Abstract
Background:
A substantial body of studies supports the view that molecular and cellular features of endometriotic lesions differ from those of eutopic endometrium. Apart from that, evidence exists that the eutopic endometrium from pa-tients with endometriosis differs from that of females without endometriosis.
Objective:
Aberrant expression profiles include a number of non-steroid signaling pathways that exert their putative influ-ence on the pathogenesis of endometriosis at least in part via crosstalk(s) with estrogen-mediated mechanisms. A rational to focus research on non-steroid signal pathways is that they might be remunerative targets for the development and selection of novel therapeutics to treat endometriosis possibly without affecting estrogen levels.
Results and Conclusion:
In this article, we describe molecular and cellular features of endometriotic lesions and focus on the canonical WNT/β-signaling pathway, a key regulatory system in biology (including stem cell homeostasis) and often in pathophysiological conditions such as endometriosis. Recently emerged novel biological concepts in signal transduction and gene regulation like exosomes and microRNAs are discussed in their putative role in the pathogenesis of endometriosis.
Keywords: WNT/β-catenin signaling, endometriosis, microenvironment, reproductive tract, stem cells, exosomes
1. INTRODUCTION
Endometriosis, a gynecological condition commonly observed in women of reproductive age, is histologically characterized by the presence and growth of endometrial-like glands and stroma outside the uterine cavity and musculature, which undergoes cyclic proliferation and breakdown similar to the endometrium. This internal bleeding, which cannot leave the body and remains on site, often results in local inflammatory reactions causing scar tissue formation and adhesions during repair processes. Endometriosis is in the majority of cases associated with dysmenorrhea, dyspareunia and/or pelvic pain, and can significantly compromise the quality of life of affected women. The prevalence in the general population is difficult to determine, largely because it can be asymptomatic or misdiagnosed, hence it has been reported to be anywhere between 5-10% in menstruating women and up to 35% in infertile women [1-4].
According to the literature, three different forms of endometriosis can occur in the pelvic cavity: peritoneal, ovarian, and deeply infiltrating lesions. The morphology and appearance of peritoneal and ovarian implants are described as white (white, yellow-brown, and peritoneal defects such as blebs), red (red, red-pink, and clear lesions), and black (black and blue lesions) [5]. Red lesions are usually highly vascularized active lesions whilst white opacification and yellow-brown lesions are latent stages of endometriosis resulting from inflammatory processes with subsequent fibrosis, total devascularization and the presence of old collagens as white plaques. Black lesions resemble enclosed implants with the presence of intraluminal debris formed by alternated tissue breakdown and healing during scarification of red lesions [5]. In rare cases, endometriosis occurs extraperitoneal in more remote sites including the colon, kidney, liver, pancreas and lungs [6-10].
1.1. Current Theories on the Pathogenesis of Endometriosis
To date the pathogenesis of endometriosis is still poorly understood and controversial despite decades of research. Several theories for its pathogenesis were proposed in recent years: i) implantation theory [11]; ii) metaplasia theory [12, 13]; iii) induction theory [14]; endometriosis disease theory [15]; and iv) endometriosis as a stem cell based condition [16-18] and reviewed in [19, 20]. Recently, Laux-Biehlmann et al. proposed another way to look at endometriosis development and associated pain based on inflammatory processes and activation of peripheral nerve endings in response to menstrual debris derived from retrograde and extra-uterine menstruation of endometriotic lesions [21].
The most widely accepted implantation theory [11] is based on the assumption that a small and early lesion is established and its subsequent growth and invasion leads to a progressive disease. Here, the origin of endometriotic tissue in the pelvic cavity is retrograde transported viable menstrual endometrial cells. These shed menstrual endometrial cells retain the ability to attach to the peritoneum, proliferate and differentiate, and invade the underlying tissue. Further dispersion of endometrial cells via the lymphatic systems [22, 23] and reviewed in [24] might be the origin of lesions at more distant locations such as thoracic or cerebellar endometriosis [10, 25, 26].
As a prerequisite to support the implantation theory several factors have to be met: i) occurrence of retrograde menstruation [27-29]; ii) presence of viable endometrial cells in the retrograde refluxed menstrual efflux [30, 31]; and iii) adhesive capacity of shed endometrial cells onto the peritoneum alongside proliferation and implantation [32].
The peritoneal cavity underlies a dynamic change of fluid (peritoneal fluid, PF) derived from e.g. macrophage secretions, ovarian exudate, refluxed tubal fluid, plasma transudate and refluxed endometrial material via retrograde menstruation and is thus an important constituent of the peritoneal environment [33, 34]. This dynamic exchange of fluid in the pelvic cavity could be one explanation for the anatomical distribution of endometriotic lesions that correlates well with principles of transplant biology [7, 35] and is thus in favor of the implantation theory. On the other hand, endometriosis is observed in only a subgroup of women, despite the fact that PF contains endometrial tissue in up to 59% of patients irrespective of endometriosis present or the stages of the menstrual cycle [32, 36-39]. However, a prolonged and heavier menstrual flow observed in women with endometriosis could increase the retrograde refluxed material in the pelvic cavity in comparison to healthy women with patent tubes [38, 40, 41].
The phenomenon of restricted endometriosis development could therefore be due to a permissive peritoneal environment favoring the implantation and growth of endometrial cells in only a certain subgroup of women. It is therefore conceivable that early endometriotic foci development depends not only on their location and depth of infiltration but also on the influence of various factors such as hormones, cytokines, growth factors and other factors present in peritoneal or ovarian fluid or the blood stream [42]. In line with this notion is the observation that eutopic and ectopic endometrial cell proliferation is enhanced in the presence of PF and follicular fluid from women with endometriosis [34, 43-45]. Tumor necrosis factor-α (TNF-α) is one factor responsible for this increased proliferative potential [45-48] but also the influence of other cytokines and steroid hormones have been investigated [47-50]. A recent study by Han et al. shows the requirement for estrogen-mediated signaling and TNF-α for apoptosis evasion and enhanced proliferation of ectopic lesions in an animal model [51]. Another possibility for generating a permissive environment for endometriosis induction could be a natural occuring microtrauma of the e.g. the uterus or the peritoneal surfaces followed by intrinsic inflammatory responses and repair mechanism. Leyendecker and colleagues proposed a new concept of tissue injury and repair mechanism (TIAR) (reviewed in [52, 53]) to explain a common pathophysiology of adenomyosis and endometriosis development. TIAR is based on the observation that women suffering from endometriosis or adenomyosis display alterations in dysperi- and hyperstalsis waves (reviewed in [54, 55]) which might attribute for more trauma. In addition, this altered uterine peristalsis could cause the dislocation of more basal endometrium und thus a greater number of stem cell-like cells present in the retrograde refluxed menstruum [56]. Furthermore, eutopic endometrium from women with endometriosis displays a reduced decidualization capacity [57] indicating that more un-differentiated cells are flushed retrogradely into the peritoneal cavity. Microtrauma could also cause the exposure of extracellular matrix components (ECM) in the peritoneal cavity which has been shown to promote adhesion and proliferation of endometrial stromal cells [58]. Furthermore, surgery in itself could aggravate the development or progression of endometriosis by repair processes under the concept of TIAR.
