Abstract
Efforts to conserve biodiversity comprise a patchwork of international goals, national-level plans, and local interventions that, overall, are failing. We discuss the potential utility of applying the mitigation hierarchy, widely used during economic development activities, to all negative human impacts on biodiversity. Evaluating all biodiversity losses and gains through the mitigation hierarchy could help prioritize consideration of conservation goals and drive the empirical evaluation of conservation investments through the explicit consideration of counterfactual trends and ecosystem dynamics across scales. We explore the challenges in using this framework to achieve global conservation goals, including operationalization and monitoring and compliance, and we discuss solutions and research priorities. The mitigation hierarchy's conceptual power and ability to clarify thinking could provide the step change needed to integrate the multiple elements of conservation goals and interventions in order to achieve successful biodiversity outcomes.
Keywords: adequacy, biodiversity, development, no net loss, sustainability
Humans’ growing demand for resources is resulting in the rapid erosion of natural habitats (Watson et al. 2016b). This is leading to an irreplaceable loss of biodiversity (Hoffmann et al. 2010) that can compromise the healthy functioning of ecosystems (Hooper et al. 2012). The primary causes of biodiversity loss include overexploitation of species, habitat modification, invasive alien species and disease, pollution, and climate change (Maxwell et al. 2016). However, although we have an increasing understanding of the causes of biodiversity loss, the main drivers can be obscured, in part, because existing frameworks for conservation planning, implementation, and evaluation do not consider conservation efforts to tackle drivers of biodiversity loss as a cohesive whole. The current patchwork of international goals and targets (e.g., the UN Convention on Biological Diversity [CBD] Aichi Targets, and Sustainable Development Goals), national plans, and local interventions can result in the gaps and weaknesses of conservation efforts being difficult to identify or articulate (Rands et al. 2010). For example, the global terrestrial protected area (PA) network now covers 14.8% of all terrestrial surfaces and 5.1% of the global ocean (UNEP–WCMC and IUCN 2016), but many of these PAs occur in residual areas, avoiding locations with high value for natural-resource extraction (Devillers et al. 2014, Venter et al. 2017). The results are a significant shortfall in protection of nature across ecoregions and important sites for biodiversity remaining unprotected (Butchart et al. 2015, Dinerstein et al. 2017).
Biodiversity loss, much like climate change, is an environmental crisis that requires a coordinated international effort if it is to be managed effectively. The 2015 Paris climate agreement specifies a clear goal to limit global warming by 2 degrees Celsius above preindustrial levels (UNFCCC 2015), and the recent publication of a roadmap for rapid decarbonization offers guidance on actions required at the national level to effectively limit carbon emissions in order to meet the goal (Rockström et al. 2017). A call has recently been made for a similar roadmap for global biodiversity conservation to guide the necessary steps to achieve goals and targets for stopping the biodiversity crisis (Watson and Venter 2017). This requires an integrated global framework, capable of being implemented at national and project levels, which would enable the quantification and subsequent reduction of humanity's impact on biodiversity. To date, no one has tried to conceptualize all human biodiversity impacts and conservation efforts within such a framework. The benefits of such an approach would be to unite all aspects of conservation under a standardized paradigm with a broad biodiversity conservation goal, supporting multiscale, evidence-based decision-making. Exploring the potential benefits of such a framework is particularly timely given that the CBD’s biodiversity strategy will be renegotiated in 2020 (CBD 2010).
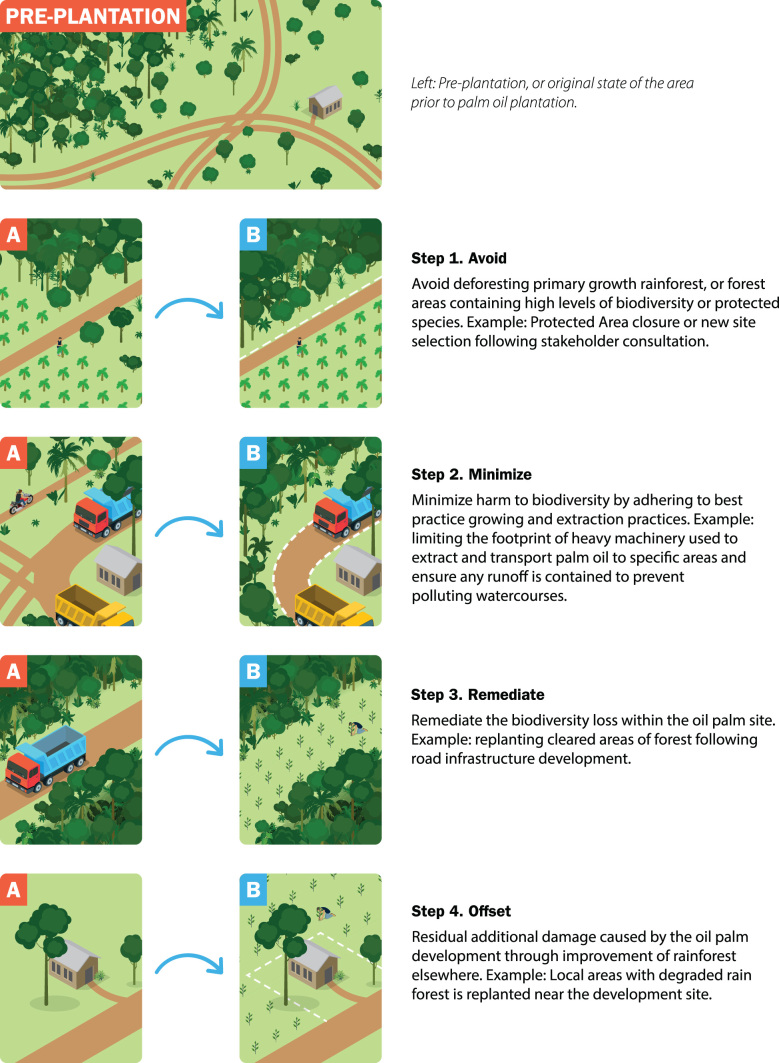
Industrial sectors such as mining, energy, and manufacturing are increasingly using a framework known as the mitigation hierarchy to guide their activities toward limiting negative impacts on biodiversity (BBOP 2012, IFC 2012). A goal either of no net loss (NNL) or net gain of biodiversity is typically set (also referred to as net neutral and net positive goals, respectively), relative to a predetermined baseline (BBOP 2012, Maron et al. 2018). The process is implemented through national planning processes and negotiations between government agencies, conservation actors, and developers, with elements of the process often formalized within an Environmental and Social Impact Assessment (ESIA). The mitigation hierarchy comprises four broad actions step that are designed to be implemented sequentially: (1) avoid, (2) minimize, (3) remediate, and (4) offset (figure 1). The first step involves avoiding impacts on biodiversity, such as screening potential risks prior to project design and selecting an alternate development site (Phalan et al. 2017). The second step of the hierarchy requires that before and during development, impacts are minimized, such as by using more environmentally friendly construction methods. The third step requires that biodiversity loss is then remediated within the footprint of the development, which could entail actions such as reseeding affected land or developing a breeding program for affected species during and after project completion. The fourth and final step requires that any residual impacts not captured by the first three steps of the hierarchy are offset elsewhere, such as through wetland restoration or the removal of invasives from ecologically important areas (Gardner et al. 2013). The four steps of the mitigation hierarchy represent broad categories of biodiversity impact reduction and compensation, meaning that most conservation actions can be categorized within these steps (table 1).
