Abstract
STUDY QUESTION
What are the effects of exposure to bisphenol A (BPA) or bisphenol S (BPS) during IVM on bovine oocyte maturation, spindle morphology and chromosome alignment?
SUMMARY ANSWER
Exposure to BPA or BPS during IVM resulted in increased spindle abnormalities and chromosome misalignment, even at very low concentrations.
WHAT IS KNOWN ALREADY
BPA is an endocrine disrupting chemical that alters oocyte maturation, spindle morphology and chromosome alignment in a range of species. The use of BPA substitutes, such as BPS, is increasing and these substitutes often display different potencies and mechanisms of action compared with BPA.
STUDY DESIGN, SIZE, DURATION
Bovine cumulus–oocyte complexes (COCs) underwent IVM with BPA or BPS for 24 h, together with vehicle-only controls. Overall, 10 different concentrations of BPA or BPS were used ranging from 1 fM to 50 μM in order to detect low dose or non-monotonic effects. An incomplete block design was utilized for the study, with at least three replicates per block. A total of 939 oocytes (250 of which were controls) were used for the BPA experiments, and 432 (110 controls) for the BPS experiments. Following the IVM period, the oocytes were denuded and fixed for immunocytochemistry.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Immunocytochemistry was used to label the chromatin, actin, and microtubules in the fixed oocytes. The meiotic stage was assessed using immunofluorescence, and the metaphase-II (MII) oocytes were further assessed for spindle morphology and chromosome alignment (in all MII oocytes regardless of spindle morphology) using immunofluorescence and confocal microscopy. Significant differences between the treatment and control groups were determined using chi-square and Fisher’s exact tests.
MAIN RESULTS AND THE ROLE OF CHANCE
There was no effect of BPA or BPS on the proportion of bovine oocytes that reached MII (P > 0.05). BPA and BPS increased spindle abnormalities in MII oocytes at almost all concentrations tested, including those as low as 1 fM (P = 0.013) or 10 fM (P < 0.0001), respectively, compared to control. Oocytes with flattened spindles with broad poles were observed at a higher frequency at some concentrations of BPA (P = 0.0002 and P = 0.002 for 10 nM and 50 μM, respectively) or BPS (P = 0.01 for 100 nM BPS), while this spindle phenotype was absent in the controls. BPA increased chromosome misalignment at concentrations of 10 fM, 10 nM and 50 μM (P < 0.0001 to P = 0.043 depending on the dose). BPS increased chromosome misalignment at concentrations of 10 fM, 100 pM, 10 nM, 100 nM and 50 μM (P < 0.0001 to P = 0.013 depending on the dose).
LIMITATIONS REASONS FOR CAUTION
Exposures to BPA or BPS were performed during the IVM of COCs to allow for determination of direct effects of these chemicals on oocyte maturation. Whole follicle culture or in vivo studies will confirm whether follicular cell interactions modify the effects of BPA or BPS on oocyte meiotic maturation. Investigation into the effects of BPA or BPS on other oocyte functions will determine whether these chemicals alter oocyte quality via mechanisms independent of the meiotic endpoints characterized here.
WIDER IMPLICATIONS OF THE FINDINGS
The findings of this study show that both BPA and BPS induce spindle abnormalities and chromosome misalignment in bovine in a non-monotonic manner, and at concentrations that are orders of magnitude below those measured in humans. Taken in context with previous studies on the effects of BPA in a range of species, our data support the literature that BPA may reduce oocyte quality and lead to subsequent infertility. Additionally, these results contribute to the burgeoning field of research on BPS and suggest that BPS may indeed be a ‘regrettable substitution’ for BPA.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by funding from the National Institutes of Health (NIH) (Grant 1R15ES024520-01). The authors declare no conflict of interest.
Keywords: bisphenol A, bisphenol S, oocyte, meiotic maturation, spindle, chromosomes, IVM
Introduction
An endocrine disrupting chemical (EDC) is defined as ‘an exogenous chemical…that interferes with any aspect of hormone action’ (Zoeller et al., 2012). Bisphenol A (BPA) is a high production volume chemical and known EDC frequently found in polycarbonate plastics, epoxy resins and a variety of consumer goods (Vandenberg et al., 2007; Geens et al., 2012). BPA has faced increasing concern over its effects on human health, including associations with adverse reproductive, developmental and metabolic conditions (Vandenberg et al., 2007; Rochester, 2013). In response to changing consumer demands and regulatory action in some countries, many manufacturers are turning to structurally related chemicals to replace BPA. Bisphenol S (BPS) is one widespread replacement chemical currently found in plastic bottles, canned food linings, receipt papers and several other sources previously known to contain BPA (Liao et al., 2012c, 2012b; Liao and Kannan, 2013, 2014). BPS has been shown to display endocrine disrupting activities (Rochester and Bolden, 2015), raising concerns that BPS may pose its own threats to human health.
BPA monomers can migrate into the environment through diffusion or when decomposition occurs, such as through exposure to heat, acids or bases; consequently, human exposure to BPA is ubiquitous. BPA is consistently detected in the urine of more than 90% of individuals sampled in developed nations (Vandenberg et al., 2010; Zhang et al., 2011; Zhou et al., 2014; CDC, 2015). BPA has also been measured in several other human fluids, including in follicular fluid at average concentrations of 2.4 ng/ml (10.5 nM) and 0.34 ng/ml (1.5 nM) (Ikezuki et al., 2002; Wang et al., 2016b). The detection of BPS in human urine has become increasingly prevalent; BPS was detected in 74% of American urine samples in 2014, as compared to 25% of samples in 2000 (Ye et al., 2015). Although average urinary BPS concentrations (0.13–0.654 ng/ml, i.e. 0.12–2.6 nM) (Liao et al., 2012a; Zhou et al., 2014; Ye et al., 2015) are lower than those for BPA (0.36–4.50 ng/ml, i.e. 1.6–19.7 nM) (Vandenberg et al., 2010; Zhang et al., 2011; Zhou et al., 2014; CDC, 2015; Ye et al., 2015), the concentrations are generally within one order of magnitude. In addition, the detection frequency and average concentration of urinary BPS have shown upward trends in recent years (Ye et al., 2015), suggesting that human exposure to BPS will continue to increase.
