Abstract
Aurora kinases are involved in the pathophysiology of several cancers including acute myeloid leukemia (AML). In this phase 1 study, we investigated the safety and efficacy of AMG 900, an orally administered, highly potent, selective, small-molecule inhibitor of both Aurora kinase A and B, in patients with AML. Patients with pathologically documented AML who either declined standard treatments or had relapsed from or were refractory to previous therapies were enrolled. Two every-2-week dose-escalation schedules using a modified 3 + 3 + 3 design were evaluated AMG 900 given daily for 4 days with 10 days off (4/10 schedule), and AMG 900 given daily for 7 days with 7 days off (7/7 schedule). Thirty-five patients were enrolled at 9 different dose levels: 22 patients on the 4/10 schedule (doses from 15 to 100 mg daily), and 13 patients on the 7/7 schedule (doses from 30 to 50 mg daily). Both schedules were tolerated; nausea (31%), diarrhea (29%), febrile neutropenia (29%), and fatigue (23%) were the most common treatment-related adverse events. Three patients (9%) achieved complete response with incomplete count recovery. Patients with higher baseline expression of a set of specific pathway-related genes (BIRC5, AURKA, TTK, CDC2, and CCNB1) were more likely to respond in an exploratory biomarker analysis. AMG 900 was tolerated in a general AML population, and pathway-specific biomarkers identified a potential target population. Future research efforts will be directed toward further exploration of biomarkers of response and combination of AMG 900 with other anticancer agents.
1 | INTRODUCTION
Acute myeloid leukemia (AML) is a biologically heterogeneous cancer of older adults, with a median age at diagnosis of 67 years. In 2016, an estimated 19 950 new cases of AML will be diagnosed in the United States, and 10 430 people are expected to die from the disease.1 Although survival rates have steadily improved over the past decades, therapeutic approaches have changed little over the same period, with survival rates of only 27% five years after diagnosis. Thus, the vast majority of AML patients will either relapse or be refractory to initial therapy, and will require additional treatment.
Aurora kinases are a family of highly conserved serine/threonine protein kinases that regulate key steps in mitosis and meiosis.2–6 Aurora A, which is present at the spindle poles during interphase, is critical to centrosome maturation, as well as spindle assembly and orientation. Inhibition of Aurora A causes cell cycle arrest in G2. Aurora B localizes to the midbody of the central spindle where it is part of the chromosomal passenger complex, which regulates chromosome condensation and orientation as well as cytokinesis. Aurora kinases A and B are amplified and/or overexpressed in many malignancies, including various types of leukemia,5,7 and are associated with high proliferation rates, poor prognosis, and therapeutic resistance.8–12 Aurora A has been shown to phosphorylate p53, impairing its tumor suppressor activity.13 Amplification/overexpression of Aurora A/B and in combination with p53 mutation may induce transformation or cause tumors to be more aggressive.5 Both Aurora A and B were significantly overexpressed in AML blasts relative to control CD34 + cells, and inhibition of Aurora A with MLN8237 inhibited proliferation and induced apoptosis of AML blasts.7 Aurora kinase inhibition is therefore a treatment approach of interest for AML.14
AMG 900 is an investigational, orally administered, highly potent, selective, small-molecule pan-aurora kinase inhibitor that inhibits both Aurora A and B.15 Aurora B phosphorylates serine 10 of histone H3,16 and therefore, inhibition of this phosphorylation step serves as a pharmacodynamic marker for AMG 900 activity.15 The molecule has demonstrated antitumor activity in taxane-resistant ovarian cancer17 and breast cancer18 cell lines, as well as single agent activity in heavily pretreated patients with chemotherapy-resistant/refractory solid tumors.19–21 It was hypothesized that an oral agent with this profile might provide a broader therapeutic window than other agents in the class for both solid and hematologic malignancies, and that biomarkers of response linked to the pathway of interest could be identified.
The primary objectives of this phase 1, open-label, multicenter, sequential dose escalation study were to evaluate the safety and tolerability of AMG 900, its pharmacokinetics after multiple oral administrations, the optimal dose schedule, and to determine the maximum tolerated dose (MTD) in patients with AML. The secondary objectives were to evaluate the antileukemic activity of AMG 900 and the pharmacodynamic effects of drug exposure on phosphorylation of histone H3 in leukemic blasts. An exploratory objective was to evaluate baseline expression of genes related to the aurora kinase pathway, p53 downstream pathways, and modulation of the G2M transition phase of the cell cycle (aurora kinase A and B [AURKA, AURKB], p53 [TP53], survivin [BIRC5], cyclin B1 [CCNB1], CDC2, TTK, and p21 [CDKN1A]) as biomarkers of response to AMG 900.
2 | METHODS
2.1 | Patients
Eligible patients were ≥ 18 years of age with pathologically documented AML who either declined standard therapy or had relapsed from or were refractory to previous therapies. Patients also had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2 and a life expectancy > 3 months in the opinion of the investigator. Patients were excluded if they had a white blood cell count greater than 20 × 103/µL; clinically significant bleeding within 2 weeks of screening (e.g., gastrointestinal bleeds, intracranial hemorrhage) or active peptic ulcer disease; prior allogeneic stem cell transplantation; or unresolved nonhematologic toxicities from prior antitumor therapy (with the exception of alopecia or toxicities that had been present and stable for > 6 months). This study (ClinicalTrials.gov: NCT01380756) was conducted in accordance with the United States Food and Drug Administration and the International Conference on Harmonisation Good Clinical Practice regulations/ guidelines. The independent ethics committee/institutional review board at each study center approved the protocol, the proposed informed consent form, and other written information provided to patients. All patients provided written informed consent.
