Abstract
Background:
Temporomandibular joint osteoarthritis (TMJ-OA) is a degenerative disease that involves changes in subchondral bone and progressive degradation of cartilage. Currently, rebamipide, a gastroprotective drug, is administered to protect gastric mucosa and accelerate ulcer healing.
Objectives:
Recent studies have shown that rebamipide also attenuates cartilage degeneration by suppressing oxidative damage and inducing homeostasis of the extracellular matrix of articular chondrocytes. Regarding the latter, reduced expression of cathepsin K, NFATc1, c-Src, and integrin β3, and increased expression of nuclear factor-kappa B, have been found to be mediated by the transcription factor, receptor activator of nuclear factor kappa-B ligand (RANKL).
Methods:
Treatment with rebamipide was also found to activate, mitogen-activated protein kinases such as p38, ERK, and JNK to reduce osteoclast differentiation. Taken together, these results strongly indicate that rebamipide mediates inhibitory effects on cartilage degradation and osteoclastogenesis in TMJ-OA.
Results and Conclusion:
Here, we highlight recent evidence regarding the potential for rebamipide to affect osteoclast differentiation and TMJ-OA pathogenesis. We also discuss the potential role of rebamipide to serve as a new strategy for the treatment of TMJ-OA.
Keywords: Rebamipide, osteoclast differentiation, ROS, chondrocyte, TMJ-OA, mitogen-activated
1. INTRODUCTION
Temporomandibular joint osteoarthritis (TMJ-OA) is a degenerative disease that reflects both non-inflammatory and inflammatory changes. TMJ-OA may involve all TMJ tissues and leads to anatomical changes [1, 2]. It is characterized by chronic inflammation in synovial tissue, progressive cartilage destruction and deterioration, and subchondral bone remodeling. The etiology is associated with multiple risk factors, complex, and sometimes unknown. However, the exact pathogenesis of TMJ-OA remains unclear and controversial. Osteoarthritis (OA) often affects the TMJ of patients with temporomandibular disorders (TMDs). Thus, TMJ-OA is an important subtype in the classification of TMDs [1, 3-5].
Patients with TMJ-OA often have pain and TMJ dysfunction with decreased quality of life [6]. The clinical signs and symptoms of TMJ-OA include a restriction in joint function and severe pain due to the presence of synovitis [3]. The pain usually associated with limitation of joint opening, stiffness, and may be relieved with rest and nonsteroid anti-inflammatory drug (NSAID) treatment [6]. Drug repositioning or reprofiling has a significant advantage over traditional drug development because a repositioned drug has already completed toxicity and safety tests and exhibited reduced toxicology. Investigations of new pleiotropic effects of drugs are very valuable and can enhance the success of pharmaceutical companies [7].
Rebamipide is an amino acid analog of 2 (1H)-quinolone and is widely used as a gastroprotective drug to treat gastric ulcers and gastritis. Rebamipide has also exhibited antibacterial effects, mucin secretagogue activity, and anti-inflammatory actions [7-11]. In endothelial inflammation, rebamipide suppresses interleukin (IL)-8 production through inhibition of IκBα phosphorylation and nuclear factor-kappa B (NF-κB) p65 [12]. Rebamipide also inhibits T cells activation, suppresses Th1 cytokines (IL-2 and interferon-γ), serum autoantibodies, IgM, and IgG1 production, and decreases NF-κB activity in autoimmune lesions of salivary glands [13].
Moreover, recent studies have demonstrated the treatment effects of rebamipide for new indications such as TMJ-OA and its potency to inhibit the formation of human osteoclasts (OCs) by inhibition of RANKL-mediated osteoclastogenesis, disruption of actin ring formation, and reduction of DC-specific transmembrane protein (DC-STAMP) [7, 14]. In OA cartilage, rebamipide attenuates matrix metalloproteinase (MMP)-13, IL-1β, hypoxia inducible factor (HIF)-2α, inducible nitric oxide synthase (iNOS), and nitrotyrosine [15]. Most of the studies suggest that rebamipide might be a potential therapeutic strategy for OA.
Bone is a tissue that is continuously remodeled via two distinct processes, bone resorption and bone formation [16]. To maintain skeletal homeostasis, these events are tightly regulated and strongly linked [17]. OCs are critical for both processes and they include bone resorbing cells which represent differentiated cells that derive from hematopoietic cells of monocyte-macrophage lineages. Conversely, osteoblasts (OBs), which derive from mesenchymal origins, are responsible for bone forming cells. When the differentiation/activity of OCs and OBs is altered, bone diseases can develop. Moreover, accumulating evidence supports the existence of a relationship between bone and the immune system, particularly in relation to pathological conditions in which activation of bone adsorption and bone resorption occurs [18].