Figure 1.
An example of the mitigation hierarchy applied to the oil palm industry in order to achieve no net loss of biodiversity for the negative impact on biodiversity (deforesting rainforest) as a result of planting oil palm monocultures, in this case African oil palm (Elaeis guineensis). The images marked with an (a) represent the types of negative impacts from planting oil palm monocultures, and the corresponding images marked (b) represent ways to address these impacts by undertaking the four steps of the mitigation hierarchy. Steps 1 to 3 occur at the site of negative impact on biodiversity, whereas step 4 occurs away from the impact site, addressing residual adverse impacts.
Table 1.
Examples of biodiversity conservation tools and actions categorized into each of the four steps of the mitigation hierarchy.
| Mitigation-hierarchy step | Examples of existing conservation tools and approaches |
|---|---|
| Avoid | Protected areas†; Alliance for Zero Extinction sites; Key Biodiversity Areas; no development in Vulnerable Marine Ecosystems (FAO vulnerable ecosystems) or critical habitat (International Finance Corporation PS6+); no damage to any listed threatened species or ecosystems (IUCN Red List of threatened species and ecosystems; national conservation list species); no damage to intact habitat, UNESCO World Heritage Sites, or Wilderness Areas. |
| Minimize | Sustainable use; agrienvironment schemes; shift from passive nonselective gear to actively targeted gear in fisheries; multiuse protected areas; payment for ecosystem services; demand reduction; certification and ecolabeling; economic incentives (market prices, taxes, subsidies, and other signals); green infrastructure; corporate environmental strategies and operations; maintenance of ecosystem resilience. |
| Remediate | Rewilding†; restoration†; natural flooding of wetlands†; artificial habitat creation†; deextinction. |
| Offset | Degraded ecosystem restoration away from impact site†; averted risk; reseeding or respawning†; captive breeding; invasive removal; species creation. |
Conservation tool or action that can shift between steps of the mitigation hierarchy depending on (a) whether the biodiversity baseline is set at a present-day or historic point in time and (b) what national and regional legislation is in place to enforce the action taken.
As it stands, the mitigation hierarchy offers transparency between stakeholders, with flexibility to address a variety of anthropogenic impacts on biodiversity, across different sectors and scales. Many regulatory and financial instruments are now in place that aim to balance biodiversity conservation with (sustainable) economic development by requiring the application of the mitigation hierarchy. For example, 69 countries have NNL policies in place or under development (Maron et al. 2016). However, taken overall, these commitments operate in a system that has allowed significant loss of biodiversity, even when development was legally compliant (BBOP 2012, Watson et al. 2016b).
The mitigation hierarchy is not widely applied to the most prevalent impacts on biodiversity that result from the direct removal of biological materials in sectors such as agriculture, fisheries, forestry, and wildlife trade (Rainey et al. 2015, Maxwell et al. 2016). Various frameworks exist to manage the impacts that result from extracting biological resources and promote sustainable use (e.g., forest certification schemes, Lattimore et al. 2013; ecosystem-based fisheries management, Pitcher et al. 2009; and agrienvironment schemes, Pretty 2008). However, these frameworks often fail to account for all the negative biodiversity impacts caused by extracting target resources. For example, in forestry, road building to access previously inaccessible trees opens up remote wilderness areas to the secondary pressures of hunting, human colonization, invasive species, and fire (Bennett 2004). Major certification schemes such as the Forest Stewardship Council have also been criticized for failing to explicitly account for incidental biodiversity impacts, such as bushmeat harvesting (FSC 2015). Applying a standardized framework such as the mitigation hierarchy to all human impact would allow for seemingly disparate impacts on biodiversity to be categorized and accounted for between sectors, scales, and nations. For example, the direct and immediate biodiversity impacts of clearing species-rich forest for an oil palm plantation, the longer-term and potentially more diffuse indirect biodiversity impacts that result from new forestry infrastructure (e.g., illegal hunting and informal clearance for settlement), and the transboundary effects of air pollution from clearance fires could be accounted for within the same framework, whereas apparently disparate mitigation efforts could be linked (figure 1).
The lack of coherence between sectors of conservation associated with sustainable use (e.g., certification), minimizing the impact of development (e.g., NNL), and direct protection (e.g., protected areas) is currently limiting the opportunities for strategic achievement of biodiversity conservation at a global scale. A far more structured approach to planning, implementing, and evaluating actions to achieve global conservation goals is needed if natural-resource extraction, industrial development, and nature conservation are to become better balanced. Here, we first describe the critical elements of the mitigation-hierarchy approach and then outline a conceptual application of the framework to integrate human biodiversity impacts with nature conservation efforts as a novel first step toward achieving a more strategic approach to achieving global biodiversity conservation goals. We then clarify how achieving a conservation goal through the mitigation hierarchy might be structured at the planetary scale by outlining four key factors for application.
Critical elements of the mitigation hierarchy approach
Developers adhering to the mitigation hierarchy are first required to set a biodiversity goal (BBOP 2012). This typically takes the form of NNL or net gain of biodiversity, although a goal such as improving trends in biodiversity could also be used (e.g., as in national species recovery plans). Next, quantitative targets and associated biodiversity metrics or indicators must be defined in order to measure achievement of the goal (BBOP 2012, Butchart et al. 2015). Undertaking this process means that assumptions surrounding what achieving the biodiversity goal would look like and the calculations required to verify it are made explicit.
The consideration of counterfactual scenarios (i.e., what would have happened in the absence of a development and its associated mitigation measure[s]) is key to evaluating whether the biodiversity goal has been met (table 2; Bull et al. 2014, Maron et al. 2016). The practice of empirically evaluating whether a specific intervention works better than alternate interventions or no action at all remains woefully lacking in conservation science (Ferraro and Pattanayak 2006), and a major benefit of the mitigation hierarchy is that it requires this critical thinking. This process requires the involvement of all stakeholders: regulators, industry, and conservationists. The wider use of the mitigation hierarchy would therefore precipitate a shift toward the routine empirical evaluation of biodiversity conservation investments.
Table 2.