2.2 | Study design
This was a phase 1, multicenter, open-label, sequential dose escalation study evaluating AMG 900 in patients with AML. The study was to be conducted in two parts: dose escalation followed by dose expansion. In the dose escalation phase, AMG 900 was to be administered daily for 4 days every 2 weeks (4 days on/10 days off or 4/10 schedule) at doses of 15, 25, 40, 60, 80, 100, 125, and 150 mg, respectively. After a protocol amendment, a second dose schedule was then added to the dose escalation phase: AMG 900 was to be administered daily for 7 days every 2 weeks (7 days on/7 days off or 7/7 schedule) at doses of 30, 40, 50, 60, and 75 mg. Patients were followed for a period of 14–21 days after the last dose of AMG 900; data beyond this point were not collected as part of this study. Patients self-administered AMG 900 on an empty stomach in the morning (no food or liquids except water 2 hours prior to drug) and refrained from food and liquid (except water) for 1 hour postdose.
2.3 | Rationale for dose selection
The 4/10 schedule was selected based on preclinical data,15,18 the desire for multiday target coverage per cycle (> 8 hours per day above the concentration at 50% maximum inhibition), available clinical data on a safe starting dose,19,20 and safety considerations. The starting dose selected (15 mg daily) was below 16 mg, a dose that did not produce dose-limiting toxicities (DLTs) in the first-in-human study.19 On observation of acceptable tolerability but low efficacy with the 4/10 schedule, the more intensive 7/7 schedule was initiated with the aim of providing a more prolonged exposure to the drug. Based on pharmacokinetic modeling and clinical safety data, the cumulative dose per month for the 7/7 schedule had to be equivalent or lower than that of the 4/10 schedule (Supporting Information Table S1).
2.4 | Dose escalation
Dose escalation was conducted using a modified 3 + 3 + 3 design (some flexibility in the number of patients enrolled in each dose cohort was allowed to avoid study delays associated with the need for patient replacement). DLT assessment (criteria listed in Supporting Information Table S2) occurred during the first 28 days (i.e., first two cycles) of AMG 900 dosing. If no DLT was observed in the initial 3–4 patients of a cohort, the dose was to be escalated in the next cohort; if one DLT was observed, 2–3 additional patients were to be enrolled at the same dose level for a total of at least six patients at that dose level; if no further DLTs were observed, the dose was to be escalated in the next cohort; however, if a second DLT was observed, additional patients were to be enrolled at the same dose level for a total of up to nine patients (consistent with the 3 + 3 + 3 design). If no further DLTs were observed, the dose was to be escalated in the next patient cohort. If ≥ 3 DLTs were observed at any dose level, enrollment was to stop. The MTD was defined as the highest dose at which < 33% of the patients enrolled in a cohort experienced a DLT.
Due to the nature of AML, hematologic adverse events (AEs) were not considered DLTs; however, prolonged pancytopenia in the presence of a hypocellular bone marrow (i.e., cellularity 5% or less without evidence of leukemia) > 42 days in duration from the start of therapy was considered dose-limiting myelosuppression. If ≥ 33% of patients in any dose cohort fit these criteria, the dose level review committee was to convene with the investigators to discuss dose or schedule modifications and/or termination of dosing at that particular dose level. In all cohorts, patients were to continue to receive treatment until disease progression, an unacceptable AE, or withdrawal of consent.
2.5 | Endpoints
The primary endpoints of this study were related to the safety (patient incidence of AEs; DLTs; clinically significant changes in vital signs, weight, electrocardiogram, and laboratory tests) and pharmacokinetic profile of AMG 900 (maximum plasma concentration [Cmax], time to Cmax [tmax], area under the concentration-time curve [AUC]). Secondary endpoints included the objective response rate (morphologic complete response [CR], cytogenetic CR, molecular CR, CR with incomplete count recovery [CRi], partial response [PR]) by Cheson response criteria22 and the change in the number of phosphorylated histone H3 (pHH3)-positive cells from baseline. Exploratory endpoints included the analysis of tumor biomarkers and RNA signatures.
2.6 | Safety and tolerability
AEs were assessed at regular study visits. The patient incidence of AEs was summarized for all treatment-emergent, serious treatment-emergent, treatment-related, serious treatment-related, and fatal AEs, and AEs leading to withdrawal of investigational product. The Medical Dictionary for Regulatory Activities version 17.1 was used to code all AEs to a system organ class and a preferred term, and the severity of each AE was graded using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
2.7 | Pharmacokinetics
Blood samples for pharmacokinetic analysis were taken predose and 1, 2, and 4 hours postdose on days 1 and 4 (4/10 schedule) or days 1 and 7 (7/7 schedule) of cycle 1, and predose and at 2 hours postdose on day 1 of cycles 2, 3, and 4. AMG 900 concentration was measured using a validated liquid chromatography-tandem mass spectroscopy method with a quantitation range of 20.0 to 10 000 ng/mL.