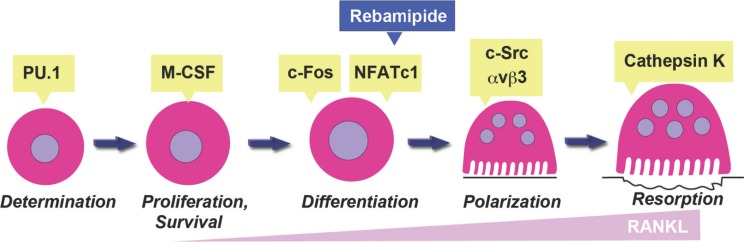
RANK is expressed by OC progenitor cells and its activation by RANKL results in the downstream stimulation of factors associated with tumor necrotic factor (TNF) receptor, as well as several signaling cascades including mitogen-activated protein kinase (MAPK), NF-κB, activating protein 1 (AP-1), and the transcription factor, nuclear factor of activated T-cells c1 (NFATc1). Cross-talk between these signaling proteins results in the formation of OCs that are multinucleated and exhibit bone resorbing activity [19, 20]. Terminal differentiation of OCs is characterized by expression of c-Src, integrin β3, cathepsin K, NFATc1, and other markers of OC differentiation [20, 21] (Fig. 1). In this review, the effect of rebamipide on the differentiation and function of OCs both in vitro and in vivo is described in an effort to develop a novel strategy to treat OC-associated bone diseases.
Fig. (1).
Rebamipide affected osteoclastogenesis. Early nonspecific differentiation of OCs, survival cytokine M-CSF, and macrophage proliferation are dependent on PU.1. Subsequently, RANKL-induced activation of RANK commits OCs to differentiation via c-Fos and NFATc1. Polarization, which requires c-Src and αvβ3 integrin, is the first step in establishing the resorptive capacity of mature OCs. Osteoclasts then mobilize the mineralized component of bone, with cathepsin K mediating degradation of the organic matrix of bone. In the present study, rebamipide treatment decreased RANKL-induced NFATc1 signaling and levels of c-Src and αvβ3 integrin. As a result, expression of cathepsin K was affected.
2. TMJ-OA
OA is the most common degenerative joint disorder that causes disability in the adult population [22]. OA patients suffer from pain during increased function and load bearing joint. The joints become tender, with decreased range of motion, and loss of articular cartilage indicated by crepitus. Radiography may show joint space narrowing, formation of osteophytes [23, 24], subchondral bone cysts, condylar head flattening, and increased subchondral cortical thickness [25, 26].
TMJ is frequently affected by OA [27, 28]. The prevalence of TMJ-OA has been observed to be 25% in 20-49 year age group and 70% in 73-75 year age group by clinical and MRI examination [27, 29]. The evidence of TMJ-OA is clinically indicated by female preponderance, with a female-to-male ratio of more than 2:1 [30, 31]. TMJ-OA has various etiologies and multifactorial factors including inflammatory, immunologic insults, biomechanical, biochemical, excessive mechanical stress, and extracartilaginous factor. The extracartilaginous factors include reduction of synovial fluid, changes in synovial membrane, changes in vascular system, and subchondral bone microfractures [32, 33].
The pathogenesis of TMJ-OA can be caused by various etiologic factors that each interact with the other and each of etiologic factor may not cause the same pathogenesis. Literally, the subchondral bone has an etiological role in the pathogenesis of TMJ-OA. Failure in internal remodeling system of mandibular condylar subchondral bone may result chondrocyte injury then leads to increase collagen degradation, with release of proteases and decrease of protease inhibitor thereby resulting in extracellular matrix (ECM) breakdown [33].
Various key mediators have been suggested to be responsible for degradation of articular cartilage in vivo and in vitro including MMP-13 and members of the closely related family of a disintegrin and metalloprotease with thrombospondin motifs 5 (ADAMTS5) [34-39]. During the process of TMJ-OA, articular chondrocytes release IL-1, TNF-α, runt-related transcription factor 2 (RUNX2), alkaline phosphatase, and type 10 collagen. Concurrently, abnormal cartilage calcification occurs and exhibits decrease levels of proteoglycan [40-45].
3. REBAMIPIDE AND THE DIFFERENTIATION OF OCS
Multinucleated cells that attach to the bone matrix via an actin rich structure are referred to as OCs. The actin rings that are formed by OCs degrade bone matrix following the secretion of protons and proteases into a space that forms between OCs and a bone surface through a specialized structure known as a ruffled border membrane [46]. OC differentiation is regulated by colony stimulating factor 1 receptor (Csf1r), also known as M-CSF receptor or c-Fms, and tumor necrosis factor receptor superfamily member 11a (Tnfrsf11a), also known as receptor activator of NF-κB (RANK) [47]. While M-CSF receptor signaling supports the survival and proliferation of OC precursor cells during osteoclastogenesis, the differentiation process of OCs is activated by RANK signaling. RANK signaling activates NF-κB and Fos, both of which are transcription factors that are essential for OC differentiation [21]. Ig-like receptors transmit signals to activate phospholipase Cγ (PLCγ) via their adaptors, DAP12 and FcRγ, with each containing an immunoreceptor tyrosine-based activation motif (ITAM) [48]. PLCγ then induces calcium oscillation, which leads to the activation of calcineurin, a Ca2+/calmodulin-dependent phosphatase. RANK and Ig-like receptor signals are finally integrated by the master transcription factor of osteoclastogenesis, NFATc1, which induces the expression of molecules that allow OCs to perform bone resorbing activities. These molecules include MMP-9, cathepsin K, the chloride channel, CLC-7, and H+-ATPase subunits [49].