Approaches to addressing the theoretical and practical challenges of applying the mitigation hierarchy, with particular focus on the offsetting step, based on practical experience to date (as articulated in, e.g., Bull et al. 2013, BBOP 2012).
| Challenge | Description | Current project-level best practice recommendations | Conceptual examples of global-level best practice |
|---|---|---|---|
| Additionality | Whether an intervention has an effect, when the intervention is compared to a baseline | Only biodiversity benefits that are additional to a baseline scenario count as valid offsets. | Nations required to account for offset-funded biodiversity protection (alongside associated biodiversity losses that triggered offset) separately from biodiversity protection going toward existing global conservation commitments (e.g., CBD Aichi target 11; Maron et al. 2015b). |
| Compliance and monitoring | Noncompliance with mitigation hierarchy; insufficient compensation resulting in lack of incentive; legislative changes during development | Ensure relevant authorities follow up with monitoring to ensure compliance. | No net loss impact to biodiversity targets are made legally binding where possible (e.g., for all UN fisheries through UNCLOS, requiring stipulation of defined baselines, indicators, and best-practices implementation); global-level monitoring and evaluation program created; requirements for national-level reporting to international body (e.g., CBD). |
| Biodiversity indicators | Unitary measures of biodiversity lost, gained, or exchanged | Use multiple or compound indicators; incorporate measure of ecological function as well as biodiversity. | Use established mechanisms to develop and test indicators (e.g., the Biodiversity Indicators Partnership, which evaluates the CBD Aichi targets and biodiversity SDGs): www.bipindicators.net. |
| Equivalency | Demonstrating equivalence between biodiversity losses and gains | Encourage “in-kind” or like-for-like trades, and prevent “out-of-kind” trading unless “trading up” from losses that have little or no conservation value; ensure that there are requirements for spatial constraints within which biodiversity offsets will and will not be considered. | An international governing body, such as the United Nations, stipulates that biodiversity offsets are restricted to “in-kind” trades implementable within a predetermined radius of the impact site, based on ecologically meaningful scales for the biodiversity concerned. |
| Least cost | Guiding actions economically by costs so that efficiency dictates that each hierarchical step be undertaken to the point at which marginal costs are equalized | Ensure offset cost is set at a sufficient level to incentivize adherence to avoidance and minimization steps higher up in the mitigation hierarchy. | Evidence that alternate scenarios representing actions higher up the mitigation hierarchy have been investigated, and their ruling out is justified prior to any offsets commencing. Require this to be recorded in Environmental and Social Impact Assessments and submitted by all signatory nations to the international governing body. Free public access to reports is granted. |
| Longevity | The length that an offset scheme should endure | Offsets should last the length of the negative impacts at a minimum; offsets should be adaptively managed in the light of ongoing external change. | Nations are required to adopt the stipulated time period for agreed-on global biodiversity goals and to enforce regulation that ensures the longevity of biodiversity offsets. Failure to successfully manage offsets for their necessary lifetime would result in censure. |
| Multipliers | A factor that increases the amount of biodiversity gains required by an offset | Calculation of multiplier is based on various factors (e.g., discount rate for future biodiversity gains and uncertainty in definition and measurement of biodiversity). | Legal requirements are put in place to ensure that appropriate biodiversity offset calculators are used for all offset projects, ensuring a minimum biodiversity offset multiplier accounts for the time discounting, additionality, and permanence of the project (e.g., Laitila et al. 2014). |
| Reversibility | Defining a development's reversibility | Ensure all biodiversity losses are reversible; otherwise, categorize the affected biodiversity as a no go. | Nations’ goals for preventing species extinction and ecosystem collapse would be required to map onto international goals, with international reporting requirements concerning compliance and monitoring. |
| Substitutability | The degree to which the “value” of a certain biodiversity type influences demand for one or more other biodiversity types | Base the value of biodiversity types on national legislation and societal value. | Clarify and justify when one ecosystem, species, or population is seen as equivalent to another and therefore tradable. |
| Thresholds | Areas or components of biodiversity that should not be compensated for because they are too important | Define explicit thresholds for biodiversity losses and gains that cannot be offset. | Internationally recognized no-go zones for biodiversity offsets such as the Protected Area network, Key Biodiversity Areas, crisis ecoregions, and the Wildlife Conservation Society's Last of the Wild places; consideration is also given to aspects of human development, which should not be traded off because of their contribution to the future of humanity, such as adequate safe water for all. |
| Time lag | Deciding whether to allow a temporal gap between development and offset gains | Incorporate a preoffset step in the form of mitigation banking. | A preimpact conservation gain requirement could be built into international funding for economic development. |
Arguably the most important step of the mitigation hierarchy is its first step, impact avoidance. This requires developers to predict and prevent negative impacts on biodiversity prior to any development actions taking place (BBOP 2012). The conservation benefits of avoiding impacts are likely to outweigh taking more uncertain remediation and offsetting measures once damage has occurred (Watson et al. 2016b, Lindenmayer et al. 2017). Actions that drive adherence to the first step of the mitigation hierarchy include following environmental regulations designed to protect biodiversity (e.g., through national planning processes and negotiations between stakeholders), giving clear guidance on critical biodiversity areas (e.g., Key Biodiversity Areas), and making political decisions to set aside areas of high societal value (e.g., World Heritage Sites). Failure to comply with the avoidance step of the mitigation hierarchy may eventuate from a lack of political or regulatory enforcement; poor process; or lack of capacity and technical knowledge of regulators, developers, and consultants (Phalan et al. 2017).
The minimization step is central to current project-level conservation activities, including sustainable use, agrienvironment schemes, alternative livelihoods, and payments for ecosystem services. At the national level, many states have adapted ESIA legislation and guidance, which feeds down into the incorporation of biodiversity concerns into economic activities at the project level (Bull et al. 2017), whereas rewilding, restoration projects, and the natural flooding of wetlands align with remediation measures for affected biodiversity. Remediation equally applies to reestablishing depleted resource stocks (table 1).
Many of the key issues regarding quantifying and compensating biodiversity, which emerge when the mitigation hierarchy is applied, have parallels with the wider challenge of defining and measuring sustainability (e.g., Heal's 2012 review on managing natural capital and the interactions between human economic activity and the environment). The most controversial element of the mitigation hierarchy is its last step, offsetting, because it is here that these challenges come into sharp relief; they can be sidestepped to some extent in the first three steps of the hierarchy. Offsetting happens when significant residual impacts from a development remain after application of the first three levels of the mitigation hierarchy (BBOP 2012). It is controversial because it requires the acceptance of a development that harms biodiversity on the assumption that this harm can be accurately quantified and balanced by benefits elsewhere (Maron et al. 2016).
The theoretical and practical challenges of achieving NNL of biodiversity from development are increasingly well described and are widely reported (table 2). For example, a nest box program in Australia intended to offset the clearing of hollow-bearing trees did not achieve the intended biodiversity outcomes for three threatened vertebrates reliant on the trees because of (a) a failure to consider equivalency (the nest boxes failed to provide habitat for the target species), (b) incorrect use of multipliers (the 1:1 offset ratio did not account for the risk of offset failure), and (c) a lack of compliance and monitoring to evaluate the true effectiveness (Lindenmayer et al. 2017). As we illustrate here, many of the issues with offsets result from poor operationalization, monitoring, and compliance rather than being inherent to the concept itself (Quétier et al. 2014).
