-day) | 0.820 | 0.359 | 1.870 | 0.64 | 0.759 | 0.49 |
| PB blasts (<12% vs ≥12%)b | 1.338 | 0.556 | 3.218 | 0.52 | 1.434 | 0.36 |
Others: better risk, intermediate risk, and not evaluable
12% was the median value for baseline PB blast %
For the 37 patients who achieved CR, the median number of cycles to achieve it was 4 (range, 1–10) for the 5-day cohort (of 20 patients) and 3 (range, 2–6) for the 10-day cohort (of 17 patients).
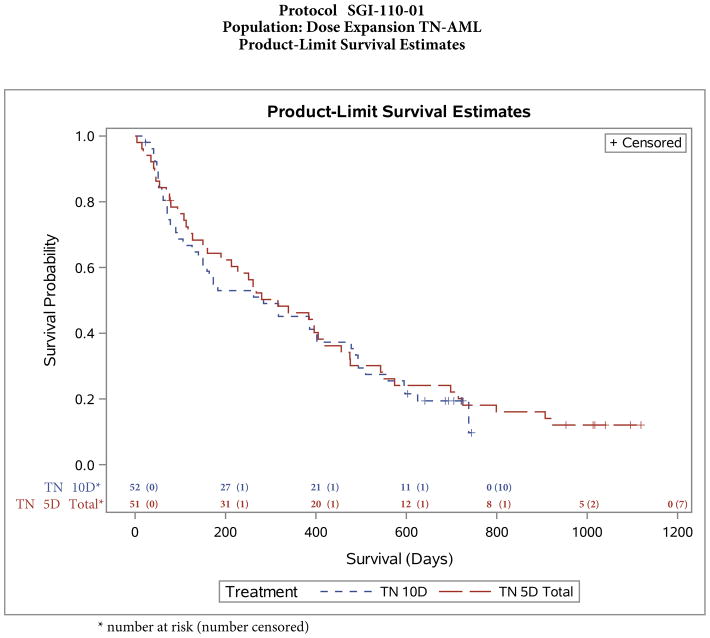
Median survival was similar for the 5-day (10·5 months [316 days], 95% CI 160–420 days; 44 patients died) and 10-day cohorts (9·5 months [284 days], 95% CI 140–478 days; 42 patients died) (figure 3). Five patients, three in the 5-day cohort and two in the 10-day cohort, underwent HCT and were not censored from the survival analysis at the time of HCT (one patient had survival duration 420 days, and the other four patients were alive at the time of analysis). Four of the five patients were in CR before HCT; the fifth (in the 10-day cohort) had achieved CRp.
Figure 3.
Survival with guadecitabine-5-day and 10-day schedules
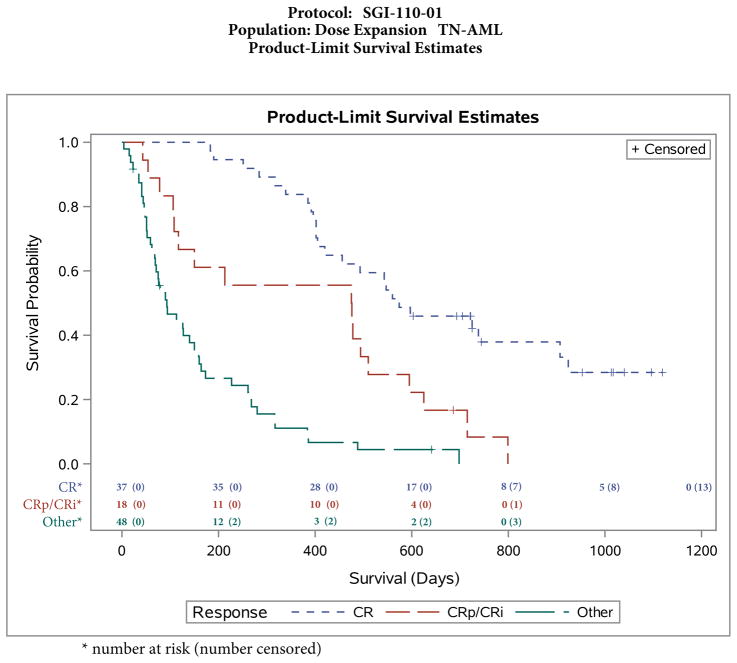
CR, CRp, and CRi were all associated with improved survival (p<0·0001, figure 4) with median survival 19·1 months (574 days, 95% CI: 420–907; based on 37 patients who achieved CR, of whom 24 died), 15·9 months (476 days, 95% CI: 108–510; based on 18 patients who achieved CRi or CRp, of whom 17 died), and 3·1 months (93 days, 95% CI: 68–150; based on 48 patients with no response, of whom 45 died). Multiple Cox regression analysis of potential predictors of survival did not reveal a statistically significant (p<0.05) predictor (appendix page 94).