BPA has been linked to reproductive abnormalities in females (Machtinger and Orvieto, 2014). During IVF, higher urinary BPA levels were associated with fewer total and mature oocytes retrieved (Mok-Lin et al., 2010; Fujimoto et al., 2011; Ehrlich et al., 2012b). While this relationship was not seen in follow-up studies with increased number of patients (Mínguez-Alarcón et al., 2015), BPA levels were associated with decreased ovarian response, decreased blastocyst formation, increased implantation failure (Bloom et al., 2011; Ehrlich et al., 2012a) and decreased clinical pregnancy and live birth in women who did not consume soy (Chavarro et al., 2016). Additionally, infertile women were significantly more likely to have detectable levels of BPA in their serum when compared to fertile women (Caserta et al., 2013), altogether suggesting that BPA may affect female fertility.
Because oocyte meiotic competence is a critical component of fertility (Albertini et al., 2003; Keefe et al., 2015), it has been suggested that BPA may affect fertility through the disruption of oocyte meiosis. Evidence that BPA affects the oocyte was first published in 2003, with mouse oocytes exposed to BPA exhibiting a higher incidence of chromosome congressional failure (Hunt et al., 2003). Subsequent studies have shown that BPA causes meiotic abnormalities, including arrest, spindle abnormalities and/or congressional failure, in the mouse (Can et al., 2005; Eichenlaub-Ritter et al., 2008; Lenie et al., 2008), pig (Mlynarčíková et al., 2009; Wang et al., 2016a), cow (Ferris et al., 2015) and human (Machtinger et al., 2013). For BPS, effects on reproductive health outcomes are largely unknown, however, preliminary studies have demonstrated that BPS exposure may increase germline apoptosis and embryonic lethality in the nematode Caenorhabditis elegans (Chen et al., 2016) and decrease egg production and impair development in the zebrafish Danio rerio (Ji et al., 2013; Naderi et al., 2014). Recently, a first study also reported on the negative impact of BPS on porcine oocytes (Žalmanová et al., 2017). There is thus a need to further investigate the effects of BPS on mammalian reproduction. Comparative studies indicate that BPS may not necessarily alter outcomes exactly as BPA does, notably depending on the cell type (Boucher et al., 2016; Chen et al., 2016; Maćczak et al., 2016). Furthermore, the experimental models under use can influence the reported effects and thus it becomes paramount to conduct direct testing of chemicals within a single study system.
The objective of this study was to conduct a direct comparison of the effects of in vitro exposure to either BPA or BPS on meiotic progression, spindle morphology and chromosome alignment in the bovine oocyte. The bovine displays similar oocyte development and follicle dynamics to humans, including hormone responsiveness and follicular wave patterns, and as such is an excellent model for human reproduction and the evaluation of the impact of EDCs on the oocyte (Mihm and Evans, 2008; Adams et al., 2012; Santos et al., 2014). Furthermore, cattle are in themselves an important agricultural species. There is significant evidence that BPA can produce low-dose effects (Vandenberg et al., 2013) and non-monotonic dose-responses (NMDRs) (Vandenberg et al., 2012) on multiple endpoints in different tissues. Due to the scarcity of data regarding low-dose effects of BPA and BPS on the oocyte, we tested a wide range of concentrations, with the aim to detect potential low-dose effects and NMDRs.
Materials and Methods
All reagents were obtained from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated.
Experimental design
Due to constraints in the number of ovaries that could be sourced on a single day, it was not possible to test all concentrations of BPA or BPS in a single experiment. Therefore, an incomplete block design was utilized that included a vehicle-only control group and three concentrations of BPA or BPS for each experiment. Each block contained one overlapping dose with another block. For BPA experiments, a total of 939 oocytes were analyzed for meiotic stage (including 250 vehicle-only control oocytes), of which a total of 767 were at MII (including 211 MII oocytes in the control) and therefore were included for analysis of spindle and chromosome configuration. For the BPS experiment, a total of 432 oocytes were analyzed for meiotic stage (including 110 vehicle-only control oocytes), of which 350 were at MII (including 91 MII oocytes in the control group) and therefore were included for analysis of spindle and chromosome configuration.
Isolation and IVM of oocytes
Ovaries were sourced from naturally cycling Holstein-Friesian and Jersey heifers from an abbatoir (Champlain Beef Co., Whitehall, NY, USA). Ethical approval was not required as this study utilized slaughterhouse materials that would otherwise be discarded. The ovaries were transported to the laboratory in 0.9% NaCl and 1× antibiotic/antimycotic, with a transit time of ~45 min. Antral follicles measuring 3–7 mm in diameter were dissected from ovaries with an active corpus luteum and maintained at 37°C in HMEM (Lonza; Walkersville, MD, USA) containing 25 mM HEPES, 1 mM sodium pyruvate, 2 mM l-glutamine, 4 μg/ml bovine serum albumin (BSA), 100 μg/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml heparin. The cumulus–oocyte complex (COC) was released following bisection of the follicle with a scalpel. COCs were graded according to the morphological guidelines published by Blondin and Sirard (1995), and only those that had multiple layers of compact cumulus cells and a homogenous ooplasm with no granulations (i.e. class one or two COCs) were selected. Groups of 5–20 COCs were washed in 450 μl of synthetic oviductal fluid (SOF) prepared as described in Tervit et al. (1972) but with the addition of 1 mM glutamine (Lonza), 1% essential amino acids, and 0.5% nonessential amino acids. The COC were subsequently transferred into SOF supplemented with 0.1 μg/ml ovine FSH (NHPP, FSH-19-SIAFPRP-2, National Hormone and Peptide Program, Torrance, CA, USA) and the appropriate treatment. Treatments included the vehicle-only control (0.025% dimethylsulfoxide) or BPA or BPS at concentrations between 1 fM and 50 μM. The COC underwent IVM at 38.5°C with 5% CO2 in air for 24 h in polystyrene 4-well plates (Nunc IVF 4-well dishes) that have been reported to be BPA-free (Gatimel et al., 2016). Following IVM, the cumulus cells were mechanically stripped from the oocytes. The denuded oocytes were fixed in freshly prepared 2% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, for 15 min in the dark at room temperature with shaking. The oocytes were then extracted in 1% Triton X-100 in PBS for 45 min at room temperature with shaking. The cells were blocked in WASH solution (0.2% Na-azide, 0.2% non-fat powdered milk, 2% normal donkey serum, 1% BSA and 0.1 M glycine in PBS) for at least 48 h at 4°C prior to immunocytochemistry.