Expanding the mitigation hierarchy to encompass all human impact on biodiversity
The direct extraction of biological resources is the dominant driver of current species loss (Maxwell et al. 2016), but the practical application of the mitigation hierarchy to the biological resource use sectors has received little attention (but see Aiama et al. 2015). In fisheries management, all four steps of the mitigation hierarchy are discussed (Wilcox and Donlan 2009, Gjertsen et al. 2014), but they have yet to be formalized into a conservation framework to manage fishing impacts. Using a mitigation hierarchy, NNL of biodiversity (or a similar goal such as population recovery) could be extended to managing the incidental impacts on biodiversity caused by extracting target resources (e.g., fisheries bycatch management; table 3; Milner-Gulland et al. 2018). A NNL goal could then be incorporated into international natural-resource management agreements such as the UN Convention on the Law of the Sea (UNCLOS) conservation and sustainable use of marine biological diversity instrument (United Nations 2015a).
Table 3.
Applying the mitigation hierarchy to the examples of housing development and commercial fisheries bycatch, to demonstrate its applicability at multiple scales and for different sectors.
| Harmful event: Housing development leading to loss of biodiversity and habitat | Harmful event: Pacific leatherback sea turtles bycaught in commercial fisheries | |||||
|---|---|---|---|---|---|---|
| Mitigation hierarchy step | Local (one house built) | National (state housing plan implemented) | Global (human urbanization footprint increasing) | Local (one turtle killed by one vessel) | National (local extinctions or population reduction in a nation's Exclusive Economic Zones) | Global (species sent to extinction) |
| Avoid | Restriction of building permissions to given areas only | Strategic plan identifies areas set aside for housing and areas for conservation | International protected-area commitments | Enforcement of small scale time or area closures | Nationally legislated caps on turtle takes for countries operating fisheries in areas frequented by turtles | Multinational no-take fishing zones tracking leatherback turtle migration |
| Minimize | Drainage areas, fence to prevent overflow of extracted dirt | Regulatory requirements for house building | International lenders require all new housing to be ecologically friendly | Gear modification resulting in increased likelihood of turtle survival | Fleetwide gear changes (e.g., implementing circle hooks, branch lines long enough to allow turtles breathing at the surface, effort restrictions) | Demand reduction through international education campaigns targeting consumers of Pacific-sourced tuna and swordfish |
| Remediate | Restoration of land along digger tracks | Land area restoration plans at the state scale | International fund for urban greening projects | Better turtle-handling and gear-removal practices resulting in higher survival rates for postcapture release | Increased marine protected area monitoring and enforcement resulting in fewer illegal fishing events, allowing turtle population to recover | Protection and reallocation of nests to increase hatching success at known Pacific leatherback turtle nesting sites throughout range |
| Offset | Protect an area of existing wetland or create a new wetland nearby | State supports protection of similar natural areas in other parts of the country | International fund for restoration of habitat types preferentially affected by urbanization | Protection of nesting turtles and their eggs at local nesting beaches and restoration of degraded nesting sites | Protection of nesting turtles and their eggs at nesting beaches within another area of the country | Protection of Atlantic leatherback sea turtles in an effort to ensure they don’t meet the same fate |
Particularly crucial to an extension of the mitigation hierarchy to global conservation is consideration of the scale at which goals and targets are evaluated (table 3). Although achieving NNL of biodiversity is often a goal for individual projects, some have suggested that net human impact on biodiversity should be evaluated at landscape or national scales considering the aggregate impact of individual developments and their associated mitigation programs (Kiesecker et al. 2010, Bull et al. 2014). Bull and Maron (2016) also considered the conceptual global application of the NNL principle to changes in species richness worldwide. A strategic approach to NNL could evaluate biodiversity gain and loss at ecologically and institutionally meaningful scales (ranging from local to global), enabling conservation efforts of different types and at a range of scales to be integrated and categorized within the hierarchy's four steps: avoid, minimize, remediate, and offset. A multiscale approach to NNL, not just a project-level one, would mean that wider goals are not contradicted by piecemeal approaches to NNL at the project level (Maron et al. 2018). Table 3 shows how the application of the mitigation hierarchy would change depending on the scale under consideration. By considering local, regional, and national actions under the same framework, we could begin to piece together a global picture of action toward an overarching net goal, offering a coherent framing for conservation efforts.
Key factors for successful application of the global mitigation hierarchy
In the next section, in order to conceptualize what biodiversity attributes could and could not be included in each step of the global mitigation hierarchy, we discuss four key factors for application.
Goals, targets and indicators
At each scale of application (global, regional, and local), there would be a need to set NNL goals (or similar goals that account for losses and gains) that focus on particular facets of biodiversity (figure 2). These could include the elements of biodiversity embodied in the essential biological variables (Pereira et al. 2013, Gonçalves et al. 2015), which are recommended to guide the setting of biodiversity goals and indicators in policymaking (Pereira et al. 2013). Ideally, these goals would be set to reflect existing aspirations for sustainable development (e.g., the sustainable development goals, SDGs; United Nations 2015b), international conservation (e.g., the CBD Aichi targets; United Nations 1992), and national legislation relating to environmental protection.
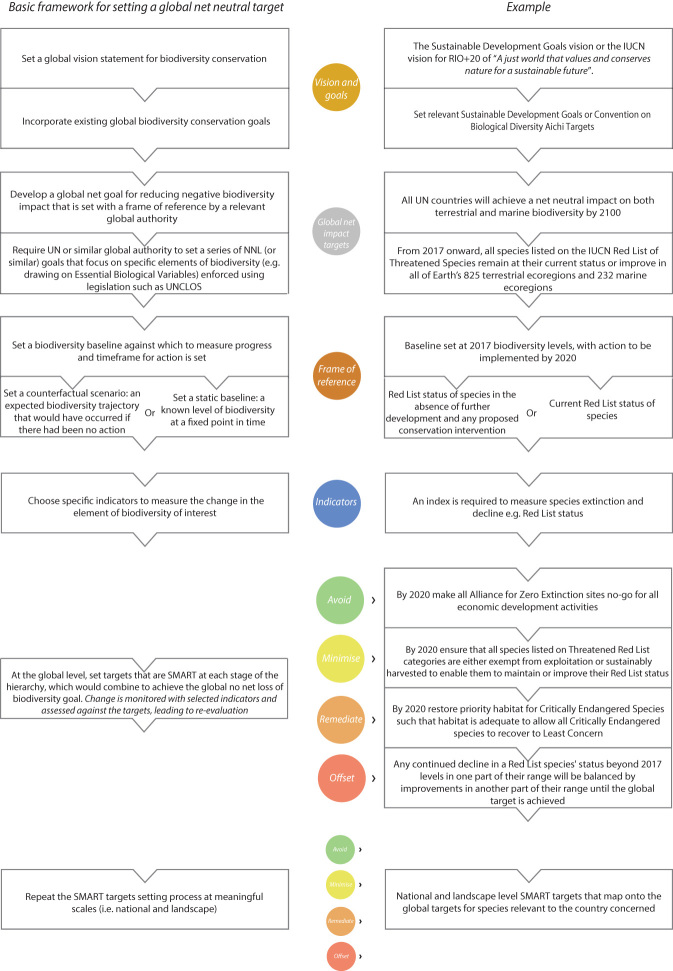
Figure 2.
The key steps required to implement a global no net loss of biodiversity target through the mitigation hierarchy, with associated goals and targets. The left column shows the basic framework for setting a global no net loss target. The right column gives a specific example focusing on the International Union for Conservation of Nature's Red List. This example shows one particular set of approaches among many that would be needed to achieve global no net loss human impact on biodiversity.