Rebamipide treatment of bone marrow macrophages (BMMs) and human monocytes inhibits RANKL-induced OC formation from precursor cells in a dose-dependent manner without cytotoxicity. Thus, rebamipide affects the generation of OCs from macrophage that are stimulated with RANKL and also the differentiation of OCs [7, 14].
4. REBAMIPIDE SUPPRESSES GENE EXPRESSION IN OCS
Previous studies have reported that rebamipide inhibits IL-8 expression that is induced by TNF-α by suppressing NF-κB signaling in human umbilical vein endothelial cells (HUVECs). Rebamipide also inhibits the adhesion of endothelial cells to endothelial cells that are stimulated by hypoxia/reoxygenation through an NF-κB-dependent pathway [12, 50]. For RANKL-induced OC differentiation, the NF-κB pathway must be activated [49]. Activation of the IκB kinase complex is a well-characterized aspect of the classical NF-κB signaling pathway, and it leads to phosphorylation of IκBα which targets it for ubiquitin-dependent degradation [51]. We have shown that rebamipide inhibits the degradation of IκBα in the cytoplasm to reduce transactivation of NF-κB [14].
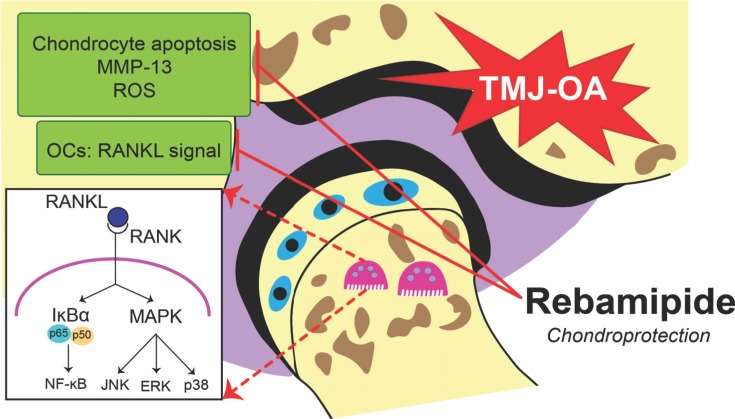
It has been reported that MAPKs (e.g. p38, JNK, and ERK) are activated by RANKL stimulation and they play a role in osteoclastogenesis [19]. In the early stages of OC generation, p38 is particularly important based on its ability to regulate microphthalmia-associated transcription factor [52]. Meanwhile, dominant-negative JNK has been shown to prevent osteoclastogenesis induced by RANKL [53]. ERK is able to induce c-Fos for osteoclastogenesis [54], while inhibition of ERK reduces OC formation [55]. In the present study, activation of these MAPKs was investigated following rebamipide treatment, and it was observed that rebamipide inhibits the phosphorylation of each. Thus, the anti-osteoclastogenic effect of rebamipide in RANKL-stimulated BMMs may be mediated via the phosphorylation of various MAPKs [20] (Fig. 2).
Fig. (2).
Rebamipide-induced chondroprotective effects in TMJ-OA. TMJ-OA that was treated with rebamipide exhibited suppressed chondrocyte apoptosis, expression of MMP-13, and oxidative damage in a dose-dependent manner. A reduction in condylar subchondral bone volume due to blocked OC activity via RANKL-induced osteoclastogenesis was also readily observed.
5. REBAMIPIDE DISRUPTS THE CYTOSKELETAL ORGANIZATION OF OCS TO INHIBIT BONE-RESORBING ACTIVITY
Degradation of the inorganic and organic matrices of bone is a crucial function of OCs. The accumulation of molecules that are able to degrade bone on the resorption surface of bone requires direct interactions between OCs and mineralized substrates. OCs create a microenvironment that is isolated from the extracellular space by restructuring the actin component of their cytoskeleton. Specifically, actin rings are formed that provide a “gasket-like” sealing of this microenvironment [56].
In the present study, assays to detect pit formation induced by RANKL show that rebamipide inhibits the ability of OCs to perform bone-resorbing activities. In addition, rebamipide treatment leads to the degradation of actin rings of mature OCs in a dose-dependent manner. However, following the exposure of bone marrow stromal cells to rebamipide, β-glycerophosphate, and osteoblastogenic medium, the mineralization and differentiation of the OCs were unaffected. Thus, rebamipide appears to mediate an anti-resorption effect, while indirectly affecting the formation of bone [14].
6. MURINE MODEL OF TMDS
As a synovial joint, the TMJ is essential for sliding and hinge movements of jaws [57]. The TMJ consists of the condyle, articular eminence, and glenoid fossa, and it provides articulation between the mandible and the cranium. These joints are surrounded by a capsule consisting of a synovial lining and fibrous material and they provide anatomic control of both occlusion and mandibular movement. An articular disk separates the joint space between the condyle and the glenoid fossa into lower and upper articular cavities and these are bounded by the condyle and the articular eminence and articular fossa, respectively [58, 59].