As an alternative to the implantation theory serves the coelomic metaplasia theory of Müllerian-type epithelium [12, 13] which could explain the rare cases of endometriosis in women without retrograde menstruation or with abnormal fallopian tubes [59] and in men undergoing high doses of estrogen treatment for prostatic carcinoma [6] or suffering from Persistent Mullerian Duct Syndrome (PMDS) [60]. An indication that endometriosis could develop by metaplasia comes from women suffering from the Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome which developed endometriosis despite the absence of menstruation [61-63]. Women with MRHK display various degrees of müllerian duct defects such as congenital absence of uterus and vagina or only a rudimentary uterus with or without functional endometrium [64]. Endometriosis could develop through metaplasia under these circumstances due to e.g. aberrant activation of genes in the peritoneum normally active during embryonic development of the female genital tract including uterine gland development [65]. The concept of metaplasia is also reflected in the embryonic rest theory as developmentally misplaced müllerian/endometrial tissue could be stimulated to undergo metaplasia. This is supported by recent evidence that displaced embryonic epithelial remnants or ectopic endometrial-like glands can be found along the fetal female reproductive tract [66-68] serving as a possible source for endometriotic lesions. However, endometriotic lesions occur also at other sites outside the course of the Müllerian ducts.
The induction theory represents a combination of the implantation and coelomic metaplasia theories and postulates that unknown substances released from shed and degenerating endometrium induces undifferentiated mesenchyma to form endometrial-like tissue [14]. In summary, the above theories focus on the onset of endometriotic lesions but are insufficient to explain the occurrence of severe endometriosis. The step-wise progression from temporary lesions to early endometriotic lesions and into severe forms resembling benign tumors might be explained by cellular modifications resulting from epigenetic or genetic alterations and is addressed in the endometriotic disease theory (EDT) [15].
In favor of this (epi)genetic concept is the observation that cystic ovarian endometriosis is clonal in origin [69, 70] and that some endometriotic cells are invasive in vitro, associated with the loss of E(pithelial)-cadherin expression, a phenomenon usually observed in tumor biology [71, 72]. In addition, there is increasing evidence of a germline predisposition to endometriosis. A familial clustering of endometriosis in humans [73] and rhesus monkeys [74] as well as increased prevalence among first-degree relatives of women with all disease severities compared to the general population [75] has been reported. Furthermore, the age at onset of symptoms is similar in affected, non-twin sisters [76] and there is concordance in monozygotic twins [74]. Additionally, environmental factors, such as chronic exposure to dioxins might also play a role in disease etiology [15, 77]. These observations lead to the conclusion that endometriosis is likely to be a complex genetic trait in which multiple genes interact with each other and the environment to produce the disease phenotype [15].
The endometrium is a highly regenerative tissue and it is not surprising that it contains cells with stem cell characteristics (reviewed in [19, 20, 78]). Evidence that endometriosis might be a stem cell-based condition comes from the observation that freshly isolated endometrial epithelial and stromal cells contain a rare population of cells with clonogenic activity visualized as colony-forming units (CFUs; [79]). The CFUs in the endometrial stromal cell fractions are comparable to mesenchymal stem cells (MSC) in their multilineage differentiation potential [80]. Enrichment of these endometrial MSC-like cells (eMSCs) is possible by their co-expression of the perivascular cell markers CD146 and PDGF-Rb. The clonogenicity of the endometrial epithelial and stromal cells showed a non-significant trend depending on the menstrual cycle stage with an increased clonogenicity in the proliferative stage for stromal cells and in the secretory stage for epithelial cells. CFUs could also be detected in noncycling endometrium [81].
Retrograde misplaced MSCs in the pelvic cavity could therefore be a critical factor in establishing an early endometriotic lesion. More importantly, menstrual blood contains cells with plasticity, namely Endometrial Regenerative Cells (ERC; [82]). ERCs resemble MSC in their appearance, growth properties and differentiation potential into various cell types. However, in contrast to MSCs, they express matrix-metalloproteases (MMP-3 and MMP-10), the angiogenic factor ANG-2 and cytokines (GM-CSF, PDGF-BB) as revealed by proteome analysis [82]. Musina et al. described the morphology of menstrual blood-derived MSC (referred to as MenSCs or MMCs) as typical fibroblast-like and similar to bone marrow-derived MSCs [83]. Another study confirmed the broad plasticity of MenSCs [84]. In general, MenSCs display a higher clonogenicity, proliferation and migration rate than bone marrow-derived MSCs and higher angiogenic potential both in vitro and in an animal model [85]. Hida et al. tested the potential of MenSCs to participate in repair processes in a rat model of Myocardial Infarction [86]. Here, MenSCs participated in the restoration of impaired cardiac function by differentiating into MenSCs-derived cardiomyocytes at the transplantation site. MenSCs can exert antimicrobial and immunomodulatory properties and secrete tissue regenerative factors in the cecal ligation and puncture (CLP) mouse sepsis model [87]. However the immunomodulatory capacities of MenSCs depend on the animal model system as e.g. lower immunosuppressive ability is observed in a chronic inflammatory arthritis (CIA) animal model whilst in an experimental xenogenic graft versus host disease (GVHD) model MenSCs caused higher survival rates independent of the degree of inflammation [88].
Thus menstrual blood contains cells with plasticity which are a novel source for cell-based replacement therapies (reviewed in [89]). These results clearly indicate that retrograde menstruation can transport cells with a stem-cell-like phenotype into the pelvic cavity and that possibly more than one cell type with putative stem/progenitor cell properties exists. The research into menstrual blood derived cells with plasticity is still at an early stage. This is also the reason why several studies report the expression of different immunophenotypic profiles of MenSCs [90]. A standardized approach to isolate and characterize the stem cell-like cells in menstrual blood is of importance to decipher their role in the pathogenesis of endometriosis.
1.2. Pathogenesis of Endometriotic Lesions
Irrespective of the mechanism, one can presume that peritoneal, ovarian and rectovaginal lesions may have discrete pathologies and etiologies [5]. The pathogenesis of peritoneal endometriotic lesions is most likely due to the implantation of retrograde refluxed menstrual endometrium through the fallopian tubes during menstruation [5, 11, 91, 92].
Ovarian endometriosis formation manifesting as typical chocolate cysts is apparently more controversial. It might be attributable to several scenarios: i) inversion and progressive invagination of the ovarian cortex after accumulation of menstrual debris derived from bleeding of superficial endometriotic implants, which are located on the ovarian surface and adherent to the peritoneum [35, 93, 94]; ii) secondary involvement of functional ovarian cysts by endometrial implants located on the ovarian surface [95-97]; or iii) metaplasia of the coelomic epithelium covering the ovary [5, 12, 98].
For the formation of deeply infiltrating endometriosis of the rectovaginal septum a natural evolution of peritoneal endometriosis of the pouch of Douglas due to secondary infiltration has been proposed [99, 100] and reviewed in [101]. It could also be an adenomyotic nodule originating by metaplasia of müllerian/embryonic remnants located in the rectovaginal septum [5].