To successfully achieve biodiversity goals, there is a need to set targets that specify a quantitative amount of change required for success. SMART (specific, measurable, ambitious, realistic, and time-bound) targets are preferred; a hypothetical example of a SMART target would be that all UN countries’ fishing fleets will achieve a NNL impact on biodiversity by 2050, set against the frame of reference of the Food and Agriculture Organization's (FAO’s) 1955 global fish stock assessments and benthic biodiversity assessments from the International Union for Conservation of Nature (IUCN; table 2). Currently, many targets suffer from ambiguity, complexity, and redundancy; lessons need to be learned from failings with the CBD Aichi targets, more than two-thirds of which were found to lack a quantifiable component (Butchart et al. 2016).
Next, relevant biodiversity indicators can be developed to measure the desired change in biodiversity in order to achieve specific goals and targets at varying scales (e.g., those developed by the Biodiversity Indicators Partnership; table 2; Butchart et al. 2007). These need to be context dependent, with best practices suggesting that they should (a) be sensitive to and respond predictably to human impact, (b) be feasible to monitor, (c) be informative at different spatial and temporal resolutions, and (d) be practical in terms of monitoring costs and data availability (Jones et al. 2011, Gonçalves et al. 2015). There are added levels of complexity surrounding indicator development that are not outlined here; for a more detailed explanation, see Jones and colleagues (2011).
The clear articulation of desirable biodiversity outcomes then drives relevant conservation actions through different levels of the mitigation hierarchy. For example, an ecosystem-focused target to drive action in the avoid part of the mitigation hierarchy might be the following: By 2020, 25% of areas currently in a predominately natural state in each of Earth's 825 terrestrial ecoregions (Olson et al. 2001) and 232 marine ecoregions (Spalding et al. 2007) will have full no-take protected area status and nondeclining biodiversity value relative to a 2017 baseline (figure 2). A species-focused target to drive action in the minimize part of the hierarchy could specify that by 2020, all fish stocks are managed according to the FAO Code of Conduct for Responsible Fisheries (FAO 1995) and all forests according to the Resolution on Sustainable Forest Management (United Nations 2008).
In this way, a global mitigation-hierarchy framework could help achieve a desired future state of biodiversity by setting multiple goals and targets at meaningful scales, measured through relevant biodiversity indicators. We present one example of goal and target setting in figure 2.
Frames of reference and counterfactuals
Assessing achievement of NNL requires specification of a frame of reference containing a biodiversity baseline or counterfactual scenario (table 2; Bull et al. 2015, Maron et al. 2018). This could take the form of a static baseline (i.e., biodiversity levels at a fixed point in time), such as the current state of biodiversity (i.e., 2017 levels) as is expressed using the chosen indicator set. Alternatively, a historic level of biodiversity could be set as a static baseline, such as species status in the year 1990 (to be compatible with the baselines used in the UN Framework Convention on Climate Change). Or a counterfactual scenario could be chosen, such as the expected state of nature in the absence of any further development or conservation interventions (figure 2).
A frame of reference is key to incorporating biological resource extraction into the framework. For example, incorporating a NNL of biodiversity goal into the management of a natural resource, such as fish stocks, does not require compensation for losses related to this harvest if the baseline is current biodiversity status and the stock in question is sustainably harvested and nondeclining. By contrast, taking a preexploitation baseline, or evaluating against a reduced human impact counterfactual, could require compensation for lost biodiversity even if harvesting is sustainable. It is also important to note that harvesting can be sustainable under a target-species-focused goal and still have adverse effects on nontarget biodiversity, which would need to be compensated under another part of the overall framework (e.g., to address the negative impact of leatherback turtle bycatch from long-line fishing; table 3).
When setting a frame of reference, there is also an essential need to clearly specify which elements of biodiversity are or are not appropriate to address at lower stages of the mitigation hierarchy (e.g., through offsetting). Some elements may be deemed too valuable to incur any human impact, and therefore, impact must be avoided (table 2; Bull et al. 2013). There are many situations in which offsets are unacceptable, regardless of whether large multipliers are applied (e.g., more than 10 units of habitat supplied elsewhere for every 1 unit destroyed; Moilanen et al. 2009). Irreplaceability is one criterion for whether biodiversity damage should be allowed and then offset. This may relate to a critically endangered or endemic species, a keystone species, an iconic area of wilderness, or biodiversity characterized by long restoration times, such as deep-sea coral systems, hydrothermal vents, and old-growth forest. For example, a recent study demonstrated that if delays between a development and the compensation of the resultant biodiversity losses through restoration are 55 or more years, then an offset is unlikely to be successful at achieving a NNL effect on biodiversity (Gibbons et al. 2016). Improving international recommendations for no-go areas to protect biodiversity (e.g., that all categories of protected areas and World Heritage Sites be considered no-go areas for large-scale development; IUCN 2016), backed up by national legislation, could help address this issue for a global mitigation hierarchy and provide a strong and agreed basis for the avoid step of the hierarchy (Phalan et al 2017).
The lack of counterfactuals remains a widespread problem in practice, both for the mitigation hierarchy as currently applied to development (e.g., Maron et al. 2015a) and in the wider conservation and environmental policy literature (e.g., Ferraro 2009). It is only just starting to be applied to measuring the impact of traditional conservation interventions (e.g., Hoffmann et al. 2015). Failure to properly consider counterfactual scenarios promotes the idea that loss in one place can be offset by “protection” in another, even if that protection involves no more than relabeling already-secure places (Maron et al. 2015b). A global mitigation hierarchy with a clear set of goals and targets would enable integration of the different commitments and legislative requirements already in place, facilitating explicit consideration of how commitments at different scales complement or conflict with each other. Transparent consideration of baselines and of where each biodiversity conservation action sits within the levels of the hierarchy would reduce the risk of indirect leakage of environmentally damaging activity to other areas following locally avoided losses (Moilanen and Laitila 2016). It would also mitigate against perverse outcomes such as governments using industry money generated by offsets to achieve existing national biodiversity commitments (Maron et al. 2015b; see “additionality” in table 2).
Ensuring equity and subsidiarity
An important consideration for a global biodiversity conservation framework is the equitable distribution of costs and benefits between stakeholders (Ives and Bekessy 2015, Bull et al. 2017). For example, the management of any global biodiversity conservation goal through the mitigation hierarchy could follow a similar framework to the United Nations’ management of carbon emissions, with nation states setting their own national goals and targets that then sum to achieve overarching planetary goals. Managing the framework in such a way could allow for equity between nations, recognizing that industrialized countries reached their present wealth through exploiting natural resources and reducing biodiversity. Mediated through the United Nations Framework Convention on Climate Change, mechanisms exist that allow for the transfer of funds and capacity from richer to poorer countries to enable the latter to meet their obligations (i.e., the Central African Forest Initiative; Müller 2016), as well as a staged process for poorer countries to reduce emissions in line with their capacity to do so. A similar framework for differential development, such that the burden of reducing impacts on biodiversity was equitably distributed, could support achievement of a global NNL of biodiversity goal. Such an adjustment could also consider the international market drivers of biodiversity loss, such as China's demand for soy (mainly as cattle feed) driving biodiversity loss in Brazil's Cerrado, a biodiversity hotspot of conservation priority (Strassburg et al. 2017).