TMDs include a heterogenous cluster of diseases [60-62]. In particular, rheumatoid arthritis (RA) and OA of the TMJ represent severe and debilitating disorders whereby the TMJ disk can undergo displacement, thickening, folding, lengthening, and disk perforation [63-65]. However, the factors responsible for the development and progression of TMD, especially OA in the TMJ, remain to be determined [57].
As compared to articular cartilage in the knee, mandibular condyle cartilage is considered secondary (e.g. from the chondroskeleton) and it derives from the cranial neural crest during embryogenesis. Compared to other synovial joints and the articular cartilage of other joints, the condyle of the mandible has less type I collagen and does not express type II collagen in the superficial layer of mandibular condylar cartilage. In addition, the articular surfaces of the mandible are composed of fibrous tissue rather than hyaline cartilage. For studies of TMDs, specific devices and methods have been applied to establish mouse and rat models of these diseases. In addition, various genetic animal models of OA have been established [66].
The TMJ-OA model established in the present study exhibited irregularities in chondrocyte alignment in the condylar cartilage layers, OA-like degenerated lesions, marked depletion of proteoglycans, and subchondral bone loss due to OC hyperactivity. Previously, it was demonstrated that forced mouth opening in mice led to a decrease in subchondral bone volume [67], while steady and repetitive jaw opening was an effective method for inducing OA-like changes in rabbits. Moreover, the latter were consistent with the presentation of TMJ-OA in patients [68]. Biomechanical stimulation from abnormal occlusion [69, 70], local application of chemicals [3], surgical manipulation of the joint [71], and genetic modifications [72, 73] have also been shown to induce early TMJ-OA.
7. REBAMIPIDE AND AUTOIMMUNITY
In a recent study, adjunct rebamipide therapy was found to effectively prevent peptic ulcers in patients that were prescribed a COX-2-selective inhibitor for arthritis [74]. Oral administration of rebamipide has also been found to reduce histological and clinical scores in animal models of RA, particularly in SKG mice and animals with arthritis induced by collagen [75, 76]. RA leads to inflammation of the synovial membrane and involves the production of IL-17, IL-1β, IL-6, and TNF-α. Expression of RANKL is also enhanced in synovial cells, thereby inducing OC differentiation. Considering these results, as well as the observation that CD4+ T cell activation is suppressed by rebamipide, it is possible that rebamipide may also be useful for the healing of bone destruction that is impaired by OCs [7, 13, 77].
Decreased apoptosis of epithelial cells in the salivary glands has been observed in rebamipide-treated mice. Rebamipide treatment has also been found to suppress the activation of Th1 cytokines (IL-2, interferon-γ) and CD4+ T-cells, thereby adversely affecting NF-κB activity and inhibiting the expression of IRF-4B, a transcription factor associated with B-cell activation and differentiation. Thus, rebamipide may represent a novel therapeutic approach for Sjorgen syndrome [13].
8. THE ROS-SCAVENGING PROPERTY OF REBAMIPIDE AND TMJ-OA
Active oxygen species that are generated by polymorphonuclear leukocytes represent a potential source of damage to cells. Meanwhile, reactive oxygen metabolites that are generated during the metabolism of arachidonic acid, smooth muscle cells, and platelet macrophages may contribute to the damage of gastric mucosa. A pharmacological effect of rebamipide includes its ability to scavenge hydroxyl radicals and attenuate the cytotoxicity of reactive oxygen metabolites. In regard to gastric mucosal damage, rebamipide has the potential to scavenge hydroxyl radicals and also suppress the production of active oxidants by modulating the activation of neutrophils [78-80].
A recent report described the inhibitory effects of rebamipide on cartilage degeneration and pain production in an experimentally induced model of OA in rat knee tissue. Oral administration of rebamipide reduced oxidative stress in the subchondral bone area and in articular cartilage. Furthermore, the chondroprotective capacity of rebamipide was associated with reduced catabolism of the articular cartilage matrix. ADAMTS5, MMPs, and HIF-2α have also been reported to mediate the ability of rebamipide to serve as an anticatabolic regulator of cartilage destruction [15].
In the present study, excessive chondrocyte apoptosis and enhanced expression of MMP-13 by chondrocytes characterized the model of TMJ-OA that was established. Then, following treatment with rebamipide, a dose-dependent attenuation of cartilage degradation was observed in the hypertrophic layer of the condylar cartilage [14].
During the cartilage degradation process, reactive oxygen species (ROS) and antioxidants may simultaneously act at different levels. Both induction of matrix degradation by enzymes and inhibition of matrix formation are involved in this process. Based on the role of ROS in mediating an increase in the apoptosis of chondrocytes during OA, ROS have been identified as a potential treatment target. In addition, inhibitor of nitrite oxide, a marker of oxidative stress, has been found to be markedly attenuated in TMJ-OA mice treated with rebamipide. Thus, the chondroprotective effects of rebamipide on cartilage affected by TMJ-OA may be mediated by its ROS-scavenging property [14, 77, 81, 82] (Fig. 2).