A permissive peritoneal environment for the onset and progression of endometriotic lesions might also be associated with altered function of immune-related cells alongside the local pelvic inflammatory processes aiding the evasion of clearance by the immune system. Evidence in the literature indicates a reduced macrophage-mediated cytolysis in women with endometriosis [102] and altered leukocyte populations within endometriotic lesions possibly secreting abnormal levels of local and systemic proinflammatory cytokines and growth factors with growth-promoting and angiogenic properties [103, 104]. Apart from the amount of refluxed menstrual endometrium present in the peritoneal cavity altered secretion of immune factors, formation of autoantibodies, impaired immune recognition and clearance of ectopic endometrial cells facilitate the onset and progression of endometriosis [105-107]. A recent viewpoint by Laschke and Menger suggests that the gut microbiome could be crucial in the pathogenesis of endometriosis by aberrant priming of immune responses [108]. Clearly, an altered immune response is a key factor in evasion of apoptosis of endometrial cells at ectopic sites in women with endometriosis (reviewed in [109]).
1.3. Classification Systems of Endometriosis
To date, a number of proposed systems to classify the different forms of endometriosis exist. These include those by Acosta et al. [110], Kistner et al. [111], the American Fertility Classification [112] and its modifications and renaming into revised American Society for Reproductive Medicine classification of endometriosis [113, 114]. Primarily, all of these classifications divide endometriosis into various stages related to stages of increasing severity with involvement of the ovaries and with adhesion formation. The number, size and location of peritoneal endometrial implants, plaques, endometriomas and/or adhesions charted with a score system translate then into various disease stages. The common goal of these classification systems is to predict based on disease severity, the chance for conception after treatment.
The rASRM classification [112-114] is used most commonly in investigative studies hence providing a tool to compare results published by different authors. It has been shown to provide a good and reproducible tool in staging endometriosis both during surgery and by a blinded reviewer using visual documentation [115]. However, other studies showed that the rASRM classification system is prone to observational error and is not as effective in predicting pregnancy [116-118]. A limitation of the rASRM staging system is the scoring of only intraperitoneal endometriosis thereby underrepresenting other manifestations of endometriosis such as extraperitoneal lesions in the bowl or bladder.
New options to classify and score endometriosis are therefore currently under investigation in order to reflect the multifaceted aspects of endometriosis and its impact on fertility. One of them is the endometriosis fertility index (EFI) which is a tool to assess pregnancy outcomes after endometriosis surgery [119]. The EFI takes into consideration the reproductive potential by scoring the fallopian tubes, fimbria and ovaries omitting uterine abnormalities. The ENZIAN-score is a staging system based on tumor grading systems which takes the localization and severity of deep infiltrating and retroperitoneal lesions into account [120]. Clinical assessment of the practicality and reproducibility of the ENZIAN-score revealed that it is helpful but requires further adaptations [121]. One critical aspect is e.g. the occurrence of duplicate scoring of lesion between rASRM and ENZIAN staging systems thus ENZIAN is in its current version not a complementation of the rASRM system. Its revisions simplified the scoring system and enhanced its benefit for staging deep infiltrating retroperitoneal endometriosis but it still lacks poor international acceptance [122-125].
1.4. Local Microenvironment: Driving Factor in the Onset and Progression of Endometriotic Lesions
Endometriotic lesions are composed of the same structural units as the lining of the uterus, the endometrium. The main components are glandular epithelial cells surrounded by stromal cells embedded at the ectopic site. Glandular epithelium is cytokeratin positive and is apparently composed of two cells types, namely E-cadherin positive and very few E-cadherin negative cells. This phenomenon of rare E-cadherin negative cells in endometriotic glands was first reported by our group for peritoneal lesions [72, 126] and has since been observed for ovarian endometrioma and rectovaginal endometriosis by others [127]. Endometriotic stromal cells express mesenchymal markers such as vimentin and THY-1 and can be distinguished from surrounding fibroblasts by e.g. expression of the membrane metallo-endopeptidase CD10 (common acute lymphocytic leukemia antigen, CALLA) [57, 128-130]. Differences in the composition of the extracellular matrix (ECM) surrounding endometriotic glands and stroma is also reported [131-133] which could influence the functional responses of e.g. endometriotic stromal cells such as their adhesive, proliferative and invasive properties [58].
The local microenvironment plays a pivotal role in the onset and progression of an endometriotic lesion, as misplaced endometrial cells need to respond to local stimuli such as factors in PF, evasion of immune detections and adhesion to the host tissue surface [109, 134]. Once an ectopic lesion is formed crosstalk between stromal and epithelial cells, paracrine signaling, hormonal responsiveness and angiogenesis are required for the persistence at the ectopic site. A study by Hull et al. identified key pathways active in the molecular interactions between ectopic endometrial tissue and its site of attachment [135]. In an elegant approach, comparing microarray data obtained from a xenotransplant model and paired eutopic versus ectopic endometrial samples, they identified alterations in four pathways: cellular injury (ubiquitin/proteasome), inflammation (NFκB), tissue remodeling (TGF-β) and cellular proliferation (KRAS). A very recent proteomic study of peritoneal endometriotic stromal cells revealed extensive metabolic reprogramming and acquisition of cancer-like changes reflected in increased cellular invasiveness and adhesiveness, reduced apoptotic potential and altered immune function [136].
The local microenvironment could also influence the growth and differentiation ability of the misplaced cells by altering gene expression through e.g. epigenetic changes (DNA methylation, histone modifications, miRNA; reviewed in [137, 138]). An increasing body of evidence exists on the role of microRNAs in endometriosis progression. In particular it was speculated by Teague et al. that endometriosis-associated molecular networks [135] are regulated by miRNA at the posttranscriptional level [139]. Indeed, they identified 22 miRNAs as aberrantly expressed in endometriotic lesions [139]. Zhao et al. performed an association study aiming to explore the relationship between these 22 miRNA and SNPs in their target sites for the risk of developing endometriosis [140]. Another study investigated the miRNome of peritoneal lesions and identified 5 unique miRNAs present in endometriotic epithelial cells which are not found in the surrounding healthy tissue [141].
Apart from differential expressed miRNAs, altered DNA methylation pattern occur during endometriosis onset and/or progression. As endometriosis is an estrogen-dependent but progesterone resistant condition it is not surprising that their respective promotor regions are affected accordingly [142, 143]. Beside epigenetic modifications, endometriotic cells display also chromosomal anomalies and instability that could alter gene expression by loss of or mutations of DNA sequences (reviewed in [144, 145]). This could be one mechanism to explain the observed deregulation of signaling pathways in endometriotic cells if for example key regulatory proteins are affected by the genetic alterations.