This raises the issue of the equivalency of biodiversity in space and time, between biodiversity types, and by type of conservation action (equivalency of offsets; table 2). We are not advocating the creation of a global market allowing the trading of biodiversity offsets toward NNL over large scales; instead, the mitigation hierarchy must be applied at biologically meaningful scales to avoid “out-of-kind” actions that allow one part of the planet to be damaged in return for enhancement of others (table 2; BBOP 2012, Bull et al. 2013). Although organizations such as the United Nations can endorse best practices at the international level, individual nations would need to implement the legal framework that would ultimately drive adherence. This is increasingly happening within the industrial development sector, with countries being supported to draft appropriate legislation and build capacity for implementation (e.g., the COMBO Project; http://combo-africa.org). In addition, as is the case for all conservation actions, effective monitoring, independent evaluation, and sanctions are required over the long term to ensure compliance with agreed-on targets and actions at all levels (table 2).
Categorizing conservation actions within the framework
The framing of global conservation efforts in terms of a mitigation hierarchy, for all human impacts on biodiversity, is novel. However, the interventions constituting the different components of such a hierarchy—at the international, national, landscape and project levels—are already in place. Presently, we know that most of the terrestrial environment is exposed to some form of human impact (Watson et al. 2016a) and that no area of the world's oceans remains free from human pressures (Halpern et al. 2015). The options for avoiding intact biodiversity (devoid of significant human impact) are already significantly constrained by the current human footprint. The benefits of complete retention of large intact areas of wilderness are self-evident to many conservationists (Watson et al. 2016b), as are the benefits of avoiding destruction of small but important areas of biodiversity value within modified settings, such as sites containing populations of very vulnerable species (e.g., Alliance for Zero Extinction sites; Ricketts et al. 2005). However, the opportunity costs of degrading many of these areas are not currently well articulated; adopting the mitigation-hierarchy framework would catalyze consideration of these costs, because it requires the comparison of relative biodiversity gains achievable at each step of the hierarchy and the associated uncertainties.
For example, the incidental environmental impacts of deep-sea fishing gear making contact with continental slopes and offshore seamounts are rarely accounted for in fisheries policy (Clark et al. 2016). Making a requirement of NNL for biodiversity targets legally binding (e.g., for all United Nations fisheries through UNCLOS) would drive the stipulation of defined baselines, indicators, and best-practices implementation concerning deep-sea fishing using National Biodiversity Strategies and Action Plans (NBSAPs), formalized through ESIA processes. This would drive stronger avoidance and minimization actions for deep-sea fishing nations because of the high level of uncertainty surrounding whether it is possible to generate biodiversity gains for benthic deep-sea organisms, such as corals, using remediation and offset measures, such as construction of artificial reefs.
What kinds of conservation action fall within a given stage of the mitigation hierarchy depends crucially on the baseline, goal, and target chosen (table 1). Taking a 2017 static baseline, for example, avoidance would comprise efforts to ensure that existing but currently unprotected areas of biodiversity value are preserved at the current status rather than being developed (e.g., to meet an area-based target; this could be done through new PAs); minimization reduces the damage of future developments on existing biodiversity in the newly developed areas (e.g., taking a species-based target; this could be done by minimizing the extent of new roads in close proximity to PAs); remediation increases the biodiversity values associated with new human impact (e.g., for an ecosystem-based target; this could be done through clean-up of new pollution in fished coastal areas); and offsetting improves biodiversity over the current status quo in ways or locations not associated with a particular new impact (e.g., for an ecosystem target by mangrove reseeding or for a species-based target by the eradication of invasives). Any entities causing new or ongoing biodiversity damage at the local, national, or international level (from road building to nontarget-fishing impacts to climate change) would need to demonstrate how they were investing in conservation in a way that would appropriately balance that damage in order to meet the goals and targets set out at the same spatiotemporal scale and institutional level as the damage.
Current protection status of terrestrial ecoregions is being mapped in order to prioritize conservation actions (Dinerstein et al. 2017). Similar mapping efforts have begun for forest restoration opportunities (Potapov et al. 2017). Maps such as these could provide the roadmap for global biodiversity conservation being called for by scientists (Watson and Venter 2017), and they could guide avoidance, mitigation, and restoration activities, as well as highlight opportunities for offsetting, within the mitigation hierarchy. For example, the effectiveness of the remediation step is open to question, with evidence suggesting that man-made or restored ecosystems do not reach the levels of ecological functionality of natural systems (Moreno-Mateos et al. 2012). At the project level, costing each step at a level that reflects biodiversity gains and losses (with associated uncertainties) could incentivize developers to move up the hierarchy, because avoiding sensitive sites could be made significantly cheaper than developing them and then offsetting.
Conclusions
Scaling up and expanding the mitigation-hierarchy concept could provide a systematic framework within which to think about what humanity wants for the planet's natural systems and how we could get there. It would help overcome the lack of cohesion in conservation efforts, which has facilitated the continuing loss of the planet's biodiversity (Rands et al. 2010). A global mitigation-hierarchy framework could also act as the foundation for a biodiversity conservation roadmap that would allow the international community to get behind a strategic goal and understand what is needed to fulfill their commitments to biodiversity. This would result in more explicit consideration of humanity's capacity to conserve different components of biodiversity. It forces the consideration of key questions such as what baseline for biodiversity we are evaluating against, how much damage could be averted or minimized given where we are now, and what this implies for the requirement for expensive and uncertain restoration and offsetting.
Nations are uniting on the issue of biodiversity loss and setting aspirational global goals and targets, and there are a number of effective systematic planning processes already in place at national levels. However, there is an obvious need for a more strategic and coherent global approach to deal with the loss of biodiversity because current efforts are manifestly failing. The mitigation hierarchy is one potential framework that would force the explicit consideration of the relationship between conservation and development and how sustainable development can be achieved. Such a reframing could be a step toward a clearer strategy for keeping within our planetary boundaries for the sake of both humanity and nature.
Acknowledgments
We thank Richard Damania, Jorn Scharlemann, and Julia Baker for comments. A special thank you to Matthew Gleeson for help creating figure 1. This article originated at a 2016 workshop, entitled the “Interdisciplinary Conservation Network” and was supported by the Pew Charitable Trusts through a Pew Marine Fellowship to EJMG, the University of Oxford, and the ARC Centre of Excellence for Environmental Decision-Making. WNSA is supported by the Commonwealth Scholarship Commission in the United Kingdom, and the University of Oxford with a PhD scholarship no. NZCR-2015-174. JWB is supported through a Marie Skłodowska-Curie Fellowship no. 655497 and acknowledges the Danish National Research Foundation for funding the Center for Macroecology, Evolution and Climate grant no. DNRF96. PFEA is supported by the Natural Environment Research Council no. NE/N005457/1. DG is supported by a PhD scholarship from the Science Sciences without Borders Program no. CNPq/Brazil, grant no. 246619/2012-0.