Figure 4.
Survival by response to guadecitabine
Note: Other=partial response or no response
All 103 treated patients were assessed for safety. The most common adverse events overall, regardless of relationship to treatment, were febrile neutropenia (67 [65%] overall; 31 [61%] and 36 [69%] in the 5-day and 10-day cohorts, respectively), constipation (59 [57%] overall; 26 [51%] and 33 [64%] in the 5-day and 10-day cohorts), diarrhoea (53 [52%] overall; 22 [43%] and 31 [60%] in the 5-day and 10-day cohorts), thrombocytopenia (52 [51%] overall; 27 [53%] and 25 [48%] in the 5-day and 10-day cohorts), and injection site events (51 [50%] overall; 34 [67%] and 17 [33%] in the 5-day and 10-day cohorts).
Common grade 1 or 2 adverse events regardless of relationship to treatment in the 5-day and 10-day cohorts, respectively, included constipation (25 [49%] and 31 [60%]), diarrhoea (22 [43%] and 29 [56%]), injection site events (33 [65%] and 17 [33%]) (table 4). Common events with incidence 20% or greater in either group, for which incidence was higher by at least 10% in the 5-day cohort compared with the 10-day cohort, respectively, were injection site events (33 [65%] and 17 [33%]), insomnia (11 [22%] and 6 [12%]), and abdominal pain (11 [22%] and 4 [8%]). Common events with incidence 20% or greater in either group, for which incidence was higher by at least 10% in the 10-day cohort compared with the 5-day cohort, respectively, were constipation (31 [60%] and 25 [49%]), diarrhoea (29 [56%] and 22 [43%]), hypomagnesaemia (23 [44%] and 14 [27%]), vomiting (16 [31%) and 9 [18%]), pain (13 [25%] and 7 [14%]), anxiety (14 [27%] and 3 [6%]), rash (11 [21%] and 5 [10%]), oedema (11 [21%] and 4 [8%]), and ecchymosis (11 [21%] and 3 [6%]).
Table 4.
Adverse events by grade regardless of relationship to study treatment (≥10% of patients with any event), by schedule
| 5-day (n=51) | 10-day (n=52) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Haematological events | ||||||||||||||||
| Febrile neutropenia | 0 | 30 | (59) | 0 | 1 | (2) | 0 | 34 | (65) | 2 | (4) | 0 | ||||
| Thrombocytopenia | 2 | (4) | 5 | (10) | 20 | (39) | 0 | 3 | (6) | 3 | (6) | 19 | (37) | 0 | ||
| Neutropenia | 1 | (2) | 5 | (10) | 15 | (29) | 0 | 0 | 1 | (2) | 17 | (33) | 0 | |||
| Anaemia | 6 | (12) | 14 | (27) | 1 | (2) | 0 | 2 | (4) | 10 | (19) | 2 | (4) | 0 | ||
| Leukopenia | 1 | (2) | 3 | (6) | 8 | (16) | 0 | 0 | 0 | 2 | (4) | 0 | ||||
| Non-haematological events | ||||||||||||||||