9. OCS AND ARTICULAR CHONDROCYTE MALFUNCTION IN TMJ-OA AND POTENTIAL EFFECTS OF REBAMIPIDE
Articular cartilage and subchondral bone are separated by a calcified cartilage zone that undergoes marked changes in structural, physical, and functional properties of the OA process [83]. OC, a multinucleated cell responsible for bone resorption, can penetrate the mineralized matrices of the bone and calcified cartilage [84].
The penetration site creates fissures and cracks in the overlying cartilage and the vascular supplies a mechanism for fluids exchange and soluble mediators between these tissues. During the OA mechanism progresses, cracks and discontinuities also develop in the subchondral bone that leads to the mechanism for exchange. There is an evidence of communication between the cartilage and subchondral bone directly via the diffusion process that allows soluble products exchange to regulate the activities of resident cells in the adjacent tissues [83, 85, 86].
A recent study reported that β-catenin signaling in chondrocytes plays an important role in postnatal bone growth and bone remodeling through OC formation in mice with conditional knockout or activation of chondrocyte-specific β-catenin [87]. A role of β-catenin is also identified in the regulation of chondrocyte differentiation and function undergo OA condition [88, 89]. Mice with β-catenin deficiency in chondrocytes exhibit increased RANKL/OPG ratios that promote OC-inducing activity. The RANKL/OPG ratio was reversed in the chondrocytes from the mice with the activating mutation, showing impaired osteoclast-inducing activity [87].
Coculture studies between chondrocyte cells and OC precursor cells with M-CSF and 1,25-dihydroxyvitamin D3 exhibited that the ability of the chondrocytes to support osteoclastogenesis could be attributed to their differential capacity to express RANKL and OPG. OPG acts as a key inhibitor of OC differentiation via interaction with RANKL, by antagonizing the function of RANKL [90, 91]. However, in addition to osteocytes and other OB lineage cells that promote RANKL and OPG expression, chondrocytes may serve as a potential regulator in osteoclastogenesis. Thus, further studies are needed to understand the potential role of chondrocytes-derived RANKL/OPG in the pathogenesis of OA [91].
This present study demonstrated that in murine TMJ-OA, rebamipide effectively attenuates the subchondral trabecular bone resorption by decreasing number of TRAP-positive cells. In OC differentiation process, rebamipide inhibits NFATc1 [14], a transcription factor that most potently induced by RANKL [49], followed by lower levels of OC function markers such as, integrin β3, c-Src, and cathepsin K as well as disrupted actin ring formations (Fig. 1). Rebamipide was also found to significantly inhibit the phosphorylation of IκBα, JNK, ERK, and p38 [14]. Therefore, rebamipide has potential effects to overcome osteoclastogenesis malfunction in murine TMJ-OA (Fig. 2).
Cellular interaction in crosstalk in vitro between chondrocytes and OBs has been investigated. OBs and articular chondrocytes derived from OA bone exhibit decreased production of aggrecan and chondrocyte markers (SOX9 and type 2 collagen), but increased the production of MMP-3 and MMP-13. This finding suggests that local factors expressed by OB initiate chondrocyte hypertrophy and matrix mineralization [92-94]. In murine TMJ-OA, increased expression of MMP-13 and excessive chondrocyte apoptosis was attenuated by treatment with rebamipide. However, in in vitro study, rebamipide was neither affected OB mineralization nor differentiation [14]. Thus, following investigations of rebamipide are needed to provide an effective treatment for TMJ-OA patients.
CONCLUSION
In the hypertrophic layer of condylar cartilage, rebamipide-treated TMJ-OA joints underwent marked degradation of cartilage, excessive chondrocyte apoptosis, and increased expression of MMP-13 by chondrocytes in a dose-dependent manner compared to vehicle-treated TMJ-OA joints. To further elucidate how these changes affect the homeostasis of cartilage ECM and induce chondroprotection, and to determine whether rebamipide affects the survival of OA chondrocytes, additional studies are needed.
Differentiation and OC activity in the rebamipide-treated TMJ-OA joints were suppressed, while highly effective anti-resorptive activity was observed, as a result of inhibited transcription factor activity in response to RANKL. Thus, the present results support further study of the potential for rebamipide to serve as a treatment for TMJ-OA.
Consent for Publication
Not applicable.