Immunocytochemistry
Each oocyte was labeled for microfilaments, microtubules and DNA. The oocytes were incubated overnight at 4°C with shaking with a 1:1 primary antibody mix containing 5 μg/ml mouse-α-tubulin antibody and 5 μg/ml mouse anti-β-tubulin antibody. All subsequent incubations were performed at 37°C with shaking. The cells were washed at 37°C three times (with WASH for 15 min) before being incubated with Alexa Fluor 488 donkey anti-mouse (3.3 μg/ml; Invitrogen; Carlsbad, CA, USA) and Texas-red phalloidin (1:20 dilution; Invitrogen) for 2 h. The cells were washed once, before being incubated with DAPI (20 μg/ml) for 1 h. Following two washes the oocytes were mounted on glass slides in 50% glycerol and 50% sodium-azide in PBS. A coverslip was added with minimal compression. Labeled oocytes were visualized and scored using conventional fluorescence microscopy. Imaging was performed at the Microscopy Imaging Center at the University of Vermont using a Zeiss LSM 510 META confocal laser scanning microscope (supported by NIH Award Number 1S10RR019246 from the National Center for Research Resources) and three-dimensional reconstructions.
Classification of oocytes
The oocytes were classified according to meiotic stage, based on organization of the microfilaments, microtubules and chromatin according to previously described criteria (Machtinger et al., 2013).
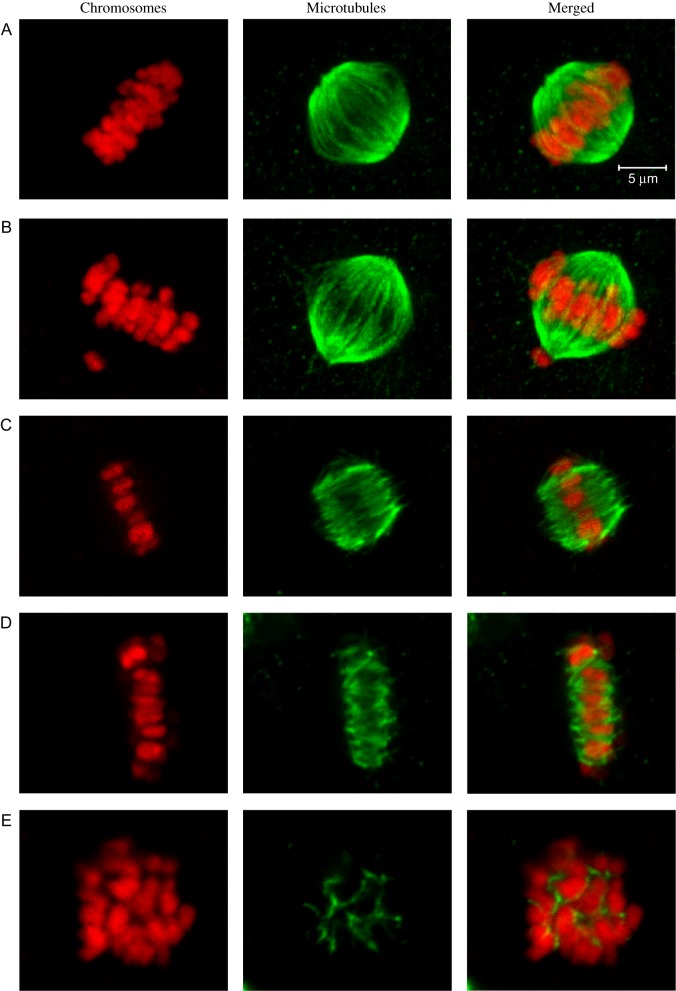
Spindle morphology and chromosome alignment (2n = 60 in the cow) were classified in metaphase II (MII) oocytes (Fig. 1). Spindles with two defined and focused poles were classified as bipolar. Spindles that had two poles but had abnormalities (e.g. splayed or disorganized microtubule fibers, broad or unfocused poles, protrusions of the spindle) were classified as bipolar with some abnormalities. Spindles that had no apparent organization, that were monopolar or tripolar were classified as non-bipolar. Chromosome alignment was determined in all MII oocytes, regardless of spindle morphology. Chromosomes that were located at the equatorial metaphase plate were classified as aligned. Where one to six chromosomes were slightly displaced from the metaphase plate, the chromosomes were classified as mostly aligned. Where more than six chromosomes were displaced from the metaphase plate, the chromosomes were classified as dispersed.
Figure 1.
Representative images of spindle and chromosome classifications in bovine metaphase II oocytes. Chromosomes are shown in red (left panels), microtubules are shown in green (center panels), and merged images are shown in the right panels. (A) Bipolar spindle with aligned chromosomes. (B) Bipolar spindle with a single misaligned chromosome at the lower pole. (C) Bipolar spindle with unfocused poles and aligned chromosomes. (D) Flattened bipolar spindle with extremely broad poles and aligned chromosomes. (E) Non-bipolar spindle with dispersed chromosomes. (The scale applies to all images.)
Statistical analysis
The data were analyzed using SAS 9.4 (SAS Inst., Inc., Cary, NC, USA), using a chi-square test to test for overall effects of treatment, followed by Fisher’s exact tests to compare between treatment and control. The P values were adjusted using the Holm–Bonferroni method to account for multiple testing (Holm, 1979). P < 0.05 was considered significant.
Results
BPA
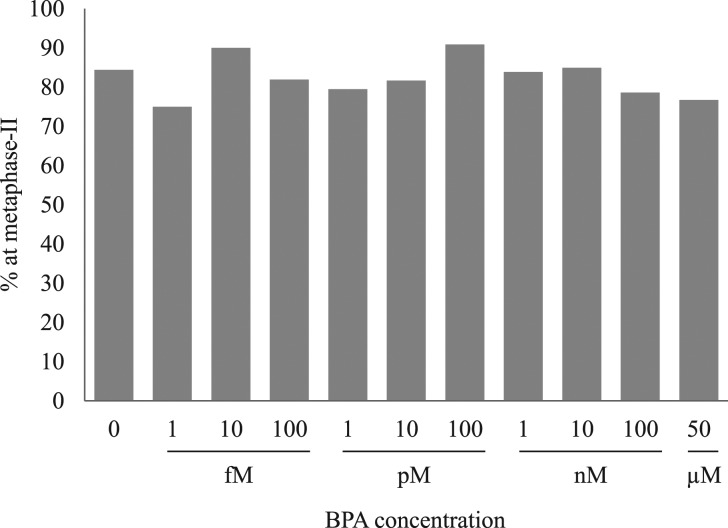
The proportion of oocytes that reached MII after 24 h of IVM in the presence of BPA is depicted in Figure 2. After 24 h of IVM, 84.4% of control oocytes had reached MII. There was no effect of treatment with BPA on the proportion of oocytes that had matured to MII at 24 h (P = 0.415).