References cited
- Aiama D, Edwards S, Bos G, Ekstrom J, Krueger L, Quétier F, Savy C, Semroc B, Sneary M, Bennun L. 2015. No Net Loss and Net Positive Impact Approaches for Biodiversity: Exploring the Potential Application of These Approaches in the Commercial Agriculture and Forestry Sectors. International Union for Conservation of Nature. [Google Scholar]
- [BBOP] Business and Biodiversity Offsets Programme 2012. Standard on Biodiversity Offsets. BBOP–Forest Trends.
- Bennett E. 2004. Seeing the Wildlife and the Trees: Improving Timber Certification to Conserve Tropical Forest Wildlife. Wildlife Conservation Society, World Bank. [Google Scholar]
- Bull J, Maron M. 2016. How humans drive speciation as well as extinction. Proceedings of the Royal Society B 283 (art. 20160600). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JW, Suttle KB, Gordon A, Singh NJ, Milner-Gulland EJ. 2013. Biodiversity offsets in theory and practice. Oryx 47: 369–380. [Google Scholar]
- Bull J, Gordon A, Law E, Suttle K, Milner-Gulland E. 2014. Importance of baseline specification in evaluating conservation interventions and achieving no net loss of biodiversity. Conservation Biology 28: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J, Singh N, Suttle K, Bykova E, Milner-Gulland E. 2015. Creating a frame of reference for conservation interventions. Land Use Policy 49: 273–286. [Google Scholar]
- Bull JW, Lloyd SP, Strange N. 2017. Implementation gap between the theory and practice of biodiversity offset multipliers. Conservation Letters 10: 656–669. doi:10.1111/conl.12335 [Google Scholar]
- Butchart SH, Akçakaya HR, Chanson J, Baillie JE, Collen B, Quader S, Turner WR, Amin R, Stuart SN, Hilton-Taylor C. 2007. Improvements to the Red List index. PLOS ONE 2 (art. e140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart SH, Clarke M, Smith RJ, Sykes RE, Scharlemann JP, Harfoot M, Buchanan GM, Angulo A, Balmford A, Bertzky B. 2015. Shortfalls and solutions for meeting national and global conservation area targets. Conservation Letters 8: 329–337. [Google Scholar]
- Butchart SHM, Di Marco M, Watson JEM. 2016. Formulating smart commitments on biodiversity: Lessons from the Aichi targets. Conservation Letters 9: 457–468. [Google Scholar]
- [CBD] Convention on Biological Diversity 2010. COP decision X/2. Strategic Plan for Biodiversity 2011–2020. CBD. [Google Scholar]
- COMBO Project 2016. The COMBO Project: COnservation, impact Mitigation and Biodiversity Offsets in Africa. COMBO Project. (15 September 2017; http://combo-africa.org).
- Devillers R, Pressey RL, Grech A, Kittinger JN, Edgar GJ, Ward T, Watson R. 2014. Reinventing residual reserves in the sea: Are we favouring ease of establishment over need for protection? Aquatic Conservation: Marine and Freshwater Ecosystems 25: 480–504. [Google Scholar]
- Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E, Hahn N, Palminteri S, Hedao P, Noss R. 2017. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 67: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [FAO] Food and Agriculture Organization of the United Nations 1995. Code of Conduct for Responsible Fisheries. FAO. [Google Scholar]
- Ferraro PJ. 2009. Counterfactual thinking and impact evaluation in environmental policy. New Directions for Evaluation 122: 75–84. [Google Scholar]
- Ferraro PJ, Pattanayak SK. 2006. Money for nothing? A call for empirical evaluation of biodiversity conservation investments. PLOS Biology 4 (art. e105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [FSC] Forest Stewardship Council 2015. FSC Principles and Criteria for Forest Stewardship. FSC. [Google Scholar]
- Gardner TA, et al. 2013. Biodiversity offsets and the challenge of achieving no net loss. Conservation Biology 27: 1254–1264. [DOI] [PubMed] [Google Scholar]
- Gibbons P, Evans MC, Maron M, Gordon A, Roux D, Hase A, Lindenmayer DB, Possingham HP. 2016. A loss–gain calculator for biodiversity offsets and the circumstances in which no net loss is feasible. Conservation Letters 9: 252–259. [Google Scholar]
- Gjertsen H, Squires D, Dutton PH, Eguchi T. 2014. Cost‐effectiveness of alternative conservation strategies with application to the Pacific leatherback turtle. Conservation Biology 28: 140–149. [DOI] [PubMed] [Google Scholar]
- Gonçalves B, Marques A, Soares AMVDM, Pereira HM. 2015. Biodiversity offsets: From current challenges to harmonized metrics. Current Opinion in Environmental Sustainability 14: 61–67. [Google Scholar]
- Halpern BS, et al. 2015. Spatial and temporal changes in cumulative human impacts on the world's ocean. Nature Communications 6 (art. 7615). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal G. 2012. Reflections: Defining and measuring sustainability. Review of Environmental Economics and Policy 6: 147–163. [Google Scholar]
- Hoffmann M, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330: 1503–1509. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Duckworth J, Holmes K, Mallon DP, Rodrigues AS, Stuart SN. 2015. The difference conservation makes to extinction risk of the world's ungulates. Conservation Biology 29: 1303–1313. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JE, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486: 105–108. [DOI] [PubMed] [Google Scholar]
- [IFC] International Finance Corporation 2012. Performance Standard 6: Biodiversity Conservation and Sustainable Management of Natural Resources. IFC. [Google Scholar]
- [IUCN] International Union for Conservation of Nature 2016. Protected Areas and Other Areas Important for Biodiversity in Relation to Environmentally Damaging Industrial Activities and Infrastructure Development. IUCN. Report no. WCC-2016-Rec-102-EN.