| Constipation | 25 | (49) | 1 | (2) | 0 | 0 | 31 | (60) | 2 | (4) | 0 | 0 | ||||
| Diarrhoea | 22 | (43) | 0 | 0 | 0 | 29 | (56) | 2 | (4) | 0 | 0 | |||||
| Injection site eventsa | 33 | (65) | 1 | (2) | 0 | 0 | 17 | (33) | 0 | 0 | 0 | |||||
| Hypokalaemia | 16 | (31) | 7 | (14) | 0 | 0 | 21 | (40) | 6 | (12) | 0 | 0 | ||||
| Nausea | 22 | (43) | 2 | (4) | 0 | 0 | 25 | (48) | 1 | (2) | 0 | 0 | ||||
| Fatigue | 18 | (35) | 3 | (6) | 0 | 0 | 23 | (44) | 3 | (6) | 0 | 0 | ||||
| Dyspnoea | 19 | (37) | 0 | 0 | 0 | 24 | (46) | 2 | (4) | 0 | 0 | |||||
| Decreased appetite | 22 | (43) | 0 | 0 | 0 | 20 | (38) | 0 | 0 | 0 | ||||||
| Pneumonia | 4 | (8) | 12 | (24) | 1 | (2) | 2 | (4) | 1 | (2) | 16 | (31) | 0 | 3 | (6) | |
| Hypomagnesaemia | 14 | (27) | 0 | 0 | 0 | 23 | (44) | 0 | 0 | 0 | ||||||
| Cough | 15 | (29) | 0 | 0 | 0 | 20 | (38) | 0 | 0 | 0 | ||||||
| Oedema peripheral | 18 | (35) | 0 | 0 | 0 | 14 | (27) | 1 | (2) | 0 | 0 | |||||
| Asthenia | 11 | (22) | 1 | (2) | 0 | 0 | 15 | (29) | 4 | (8) | 0 | 0 | ||||
| Dizziness | 14 | (27) | 0 | 0 | 0 | 14 | (27) | 0 | 0 | 0 | ||||||
| Stomatitis | 13 | (25) | 0 | 0 | 0 | 14 | (27) | 1 | (2) | 0 | 0 | |||||
| Vomiting | 9 | (18) | 2 | (4) | 0 | 0 | 16 | (31) | 0 | 0 | 0 | |||||
| Pyrexia | 10 | (20) | 5 | (10) | 0 | 0 | 9 | (17) | 2 | (4) | 0 | 0 | ||||
| Epistaxis | 10 | (20) | 0 | 0 | 0 | 13 | (25) | 2 | (4) | 0 | 0 | |||||
| Contusion | 13 | (25) | 0 | 0 | 0 | 11 | (21) | 0 | 0 | 0 | ||||||
| Cellulitis | 3 | (6) | 5 | (10) | 0 | 0 | 10 | (19) | 5 | (10) | 0 | 0 | ||||
| Sepsis | 0 | 4 | (8) | 2 | (4) | 2 | (4) | 0 | 5 | (10) | 3 | (6) | 6 | (12) | ||
| Back pain | 8 | (16) | 1 | (2) | 0 | 0 | 12 | (23) | 0 | 0 | 0 | |||||
| Headache | 11 | (22) | 1 | (2) | 0 | 0 | 8 | (15) | 0 | 0 | 0 | |||||
| Hypotension | 1 | (2) | 9 | (18) | 0 | 0 | 9 | (17) | 1 | (2) | 0 | 0 | ||||
| Oropharyngeal pain | 7 | (14) | 0 | 0 | 0 | 13 | (25) | 0 | 0 | 0 | ||||||
| Pain | 7 | (14) | 0 | 0 | 0 | 13 | (25) | 0 | 0 | 0 | ||||||
| Confusional state | 5 | (10) | 4 | (8) | 0 | 0 | 10 | (19) | 0 | 0 | 0 | |||||
| Abdominal pain | 11 | (22) | 0 | 0 | 0 | 4 | (8) | 2 | (4) | 0 | 0 | |||||
| Anxiety | 3 | (6) | 0 | 0 | 0 | 14 | (27) | 0 | 0 | 0 | ||||||
| Insomnia | 11 | (22) | 0 | 0 | 0 | 6 | (12) | 0 | 0 | 0 | ||||||
| Arthralgia | 7 | (14) | 1 | (2%) | 0 | 0 | 7 | (13) | 1 | (2) | 0 | 0 | ||||
| Oedema | 4 | (8) | 0 | 0 | 0 | 11 | (21) | 1 | (2) | 0 | 0 | |||||
| Rash | 5 | (10) | 0 | 0 | 0 | 11 | (21) | 0 | 0 | 0 | ||||||