Figure 2.
Effects of bisphenol A on the percentage of bovine cumulus-enclosed oocytes to reach metaphase II at 24 h. The data are from at least three independent experiments per concentration. The total number of oocytes used for each concentration was: 250 (no bisphenol A (BPA)), 48 (1 fM), 50 (10 fM), 105 (100 fM), 39 (1 pM), 49 (10 pM), 22 (100 pM), 62 (1 nM), 146 (10 nM), 84 (100 nM), 73 (50 μM).
Spindle morphology and chromosome alignment were determined in the MII oocytes exposed to BPA, as depicted in Tables I and II, respectively. In the control group, 82.9% of MII stage oocytes had a bipolar spindle and 90.0% had aligned chromosomes. Overall effects of BPA treatment on spindle morphology (P < 0.0001) and chromosomes alignment (P = 0.0037) were detected.
Table I.
Effects of bisphenol A on spindle morphology in bovine metaphase II oocytes.
| Ctrl | fM (10−15 M) | pM (10−12 M) | nM (10−9 M) | uM (10−6 M) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 50 | ||
| Bipolar | 175 (82.9) | 21(58.3) | 22 (48.9) | 60 (69.8) | 24 (77.4) | 27 (67.5) | 16 (80.0) | 26 (50.0) | 68 (54.8) | 37 (56.1) | 27 (48.2) |
| Bipolar abn | 26 (12.3) | 12 (33.3) | 19 (42.2) | 23 (26.7) | 7 (22.6) | 13 (32.5) | 4 (20.0) | 19 (36.5) | 41 (33.1) | 23 (34.8) | 24 (42.9) |
| Non-bipolar | 10 (4.7) | 3 (8.3) | 4 (8.9) | 3 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (13.5) | 15 (12.1) | 6 (9.1) | 5 (8.9) |
| P value | – | 0.0129 | <0.0001 | 0.0293 | 0.3216 | 0.0129 | 0.4074 | <0.0001 | <0.0001 | 0.0002 | <0.0001 |
The number of oocytes (percentage in parentheses) with bipolar spindles, bipolar spindles with abnormalities and non-bipolar spindles are shown from at least three independent experiments per concentration. P values < 0.05 indicate a significant difference compared to control as determined by Fisher’s exact test.
Table II.
Effects of bisphenol A on chromosome alignment in bovine metaphase II oocytes.
| Ctrl | fM (10−15 M) | pM (10−12 M) | nM (10−9 M) | uM (10−6 M) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 50 | ||
| Aligned | 190 (90.0) | 30 (83.3) | 33 (75.0) | 72 (83.7) | 27 (87.1) | 36 (90.0) | 19 (95.0) | 43 (82.7) | 85 (68.5) | 54 (81.8) | 39 (69.6) |
| Most aligned | 11 (5.2) | 2 (5.6) | 3 (6.8) | 4 (4.7) | 2 (6.5) | 2 (5.0) | 0 (0.0) | 3 (5.8) | 12 (9.7) | 3 (4.5) | 8 (14.3) |
| Dispersed | 10 (4.7) | 4 (11.1) | 8 (18.2) | 10 (11.6) | 2 (6.5) | 2 (5.0) | 1 (5.0) | 6 (11.5) | 27 (21.8) | 9 (13.6) | 9 (16.1) |
| P value | – | 1.0000 | 0.0434 | 0.5953 | 1.0000 | 1.0000 | 1.0000 | 0.8913 | <0.0001 | 0.3102 | 0.0044 |
The number of oocytes (percentage in parentheses) with aligned chromosomes, mostly aligned chromosomes and dispersed chromosomes are shown from at least three independent experiments per concentration. P values < 0.05 indicate a significant difference compared to control as determined by Fisher’s exact test.
Significantly fewer oocytes with bipolar spindles were seen following exposure to BPA at concentrations of 1 fM, 10 fM, 100 fM, 10 pM, 1 nM, 10 nM, 100 nM and 50 μM, compared to the control. There was no effect of BPA on spindle morphology at concentrations of 1 or 100 pM. We observed a particularly distinctive spindle abnormality that occurred more frequently in oocytes exposed to the higher concentrations of BPA (Fig. 1D) when compared to oocytes in the control group, in which the abnormality was not observed. In the 100 nM and 50 μM groups, flattened spindles with broad poles constituted 17.2% (P = 0.0002) and 10.3% (P = 0.0022) of all abnormalities, respectively.
Significantly fewer oocytes had aligned chromosomes following treatment with BPA at 10 fM, 10 nM and 50 μM. There was no effect of BPA on chromosome alignment at any other concentration.
BPS
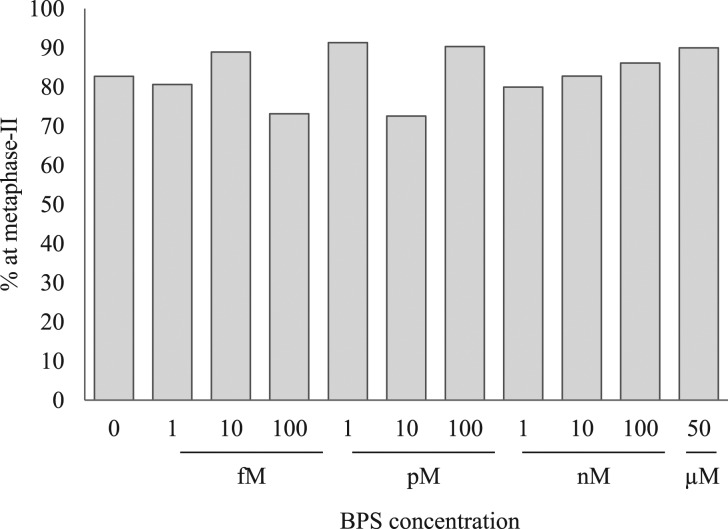
The proportion of oocytes that reached MII after 24 h of IVM in the presence of BPS is depicted in Fig. 3. After 24 h of IVM, 82.7% of oocytes in the control group had reached MII. There was no effect of BPS on the proportion of oocytes that had matured to MII by 24 h.