2.8 | Pharmacodynamics
The pharmacodynamic effect of AMG 900 was measured in peripheral blood leukemic blasts using a flow cytometric assay to assess pHH3 and cell cycle in whole blood. Briefly, red blood cell-lysed/fixed/permeabilized blood was labeled with antibodies specific for pHH3 (mitosis marker, Alexa Fluor 647, Cell Signaling Technologies, Danvers, MA) and DAPI (DNA content, Cell Signaling Technologies). The change from baseline in the percentage of pHH3-positive cells in the G2/M phase was summarized by dose.
2.9 | Biomarkers
Bone marrow mononuclear cells collected at baseline were analyzed using the Agilent microarray (Agilent Human Whole Genome 4×180K v2, Agilent Technologies, Santa Clara, CA). Gene expression results were log2 transformed and quantile normalized prior to analysis. A subset of genes was analyzed for the primary biomarker objective of determining the association of baseline expression and response. The genes of primary interest were AURKA, AURKB, TP53, CDKN1A, BIRC5, CDC2, TTK, and CCNB1.
2.10 | Statistical analysis
Pharmacokinetic parameters were estimated using standard noncompartmental methods and summarized by dose and across cycle. Individual plasma AMG 900 concentration-time profiles were summarized by dose. The proportion of patients with an objective response (PR + CR) and corresponding 2-sided 80% confidence intervals were calculated.23 A univariate logistic regression model of objective response (CR or PR) was fit to the baseline biomarker results. Model significance was determined by the likelihood ratio test P-value being less than the Benjamini-Hochberg threshold for 5% false discovery. The effect sizes of gene expression were estimated using odds ratios with 95% confidence intervals, along with a P-value for the estimate. Principal component analyses24 of the z-scores for genes of interest were used to further explore expression signatures.
3 | RESULTS
3.1 | Patient enrollment and disposition
This study was conducted between October 2011 (first patient enrolled) and September 2014 (last patient completed follow-up). A total of 49 patients were screened and 35 were enrolled: 22 patients to the 4/10 dose schedule and 13 patients to the 7/7 dose schedule. All 35 patients discontinued treatment. The median (range) duration of treatment was 5 (1, 27) weeks. The most common reasons for discontinuation were disease progression (66%), AEs (11%), death (9%), withdrawal of consent (6%), other reasons (6%), and requirement for alternative therapy (3%). The dose expansion phase was not initiated for reasons discussed below.
3.2 | Baseline demographics and disease characteristics
The median (Q1, Q3) age of patients was 69 (61, 73) years, and over two-thirds (69%) were ≥ 65 years of age (Table 1). Most patients had an ECOG status of 0 or 1 (83%), and the median (Q1, Q3) time since diagnosis of AML was 7 (4, 17) months. Nineteen (54%) patients had received 3 or more lines of therapy, 9 (26%) had received 2 lines, and 7 (20%) had received 1 line. Cytogenetic categories were as follows: normal (34%), complex (3 or more abnormalities) (26%), 27 and/or inv(3) (11%), and other abnormality/ies (23%). One patient (3%) had insufficient metaphases to allow determination of abnormalities. See Table 1 for distribution by dose and schedule.
TABLE 1.
Baseline demographics and disease characteristics
| 4 days on/10 days off | 7 days on/7 days off | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 15 mg (n = 4) |
25 mg (n = 3) |
40 mg (n = 7) |
60 mg (n = 4) |
80 mg (n = 3) |
100 mg (n = 1) |
30 mg (n = 3) |
40 mg (n = 4) |
50 mg (n = 6) |
All (n = 35) |
|
| Male, n (%) | 3 (75) | 2 (67) | 6 (86) | 1 (25) | 3 (100) | 1 (100) | 2 (67) | 0 (0) | 5 (83) | 23 (66) |
|
| ||||||||||
| Race, n (%) | ||||||||||
| White | 3 (75) | 1 (33) | 6 (86) | 4 (100) | 3 (100) | 1 (100) | 2 (67) | 3 (75) | 4 (67) | 27 (77) |
| Black/African American | 1 (25) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | 4 (11) |
| Other | 0 (0) | 1 (33) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (17) | 4 (11) |
|
| ||||||||||
| Age, years | ||||||||||
| Median | 68 | 69 | 72 | 69 | 66 | 71 | 71 | 59 | 68 | 69 |
| Q1, Q3 | 64, 73 | 55, 73 | 54, 80 | 65, 78 | 43, 71 | 71, 71 | 61, 73 | 48, 77 | 58, 75 | 61, 73 |
| ≥ 65, n (%) | 3 (75) | 2 (67) | 5 (71) | 3 (75) | 2 (67) | 1 (100) | 2 (67) | 2 (50) | 4 (67) | 24 (69) |
|
| ||||||||||
| ECOG PS, n (%) | ||||||||||
| 0 | 1 (25) | 0 (0) | 3 (43) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (17) | 7 (20) |
| 1 | 3 (75) | 3 (100) | 3 (43) | 3 (75) | 3 (100) | 1 (100) | 2 (67) | 1 (25) | 3 (50) | 22 (63) |
| 2 | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 2 (50) | 2 (33) | 6 (17) |
|
| ||||||||||
| Lines of prior therapy, n (%) | ||||||||||
| 1 | 3 (75) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 1 (17) | 7 (20) |
| 2 | 1 (25) | 0 (0) | 1 (14) | 1 (25) | 1 (33) | 1 (100) | 0 (0) | 2 (50) | 2 (33) | 9 (26) |
| 3 or more | 0 (0) | 3 (100) | 5 (71) | 3 (75) | 2 (67) | 0 (0) | 1 (33) | 2 (50) | 3 (50) | 19 (54) |
|
| ||||||||||
| Cytogenetic category, n (%) | ||||||||||
| Complex (3 or more abnormalities) | 0 (0) | 1 (33) | 1 (14) | 1 (25) | 1 (33) | 0 (0) | 1 (33) | 1 (25) | 3 (50) | 9 (26) |
| −7/inv(3) | 0 (0) | 0 (0) | 2 (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 4 (11) |
| Other abnormality(ies) | 2 (50) | 1 (33) | 2 (29)a | 1 (25) | 1 (33) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | 9 (26) |
| Normal | 2 (50) | 1 (33) | 2 (29) | 2 (50) | 0 (0) | 1 (100) | 1 (33) | 1 (25) | 2 (33) | 12 (34) |
| Insufficient metaphases | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
Cytogenetic report from one patient was from 2 months before baseline.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status.