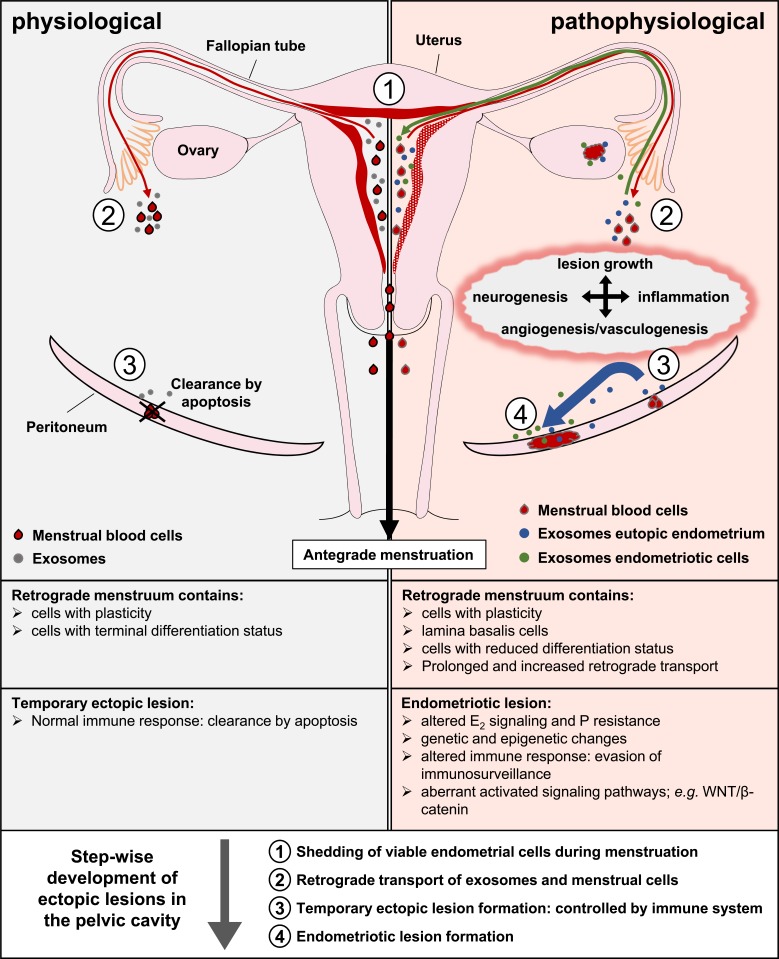
With the discovery that cells release extracellular vesicles (EVs) such as exosomes and microvesicles composed of diverse types of membranes of plasma and endosomal membrane origin researchers focused on extracting these EVs from various body fluids. EVs are an alternative source for intercellular communication as they contain e.g. miRNAs or enzymes and are able to modulate cellular responses e.g. survival, differentiation or modulation of immunogenic responses (reviewed in [146, 147]). Thus, exosomes could also be important for endometriosis as endometrial epithelial cell-derived exosomes contain miRNAs with target genes in signaling pathways connected to successful embryo-endometrial crosstalk during implantation such as adherence junctions, ECM-receptor interactions, the VEGF-signaling pathway, the Jak-STAT pathway and the Toll-like receptor signaling pathway [148]. Texidó et al. could show the presence of ectonucleotidase containing exosomes in aspirates from endometriomas which could contribute to endometriosis progression and local suppression of immune responses by regulating extracellular ATP and rising extracellular adenosine levels [149]. A study by Harp et al. showed that endometriotic stromal cell derived exosomes could exert enhanced angiogenic effects [150]. Another study by Braza-Boïls et al. observed a modified miRNA expression profile in endometrial stromal cells from women with endometriosis including miRNAs involved in angiogenesis mediated by peritoneal fluid (PF) from endometriosis patients [151]. It is conceivable that endometrial exosomes could be flushed retrograde into the pelvic cavity or be shed there by menstrual cells and influence the fate of ectopic cells. Exosomes could therefore be one important factor to enable a temporary endometriotic lesion to establish a sufficient blood supply in order to grow and survive at the ectopic site as they act in an autocrine, paracrine and endocrine manner in intercellular communication. Endometrial exosomes from women with endometriosis might also play a role in endometriosis manifestation as a disease. The onset and progression of endometriosis could therefore be a combination of several steps and factors. The seeding endometrial tissue in women with endometriosis displays intrinsic (epi)genetic, biochemical and structural changes [57, 152-158] and their shed endometrial-derived exosomes could prime the soil for attachment at ectopic sites by retrograde flushed exosomes into the peritoneal cavity and local modulation of cells and tissue via intercellular communication. Retrograde transported menstrual cells could attach to this primed soil and form temporary lesions. The released exosomes of ectopic endometrial cells could facilitate immune evasion; enhance proliferation, invasion and angiogenesis of the lesion and subsequent progression into a persistent endometriotic lesion (Fig. 1). Thereby intercellular communication mediated via exosomes could also represent a missing link between the different theories on the pathogenesis of endometriosis. Exosomes released by eutopic or ectopic endometrium or shed endometrial cells could e.g. induce metaplasia of cells at ectopic sites (coelomic metaplasia and induction theories) or aid in tissue remodeling after injury (TIAR concept). Furthermore, exosomes could exert morphoregulatory function by altering signal transduction pathways.
Fig. (1).
Schematic overview of endometriosis development. Shed menstrual endometrium leaves the cavity mainly antegradely (black arrow) but is also flushed retrograde into the pelvic cavity (red arrow). Endometrial derived exosomes can also be transported retrograde into the pelvic cavity even in the absence of menstruation (grey and blue filled circles). Once in the pelvic cavity, menstrual cells can attach by gravity to the peritoneal surfaces and form temporary lesions. In healthy women, temporary ectopic lesions are removed by the immune system through apoptosis induction. In women developing endometriosis as a disease, these ectopic lesions evade the immunosurveillance and progress in response to e.g. locally present cytokines and/or growth factors (blue arrow). Exosomes derived from endometriotic cells (green filled circles) could act in an autocrine/paracrine manner but could also be transported back through the fallopian tubes into the uterine cavity (green arrow) and modulate signaling events in eutopic endometrium. Albeit the implantation theory is the most likely explanation for the pathophysiological lesion formation, the step-wise development of ectopic lesions could also be explained in part by other proposed theories. For example, the TIAR concept suggests that trauma could cause the displacement of lamina basalis cells (altered uterine contractility) but also the exposure of ectopic attachment sites (injury). This could allow the attachment and invasion of ectopic cells followed by wound closure. It is also conceivable that exosomes could mediate metaplasia of peritoneal cells in other cases.
1.5. Non-Steroid Signaling: The WNT Connection in Endometriosis
As outlined in this article and by other authors in this issue, the establishment and progression of endometriosis as a disease requires a number of biological processes that appear aberrant in ectopic endometrium if compared to eutopic endometrium. These differences concern for example the responsiveness of the ectopic cells to a variety of signaling peptides, subsequently their adhesion to and invasion into the peritoneum, their morphogenesis, development and dysregulation of apoptosis as well as the angiogenesis in the ectopic endometrium. Since endometriosis is frequently a relapsing disease (eventually even after hysterectomy), a subpopulation of endometriotic cells exhibits most likely stem cell characteristics and/or plasticity, prerequisites for the recurrence of the disease (see outlined above). Apart from the morphological similarities between eutopic and ectopic endometrium, numerous studies revealed differences in the transcriptome of the two tissues. For example, comparative analyses of gene expression patterns between eutopic and ectopic endometrium [159] discovered many dysregulated genes assigned to particular pathways. Among others and as anticipated, these were genes of the cell cycle, adherence and tight junctions as well as those of MAP kinase, TGF-β, WNT, Jak-STAT and mTOR signaling pathways. In addition, a number of cytokine-cytokine receptor interactions appeared dysregulated. Whether and how far the indicated pathways contribute to the pathogenesis of endometriosis by integrating different signals and activities is poorly understood and under intensive investigation. Although considered an estrogen-dependent disorder, several non-steroid pathways are apparently important for the pathogenesis of endometriosis. Therefore, it is the hope that effectors of signaling pathways possibly involved in the pathogenesis of endometriosis, particularly kinases, may serve as potential targets for non-steroid therapeutics in the future (reviewed in [160]).