Figure 3.
Effects of bisphenol S on the percentage of bovine cumulus-enclosed oocytes to reach metaphase II at 24 h. The data are from at least three independent experiments per concentration. The total number of oocytes used for each concentration were: 110 (no bisphenol S (BPS)), 31 (1 fM), 18 (10 fM), 41 (100 fM), 23 (1 pM), 51 (10 pM), 31 (100 pM), 25 (1 nM), 29 (10 nM), 36 (100 nM), 30 (50 μM).
Spindle morphology and chromosome alignment were determined in the MII oocytes exposed to BPS, as depicted in Tables III and IV, respectively. In the control group, 85.7% of metaphase-II oocytes had a bipolar spindle and 93.4% had aligned chromosomes. There were overall effects of BPS on spindle morphology (P < 0.0001) and chromosome alignment (P < 0.0001).
Table III.
Effects of bisphenol S on spindle morphology in bovine metaphase II oocytes.
| Ctrl | fM (10−15 M) | pM (10−12 M) | nM (10−9 M) | uM (10−6 M) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 50 | ||
| Bipolar | 78 (85.7) | 20 (80.0) | 5 (31.3) | 23 (76.7) | 13 (61.9) | 24 (64.9) | 7 (25.0) | 6 (30.0) | 18 (75.0) | 13 (41.9) | 7 (25.9) |
| Bipolar abn | 10 (11.0) | 5 (20.0) | 8 (50.0) | 7 (23.3) | 8 (38.1) | 11 (29.7) | 13 (46.4) | 12 (60.0) | 0 (0.0) | 11 (35.5) | 11 (40.7) |
| Non-bipolar | 3 (3.3) | 0 (0.0) | 3 (18.8) | 0 (0.0) | 0 (0.0) | 2 (5.4) | 8 (28.6) | 2 (10.0) | 6 (25.0) | 7 (22.6) | 9 (33.3) |
| P value | – | 0.3445 | <0.0001 | 0.3213 | 0.0316 | 0.0755 | <0.0001 | <0.0001 | 0.0039 | <0.0001 | <0.0001 |
The number of oocytes (percentage in parentheses) with bipolar spindles, bipolar spindles with abnormalities and non-bipolar spindles are shown from at least three independent experiments per concentration. P values < 0.05 indicate a significant difference compared to control as determined by Fisher’s exact test.
Table IV.
Effects of bisphenol S on chromosome alignment in bovine metaphase II oocytes.
| Ctrl | fM (10−15 M) | pM (10−12 M) | nM (10−9 M) | uM (10−6 M) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 50 | ||
| Aligned | 85 (93.4) | 24 (96.0) | 9 (56.3) | 27 (90.0) | 17 (81.0) | 30 (81.1) | 18 (64.3) | 16 (80.0) | 17 (70.8) | 24 (77.4) | 19 (70.4) |
| Most aligned | 3 (3.3) | 1 (4.0) | 3 (18.8) | 2 (6.7) | 4 (19.0) | 5 (13.5) | 0 (0.0) | 2 (10.0) | 1 (4.2) | 0 (0.0) | 1 (3.7) |
| Dispersed | 3 (3.3) | 0 (0.0) | 4 (25.0) | 1 (3.3) | 0 (0.0) | 2 (5.4) | 10 (35.7) | 2 (10.0) | 6 (25.0) | 7 (22.6) | 7 (25.9) |
| P value | – | 1.0000 | 0.0013 | 1.0000 | 0.1035 | 0.3065 | <0.0001 | 0.4971 | 0.0131 | 0.0136 | 0.0079 |
The number of oocytes (percentage in parentheses) with aligned chromosomes, mostly aligned chromosomes, and dispersed chromosomes are shown from at least three independent experiments per concentration. P values < 0.05 indicate a significant difference compared to control as determined by Fisher’s exact test.
A reduction in the proportion of oocytes with a bipolar spindle was seen when oocytes were treated with 10 fM, 1 pM, 100 pM, 1 nM, 10 nM, 100 nM or 50 μM BPS. There was no effect of 1 fM, 100 fM or 10 pM BPS on spindle morphology. Similar to BPA, we observed that exposure to BPS resulted in a significant increase in flattened spindles with broad poles (Fig. 1D). Here again, spindles with broad poles were absent altogether in the control group, but represented 17% of abnormalities in the 100 nM BPS group (P = 0.0106).
A reduction in the proportion of oocytes with properly aligned chromosomes was seen following incubation with 10 fM, 100 pM, 10 nM, 100 nM and 50 μM BPS. There was no effect of BPS on chromosome alignment at any other concentration.
Discussion
To our knowledge, this is the first study that compares the effects of BPA and BPS on oocytes. This study shows that BPA and BPS both have significantly negative effects on cytoskeletal organization and chromosome alignment in bovine oocytes. We demonstrate that the relationship between BPA or BPS concentration and spindle morphology and chromosome alignment was non-linear. Furthermore, effects of BPA and BPS were detected at concentrations several orders of magnitude lower than what has been reported in human populations.