| Bacteraemia | 0 | 5 | (10) | 1 | (2) | 0 | 0 | 7 | (13) | 1 | (2%) | 0 | ||||
| Ecchymosis | 3 | (6) | 0 | 0 | 0 | 11 | (21) | 0 | 0 | 0 | ||||||
| Erythema | 4 | (8) | 1 | (2%) | 0 | 0 | 9 | (17) | 0 | 0 | 0 | |||||
| Fall | 4 | (8) | 0 | 0 | 0 | 9 | (17) | 1 | (2%) | 0 | 0 | |||||
| Fluid overload | 4 | (8) | 0 | 0 | 0 | 10 | (19) | 0 | 0 | 0 | ||||||
| Hyponatraemia | 6 | (12) | 0 | 0 | 0 | 5 | (10) | 3 | (6) | 0 | 0 | |||||
| Hypophosphataemia | 6 | (12) | 2 | (4) | 0 | 0 | 4 | (8) | 2 | (4) | 0 | 0 | ||||
| Depression | 8 | (16) | 0 | 0 | 0 | 5 | (10) | 0 | 0 | 0 | ||||||
| Urinary tract infection | 2 | (4) | 4 | (8) | 0 | 0 | 5 | (10) | 2 | (4) | 0 | 0 | ||||
| Pain in extremity | 5 | (10) | 0 | 0 | 0 | 7 | (13) | 0 | 0 | 0 | ||||||
| Petechiae | 7 | (14) | 0 | 0 | 0 | 5 | (10) | 0 | 0 | 0 | ||||||
| Pleural effusion | 3 | (6) | 0 | 0 | 0 | 9 | (17) | 0 | 0 | 0 | ||||||
| Chills | 5 | (10) | 0 | 0 | 0 | 6 | (12) | 0 | 0 | 0 | ||||||
| Dry mouth | 3 | (6) | 0 | 0 | 0 | 7 | (13) | 0 | 0 | 0 | ||||||
| Hypocalcaemia | 4 | (8) | 2 | (4) | 0 | 0 | 3 | (6) | 1 | (2) | 0 | 0 | ||||
| Rhinorrhoea | 3 | (6) | 0 | 0 | 0 | 7 | (13) | 0 | 0 | 0 | ||||||
| Syncope | 1 | (2) | 6 | (12) | 0 | 0 | 2 | (4) | 1 | (2) | 0 | 0 | ||||
| Transfusion reaction | 3 | (6) | 2 | (4) | 0 | 0 | 5 | (10) | 0 | 0 | 0 | |||||
| Upper respiratory tract infection | 4 | (8) | 2 | (4) | 0 | 0 | 4 | (8) | 0 | 0 | 0 | |||||
Injection site events is a group term comprising the individual terms: injection site pain, nodule, reaction, haematoma, haemorrhage, erythema, discomfort, infection, inflammation, joint redness
Common grade 3 or higher adverse events, regardless of relationship to treatment, for the 5- and 10-day schedules, respectively, included febrile neutropenia (31 [61%] and 36 [69%]), thrombocytopenia (25 [49%] and 22 [42%]), neutropenia (20 [39%] and 18 [35%]), pneumonia (15 [29%] and 19 [37%]), anaemia (15 [29%] and 12 [23%]), and sepsis (8 [16%] and 14 [27%]; appendix page 95). On the 5-day schedule, grade 3 or higher adverse events with clinically higher incidence for 90 than 60 mg/m2 were neutropenia (12 [44%] v 8 [33%]), pneumonia (10 [37%] v 5 [21%]), and sepsis (6 [22]% v 2 [8%]). The 10-day schedule had more grade 3 or higher adverse events than the 5-day schedule for pneumonia (19 [37%] v 15 [29%]) and sepsis (14 [27% v 8 [16%]; appendix page 95). All adverse events by grade are presented in the appendix (page 96).