Hence, a highly relevant issue related to the analyses of different signaling pathways in endometriosis is whether and how female sex hormones, the estrogens (E2), possibly connect to and affect non-steroid signaling activities. In recent years, several studies have searched for such interactions and for example found them in the context of the WNT/β-catenin signal pathway. This part of the paragraph will focus on such findings.
The canonical WNT/β-catenin signal pathway, stimulated by individual WNT ligands binding to the frizzled and LRP5/6 receptors, is a key regulatory system in biology and often in pathophysiology. It is essential for the development of multicellular organisms (from hydra to mammals) as well as for the homeostasis of many of their regenerating tissues. A substantial body of literature presents findings that prenatal as well as postnatal processes are orchestrated by WNT signaling, in particular those which depend on the proper renewal (and thus controlled proliferation) of somatic stem cells. Examples are endometrium, mammary gland, blood vessels and intestine. Notably, dysregulated and/or mutated components of the WNT/β-catenin pathway often contribute to formation and/or progression of different types of tumors but also other human diseases such as osteoporosis or type 2 diabetes (reviewed in [161]). Although WNT signaling is subject of intensive studies in many research areas, it is only limited in the focus of investigations in endometriosis.
The central signal-transducing molecule of the WNT pathway is the multifunctional protein β-catenin, belonging to the family of armadillo-repeat proteins. It is a direct binding partner of the intercellular adhesion and metastasis suppressor protein E-cadherin thereby exerting a morphoregulatory function. Hence, it is important for the structure and stabilization of the adherence junctions (AJ) and thus for formation of functional epithelial tissues. Disruption of AJs by stimulation of epithelial mesenchymal transition (EMT) through signaling of for example receptor tyrosine kinases (RTK; e.g. EGF receptors) induces loss of epithelial cell architecture. Consequently, cells become more motile and may invade surrounding tissue (reviewed in [162]).
Cytoplasmic ß-catenin not binding to E-cadherin underlies constant degradation, a process highly controlled by different proteins and a cascade of phosphorylation events at β-catenin itself. Within this process, phosphorylation of β-catenin through kinase GSK3β finally initiates its degradation. In turn, inhibition of GSK3β leads to stabilization of β-catenin allowing its transcriptional activation as described below.
Stabilized β-catenin can reach the nucleus and interact with members of the transcription factor family TCF-LEF. This complex regulates transcription of its target genes. These are for example players in the regulation of proliferation, tissue development and architecture as well as angiogenesis [161].
In a recent paper, Xiong et al. hypothesized a link between estrogen and β-catenin in the pathogenesis of endometriosis. They showed that estradiol (E2) treatment of human endometrial stromal cells (HESC) from patients with endometriosis enhances the level of β-catenin, its nuclear localization as well as the cells’ invasiveness. Downregulation of β-catenin in HESCs decreases invasiveness. Along these lines, implantation of human endometrium into the pelvic cavity of immune-compromised NOD-SCID mice under E2 injection led to upregulation of vascular vascular endothelial growth factor (VEGF) and MMP-9 as well as the formation of adhesive and invasive endometriotic lesions. Downregulation of β-catenin prevented such lesions and repressed VEGF and MMP-9 expression. These data imply that E2 might accelerate disease progression by upregulating β-catenin and thus it target genes possibly under conditions of abnormal estrogen levels [163].
In a another report, Zang et al. addressed the question whether the neovascularization of endometriotic lesions as one important step of lesion survival is enhanced by upregulation of VEGF through E2 [164]. The authors present an interesting set of data indicating how VEGF, E2 and canonical WNT signal transduction depend on each other mechanistically in the pathogenesis of endometriosis. E2 first enhances the level of β-catenin protein possibly through binding of the estrogen receptor α (ERα) to ERE sites in the β-catenin promoter thereby stimulating its transcription. Subsequently, nuclear β-catenin/TCF-LEF complexes target to the TCF-LEF binding sites in the VEGF gene promotor finally enhancing the expression of VEGF mRNA.
The results and conclusions as presented [164] are supported by a paper published by de Mattos et al. studying components of the WNT/β-catenin pathway in a rat model of peritoneal endometriosis [165]. In summary, these results imply effects on cell proliferation and angiogenesis by activation of the WNT pathway. More in detail, they identified decreased levels of GSK3β and E-cadherin as well as a higher amount of nuclear β-catenin in endometriotic lesions when compared to uterine endometrial tissue. Such data are consistent with the idea that a decrease in GSK3β leads to stabilization of β-catenin, which then exhibits elevated expression and presence in the nucleus. This in turn should lead to enhanced expression of β-catenin target genes of which VEGF is required for angiogenesis. Why endometriotic lesions exhibit a decrease in E-cadherin mRNA as compared to endometrial tissue is not clear. A rather trivial explanation for this observation is that ectopic endometrium contains more stromal than epithelial cells. Nevertheless, E-cadherin appears dysregulated in a number of cells of ectopic lesions as shown by our group and others [72, 126, 127]. Complete or partial EMT taking place in the lesions might be the responsible mechanism resulting in the downregulation of E-cadherin. This would alter the molecular composition and thus functional features of the epithelial cells in the ectopic endometrial lesions.
de Mattos et al. also found that elevated levels of WNT4 and WNT7b in the ectopic lesions [165]. This is in agreement with previous reports by Gaetje et al. showing increased WNT4 and WNT7a levels in human endometriotic lesions [65, 166]. Despite the fact that these WNT ligands are obviously important for the normal development of endometrium, it seems hitherto difficult to find a coherent explanation for their role in the establishment and/or maintenance of endometriosis. Since WNT ligands such as WNT4 act as regulators of cell proliferation and differentiation, it appears however likely that they influence such processes also in pathophysiological events like endometriosis or tumor development.
Based on the reports referred to above it might be anticipated that inhibition of the WNT/β-catenin pathway reduces for example cell migration, invasion and matrix metalloproteinase expression (necessary for invasion). Such cell features are prerequisites to develop and possibly maintain endometriotic lesions. Indeed, Matsuzaki and Darcha showed inhibitory effects of the small molecule PKF 115-584 on cell migration and invasion in epithelial and stromal cells in vitro from patients with endometriosis prepared at the menstrual phase [167]. This inhibition of TCF-LEF/β-catenin-mediated effects and therefore target gene expression was weaker in epithelial and stromal cells from patients without endometriosis than from patients with endometriosis.
Taken together, the current knowledge of the WNT/β-catenin pathway in endometriosis favors the idea that it might be a remunerative target for the development of novel therapeutics. This is in line with a recent report describing screenings of naturally derived WNT signal modulators anticipated to bind to and affect mainly β-catenin activity in pathophysiological but not physiological conditions [168].