Exposure of oocytes to concentrations as low as 1 fM BPA and 10 fM BPS resulted in spindle abnormalities. Additionally, effects of BPA and BPS on chromosome alignment were seen at concentrations as low as 10 fM. The mean concentrations of BPA in follicular fluid from women undergoing IVF was 2.4 and 0.34 ng/ml in two different studies (Ikezuki et al., 2002; Wang et al., 2016b). BPS was not measurable in porcine follicular fluid where the assay detection limit was 0.125 ng/ml (Žalmanová et al., 2017), however, concentrations in human follicular fluid are likely to be higher than in the porcine due to bioaccumulation when moving up the food chain (Rhind et al., 2010). Our study adds to others that detected effects of low-dose concentrations of BPA or BPS on the spindle, although we now document effects at even lower doses than previously reported (i.e. in the fM range, as reported herein). For example, the single study to date on BPS in mammalian oocytes (in the pig) reported that exposure of COCs to 3 nM BPS had negative effects on oocyte spindle organization, although there were no effects at the next lower tested dose of 30 pM (Žalmanová et al., 2017). Exposure of partially cumulus-enclosed human oocytes discarded from IVF to 20 ng/ml (87 nM) BPA caused a reduction in oocytes with bipolar spindles and aligned chromosome (Machtinger et al., 2013). Similarly, Ferris et al. (2015) found that exposure of bovine COCs to 30 ng/ml (131 nM) BPA during IVM caused spindle abnormalities and chromosome dispersal in the oocyte but that there was no effect of 15 ng/ml (65 nM) BPA. Although we did not test the exact concentrations used in the Ferris et al. (2015) study, we did test concentrations approximating these doses (i.e. 10 and 100 nM). Our study found that these doses of BPA resulted in significantly increased oocytes with spindle abnormalities at both concentrations, and an increase in oocytes with dispersed chromosomes at 10 nM, but not at the higher 100 nM dose. Although these discrepancies may be explained by differences in the IVM systems used, they may also point to a complex mechanism of action of BPA on spindle morphology and chromosome alignment.
Endocrine disruptors are well established to elicit NMDRs, depending upon the endpoint being measured (Vandenberg, 2013). Given our finding that the dose–response relationship between BPA or BPS concentration and spindle morphology or chromosome alignment was not linear, it seems plausible that a NMDR exists within our data. Several mechanisms that may elicit NMDRs have been proposed. These include the possibility of multiple molecular targets, negative feedback or desensitization of the receptor, toxicity at high doses, and induction of dose-dependent metabolism or detoxification (Lagarde et al., 2015). Additionally, BPA has been reported to have a multitude of actions including acting as an androgen agonist and antagonist, estrogen agonist and antagonist, and thyroid hormone antagonist (Rubin, 2011), which may contribute to the phenomenon of NMDRs. Two previous studies have suggested NMDRs with regards to BPA exposure and morphology of the oocyte spindle. Machtinger et al. (2013) found that low (20 ng/ml, i.e. 87 nM) and high (20 μg/ml, i.e. 87 μM) concentrations of BPA reduced the proportion of human oocytes with a bipolar spindle, whereas a middle concentration (200 ng/ml, i.e. 877 nM) had no effect. In an in vitro follicle culture system, Lenie et al. (2008) reported the highest abnormalities in oocyte meiosis II for whole mouse follicles exposed to lower (3 and 30 nM) when compared to higher (30 μM) doses of BPA. It is relevant to note that not all studies have documented the existence of non-linear effects in oocytes, likely due in part to experimental designs that do not always permit their detection. Distinguishing itself from all prior studies, our experimental design (with a total of 10 doses from pM to μM ranges) permitted identification of NMDR effects of BPA and BPS. Continued attention to NMDRs remains essential when assessing actual risks in mammalian oocytes, as opposed to the focus on traditional toxicological dogma that dose effects are linear (Vandenberg, 2013).
Our finding that BPA had no effect on the proportion of oocytes at MII is in contrast to prior studies which found that BPA arrested oocyte meiotic maturation in humans (Machtinger et al., 2013), mice (Can et al., 2005; Eichenlaub-Ritter et al., 2008; Lenie et al., 2008), porcine (Mlynarčíková et al., 2009; Wang et al., 2016a) and bovine (Ferris et al., 2015). However, the previously reported difference in the bovine (Ferris et al., 2015), although significant, was only a 15% reduction in the proportion of oocytes reaching MII at 30 ng/ml (131 nM), a reduction that was in line with the 10–12% nonsignificant one reported herein for 100 nM and 50 μM (Fig. 2). Differences in maturation effects may reflect species-specific differences in timing of meiotic progression, and therefore sensitivity to endocrine disruptors (e.g. germinal vesicle breakdown occurs within 2 h of IVM in mouse oocytes (Downs et al., 1988), 6 h in bovine oocytes (Sirard et al., 1989) and 22 h in porcine oocytes (Wehrend and Meinecke, 2001)). Additionally, the IVM culture systems used (e.g. media composition, the addition or omission of serum, the extent of cumulus cell support) may influence the sensitivity of oocyte maturation to endocrine disruptors. Another significant source of variability stems from the source material that often varies across studies (including genetically similar lab animals, or genetically diverse slaughterhouse individuals or even developmentally unique oocytes that come from otherwise discarded material during human IVF). Our findings, together with studies by others, attest to the complexity of the effects and interactions, in turn justifying the need to explore reasons for these differences. In vitro studies (such as ours) consistently support the conclusions that endocrine disruptors (such as BPA and now BPS) impair in several mammalian species one essential aspect of oocyte development, namely, normal female meiotic processes.
There are two likely scenarios where COCs may be exposed to endocrine disruptors. First, exposure may occur during ART via plastic consumables (i.e. plastic tubing, culture dishes, commercial media and syringes). Recent studies have demonstrated that commonly used plastic consumables do not leach detectable levels of BPA (Mahalingaiah et al., 2012; Gatimel et al., 2016). However, our findings demonstrate effects at concentrations far lower than the limit of detection in those studies (2–4 nM), substantiating the need to reduce potential sources of exposure where possible. Second, exposure may occur while the COC is contained within the follicle inside the body. The repeatability and relevance of in vitro studies must be examined in vivo to confirm that BPA and BPS elicit the same effects during systemic exposures. Several studies have examined the correlation between urinary BPA concentrations and ART outcomes (Chavarro et al., 2007; Mok-Lin et al., 2010; Bloom et al., 2011; Fujimoto et al., 2011; Ehrlich et al., 2012a, 2012b; Mínguez-Alarcón et al., 2015), providing valuable information regarding the effects of BPA in an infertile population. However, larger population-based studies are needed to ascertain effects of EDCs on female fertility in the general population and to perhaps provoke regulatory changes in terms of their widespread use.