The most common serious adverse events, regardless of relationship to treatment, for the 5- and 10-day cohorts, respectively, were febrile neutropenia (27 [53%] and 25 [48%]), pneumonia (14 [27%] and 16 [31%]), and sepsis (8 [16%] and 14 [27%]). Twenty-two patients (21%) had drug-related serious adverse events (10 [20%] and 12 [23%] in the 5-day and 10-day cohorts, respectively); those that occurred in more than one patient included febrile neutropenia (6 [12%] and 10 [19%]), pneumonia (2 [4%] and 5 [10%]), sepsis (3 [6%], 10-day cohort only), and bacteremia (2 [4%], 10-day cohort only). A total of 23 patients (22%) died due to adverse events, regardless of relationship to treatment (9 [18%] and 14 [27%] in the 5-day and 10-day cohorts, respectively). Adverse events that led to death were sepsis (8 [8%] overall; 2 [4%] and 6 [12%] in 5- and 10-day cohorts), pneumonia (5 [5%] overall; 2 [4%] and 3 [6%] in 5- and 10-day cohorts), multi-organ failure (2 [2%] overall; one each 5- and 10-day), respiratory failure (2 [2%] overall; both 10-day), intracranial haemorrhage (2 [2%] overall; one each 5- and 10-day), febrile neutropenia (1 [1%] overall; 5-day cohort), pericardia effusion (1 [1%] overall; 5-day), cardiorespiratory arrest (1 [1%] overall; 10-day), and myocardial ischaemia (1 [1%] overall; 5-day). Four of the 23 patients died due to adverse events (4% overall; all in the 10-day cohort) considered treatment-related : pneumonia (2 [2%]), multi-organ failure (1 [1%]), and sepsis (1 [1%]). One patient (1%; 60 mg/m2 5-day regimen) discontinued treatment due to drug-related toxicity (febrile neutropenia, not serious). 30-day, 60-day, and 90-day all-cause mortality were similar across doses and schedules with no significant differences (table 5).
Table 5.
30-, 60-, and 90-day all-cause mortality rates
| Mortality | Number (%) of Subjects
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5-Day
|
10-Day
|
Total (N=103) | ||||||||
| 60 mg/m2 (N=24) | 90 mg/m2 (N=27) | Total (N=51) | 60 mg/m2 (N=52) | |||||||
|
|
|
|||||||||
| 30-day | 2 | (8·3) | 1 | (3·7) | 3 | (5·9) | 1 | (1·9) | 4 | (3·9) |
| 60-day | 4 | (16·7) | 4 | (14·8) | 8 | (15·7) | 9 | (17·3) | 17 | (16·5) |
| 90-day | 5 | (20.8) | 6 | (22.2) | 11 | (21.6) | 15 | (28.8) | 26 | (25.2) |
DISCUSSION
We analysed outcomes of treatment-naïve older patients with AML not candidates for intensive chemotherapy; these patients were treated with guadecitabine at 60 or 90 mg/m2 on a 5-day schedule, or 60 mg/m2 on a 10-day schedule. The primary endpoint for this phase 2 trial was CRc, as opposed to the more standard endpoint of overall survival, to facilitate a relatively quick determination of clinical activity of the different doses and schedules. These three regimens produce similar efficacy and safety results. CR rates were 39% (20 patients) and 33% (17 patients) in 5-day and 10-day groups, respectively, and CRc rates were 57% (29 patients) and 50% (26 patients), respectively. Considering the age of this population (mean 77 years) and the poor prognostic features (ECOG status ≥2 [39 patients, 38%], poor risk cytogenetics [43 patients, 42%], secondary AML [37 patients, 36%]), the guadecitabine efficacy results compare favourably with published data in treatment-naïve older patients with AML treated with azacitidine or decitabine. In studies of azacitidine or decitabine, the reported CR rates were 16%–20% and CRc rate was 28% in both studies (5,6). 30- and 60-day all-cause early mortality with guadecitabine (3·9% and 16·5%, respectively) was similar to that reported for decitabine (9% and 19·7%) or azacitidine (6·6% and 16·2%) in similar clinical trials (5,6); thus guadecitabine treatment does not seem more toxic, despite the higher CR and CRc rates. Grade 3 or higher adverse events on this study were reported regardless of relationship to study therapy, and most event incidence was similar across cohorts.