CONCLUSION
The molecular hallmarks of endometriosis comprise a hormone-dependent (estrogen-dependence, progesterone resistance) and inflammatory condition with a (epi)genetic predisposition driven most likely by cells with plasticity. Newly discovered biological concepts of general relevance (e.g. exosomes and miRNAs) are also relevant for the pathogenesis of endometriosis. Intercellular communication mediated by exosomes could be viewed as a novel mechanistic tool to orchestrate cell fate by e.g. modulating signaling pathways. A challenge in endometriosis research will be the assessment of non-steroid signaling pathways as targets for novel therapeutics to treat endometriosis. This might be a chance to replace E2-depletion therapies to minimize adverse side effects such as early menopause.
Consent for Publication
Not applicable.
Acknowledgements
Both authors contributed equally.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Cramer D.W. 1995. Epidemiology of endometriosis in adolescents. [Google Scholar]
- 2.Olive D.L., Schwartz L.B. Endometriosis. N. Engl. J. Med. 1993;328(24):1759–1769. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 3.Osteen K.G., Bruner-Tran K.L., Eisenberg E. The disease endometriosis. In: Mp D., Kg O., editors. Endometrium & Endometriosis. 1995. [Google Scholar]
- 4.Wellbery C. Diagnosis and treatment of endometriosis. 1999. [PubMed]
- 5.Nisolle M., Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997;68(4):585–596. doi: 10.1016/s0015-0282(97)00191-x. [DOI] [PubMed] [Google Scholar]
- 6.Oliker A.J., Harris A.E. Endometriosis of the bladder in a male patient. J. Urol. 1971;106(6):858–859. doi: 10.1016/s0022-5347(17)61418-6. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins S., Olive D.L., Haney A.F. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet. Gynecol. 1986;67(3):335–338. [PubMed] [Google Scholar]
- 8.Halme J.D.S. 1995. Endometriosis and its medical management. [Google Scholar]
- 9.Machairiotis N., Stylianaki A., Dryllis G., et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn. Pathol. 2013;8(1):1–12. doi: 10.1186/1746-1596-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augoulea A., Lambrinoudaki I., Christodoulakos G. Thoracic endometriosis syndrome. Respiration. 2008;75(1):113–119. doi: 10.1159/000105102. [DOI] [PubMed] [Google Scholar]
- 11.Sampson J. The development of the implantation theory for the origin of peritoneal endometriosis. Am. J. Obstet. Gynecol. 1940;40:549–556. [Google Scholar]
- 12.Meyer R. Ueber den stand der frage der adenomyositis und adenomyome im allgemeinem und insbesondere ueber adenomyositis serosoepithelialis und adenomyometritis sarcomatosa. Zentralbibliothek Gynaecologie. 1919;43:745–750. [Google Scholar]
- 13.Fujii S. Secondary mullerian system and endometriosis. Am. J. Obstet. Gynecol. 1991;165(1):219–225. doi: 10.1016/0002-9378(91)90255-p. [DOI] [PubMed] [Google Scholar]
- 14.Levander G., Normann P. The pathogenesis of endometriosis; an experimental study. Acta Obstet. Gynecol. Scand. 1955;34(4):366–398. doi: 10.3109/00016345509158287. [DOI] [PubMed] [Google Scholar]
- 15.Koninckx P.R., Barlow D., Kennedy S. Implantation versus infiltration: the Sampson versus the endometriotic disease theory. Gynecol. Obstet. Invest. 1999;47(Suppl. 1):3–9. doi: 10.1159/000052853. [DOI] [PubMed] [Google Scholar]
- 16.Starzinski-Powitz A., Zeitvogel A., Schreiner A., Baumann R. Zentralbl. Gynakol. 2003;125(7-8):235–238. doi: 10.1055/s-2003-42276. [Endometriosis--a stem cell disease?]. [DOI] [PubMed] [Google Scholar]
- 17.Starzinski-Powitz A., Zeitvogel A., Schreiner A., Baumann R. In search of pathogenic mechanisms in endometriosis: the challenge for molecular cell biology. Curr. Mol. Med. 2001;1(6):655–664. doi: 10.2174/1566524013363168. [DOI] [PubMed] [Google Scholar]
- 18.Gargett C.E. Stem cells in gynaecology. Aust. N. Z. J. Obstet. Gynaecol. 2004;44(5):380–386. doi: 10.1111/j.1479-828X.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 19.Gargett C.E., Schwab K.E., Deane J.A. Endometrial stem/progenitor cells: the first 10 years. Hum. Reprod. Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Huang F. Stem cell and endometriosis: new knowledge may be producing novel therapies. Int. J. Clin. Exp. Med. 2014;7(11):3853–3858. [PMC free article] [PubMed] [Google Scholar]
- 21.Laux-Biehlmann A., d’Hooghe T., Zollner T.M. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol. Sci. 2015;36(5):270–276. doi: 10.1016/j.tips.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Tempfer C.B., Wenzl R., Horvat R., et al. Lymphatic spread of endometriosis to pelvic sentinel lymph nodes: a prospective clinical study. Fertil. Steril. 2011;96(3):692–696. doi: 10.1016/j.fertnstert.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 23.Gong Y., Tempfer C.B. Regional lymphatic spread in women with pelvic endometriosis. Med. Hypotheses. 2011;76(4):560–563. doi: 10.1016/j.mehy.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Jerman LF, Hey-Cunningham AJ. The role of the lymphatic system in endometriosis: A comprehensive review of the literature. 2015. [DOI] [PubMed]
- 25.Sarma D., Iyengar P., Marotta T.R., terBrugge K.G., Gentili F., Halliday W. Cerebellar endometriosis. AJR Am. J. Roentgenol. 2004;182(6):1543–1546. doi: 10.2214/ajr.182.6.1821543. [DOI] [PubMed] [Google Scholar]
- 26.Thibodeau L.L., Prioleau G.R., Manuelidis E.E., Merino M.J., Heafner M.D. Cerebral endometriosis. J. Neurosurg. 1987;66(4):609–610. doi: 10.3171/jns.1987.66.4.0609. [DOI] [PubMed] [Google Scholar]
- 27.Beyth Y., Yaffe H., Levij S., Sadovsky E. Retrograde seeding of endometrium: a sequela of tubal flushing. Fertil. Steril. 1975;26(11):1094–1097. doi: 10.1016/s0015-0282(16)41476-7. [DOI] [PubMed] [Google Scholar]
- 28.Halme J., Hammond M.G., Hulka J.F., Raj S.G., Talbert L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 29.Liu D.T., Hitchcock A. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br. J. Obstet. Gynaecol. 1986;93(8):859–862. doi: 10.1111/j.1471-0528.1986.tb07995.x. [DOI] [PubMed] [Google Scholar]
- 30.Keettel W.C., Stein R.J. The viability of the cast-off menstrual endometrium. Am. J. Obstet. Gynecol. 1951;61(2):440–442. doi: 10.1016/0002-9378(51)90266-9. [DOI] [PubMed] [Google Scholar]
- 31.Koks C.A., Demir Weusten A.Y., Groothuis P.G., Dunselman G.A., de Goeij A.F., Evers J.L. Menstruum induces changes in mesothelial cell morphology. Gynecol. Obstet. Invest. 2000;50(1):13–18. doi: 10.1159/000010271. [DOI] [PubMed] [Google Scholar]