Acknowledgements
This work was supported by the grants provided by JSPS KAKENHI (Grant Numbers. 25713063, 15K15757, 17K19758 to T.I.), The Ichiro Kanehara Foundation, Suzuken Memorial Foundation, The Nakatomi Foundation, Smoking Research Foundation to T.I., and Otsuka Toshimi Scholarship Foundation to I.H.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Scrivani S.J., Keith D.A., Kaban L.B. Temporomandibular disorders. N. Engl. J. Med. 2008;359:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 2.Wang X.D., Kou X.X., Mao J.J., Gan Y.H., Zhou Y.H. Sustained inflammation induces degeneration of the temporomandibular joint. J. Dent. Res. 2012;91:499–505. doi: 10.1177/0022034512441946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X.D., Kou X.X., He D.Q., et al. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.D., Zhang J.N., Gan Y.H., Zhou Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015;94:666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 5.McNeill C., Mohl N.D., Rugh J.D., Tanaka T.T. Temporomandibular disorders: diagnosis, management, education, and research. J. Am. Dent. Assoc. 1990;120:253–, 255, 257 passim. doi: 10.14219/jada.archive.1990.0049. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.J., Shih T.T., Wang J.S., Wang H.Y., Shiau Y.Y. Magnetic resonance images of the temporomandibular joints of patients with acquired open bite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005;99:734–742. doi: 10.1016/j.tripleo.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Nanke Y., Kobashigawa T., Yago T., et al. Rebamipide, an Amino Acid Analog of 2(1H)-Quinolinone, Inhibits the Formation of Human Osteoclasts. BioMed Res. Int. 2016:6824719. doi: 10.1155/2016/6824719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iijima K., Ichikawa T., Okada S., et al. Rebamipide, a cytoprotective drug, increases gastric mucus secretion in human: evaluations with endoscopic gastrin test. Dig. Dis. Sci. 2009;54:1500–1507. doi: 10.1007/s10620-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka H., Fukuda K., Ishida W., et al. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br. J. Ophthalmol. 2013;97:912–916. doi: 10.1136/bjophthalmol-2012-302868. [DOI] [PubMed] [Google Scholar]
- 10.Naito Y., Yoshikawa T. Rebamipide: a gastrointestinal protective drug with pleiotropic activities. Expert Rev. Gastroenterol. Hepatol. 2010;4:261–270. doi: 10.1586/egh.10.25. [DOI] [PubMed] [Google Scholar]
- 11.Urashima H., Takeji Y., Okamoto T., Fujisawa S., Shinohara H. Rebamipide increases mucin-like substance contents and periodic acid Schiff reagent-positive cells density in normal rabbits. J. Ocul. Pharmacol. Ther. 2012;28:264–270. doi: 10.1089/jop.2011.0147. [DOI] [PubMed] [Google Scholar]
- 12.Choe J.Y., Park K.Y., Lee S.J., Park S.H., Kim S.K. Rebamipide inhibits tumor necrosis factor-alpha-induced interleukin-8 expression by suppressing the NF-kappaB signal pathway in human umbilical vein endothelial cells. Inflamm. Res. 2010;59:1019–1026. doi: 10.1007/s00011-010-0221-5. [DOI] [PubMed] [Google Scholar]
- 13.Kohashi M., Ishimaru N., Arakaki R., Hayashi Y. Effective treatment with oral administration of rebamipide in a mouse model of Sjogren’s syndrome. Arthritis Rheum. 2008;58:389–400. doi: 10.1002/art.23163. [DOI] [PubMed] [Google Scholar]
- 14.Izawa T., Mori H., Shinohara T., et al. Rebamipide Attenuates Mandibular Condylar Degeneration in a Murine Model of TMJ-OA by Mediating a Chondroprotective Effect and by Downregulating RANKL-Mediated Osteoclastogenesis. PLoS One. 2016;11:e0154107. doi: 10.1371/journal.pone.0154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon S.J., Woo Y.J., Jeong J.H., et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthritis Cartilage. 2012;20:1426–1438. doi: 10.1016/j.joca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Raggatt L.J., Partridge N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamma R., Zallone A. Osteoblast and osteoclast crosstalks: from OAF to Ephrin. Inflamm. Allergy Drug Targets. 2012;11:196–200. doi: 10.2174/187152812800392670. [DOI] [PubMed] [Google Scholar]
- 18.Mori G., D’Amelio P., Faccio R., Brunetti G. The Interplay between the bone and the immune system. Clin. Dev. Immunol. 2013:720504. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 20.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 21.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 22.Hootman J.M., Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 23.Pitsillides A.A., Beier F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nat. Rev. Rheumatol. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- 24.Messent E.A., Ward R.J., Tonkin C.J., Buckland-Wright C. Osteophytes, juxta-articular radiolucencies and cancellous bone changes in the proximal tibia of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:179–186. doi: 10.1016/j.joca.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Goldring M.B., Goldring S.R. Osteoarthritis. J. Cell. Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 26.Goldring S.R. Role of bone in osteoarthritis pathogenesis. Med. Clin. North Am. 2009;93:25–35. doi: 10.1016/j.mcna.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Schmitter M., Essig M., Seneadza V., et al. Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofac. Radiol. 2010;39:231–234. doi: 10.1259/dmfr/16270943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahamsson A.K., Kristensen M., Arvidsson L.Z., et al. Frequency of temporomandibular joint osteoarthritis and related symptoms in a hand osteoarthritis cohort. Osteoarthritis Cartilage. 2017;25:654–657. doi: 10.1016/j.joca.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Bernhardt O, Biffar R, Kocher T, Meyer G. Prevalence and clinical signs of degenerative temporomandibular joint changes validated by magnetic resonance imaging in a non-patient group. 2007. [DOI] [PubMed]