Our findings document the sensitivity of meiotic events, specifically the organization of spindles and arrangement of chromosomes, to both BPA and BPS in bovine oocytes. Depending on the species, the exact types of spindle and chromosome derangements vary (Ikezuki et al., 2002; Machtinger et al., 2013; Ferris et al., 2015; Žalmanová et al., 2017); however, and as reported in the only other study on BPA in bovine oocytes (Ferris et al., 2015), we observed a unique and striking phenotype of flat spindles with remarkably broad ends (Fig. 1D) that may suggest impairments in spindle pole formation and/or maintenance by BPA in the bovine oocyte. Of potential relevance, BPA has been reported to alter the localization of a key microtubule organizing center protein, pericentrin, in mouse oocytes (Can et al., 2005; Eichenlaub-Ritter et al., 2008). Interestingly, we observed similar patterns of abnormalities (in both types and incidences) for either BPA or BPS, pointing to common targets of disruption. Even if a common target (i.e. the oocyte spindle) is herein identified for BPA and BPS, it remains possible that distinct mechanisms of action are at play, as previously shown in other study systems (Boucher et al., 2016; Chen et al., 2016; Maćczak et al., 2016). It is also pertinent to note that in our study, the decreases in the proportion of bipolar spindles in MII oocytes were greater for BPS than for BPA when compared to untreated oocytes (with, e.g. a 64% decrease in bipolar spindles at 50 μM BPS when compared to the control versus a 34% decrease at 50 μM BPA). These findings suggest differences in potencies between the two chemicals, specifically with an increased potency of BPS. In addition, oocytes are exposed in vivo to a complex mixture of chemicals, and thus future studies need to ascertain the actual impact of co-exposures to several bisphenol types.
Given that impairments in spindle organization may result in improper chromosome segregation and subsequent aneuploidy, spindle assembly and conformation is an important contributor to oocyte quality. In humans oocytes, flattened spindle poles and altered pole-to-pole length were associated with misalignment of the chromosomes at the metaphase plate (Bromfield et al., 2009; Coticchio et al., 2013). Additionally, chromosome alignment is also regulated epigenetically through post-translational modifications of histones (Huang et al., 2012; Ma and Schultz, 2016). EDCs, including BPA, can alter epigenetic marks (Xin et al., 2015), and interestingly, exposure of mouse follicles to 3 nM BPA altered the trimethylation pattern on histone 3 at residues associated with chromosome misalignment (Trapphoff et al., 2013). Thus, the reported associations between BPA levels and miscarriage risks in human (Sugiura-Ogasawara et al., 2005; Lathi et al., 2014), may be mediated by multiple mechanisms including spindle conformation and epigenetic changes. Our findings now support the need to examine similar relationships for BPS. It is relevant to note that even though this study showed that BPA and BPS induced statistically significant reductions in the proportions of oocytes with aligned chromosomes, the magnitude of the effects were generally rather small. Further research is needed to determine how these relatively small increases in chromosome misalignment carry over to an in vivo system to affect oocyte competence and embryo development. Furthermore, evaluations also need to extend beyond the spindle apparatus so as to include the complexity of events that are essential during oocyte development for the formation of a gamete, and subsequently an embryo, of high competency and health.
Our study identified the effects of BPA or BPS during a narrow specific period of development (namely, the 24 h window of oocyte maturation), irrespective of potential prior historical exposures in vivo. While other studies need to address the prolonged impact of continuous exposures, there are several notable strengths to our study model. As ovaries were sourced from an abattoir, the cellular material originated from a genetically diverse population of cows with different life and exposure histories; this experimental model represents a relevant situation akin to considering the impact on EDCs in an unselected group of humans. Our IVM conditions also employed carefully selected COCs that were cultured in a defined medium without serum supplementation. The impact of BPA and BPS was also directly comparable since it was tested within the same study using identical experimental design and conditions. The ability for such direct comparison is otherwise not possible across publications due to inherent variations in one or more aspects of the study design.
In summary, we report for the first time on a direct comparison of the effects of BPA and BPS in bovine oocytes during IVM. Both chemicals negatively impacted spindle and chromosome organizations at very low doses and in a non-linear fashion. Similarly to BPA, BPS also poses a threat to oocyte development, and future empirical evidence must continue evaluating the actual safety of the multitude of existing BPA substitutes.
Authors’ roles
K.A.C. was involved in performing the research, analysis and interpretation of the data, and drafting and editing the article. K.M.K. was involved in performing the research, and drafting and editing the article. B.B. and J.M.E. were involved in performing the research and editing the article. C.M.H.C. was involved in the conception of the study and experimental design, performing the research and editing the article.
Funding
The National Institute of Environmental Health Sciences (Grant 1R15ES024520-01) awarded to C.M.H.C.
Conflict of interest
The authors do not have any conflicts of interest to declare.