- 32.van der Linden P.J., de Goeij A.F., Dunselman G.A., van der Linden E.P., Ramaekers F.C., Evers J.L. Expression of integrins and E-cadherin in cells from menstrual effluent, endometrium, peritoneal fluid, peritoneum, and endometriosis. Fertil. Steril. 1994;61(1):85–90. doi: 10.1016/s0015-0282(16)56457-7. [DOI] [PubMed] [Google Scholar]
- 33.Oral E., Olive D., Arici A. The peritoneal environment in endometriosis. Hum. Reprod. Update. 1996;2(5):385–398. doi: 10.1093/humupd/2.5.385. [DOI] [PubMed] [Google Scholar]
- 34.Bahtiyar M.O., Seli E., Oral E., Senturk L.M., Zreik T.G., Arici A. Follicular fluid of women with endometriosis stimulates the proliferation of endometrial stromal cells. Hum. Reprod. 1998;13(12):3492–3495. doi: 10.1093/humrep/13.12.3492. [DOI] [PubMed] [Google Scholar]
- 35.Vercellini P., Aimi G., De Giorgi O., Maddalena S., Carinelli S., Crosignani P.G. Is cystic ovarian endometriosis an asymmetric disease? Br. J. Obstet. Gynaecol. 1998;105(9):1018–1021. doi: 10.1111/j.1471-0528.1998.tb10267.x. [DOI] [PubMed] [Google Scholar]
- 36.Koninckx P.R., Ide P., Vandenbroucke W., Brosens I.A. New aspects of the pathophysiology of endometriosis and associated infertility. J. Reprod. Med. 1980;24(6):257–260. [PubMed] [Google Scholar]
- 37.Badawy S.Z., Cuenca V., Marshall L., Munchback R., Rinas A.C., Coble D.A. Cellular components in peritoneal fluid in infertile patients with and without endometriosis. Fertil. Steril. 1984;42(5):704–708. [PubMed] [Google Scholar]
- 38.Bartosik D., Jacobs S.L., Kelly L.J. Endometrial tissue in peritoneal fluid. Fertil. Steril. 1986;46(5):796–800. doi: 10.1016/s0015-0282(16)49813-4. [DOI] [PubMed] [Google Scholar]
- 39.Kulenthran A., Jeyalakshmi N. Dissemination of endometrial cells at laparoscopy and chromotubation--a preliminary report. Int. J. Fertil. 1989;34(4):256–258. [PubMed] [Google Scholar]
- 40.Bulletti C., De Ziegler D., Polli V., Del Ferro E., Palini S., Flamigni C. Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertil. Steril. 2002;77(6):1156–1161. doi: 10.1016/s0015-0282(02)03087-x. [DOI] [PubMed] [Google Scholar]
- 41.Vercellini P., De Giorgi O., Aimi G., Panazza S., Uglietti A., Crosignani P.G. Menstrual characteristics in women with and without endometriosis. Obstet. Gynecol. 1997;90(2):264–268. doi: 10.1016/S0029-7844(97)00235-4. [DOI] [PubMed] [Google Scholar]
- 42.Koninckx P.R., Kennedy S.H., Barlow D.H. Pathogenesis of endometriosis: the role of peritoneal fluid. Gynecol. Obstet. Invest. 1999;47(Suppl. 1):23–33. doi: 10.1159/000052856. [DOI] [PubMed] [Google Scholar]
- 43.Surrey E.S., Halme J. Effect of peritoneal fluid from endometriosis patients on endometrial stromal cell proliferation in vitro. Obstet. Gynecol. 1990;76(5 Pt 1):792–797. doi: 10.1097/00006250-199011000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Overton C.E., Fernandez-Shaw S., Hicks B., Barlow D.H., Starkey P. In vitro culture of endometrial stromal and gland cells as a model for endometriosis: the effect of peritoneal fluid on proliferation. Fertil. Steril. 1997;67(1):51–56. doi: 10.1016/s0015-0282(97)81855-9. [DOI] [PubMed] [Google Scholar]
- 45.Braun D.P., Ding J., Dmowski W.P. Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil. Steril. 2002;78(4):727–732. doi: 10.1016/s0015-0282(02)03318-6. [DOI] [PubMed] [Google Scholar]
- 46.Hammond M.G., Oh S.T., Anners J., Surrey E.S., Halme J. The effect of growth factors on the proliferation of human endometrial stromal cells in culture. Am. J. Obstet. Gynecol. 1993;168(4):1131–1136. doi: 10.1016/0002-9378(93)90356-n. [DOI] [PubMed] [Google Scholar]
- 47.Badawy S.Z., Holland J., Landas S., Frankel L., Cuenca V., Khan S. The role of estradiol, progesterone, and transforming growth factor on human endometrioma cell culture. Am. J. Reprod. Immunol. 1996;36(1):58–63. doi: 10.1111/j.1600-0897.1996.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 48.Iwabe T., Harada T., Tsudo T., et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J. Clin. Endocrinol. Metab. 2000;85(2):824–829. doi: 10.1210/jcem.85.2.6335. [DOI] [PubMed] [Google Scholar]
- 49.Holinka C.F. Growth and hormonal responsiveness of human endometrial stromal cells in culture. Hum. Cell. 1988;1(2):207–217. [PubMed] [Google Scholar]
- 50.Yoshioka H., Harada T., Iwabe T., et al. Menstrual cycle-specific inhibition of the proliferation of endometrial stromal cells by interleukin 6 and its soluble receptor. Am. J. Obstet. Gynecol. 1999;180(5):1088–1094. doi: 10.1016/s0002-9378(99)70599-5. [DOI] [PubMed] [Google Scholar]
- 51.Han S.J., Jung S.Y., Wu S.P., et al. Estrogen receptor beta modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leyendecker G., Wildt L., Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch. Gynecol. Obstet. 2009;280(4):529–538. doi: 10.1007/s00404-009-1191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leyendecker G., Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011;5(2):125–142. doi: 10.1515/HMBCI.2011.002. [DOI] [PubMed] [Google Scholar]
- 54.van Gestel I. MM IJ, Hoogland HJ, Evers JL. Endometrial wave-like activity in the non-pregnant uterus. Hum. Reprod. Update. 2003;9(2):131–138. doi: 10.1093/humupd/dmg011. [DOI] [PubMed] [Google Scholar]
- 55.Leyendecker G., Kunz G., Herbertz M., et al. Uterine peristaltic activity and the development of endometriosis. Ann. N. Y. Acad. Sci. 2004;1034:338–355. doi: 10.1196/annals.1335.036. [DOI] [PubMed] [Google Scholar]
- 56.Leyendecker G. Evidence that endometriosis results from the dislocation of basal endometrium? Hum. Reprod. 2003;18(5):1130–a-31. doi: 10.1093/humrep/deg182. [DOI] [PubMed] [Google Scholar]
- 57.Klemmt P.A., Carver J.G., Kennedy S.H., Koninckx P.R., Mardon H.J. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil. Steril. 2006;85(3):564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klemmt P.A., Carver J.G., Koninckx P., McVeigh E.J., Mardon H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007;22(12):3139–3147. doi: 10.1093/humrep/dem262. [DOI] [PubMed] [Google Scholar]
- 59.Suginami H. A reappraisal of the coelomic metaplasia theory by reviewing endometriosis occurring in unusual sites and instances. Am. J. Obstet. Gynecol. 1991;165(1):214–218. doi: 10.1016/0002-9378(91)90254-o. [DOI] [PubMed] [Google Scholar]