3.3 | Exposure
All 35 patients enrolled in the study received ≥ 1 dose of AMG 900. Patients received a median (range) of 14 (4, 49) doses, and the median (range) cumulative dose was 480 (120, 2240) mg. The median (Q1, Q3) number of cycles started was 3 (2, 4) and the median (range) dose delivered per cycle was 210 (53, 400) mg.
3.4 | Safety and tolerability
AMG 900 was evaluated at nine different levels across the two dose schedules (up to 100 mg for the 4/10 schedule and up to 50 mg for the 7/7 schedule). The 125 mg and 150 mg doses in the 4/10 schedule and the 60 mg and 75 mg doses in the 7/7 schedule were not tested. There were two DLTs based on the protocol definitions: one patient who received 40 mg AMG 900 on the 4/10 schedule had a DLT of grade 3 pancytopenia on day 22 that was considered by the investigator to be treatment-related; and one patient who received 50 mg AMG 900 on the 7/7 schedule had two DLTs (grade 3 treatment-related febrile neutropenia and grade 3 abdominal pain on day 82) (Table 2). None of the DLTs met the criteria for a serious AE by the CTCAE definition.
TABLE 2.
Dose-limiting toxicities and most common treatment-related adverse events of all grades (≥ 10% frequency overall) and grade ≥ 3 (≥ 5% frequency overall)
| 4 days on/10 days off | 7 days on/7 days off | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 15 mg (n = 4) n (%) |
25 mg (n = 3) n (%) |
40 mg (n = 7) n (%) |
60 mg (n = 4) n (%) |
80 mg (n = 3) n (%) |
100 mg (n = 1) n (%) |
30 mg (n = 3) n (%) |
40 mg (n = 4) n (%) |
50 mg (n = 6) n (%) |
Total (n = 35) n (%) |
|
| Dose-limiting toxicity | ||||||||||
| Grade 3 treatment-related pancytopenia | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Grade 3 treatment-related neutropenia and grade 3 abdominal pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (3) |
|
| ||||||||||
| Any treatment-related adverse events | 4 (100) | 1 (33) | 5 (71) | 3 (75) | 3 (100) | 1 (100) | 3 (100) | 4 (100) | 6 (100) | 30 (86) |
| Nausea | 2 (50) | 0 (0) | 3 (43) | 1 (25) | 0 (0) | 1 (100) | 1 (33) | 1 (25) | 2 (33) | 11 (31) |
| Diarrhea | 0 (0) | 0 (0) | 2 (29) | 1 (25) | 1 (33) | 0 (0) | 1 (33) | 2 (50) | 3 (50) | 10 (29) |
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (14) | 3 (75) | 2 (67) | 1 (100) | 0 (0) | 1 (25) | 2 (33) | 10 (29) |
| Fatigue | 2 (50) | 0 (0) | 1 (14) | 1 (25) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 2 (33) | 8 (23) |
| Vomiting | 0 (0) | 0 (0) | 1 (14) | 1 (25) | 0 (0) | 1 (100) | 1 (33) | 0 (0) | 2 (33) | 6 (17) |
| Alopecia | 0 (0) | 0 (0) | 1 (14) | 1 (25) | 1 (33) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | 5 (14) |
| Dyspnea | 1 (25) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | 4 (11) |
| Epistaxis | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 2 (33) | 4 (11) |
| Mucosal inflammation | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 1 (33) | 1 (25) | 0 (0) | 4 (11) |
| Rash | 1 (25) | 0 (0) | 0 (0) | 1 (25) | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (11) |
|
| ||||||||||
| Treatment-related adverse events by grade | ||||||||||
| Grade ≥ 3 | 2 (50) | 0 (0) | 3 (43) | 3 (75) | 2 (67) | 1 (100) | 1 (33) | 1 (25) | 3 (50) | 16 (46) |
| Grade ≥ 4 | 1 (25) | 0 (0) | 1 (14) | 1 (25) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (17) | 5 (14) |
| Fatal | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 2 (6) |
|
| ||||||||||
| Grade ≥ 3 treatment-related adverse events | ||||||||||
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (14) | 3 (75) | 2 (67) | 1 (100) | 0 (0) | 1 (25) | 2 (33) | 10 (29) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 2 (6) |
| Device-related infection | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| Pancytopenia | 0 (0) | 0 (0) | 2 (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| Septic shock | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 2 (6) |
| Thrombocytopenia | 1 (25) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
A total of 30 patients (86%) had treatment-related AEs (Table 2). The most common treatment-related AEs (in ≥ 10% of patients overall, any grade) were nausea (31%), diarrhea (29%), febrile neutropenia (29%), fatigue (23%), vomiting (17%), alopecia (14%), dyspnea (11%), epistaxis (11%), mucosal inflammation (11%), and rash (11%). The most common grade ≥ 3 treatment-related AE was neutropenia in 10 patients (29%); all other grade ≥ 3 treatment-related AEs occurred in two patients each (6%). A total of 11 patients (31%) had serious treatment-related AEs; the most common (occurring in ≥ 5% of patients) were febrile neutropenia (26%), device-related infection (6%), and septic shock (6%). The median (range) duration of febrile neutropenia was 8 (1, 45) days.