- 30.Israel H.A., Diamond B., Saed-Nejad F., Ratcliffe A. Osteoarthritis and synovitis as major pathoses of the temporomandibular joint: comparison of clinical diagnosis with arthroscopic morphology. J. Oral Maxillofac. Surg. 1998;56:1023–1027. doi: 10.1016/s0278-2391(98)90246-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y.P., Ma X.C. Temporomandibular disorders related pain interaction with age, sex and imaging changes of osteoarthrosis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:757–758. [PubMed] [Google Scholar]
- 32.Israel H.A., Langevin C.J., Singer M.D., Behrman D.A. The relationship between temporomandibular joint synovitis and adhesions: pathogenic mechanisms and clinical implications for surgical management. J. Oral Maxillofac. Surg. 2006;64:1066–1074. doi: 10.1016/j.joms.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Dijkgraaf L.C., de Bont L.G., Boering G., Liem R.S. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J. Oral Maxillofac. Surg. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 34.Glasson S.S., Askew R., Sheppard B., et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 35.Kozaci L.D., Buttle D.J., Hollander A.P. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–174. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 36.Neuhold L.A., Killar L., Zhao W., et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem. J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton H., Rogerson F.M., East C.J., et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 39.Tortorella M.D., Malfait A.M., Deccico C., Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 40.Amano K., Densmore M., Nishimura R., Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. J. Biol. Chem. 2014;289:24898–24910. doi: 10.1074/jbc.M114.570507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldring M.B. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 42.He Y., Siebuhr A.S., Brandt-Hansen N.U., et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet. Disord. 2014;15:309. doi: 10.1186/1471-2474-15-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell P.G., Magna H.A., Reeves L.M., et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhen G., Wen C., Jia X., et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Q., Zhou G., Morello R., et al. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinohara M., Chang B.Y., Buggy J.J., et al. The orally available Btk inhibitor ibrutinib (PCI-32765) protects against osteoclast-mediated bone loss. Bone. 2014;60:8–15. doi: 10.1016/j.bone.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 48.Koga T., Inui M., Inoue K., et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi H., Kim S., Koga T., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 50.Kim C.D., Kim Y.K., Lee S.H., Hong K.W. Rebamipide inhibits neutrophil adhesion to hypoxia/reoxygenation-stimulated endothelial cells via nuclear factor-kappaB-dependent pathway. J. Pharmacol. Exp. Ther. 2000;294:864–869. [PubMed] [Google Scholar]
- 51.Asagiri M., Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto M., Sudo T., Saito T., Osada H., Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J. Biol. Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda F., Nishimura R., Matsubara T., et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 2004;114:475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monje P., Hernandez-Losa J., Lyons R.J., Castellone M.D., Gutkind J.S. Regulation of the transcriptional activity of c-Fos by ERK: A novel role for the prolyl isomerase PIN1. J. Biol. Chem. 2005;280:35081–35084. doi: 10.1074/jbc.C500353200. [DOI] [PubMed] [Google Scholar]
- 55.Ang E., Liu Q., Qi M., et al. Mangiferin attenuates osteoclastogenesis, bone resorption, and RANKL-induced activation of NF-kappaB and ERK. J. Cell. Biochem. 2011;112:89–97. doi: 10.1002/jcb.22800. [DOI] [PubMed] [Google Scholar]
- 56.Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki A., Iwata J. Mouse genetic models for temporomandibular joint development and disorders. Oral Dis. 2016;22:33–38. doi: 10.1111/odi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nozawa-Inoue K., Amizuka N., Ikeda N., et al. Synovial membrane in the temporomandibular joint--its morphology, function and development. Arch. Histol. Cytol. 2003;66:289–306. doi: 10.1679/aohc.66.289. [DOI] [PubMed] [Google Scholar]
- 59.Christo J.E., Bennett S., Wilkinson T.M., Townsend G.C. Discal attachments of the human temporomandibular joint. Aust. Dent. J. 2005;50:152–160. doi: 10.1111/j.1834-7819.2005.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 60.Guarda-Nardini L., Piccotti F., Mogno G., Favero L., Manfredini D. Age-related differences in temporomandibular disorder diagnoses. Cranio. 2012;30:103–109. doi: 10.1179/crn.2012.015. [DOI] [PubMed] [Google Scholar]
- 61.Manfredini D., Bucci M.B., Montagna F., Guarda-Nardini L. Temporomandibular disorders assessment: medicolegal considerations in the evidence-based era. J. Oral Rehabil. 2011;38:101–119. doi: 10.1111/j.1365-2842.2010.02131.x. [DOI] [PubMed] [Google Scholar]
- 62.Smith S.B., Maixner D.W., Greenspan J.D., et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J. Pain. 2011;12:T92–T101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuribayashi A., Okochi K., Kobayashi K., Kurabayashi T. MRI findings of temporomandibular joints with disk perforation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008;106:419–425. doi: 10.1016/j.tripleo.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Melchiorre D., Calderazzi A., Maddali Bongi S., et al. A comparison of ultrasonography and magnetic resonance imaging in the evaluation of temporomandibular joint involvement in rheumatoid arthritis and psoriatic arthritis. Rheumatology (Oxford) 2003;42:673–676. doi: 10.1093/rheumatology/keg181. [DOI] [PubMed] [Google Scholar]