This study’s limitations include relatively small cohorts and lack of randomization against a treatment comparator. Based on limited sample size, statistical results should be interpreted with caution. Lack of a randomized control group confounds comparison of results to those from other trials due to selection bias. Minimal residual disease (MRD) was not assessed in this study. Further study evaluating the depth of CR assessed by MRD should be performed in patients treated with guadecitabine to assess the frequency (if any) of MRD negativity and its potential effect on response duration and survival. The univariate and multivariate analyses attempted to identify predictors of response and survival but were not predefined in the protocol.
Median survival was similar with guadecitabine 5-day and 10-day exposure at 10·5 and 9·5 months, respectively. These data compare with the previously reported median survival time of 7·7 months associated with decitabine treatment in similar patients (5) and a median survival time of 10·4 months reported with azacitidine in a better-risk population (6). In the azacitidine study, patients were younger (median age 75 years) and those with a WBC count over 15,000 per microliter were excluded; additionally patients had fewer adverse features (ECOG status 2, adverse karyotype, or secondary AML) compared with our guadecitabine study (6). Critically, the azacitidine study included patients who were eligible to receive intensive chemotherapy, as it was offered as one of the conventional care regimens in the control group (in addition to subcutaneous cytarabine or best supportive care). While these differences only allow estimates, the results of our analysis are encouraging and prompted an ongoing phase 3 study to compare guadecitabine with standard-of-care low intensity therapy (decitabine, azacitidine, or low dose cytarabine) in 800 treatment-naïve older patients with AML not candidates for intensive chemotherapy.
Early study of azacitidine and decitabine included limited investigations of dose intensity and dose schedules (17). Based on these investigations, decitabine was developed with the regimen of 20 mg/m2 daily×5 every month. This concept was later challenged by studies suggesting that decitabine given over a longer schedule of 10 days might produce better results in AML (13,14). In the phase 1 and extended phase 2 studies of guadecitabine, we attempted to address these questions of dose intensity and schedule dependency. From the phase 1 study, it appeared that guadecitabine 60 mg/m2 resulted in demethylation as effective as higher doses of 90–125 mg/m2 (12). Clinical results in this report confirm that higher intensity regimens may cause more adverse events (such as grade 3 or higher pneumonia and sepsis) without improving efficacy in treatment-naïve older patients with AML not candidates for intensive chemotherapy. A similar study with guadecitabine suggested better tolerability of the higher intensity regimen in relapsed/refractory AML patients who are younger, have a more resistant disease, and are probably more in need of initial intensification (data on file).
After guadecitabine therapy, patients who achieved CR did so after a median of 4 cycles (range, 1–10) in the 5-day cohort and 3 cycles (range, 2–6) in the 10-day cohort. These results highlight the importance of continuing treatment with HMA therapy beyond the 1–2 cycles usually administered with intensive induction chemotherapy, as HMA therapy may produce CR after as many as 10 cycles, as evidenced in these results.
With intensive chemotherapy in AML, CR was considered the only response category associated with improved survival. This notion has been questioned with epigenetic therapy in both MDS and AML based on the hypothesis that haematologic improvements may stabilise disease and allow sufficient normal stem cell recovery to forestall mortality from myelosuppression and leukaemia progression. While the association between response and survival should always be interpreted with caution since responders tend to live long enough to achieve a response status, nevertheless as expected, in this study we observed that CR was associated with the longest survival (median 19·1 months), and CR without complete normal count recovery (CRi or CRp) was also associated with survival more than five times longer than that associated with no response (median 15·9 months among patients achieving CRi or CRp vs 3·1 months among patients with no response). This interesting observation needs to be considered in the evaluation of novel targeted therapies in AML that are not cytotoxic chemotherapy.
Supplementary Material
Research in context.