Five patients (14%) discontinued AMG 900 treatment due to AEs of cellulitis (left lower extremity, considered related to treatment), septic shock, lung infection, and acute myocardial infarction in one patient each (considered not related to treatment), and bronchopulmonary aspergillosis (considered related to treatment) and pneumonia in one patient (considered not related to treatment). The patient with pneumonia and bronchopulmonary aspergillosis had in addition a serious AE of febrile neutropenia during the same period that was considered related to treatment.
Nine patients (26%) died during the study. These deaths occurred due to lung infection and respiratory failure (two patients each), and acute respiratory failure, cardiorespiratory arrest, respiratory distress, sepsis, and septic shock (one patient each). Only the deaths due to respiratory failure and septic shock (one patient each) were considered treatment-related.
3.5 | Pharmacokinetics
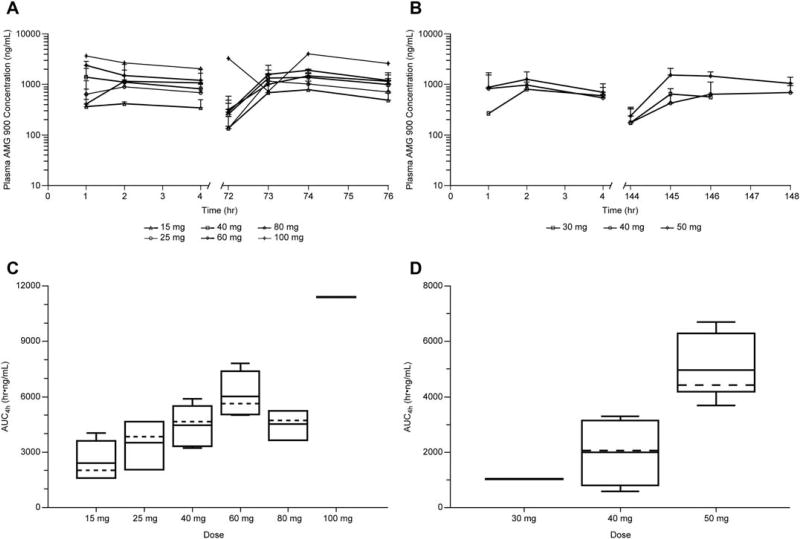
The mean plasma concentration, Cmax, and AUC of AMG 900 generally increased with increasing dose (Figure 1 and Supporting Information Table S3). At dose levels of 15, 25, 40, 60, 80, and 100 mg, mean Cmax on cycle 1 day 1 was 501, 951, 1600, 1890, 1430, and 3670 ng/mL, respectively, and mean AUC4h on cycle 1 day 1 was 1380, 2740, 4360, 5620, 3210, and 10200 h·ng/mL, respectively. Minimal accumulation was observed in patients on both dose schedules: the mean accumulation ratio (cycle 1 day 4/cycle 1 day 1) for Cmax ranged from 1.03 to 1.85, while the mean accumulation ratio for AUC4h ranged from 1.11 to 1.72. Clearance and half-life could not be estimated due to the limited duration of monitoring.
FIGURE 1.
Mean plasma concentration-time profiles following multiple oral administrations for the 4 days on/10 days off schedule (A) or the 7 days on/7 days off schedule (B); area under the concentration-time curve by dose group after the first 4 days of the 4 days on/10 days off schedule (C) or after the first 7 days of the 7 days on/7 days off schedule (D). AUC4h, area under the concentration-time curve from time zero to 4 hours postdose
3.6 | Pharmacodynamics
Peripheral blood samples from 31 of the 35 patients were analyzed for characterization of pHH3 and DNA content. Baseline numbers of pHH3-positive leukemia cells before treatment were highly variable between patients; however, a clear inhibition of pHH3 expression was observed in whole blood as early as 1 hour after treatment with AMG 900 (Supporting Information Table S4). This inhibition was most prominent in cases where higher numbers of pHH3-positive cells were present at baseline, regardless of the dosing schedule (4/10 or 7/7) (Supporting Information Figure S1). Inhibition was transient, and the numbers of pHH3-positive cells increased during the off-treatment days.
3.7 | Efficacy
Three patients (9%) in the 4/10 dose schedule (two patients at 40 mg and one patient at 60 mg) had a best response of complete response with CRi by Cheson criteria; no other responses were observed.
Overall, the objective response rate for all patients treated with AMG 900 at any dose and in either schedule was 9% (80% confidence interval: 3%, 18%). In the three patients who responded to AMG 900, the duration of response was 3 months, 1.5 months (censored observation due to discontinuation of AMG 900 due to requirement for other therapy), and < 1 month.