- 65.Taskaya-Yilmaz N., Ogutcen-Toller M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J. Oral Maxillofac. Surg. 2001;59:860–865. doi: 10.1053/joms.2001.25015. [DOI] [PubMed] [Google Scholar]
- 66.Hinton R.J., Serrano M., So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod. Craniofac. Res. 2009;12:168–177. doi: 10.1111/j.1601-6343.2009.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sobue T., Yeh W.C., Chhibber A., et al. Murine TMJ loading causes increased proliferation and chondrocyte maturation. J. Dent. Res. 2011;90:512–516. doi: 10.1177/0022034510390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujisawa T., Kuboki T., Kasai T., et al. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J. Dent. Res. 2003;82:731–735. doi: 10.1177/154405910308200914. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Jiao K., Zhang M., et al. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J. Dent. Res. 2013;92:253–259. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiao K., Niu L.N., Wang M.Q., et al. Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats. Bone. 2011;48:362–371. doi: 10.1016/j.bone.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Xu L., Polur I., Lim C., et al. Early-onset osteoarthritis of mouse temporomandibular joint induced by partial discectomy. Osteoarthritis Cartilage. 2009;17:917–922. doi: 10.1016/j.joca.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Embree M., Ono M., Kilts T., et al. Role of subchondral bone during early-stage experimental TMJ osteoarthritis. J. Dent. Res. 2011;90:1331–1338. doi: 10.1177/0022034511421930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wadhwa S., Embree M.C., Kilts T., Young M.F., Ameye L.G. Accelerated osteoarthritis in the temporomandibular joint of biglycan/fibromodulin double-deficient mice. Osteoarthritis Cartilage. 2005;13:817–827. doi: 10.1016/j.joca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Hasegawa M., Horiki N., Tanaka K., et al. The efficacy of rebamipide add-on therapy in arthritic patients with COX-2 selective inhibitor-related gastrointestinal events: a prospective, randomized, open-label blinded-endpoint pilot study by the GLORIA study group. Mod. Rheumatol. 2013;23:1172–1178. doi: 10.1007/s10165-012-0819-2. [DOI] [PubMed] [Google Scholar]
- 75.Moon S.J., Park J.S., Woo Y.J., et al. Rebamipide suppresses collagen-induced arthritis through reciprocal regulation of th17/treg cell differentiation and heme oxygenase 1 induction. Arthritis Rheumatol. 2014;66:874–885. doi: 10.1002/art.38310. [DOI] [PubMed] [Google Scholar]
- 76.Byun J.K., Moon S.J., Jhun J.Y., et al. Rebamipide attenuates autoimmune arthritis severity in SKG mice via regulation of B cell and antibody production. Clin. Exp. Immunol. 2014;178:9–19. doi: 10.1111/cei.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kotake S., Nanke Y., Mogi M., et al. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur. J. Immunol. 2005;35:3353–3363. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 78.Hahm K.B., Park I.S., Kim Y.S., et al. Role of rebamipide on induction of heat-shock proteins and protection against reactive oxygen metabolite-mediated cell damage in cultured gastric mucosal cells. Free Radic. Biol. Med. 1997;22:711–716. doi: 10.1016/s0891-5849(96)00406-6. [DOI] [PubMed] [Google Scholar]
- 79.Rosen G.M., Pou S., Ramos C.L., Cohen M.S., Britigan B.E. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 80.Yamasaki K., Kanbe T., Chijiwa T., Ishiyama H., Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur. J. Pharmacol. 1987;142:23–29. doi: 10.1016/0014-2999(87)90649-2. [DOI] [PubMed] [Google Scholar]
- 81.Alcaraz M.J., Megias J., Garcia-Arnandis I., Clerigues V., Guillen M.I. New molecular targets for the treatment of osteoarthritis. Biochem. Pharmacol. 2010;80:13–21. doi: 10.1016/j.bcp.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 82.Pelletier J.P., Jovanovic D.V., Lascau-Coman V., et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43:1290–1299. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 83.Bullough PG. The role of joint architecture in the etiology of arthritis. 2004. [DOI] [PubMed]
- 84.Salo J., Lehenkari P., Mulari M., Metsikko K., Vaananen H.K. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 85.Amin A.K., Huntley J.S., Simpson A.H., Hall A.C. Chondrocyte survival in articular cartilage: the influence of subchondral bone in a bovine model. J. Bone Joint Surg. Br. 2009;91:691–699. doi: 10.1302/0301-620X.91B5.21544. [DOI] [PubMed] [Google Scholar]
- 86.Pan J., Zhou X., Li W., et al. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 2009;27:1347–1352. doi: 10.1002/jor.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang B., Jin H., Zhu M., et al. Chondrocyte beta-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model. Arthritis Rheumatol. 2014;66:107–120. doi: 10.1002/art.38195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu M., Chen M., Zuscik M., et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu M., Tang D., Wu Q., et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J. Bone Miner. Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glass D.A., II, Bialek P., Ahn J.D., et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Calatrava M.J., Prieto-Potin I., Roman-Blas J.A., et al. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res. Ther. 2012;14:R149. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan X.L., Meng H.Y., Wang T.C., et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage. 2014;22:1077–1089. doi: 10.1016/j.joca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez C., Deberg M.A., Piccardi N., et al. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1beta and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage. 2005;13:979–987. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Sanchez C., Deberg M.A., Piccardi N., et al. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005;13:988–997. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]