Evidence before this study
We did not do a systematic review before starting this trial. First-generation hypomethylating agents (azacitidine and decitabine) have been studied and used in the clinic for more than 20 years and approved worldwide with a standard dose and schedule. The purpose of our trial was to define the best dose and schedule for the next-generation hypomethylating agent, guadecitabine, building on the findings of the guadecitabine phase 1 trial (12). That study did not include treatment-naïve AML patients. In this phase 2 study, we document the efficacy and safety of guadecitabine, a new HMA, for the first time in treatment-naïve AML patients not candidates for intensive chemotherapy using different doses and schedules as guided by the phase 1 results (12) and previously published resulted from decitabine, the active metabolite of guadecitabine (13,14). Phase 1 data with guadecitabine identified 60 mg/m2 as the biologically effective dose (BED) resulting in maximal demethylation of LINE-1 elements. Patients with relapsed/refractory AML were treated up to 125 mg/m2; 90 mg/m2 was the highest tolerated dose for MDS patients, but AML patients tolerated all doses. In the phase 1 study, guadecitabine was given sequentially for 5 days or on two different weekly schedules. The daily schedule was found to be better than the weekly schedules. The phase 1 study could not address whether BED or higher doses had different efficacy and safety, or whether a prolonged daily administration (10 days) would be better than the 5-day schedule. These questions also remain inadequately addressed with the first-generation HMAs decitabine and azacitidine. Treatment doses and schedules for these agents were largely based on MDS studies without clear identification of the BED. The value of longer daily schedules in AML patients for decitabine came from single-arm, single-centre studies that included a heterogeneous population of relapsed/refractory and treatment-naïve AML (13,14).
Added value of this study
We assessed dose intensity in a randomised comparison between the BED and the highest tolerated dose and subsequently assessed the value of longer daily administration. While the 5-day and 10-day schedule comparison was not randomised, it was conducted using the same protocol, ensuring identical eligibility and centres, thus minimising patient heterogeneity. This was also a multicentre study minimising patient selection bias.
Implications of all the available evidence
In the absence of any signal or trend for superior efficacy in the higher dose (90 mg/m2) or the longer daily administration (10-day schedule) cohorts of treatment-naïve AML patients not candidates for intensive chemotherapy, the recommended guadecitabine regimen in this population is 60 mg/m2/day in a 5-day schedule. A similar study was conducted in relapsed/refractory AML patients and the results will be reported separately. The high CRc rate with guadecitabine (≥50%) warrants further investigation of this new HMA in a phase 3 study, which is currently ongoing.
Acknowledgments
The trial was supported by Astex Pharmaceuticals, Inc. and Stand Up To Cancer (SU2C). The authors thank Sherry Stinn, Renee Hansen, and Laurie Haynes for medical writing and administrative support; Sue Naim for managing overall study conduct; Xiang Yao Su for statistical support; and Woonbok Chung and Pietro Taverna for assistance with LINE-1 analysis. SS is a medical writer supported by funding from Astex Pharmaceuticals; WC is a scientist funded through Fels Institute for Cancer Research and Molecular Biology; RH, LH, SN, XYS, and PT are present or past employees of Astex Pharmaceuticals.
Footnotes
Contributors
HMK and GJR coauthored the report. MA and J-PJI conceived and designed the study. HMK, GJR, PLK, KWLY, CLO’C, RT, KJW, NAP, EAG, EJ, GG-M, DR, WS, MRS, TLR, JGB, and FR enrolled and treated patients and gathered data. YH gathered data. HMK, J-PJI, YH, EPR, and MA analysed and interpreted the data. HMK, GJR, EPR, and MA wrote the report. All authors reviewed the report and approved the final version.
Declaration of interests
HMK has received institutional research funds from Amgen, Ariad, Astex, BMS, Novartis and Pfizer; and honoraria from AbbVie, Actinium, Amgen, ARIAD, BMS, Immunogen, Orsinex and Pfizer.
GJR has served as a consultant for AbbVie, Agios Pharmaceuticals, Amgen, Amphivena Therapeutics, Astellas Pharma, Astex Pharmaceuticals, AstraZeneca, Array BioPharma, Celator Pharmaceuticals, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceutica, Juno Therapeutics, MEI Pharma, MedImmune, Novartis, Onconova Therapeutics, Pfizer, Roche/Genentech, and Sunesis Pharmaceuticals. GJR has received research support from Cellectis.
PLK has served as a consultant for Celgene and Takeda.