3.8 | Biomarkers
At baseline, gene expression results (Agilent, Human Whole Genome) were available for 24 patients, and bone marrow aspirates (pHH3) were available for 14 patients. Higher baseline expression of five biomarkers, BIRC5, AURKA, TTK, CDC2, and CCNB1, was associated with the three CRi responses in univariate logistic regression models (all P < .021, the false discovery threshold) (Table 3). These analyses were exploratory and hypothesis generating, and the study was not powered to detect a certain OR with a confidence interval excluding 1. Nevertheless, the univariate analyses did produce well-fitting models of response, and the P-values were robust to multiple testing. Given the small number of responders, additional data would be needed to confirm the effects observed.
TABLE 3.
P-values for logistic regression model of objective response vs. baseline gene expression
| BIRC5* | CCNB1*a | AURKB | AURKA* | CDC2* | TTK* | TP53b | CDKN1A | TP53c | CCNB1*d | |
|---|---|---|---|---|---|---|---|---|---|---|
| p model | 0.01 | 0.01 | 0.04 | 0.02 | 2.90E−3 | 0.01 | 0.77 | 0.82 | 0.14 | 0.01 |
| OR estimate | 13.50 | 17.71 | 5.63 | 8.33 | 824.32 | 21.52 | 1.39 | 0.90 | 0.24 | 260.61 |
| Lower 95% CL | 0.38 | 0.28 | 0.48 | 0.36 | 0.01 | 0.12 | 0.13 | 0.37 | 0.03 | 0.07 |
| Upper 95% CL | 475.53 | 1138.35 | 66.39 | 193.19 | 1.25E+08 | 3789.51 | 14.47 | 2.21 | 1.92 | 1.03E+06 |
| p OR | 0.15 | 0.18 | 0.17 | 0.19 | 0.27 | 0.24 | 0.78 | 0.82 | 0.18 | 0.19 |
Results from a univariate logistic regression of response (CR or PR) versus gene expression are tabulated. Model significance was determined by the likelihood ratio test p-value (p model) being less than the Benjamini-Hochberg threshold for 5% false discovery (0.0211); this is indicated by an asterisk (*) next to the gene name. Also tabulated are the effect sizes of gene expression, as indicated by the odds ratio (OR) estimate and its 95% confidence interval and p-value of the estimate (p OR). The OR is interpreted as the change in odds of response for each doubling of gene expression.
CCNB1 results from probe A_23_P122197.
TP53 results from probe A_23_P26810.
TP53 results from probe A_33_P3315764.
CCNB1 results from probe A_33_P3401621.
Abbreviations: CL, confidence limit.
Baseline gene expression was used to look for multivariate stratifying signatures with principal component analysis. The first two principal components accounted for at least 80% of the expression variance. Patients with AML clustered into two groups, one of which (higher levels of expression of BIRC5, CCNB1, AURKB, AURKA, CDC2, and TTK) was enriched for responders (Supporting Information Figure S2).
4 | DISCUSSION
Patients with relapsed/refractory AML have limited treatment options. Response rates to aggressive re-induction therapy are 20% to 30%, but as AML is primarily a disease of older adults, few patients can tolerate these approaches, and they opt instead for less aggressive treatment or palliative care. In this phase 1 study in a heavily pretreated group of patients with AML who had declined standard therapy or had relapsed from or were refractory to previous therapies, AMG 900 monotherapy was associated with manageable nonhematologic toxicity and modest antileukemic activity, although patients whose leukemia cells expressed a specific set of genes related to the aurora kinase and downstream pathways were enriched for responders. Prolonged cytopenias prevented further dose escalation, and the dose expansion phase of the study was not initiated. The results highlight the challenge of identifying a therapeutic window for AMG 900 in which a given dose and a dosing schedule can achieve selective antitumor activity without excessive myelosuppression.
AMG 900 was engineered to have a high specificity for both of the target aurora kinases while evading the ATP-binding cassette transporters,25 to have efficacy in multiple tumor xenograft models at reasonable doses, and to have predicted human pharmacokinetic properties that would provide adequate target coverage.26 Aurora B phosphorylates histone H3 on serine 10 during mitosis; hence the inhibition of pHH3 observed in this study suggests on-target modulation of aurora kinase B by AMG 900. In an effort to achieve maximal target coverage, we evaluated escalating doses using two administration schedules: 4 days on/10 days off and 7 days on/7 days off. Pharmacokinetic data were insufficient to accurately determine the linearity and exposure of AMG 900; however, the mean Cmax and AUC generally increased with increasing dose for both schedules and minimal accumulation was observed. Clearance and elimination half-life could not be accurately estimated with noncompartmental analysis at the individual dose levels due to the limited plasma sampling. Further analysis with a population modeling approach would be needed to fully evaluate the pharmacokinetic characteristics of AMG 900.
Two patients had DLTs in this study, including grade 3 pancytopenia and grade 3 neutropenia/abdominal pain. The MTD of AMG 900 was not formally reached in either dosing schedule because the criterion of > 33% of patients in a single dose cohort experiencing a DLT was not met. The highest doses evaluated were 100 mg daily for the 4/10 schedule and 50 mg daily for the 7/7 schedule, both of which were tolerated, apart from hematologic AEs. The incidence of grade ≥ 3 febrile neutropenia was 29% overall in this study, a rate comparable to those reported in studies of other aurora kinase inhibitors, including the selective Aurora kinase B inhibitor barasertib27,28 and the selective Aurora kinase A inhibitor alisertib.29 The findings of pancytopenias are also consistent with aurora kinase inhibitor class effects.30
The response rate observed in this study was 9% consisting of three patients with CRi, generally comparable to that observed with other molecules in this class, though lower than that observed with alisertib.27,29,31–33 We did not observe a dose effect on efficacy or on pHH3 inhibition despite a large range of explored doses in the 4/10 regimen and a clear impact of AMG 900 dose on AUC. Thus, despite the described dose-concentration effect, we were not able to observe a concentration-effect relationship within the patient sample evaluated in this study.