- Ives CD, Bekessy SA. 2015. The ethics of offsetting nature. Frontiers in Ecology and the Environment 13: 568–573. [Google Scholar]
- Jones J, Collen B, Atkinson G, Baxter P, Bubb P, Illian J, Katzner T, Keane A, Loh J, McDonald-Madden E. 2011. The why, what, and how of global biodiversity indicators beyond the 2010 target. Conservation Biology 25: 450–457. [DOI] [PubMed] [Google Scholar]
- Kiesecker JM, Copeland H, Pocewicz A, McKenney B. 2010. Development by design: Blending landscape-level planning with the mitigation hierarchy. Frontiers in Ecology and the Environment 8: 261–266. [Google Scholar]
- Laitila J, Moilanen A, Pouzols FM. 2014. A method for calculating minimum biodiversity offset multipliers accounting for time discounting, additionality and permanence. Methods in Ecology and Evolution 5: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimore B, Smith CT, Titus B, Stupak I, Egnell G. 2013. Woodfuel harvesting: A review of environmental risks, criteria and indicators, and certification standards for environmental sustainability. Journal of Sustainable Forestry 32: 58–88. [Google Scholar]
- Lindenmayer DB, Crane M, Evans MC, Maron M, Gibbons P, Bekessy S, Blanchard W. 2017. The anatomy of a failed offset. Biological Conservation 210: 286–292. [Google Scholar]
- Maron M, Bull JW, Evans MC, Gordon A. 2015a. Locking in loss: Baselines of decline in Australian biodiversity offset policies. Biological Conservation 192: 504–512. [Google Scholar]
- Maron M, Gordon A, Mackey BG, Possingham H, Watson JE. 2015b. Stop misuse of biodiversity offsets. Nature 523 (art. 401). [DOI] [PubMed] [Google Scholar]
- Maron M, Ives CD, Kujala H, Bull JW, Maseyk FJ, Bekessy S, Gordon A, Watson JE, Lentini PE, Gibbons P. 2016. Taming a wicked problem: Resolving controversies in biodiversity offsetting. BioScience 66: 489–498. [Google Scholar]
- Maron M, Brownlie S, Bull JW, Evans MC, von Hase A, Quétier F, Watson JE, Gordon A. 2018. The many meanings of no net loss in environmental policy. Nature Sustainability 1: 19–27. [Google Scholar]
- Maxwell SL, Fuller RA, Brooks TM, Watson JE. 2016. The ravages of guns, nets and bulldozers. Nature 536: 144–145. [DOI] [PubMed] [Google Scholar]
- Milner-Gulland EJ, et al. 2018. Translating the terrestrial mitigation hierarchy to marine megafauna bycatch. Fish and Fisheries. doi:10.1111/faf.12273 [Google Scholar]
- Moilanen A, Laitila J. 2016. FORUM: Indirect leakage leads to a failure of avoided loss biodiversity offsetting. Journal of Applied Ecology 53: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen A, van Teeffelen AJA, Ben-Haim Y, Ferrier S. 2009. How much compensation is enough? A framework for incorporating uncertainty and time discounting when calculating offset ratios for impacted habitat. Restoration Ecology 17: 470–478. [Google Scholar]
- Moreno-Mateos D, Power ME, Comín FA, Yockteng R. 2012. Structural and functional loss in restored wetland ecosystems. PLOS Biology 10 (art. e1001247). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F. 2016. “Save the planet, plant a tree!”: REDD+ and global/local forest governance in the Anthropocene. Resilience 5: 182–200. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood EC, D’amico JA, Itoua I, Strand HE, Morrison JC. 2001. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 51: 933–938. [Google Scholar]
- Pereira HM, Ferrier S, Walters M, Geller GN, Jongman R, Scholes RJ, Bruford MW, Brummitt N, Butchart S, Cardoso A. 2013. Essential biodiversity variables. Science 339: 277–278. [DOI] [PubMed] [Google Scholar]
- Phalan B, Hayes G, Brooks S, Marsh D, Howard P, Costelloe B, Vira B, Kowalska A, Whitaker S. 2017. Avoiding impacts on biodiversity through strengthening the first stage of the mitigation hierarchy. Oryx 2017: 1–9. (5 March 2018; https://doi.org/10.1017/S0030605316001034) [Google Scholar]
- Pitcher TJ, Kalikoski D, Short K, Varkey D, Pramod G. 2009. An evaluation of progress in implementing ecosystem-based management of fisheries in 33 countries. Marine Policy 33: 223–232. [Google Scholar]
- Potapov P, et al. 2017. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Science Advances 3 (art. e1600821). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretty J. 2008. Agricultural sustainability: Concepts, principles and evidence. Philosophical Transactions of the Royal Society B 363: 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quétier F, Regnery B, Levrel H. 2014. No net loss of biodiversity or paper offsets? A critical review of the French no net loss policy. Environmental Science and Policy 38: 120–131. [Google Scholar]
- Rainey HJ, Pollard EH, Dutson G, Ekstrom JM, Livingstone SR, Temple HJ, Pilgrim JD. 2015. A review of corporate goals of no net loss and net positive impact on biodiversity. Oryx 49: 232–238. [Google Scholar]
- Rands MR, Adams WM, Bennun L, Butchart SH, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JP. 2010. Biodiversity conservation: Challenges beyond 2010. Science 329: 1298–1303. [DOI] [PubMed] [Google Scholar]
- Ricketts TH, Dinerstein E, Boucher T, Brooks TM, Butchart SH, Hoffmann M, Lamoreux JF, Morrison J, Parr M, Pilgrim JD. 2005. Pinpointing and preventing imminent extinctions. Proceedings of the National Academy of Sciences 102: 18497–18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockström J, Gaffney O, Rogelj J, Meinshausen M, Nakicenovic N, Schellnhuber HJ. 2017. A roadmap for rapid decarbonization. Science 355: 1269–1271. [DOI] [PubMed] [Google Scholar]
- Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA. 2007. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 57: 573–583. [Google Scholar]
- Strassburg BB, Brooks T, Feltran-Barbieri R, Iribarrem A, Crouzeilles R, Loyola R, Latawiec AE, Oliveira Filho FJ, Scaramuzza CAdM, Scarano FR. 2017. Moment of truth for the Cerrado hotspot. Nature Ecology and Evolution 1 (art. 0099). [DOI] [PubMed] [Google Scholar]
- [UNEP–WCMC] UN Environment World Conservation Monitoring Centre, [IUCN] International Union for Conservation of Nature 2016. Update on Global Statistics December 2016. UNEP–IUCN. [Google Scholar]
- [UNFCCC] United Nations Framework Convention on Climate Change 2015. Adoption of the Paris Agreement. I: Proposal by the President (Draft Decision). United Nations. [Google Scholar]
- United Nations 1992. Convention on Biological Diversity. United Nations. [Google Scholar]
- United Nations 2008. Non-Legally Binding Instrument on All Types of Forests. United Nations. [Google Scholar]
- United Nations 2015a. Development of an International Legally Binding Instrument under the United Nations Convention on the Law of the Sea on the Conservation and Sustainable Use of Marine Biological Diversity of Areas beyond National Jurisdiction. United Nations. [Google Scholar]
- United Nations 2015b. The Sustainable Development Goals 2015. United Nations. [Google Scholar]
- Venter O, Magrach A, Outram N, Klein CJ, Marco MD, Watson JEM. 2017. Bias in protected-area location and its effects on long-term aspirations of biodiversity conventions. Conservation Biology 32: 127–134. doi:10.1111/cobi.12970 [DOI] [PubMed] [Google Scholar]
- Watson J, Jones K, Fuller R, Marco M, Segan D, Butchart S, Allan J, McDonald‐Madden E, Venter O. 2016a. Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conservation Letters 9: 413–421. [Google Scholar]
- Watson J, Shanahan D, Di Marco M, Allan J, Laurance W, Sanderson E, Mackey B, Venter O. 2016b. Catastrophic declines in wilderness areas undermine global environment targets. Current Biology 26: 2929–2934. [DOI] [PubMed] [Google Scholar]
- Watson JE, Venter O. 2017. Ecology: A global plan for nature conservation. Nature 550: 48–49. [DOI] [PubMed] [Google Scholar]
- Wilcox C, Donlan CJ. 2009. Need for a clear and fair evaluation of biodiversity offsets for fisheries bycatch. Conservation Biology 23: 770–772. [DOI] [PubMed] [Google Scholar]