References
- Adams GP, Singh J, Baerwald AR. Large animal models for the study of ovarian follicular dynamics in women. Theriogenology 2012;78:1733–1748. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Sanfins A, Combelles CMH. Origins and manifestations of oocyte maturation competencies. Reprod Biomed Online 2003;6:410–415. [DOI] [PubMed] [Google Scholar]
- Blondin P, Sirard M-A. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev 1995;41:54–62. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Kim D, vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 2011;96:672–677.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, Gagné R, Rowan-Carroll A, Boudreau A, Yauk CL, Atlas E, Bisphenol A, Bisphenol S. Induce distinct transcriptional profiles in differentiating human primary preadipocytes. PLoS One 2016;11:e0163318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod 2009;24:2114–2123. [DOI] [PubMed] [Google Scholar]
- Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod 2005;11:389–396. [DOI] [PubMed] [Google Scholar]
- Caserta D, Bordi G, Ciardo F, Marci R, Rocca CL, Tait S, Bergamasco B, Stecca L, Mantovani A, Guerranti C et al. The influence of endocrine disruptors in a selected population of infertile women. Gynecol Endocrinol 2013;29:444–447. [DOI] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences. Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables, February 2015) [Internet]. 2015http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf
- Chavarro JE, Mínguez-Alarcón L, Chiu Y-H, Gaskins AJ, Souter I, Williams PL, Calafat AM, Hauser R, EARTH Study Team . Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. J Clin Endocrinol Metab 2016;101:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro J, Rich-Edwards J, Rosner B, Willett W. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol 2007;110:1050. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shu L, Qiu Z, Lee DY, Settle SJ, Que Hee S, Telesca D, Yang X, Allard P. Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLoS Genet 2016;12:e1006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coticchio G, Guglielmo MC, Dal Canto M, Fadini R, Mignini Renzini M, De Ponti E, Brambillasca F, Albertini DF. Mechanistic foundations of the metaphase II spindle of human oocytes matured in vivo and in vitro. Hum Reprod 2013;28:3271–3282. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SAJ, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 1988;245:86–96. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect 2012. a;120:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 2012. b;27:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat Res Toxicol Environ Mutagen 2008;651:82–92. [DOI] [PubMed] [Google Scholar]
- Ferris J, Favetta LA, King WA. Bisphenol A exposure during oocyte maturation in vitro results in spindle abnormalities and chromosome misalignment in Bos taurus. Cytogenet Genome Res 2015;145:50–58. [DOI] [PubMed] [Google Scholar]
- Fujimoto VY, Kim D, Saal FS, vom Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril 2011;95:1816–1819. [DOI] [PubMed] [Google Scholar]
- Gatimel N, Lacroix MZ, Chanthavisouk S, Picard-Hagen N, Gayrard V, Parinaud J, Léandri RD. Bisphenol A in culture media and plastic consumables used for ART. Hum Reprod 2016;31:1436–1444. [DOI] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon J-P, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet A-M, Pussemier L, Scippo M-L et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 2012;50:3725–3740. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- Huang J, Li T, Ding C-H, Brosens J, Zhou C-Q, Wang H-H, Xu Y-W. Insufficient histone-3 lysine-9 deacetylation in human oocytes matured in vitro is associated with aberrant meiosis. Fertil Steril 2012;97:178–184.e3. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 2003;13:546–553. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002;17:2839–2841. [DOI] [PubMed] [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol 2013;47:8793–8800. [DOI] [PubMed] [Google Scholar]
- Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril 2015;103:317–322. [DOI] [PubMed] [Google Scholar]
- Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health 2015;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathi RB, Liebert CA, Brookfield KF, Taylor JA, Saal FS, vom Fujimoto VY, Baker VL. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril 2014;102:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res Toxicol Environ Mutagen 2008;651:71–81. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 2013;61:4655–4662. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol 2014;67:50–59. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon H-B, Nakata H, Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 2012. a;46:6860–6866. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon H-B, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 2012. b;46:9138–9145. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Kannan K. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environ Sci Technol 2012. c;46:6515–6522. [DOI] [PubMed] [Google Scholar]
- Ma P, Schultz RM. HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: specificity versus compensation. Cell Death Differ 2016;23:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maćczak A, Cyrkler M, Bukowska B, Michałowicz J. Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J Hazard Mater 2016;307:328–335. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Combelles CMH, Missmer SA, Correia KF, Williams P, Hauser R, Racowsky C. Bisphenol-A and human oocyte maturation in vitro. Hum Reprod 2013;28:2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R, Orvieto R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: review of animal and human data. Reprod Biomed Online 2014;29:404–410. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Hauser R, Patterson DG Jr., Woudneh M, Racowsky C. Bisphenol A is not detectable in media or selected contact materials used in IVF. Reprod Biomed Online 2012;25:608–611. [DOI] [PubMed] [Google Scholar]
- Mihm M, Evans A. Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod Domest Anim 2008;43:48–56. [DOI] [PubMed] [Google Scholar]
- Mlynarčíková A, Nagyová E, Ficková M, Scsuková S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte–cumulus complexes. Toxicol In Vitro 2009;23:371–377. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010;33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Gaskins AJ, Chiu Y-H, Williams PL, Ehrlich S, Chavarro JE, Petrozza JC, Ford JB, Calafat AM, Hauser R. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod 2015;30:2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi M, Wong MYL, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol 2014;148:195–203. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Evans NP, Bellingham M, Sharpe RM, Cotinot C, Mandon-Pepin B, Loup B, Sinclair KD, Lea RG, Pocar P et al. Effects of environmental pollutants on the reproduction and welfare of ruminants. Animal 2010;4:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 2015;123:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 2011;127:27–34. [DOI] [PubMed] [Google Scholar]
- Santos RR, Schoevers EJ, Roelen BA. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol 2014;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard MA, Florman HM, Leibfried-Rutledge ML, Barnes FL, Sims ML, First NL. Timing of nuclear progression and protein synthesis necessary for meiotic maturation of bovine oocytes. Biol Reprod 1989;40:1257–1263. [DOI] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod 2005;20:2325–2329. [DOI] [PubMed] [Google Scholar]
- Tervit HR, Whittingham DG, Rowson LEA. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil 1972;30:493–497. [DOI] [PubMed] [Google Scholar]
- Trapphoff T, Heiligentag M, El Hajj N, Haaf T, Eichenlaub-Ritter U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil Steril 2013;100:1758–1767.e1. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response 2013;12:259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010;118:1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, Saal FS, vom Welshons WV et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012;33:378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS et al. Low dose effects of bisphenol A. Endocr Disruptors 2013;1:e26490. [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–177. [DOI] [PubMed] [Google Scholar]
- Wang T, Han J, Duan X, Xiong B, Cui X-S, Kim N-H, Liu H-L, Sun S-C, Wang T, Han J et al. The toxic effects and possible mechanisms of bisphenol A on oocyte maturation of porcine in vitro. Oncotarget 2016. a;7:32554–32565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu Q, Dang X, He Y, Li X, Sun Y. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecol Endocrinol 2016. b;0:1–5. [DOI] [PubMed] [Google Scholar]
- Wehrend A, Meinecke B. Kinetics of meiotic progression, M-phase promoting factor (MPF) and mitogen-activated protein kinase (MAP kinase) activities during in vitro maturation of porcine and bovine oocytes: species specific differences in the length of the meiotic stages. Anim Reprod Sci 2001;66:175–184. [DOI] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol 2015;43:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol 2015;49:11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Alomirah H, Cho H-S, Li Y-F, Liao C, Minh TB, Mohd MA, Nakata H, Ren N, Kannan K. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol 2011;45:7044–7050. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B 2014;944:152–156. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 2012;153:4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žalmanová T, Hošková K, Nevoral J, Adámková K, Kott T, Šulc M, Kotíková Z, Prokešová Š, Jílek F, Králíčková M et al. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci Rep 2017;7:485. [DOI] [PMC free article] [PubMed] [Google Scholar]