Highlighting the importance of identifying biomarkers of response in heterogeneous cancers such as AML, AMG 900 elicited significant changes in pathway-related genes, and baseline levels of a subset of genes allowed enrichment for the three responders. Due to the small sample size and limited number of responders, these results must be interpreted with caution and explored further in larger trials.
Though antileukemic responses in this single-agent setting were modest, it is possible that combining tolerable doses of AMG 900 with additional agents that inhibit cell division through other mechanisms may produce more profound effects. Preclinical data suggest AMG 900 enhances the effect of microtubule-targeting agents such as paclitaxel, carboplatin, and doxorubicin17,18 and this approach is supported by data from combination therapy with other aurora kinase inhibitors.34–37 Evaluation of AMG 900 in combination with other agents is an appropriate next step in the development of this molecule as a treatment for AML.
5 | CONCLUSIONS
AMG 900 was associated with manageable extra-hematological toxicity and modest response in patients with recurrent/refractory AML, although prolonged cytopenias hampered further dose escalation in this single-agent treatment setting. Combination of low doses of AMG 900 with other anticancer agents, or additional sequencing and scheduling evaluations should be explored in future studies.
Supplementary Material
Acknowledgments
This work was supported by Amgen Inc. Wanda Krall, PhD, on behalf of Amgen, and Micah Robinson, PhD, of Amgen, assisted with the writing of this manuscript. Darrin Beaupre, MD PhD, and John Hill, PhD, both of Amgen at the time the work was conducted, assisted with the design, data interpretation, and execution of the study.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Hagop M. Kantarjian reports research funding from BMS, Ariad, Pfizer, Amgen, and Novartis. Michael W. Schuster reports honoraria and speakers’ bureau participation from Amgen, and equity ownership in Amgen. Nitin Jain reports nothing to disclose. Anjali Advani reports research funding from Amgen. Elias Jabbour reports research funding and consultancy fees from Amgen. Mikkael A. Sekeres reports advisory board participation from Amgen and Celgene. Erik Rasmussen, Gloria Juan, Abraham Anderson, Vincent F. Chow, and Gregory Friberg are employees of Amgen and hold stock in Amgen. Erick Gamelin is a former employee of Amgen, a current employee of Pfizer, and holds stock in Amgen and Pfizer. Florian D. Vogl is a former employee of Amgen and holds stock in Amgen.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Surveilance, Epidemiology, and End Results (SEER) Program. Cancer Stat Fact Sheet: Acute Myeloid Leulemia. National Institutes of Health, National Cancer Institute; 2016. [Google Scholar]
- 2.van der Waal MS, Hengeveld RC, van der Horst A, et al. Cell division control by the Chromosomal Passenger Complex. Exp Cell Res. 2012;318:1407–1420. doi: 10.1016/j.yexcr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 4.Afonso O, Figueiredo AC, Maiato H. Late mitotic functions of Aurora kinases. Chromosoma. 2017;126:93–103. doi: 10.1007/s00412-016-0594-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldenson B, Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34:537–545. doi: 10.1038/onc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmena M, Earnshaw WC, Glover DM. The Dawn of Aurora Kinase research: From fly genetics to the clinic. Front Cell Dev Biol. 2015;3:73. doi: 10.3389/fcell.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Ikezoe T, Nishioka C, et al. CD34(+)/CD38(−) acute myelogenous leukemia cells aberrantly express Aurora kinase A. Int J Cancer. 2013;133:2706–2719. doi: 10.1002/ijc.28277. [DOI] [PubMed] [Google Scholar]
- 8.Cirak Y, Furuncuoglu Y, Yapicier O, et al. Aurora A overexpression in breast cancer patients induces taxane resistance and results in worse prognosis. J Buon. 2015;20:1414–1419. [PubMed] [Google Scholar]
- 9.Gautschi O, Heighway J, Mack PC, et al. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 10.Mignogna C, Staropoli N, Botta C, et al. Aurora Kinase A expression predicts platinum-resistance and adverse outcome in high-grade serous ovarian carcinoma patients. J Ovarian Res. 2016;9:31. doi: 10.1186/s13048-016-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portella G, Passaro C, Chieffi P, Aurora B. a new prognostic marker and therapeutic target in cancer. Curr Med Chem. 2011;18:482–496. doi: 10.2174/092986711794480203. [DOI] [PubMed] [Google Scholar]
- 12.Lucena-Araujo AR, de Oliveira FM, Leite-Cueva SD, et al. High expression of AURKA and AURKB is associated with unfavorable cytogenetic abnormalities and high white blood cell count in patients with acute myeloid leukemia. Leuk Res. 2011;35:260–264. doi: 10.1016/j.leukres.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 14.Moore AS, Blagg J, Linardopoulos S, et al. Aurora kinase inhibitors: novel small molecules with promising activity in acute myeloid and Philadelphia-positive leukemias. Leukemia. 2010;24:671–678. doi: 10.1038/leu.2010.15. [DOI] [PubMed] [Google Scholar]