KWLY has participated in advisory board meetings for Celgene Inc., Novartis Inc., Hoffman-LaRoche Inc., Tolero Pharmaceuticals, Otsuka Pharmaceuticals, and Pfizer and has received research funding from Oncoethix, Merck, Agensys, Inc, Hoffmann-la Roche Limited / Genentech, Astex Pharmaceuticals, Inc., and GlaxoSmithKline.
CLO’C has participated in a scientific advisory board for Astex.
RT has received institutional research funds from Astex Pharmaceuticals, Inc. for clinical trial.
KJW has nothing to disclose.
NAP has served as an advisory board member for Alexion, Ariad, Incyte, CTI BioPharma, and Spectrum.
EAG has participated in advisory board meetings for Celgene Inc., Pfizer Inc., Alexion Pharmaceuticals, and Novartis Inc.; received honoraria from Alexion Pharmaceuticals and Celgene Inc. for lecturing; and has received research funding from Astex Pharmaceuticals, Inc., and Celgene Inc.
EJ has nothing to disclose.
GG-M has nothing to disclose.
DR is on a speaker’s bureau supported by Celgene, Incyte, Gilead, and Seattle Genetics; Advisory Boards for Novartis, Kite, Gilead, Celgene, Seattle Genetics, Spectrum, Ariad, Astellas, Amgen, Pfizer, Agios, and Teva.
WS has served on advisory boards for Pfizer and Amgen.
MRS has received research grants from Astex Pharmaceuticals, Inc., Incyte, Sunesis, and TG Therapeutics; has served on advisory boards for Amgen, Astex, Bayer, Celgene, CTI, Gilead, Incyte, Karyopharm, Sunesis, and TG Therapeutics; consultancy and equity ownership in Karyopharm.
TLR has participated in advisory board meetings for Astellas Pharma, Incyte, Pfizer Inc., Alexion Pharmaceuticals, Boerhringer Ingelheim Pharmaceuticals, and Sunesis Pharmaceuticals.
JGB has received research funding from Astex Pharmaceuticals, Inc., Celgene, Amgen, BMS, Janssen, Novartis, and Takeda.
FR has received honoraria from Astex Pharmaceuticals, Inc.; no other relevant disclosures.
JPJI has received research funding from Astex Pharmaceuticals, Inc. and is a consultant to Astex and Teva. He has served as an advisory board member for Janssen and GSK.
MA, YH, and EPR are employees of Astex Pharmaceuticals, Inc.
References
- 1.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 2.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–46. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of higher-risk myelodysplastic syndromes: a randomized, open-label phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care of low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–77. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombret H, Seymour FJ, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–99. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karahoca M, Momparler RL. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2′-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigenetics. 2013;5:3. doi: 10.1186/1868-7083-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcucci G, Silverman L, Eller M, Lintz L, Beach CL. Bioavailability of azacitidine subcutaneous versus intravenous in patients with myelodysplastic syndromes. J Clin Pharmacol. 2005;45:597–602. doi: 10.1177/0091270004271947. [DOI] [PubMed] [Google Scholar]
- 9.Yoo CB, Jeong S, Egger G, et al. Delivery of 5-aza-2′-deoxycytidine to cells using oligodeoxynucleotides. Cancer Res. 2007;67:6400–08. doi: 10.1158/0008-5472.CAN-07-0251. [DOI] [PubMed] [Google Scholar]
- 10.Chuang JC, Warner SL, Vollmer D, et al. S110, a 5-aza-2′-deoxycytidine-containing dinucleotide, is an effective DNA methylation inhibitor in vivo and can reduce tumor growth. Mol Cancer Ther. 2010;9:1443–50. doi: 10.1158/1535-7163.MCT-09-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava P, Paluch BE, Matsuzaki J, et al. Immunomodulatory action of SGI-110, a hypomethylating agent, in acute myeloid leukemia cells and xenografts. Leuk Res. 2014;38:1332–41. doi: 10.1016/j.leukres.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Issa J-P, Roboz G, Rizzieri D, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol. 2015;16:1099–110. doi: 10.1016/S1470-2045(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473–78. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54:2003–07. doi: 10.3109/10428194.2012.762093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang AS, Estécio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–49. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocyic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.