- 15.Payton M, Bush TL, Chung G, et al. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010;70:9846–9854. doi: 10.1158/0008-5472.CAN-10-3001. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JY, Sun ZW, Li X, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 17.Kalous O, Conklin D, Manivong K, et al. Preclinical characterization of AMG 900, a pan-aurora kinase inhibitor, alone and in combination with taxanes in ovarian cancer; AACR Annual Meeting; New Orleans, LA. 2016; p Abstract 3008. [Google Scholar]
- 18.Bush TL, Payton M, Heller S, et al. AMG 900, a small-molecule inhibitor of aurora kinases, potentiates the activity of microtubule-targeting agents in human metastatic breast cancer models. Mol Cancer Ther. 2013;12:2356–2366. doi: 10.1158/1535-7163.MCT-12-1178. [DOI] [PubMed] [Google Scholar]
- 19.Carducci MA, Shaheen MF, Paller CJ, et al. First-in-human study of AMG 900, an oral pan-aurora kinase inhibitor, in adult patients (pts) with advanced solid tumors. J Clin Oncol. 2012;30(suppl) doi: 10.1007/s10637-018-0625-6. Abstract 3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markman B, Mahadevan D, Hurvitz S, et al. Phase 1 dose-expansion study of AMG 900, a pan-aurora kinase inhibitor, in adult patients with advanced taxane-resistant solid tumors. Eur J Cancer. 2014;50:197–198. [Google Scholar]
- 21.Shaheen M, Markman B, Carducci M, et al. Phase 1, open label, first-in-human study of AMG 900, an orally administered pan-aurora kinase inhibitor, in adult patients (pts) with advanced solid tumors; AACR Annual Meeting; New Orleans, LA. 2016; p Abstract CT045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Collett D. Modelling Binary Data. London: Chapman & Hall; 1991. [Google Scholar]
- 24.Dunteman GH. Principal Components Analysis. 1. London: SAGE Publications; 1989. p. 96. [Google Scholar]
- 25.Michaelis M, Selt F, Rothweiler F, et al. ABCG2 impairs the activity of the aurora kinase inhibitor tozasertib but not of alisertib. BMC Res Notes. 2015;8:484. doi: 10.1186/s13104-015-1405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geuns-Meyer S, Cee VJ, Deak HL, et al. Discovery of N-(4-(3-(2-aminopyrimidin-4-yl)pyridin-2-yloxy)phenyl)-4-(4-methylthiophen-2-yl)p hthalazin-1-amine (AMG 900), a highly selective, orally bioavailable inhibitor of aurora kinases with activity against multidrug-resistant cancer cell lines. J Med Chem. 2015;58:5189–5207. doi: 10.1021/acs.jmedchem.5b00183. [DOI] [PubMed] [Google Scholar]
- 27.Lowenberg B, Muus P, Ossenkoppele G, et al. Phase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood. 2011;118:6030–6036. doi: 10.1182/blood-2011-07-366930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuboi K, Yokozawa T, Sakura T, et al. A Phase I study to assess the safety, pharmacokinetics and efficacy of barasertib (AZD1152), an Aurora B kinase inhibitor, in Japanese patients with advanced acute myeloid leukemia. Leuk Res. 2011;35:1384–1389. doi: 10.1016/j.leukres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg SL, Fenaux P, Craig MD, et al. An exploratory phase 2 study of investigational Aurora A kinase inhibitor alisertib (MLN8237) in acute myelogenous leukemia and myelodysplastic syndromes. Leuk Res Rep. 2014;3:58–61. doi: 10.1016/j.lrr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 31.Foran J, Ravandi F, Wierda W, et al. A phase I and pharmacodynamic study of AT9283, a small-molecule inhibitor of aurora kinases in patients with relapsed/refractory leukemia or myelofibrosis. Clin Lymphoma Myeloma Leuk. 2014;14:223–230. doi: 10.1016/j.clml.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarjian HM, Sekeres MA, Ribrag V, et al. Phase I study assessing the safety and tolerability of barasertib (AZD1152) with low-dose cytosine arabinoside in elderly patients with AML. Clin Lymphoma Myeloma Leuk. 2013;13:559–567. doi: 10.1016/j.clml.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarjian HM, Martinelli G, Jabbour EJ, et al. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer. 2013;119:2611–2619. doi: 10.1002/cncr.28113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71:3912–3920. doi: 10.1158/0008-5472.CAN-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amin M, Minton SE, LoRusso PM, et al. A phase I study of MK-5108, an oral aurora a kinase inhibitor, administered both as monotherapy and in combination with docetaxel, in patients with advanced or refractory solid tumors. Invest New Drugs. 2016;34:84–95. doi: 10.1007/s10637-015-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond E, Alexandre J, Faivre S, et al. A phase I schedule dependency study of the aurora kinase inhibitor MSC1992371A in combination with gemcitabine in patients with solid tumors. Invest New Drugs. 2014;32:94–103. doi: 10.1007/s10637-013-9950-y. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal A, Kumar S, Hofmeister C, et al. A Phase Ib Study of the combination of the Aurora Kinase Inhibitor Alisertib (MLN8237) and Bortezomib in Relapsed Multiple Myeloma. Br J Haematol. 2016;174:323–325. doi: 10.1111/bjh.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.