- 60.Nerune S.M., Hippargi S.B., Mestri N.B., Mehrotra N.M. Persistent Mullerian Duct Syndrome with Ovarian Endometriosis-A Rare Case Report. Print; 2016. pp. 2249–782X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troncon J.K., Zani A.C.T., Vieira A.D.D., Poli-Neto O.B., Nogueira A.A., Rosa-e-Silva J.C. Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser Syndrome. Case Rep. Obstet. Gynecol. 2014;2014:4. doi: 10.1155/2014/376231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mok-Lin E.Y., Wolfberg A., Hollinquist H., Laufer M.R. Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser syndrome and complete uterine agenesis: Evidence to support the theory of coelomic metaplasia. J. Pediatr. Adolesc. Gynecol. 2010;23(1):e35–e37. doi: 10.1016/j.jpag.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Cho M.K., Kim C.H., Oh S.T. Endometriosis in a patient with Rokitansky-Kuster-Hauser syndrome. J. Obstet. Gynaecol. Res. 2009;35(5):994–996. doi: 10.1111/j.1447-0756.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 64.Guerrier D., Mouchel T., Pasquier L., Pellerin I. The Mayer-Rokitansky-Küster-Hauser syndrome (congenital absence of uterus and vagina) – phenotypic manifestations and genetic approaches. J Negat Res BioMed. 2006;5(1):1–8. doi: 10.1186/1477-5751-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaetje R., Holtrich U., Engels K., et al. Endometriosis may be generated by mimicking the ontogenetic development of the female genital tract. Fertil. Steril. 2007;87(3):651–656. doi: 10.1016/j.fertnstert.2006.07.1533. [DOI] [PubMed] [Google Scholar]
- 66.Bouquet De Joliniere J., Ayoubi J.M., Lesec G., et al. Identification of displaced endometrial glands and embryonic duct remnants in female fetal reproductive tract: Possible pathogenetic role in endometriotic and pelvic neoplastic processes. Front. Physiol. 2012;3:444. doi: 10.3389/fphys.2012.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Signorile P.G., Baldi F., Bussani R., et al. New evidence of the presence of endometriosis in the human fetus. Reprod. Biomed. Online. 2010;21(1):142–147. doi: 10.1016/j.rbmo.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Signorile P.G., Baldi F., Bussani R., D’Armiento M., De Falco M., Baldi A. Ectopic endometrium in human foetuses is a common event and sustains the theory of mullerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J. Exp. Clin. Cancer Res. 2009;28:49. doi: 10.1186/1756-9966-28-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yano T., Jimbo H., Yoshikawa H., Tsutsumi O., Taketani Y. Molecular analysis of clonality in ovarian endometrial cysts. Gynecol. Obstet. Invest. 1999;47(Suppl. 1):41–45. doi: 10.1159/000052858. [DOI] [PubMed] [Google Scholar]
- 70.Nilbert M., Pejovic T., Mandahl N., Iosif S., Willen H., Mitelman F. Monoclonal origin of endometriotic cysts. Int. J. Gynecol. Cancer. 1995;5(1):61–63. doi: 10.1046/j.1525-1438.1995.05010061.x. [DOI] [PubMed] [Google Scholar]
- 71.Gaetje R., Kotzian S., Herrmann G., Baumann R., Starzinski-Powitz A. Invasiveness of endometriotic cells in vitro. Lancet. 1995;346(8988):1463–1464. doi: 10.1016/s0140-6736(95)92474-4. [DOI] [PubMed] [Google Scholar]
- 72.Gaetje R., Kotzian S., Herrmann G., Baumann R., Starzinski-Powitz A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 1997;150(2):461–467. [PMC free article] [PubMed] [Google Scholar]
- 73.Kennedy S., Mardon H., Barlow D. Familial endometriosis. J. Assist. Reprod. Genet. 1995;12(1):32–34. doi: 10.1007/BF02214126. [DOI] [PubMed] [Google Scholar]
- 74.Hadfield R.M., Yudkin P.L., Coe C.L., et al. Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): a case-control study. Hum. Reprod. Update. 1997;3(2):109–115. doi: 10.1093/humupd/3.2.109. [DOI] [PubMed] [Google Scholar]
- 75.Moen M.H., Magnus P. The familial risk of endometriosis. Acta Obstet. Gynecol. Scand. 1993;72(7):560–564. doi: 10.3109/00016349309058164. [DOI] [PubMed] [Google Scholar]
- 76.Kennedy S., Hadfield R., Mardon H., Barlow D. Age of onset of pain symptoms in non-twin sisters concordant for endometriosis. Hum. Reprod. 1996;11(2):403–405. doi: 10.1093/humrep/11.2.403. [DOI] [PubMed] [Google Scholar]
- 77.Koninckx P.R., Braet P., Kennedy S.H., Barlow D.H. Dioxin pollution and endometriosis in Belgium. Hum. Reprod. 1994;9(6):1001–1002. doi: 10.1093/oxfordjournals.humrep.a138623. [DOI] [PubMed] [Google Scholar]
- 78.Gargett C.E., Chan R.W., Schwab K.E. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol. Cell. Endocrinol. 2008;288(1-2):22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 79.Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004;70(6):1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 80.Schwab K.E., Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 81.Schwab K.E., Chan R.W., Gargett C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 2005;84(Suppl. 2):1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 82.Meng X., Ichim T.E., Zhong J., et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007;5:57–7. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Musina R.A., Belyavski A.V., Tarusova O.V., Tarusova O.V., Solovyova E.V., Sukhikh G.T. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 2008;145(4):539–543. doi: 10.1007/s10517-008-0136-0. [DOI] [PubMed] [Google Scholar]
- 84.Patel A.N., Park E., Kuzman M., Benetti F., Silva F.J., Allickson J.G. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 85.Alcayaga-Miranda F., Cuenca J., Luz-Crawford P., et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hida N., Nishiyama N., Miyoshi S., et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26(7):1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 87.Alcayaga-Miranda F., Cuenca J., Martin A., Contreras L., Figueroa F.E., Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res. Ther. 2015;6:199. doi: 10.1186/s13287-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luz-Crawford P., Torres M.J., Noel D., et al. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34(2):456–469. doi: 10.1002/stem.2244. [DOI] [PubMed] [Google Scholar]
- 89.Toyoda M., Cui C.H., Umezawa A. Myogenic transdifferentiation of menstrual blood-derived cells. Acta Mycol. 2007;26(3):176–178. [PMC free article] [PubMed] [Google Scholar]
- 90.Khoury M., Alcayaga-Miranda F., Illanes S.E., Figueroa F.E. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front. Immunol. 2014;5:205. doi: 10.3389/fimmu.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donnez J., Van Langendonckt A., Casanas-Roux F., et al. Current thinking on the pathogenesis of endometriosis. Gynecol. Obstet. Invest. 2002;54(Suppl. 1):52–58. doi: 10.1159/000066295. [DOI] [PubMed] [Google Scholar]
- 92.Witz C.A., Monotoya-Rodriguez I.A., Schenken R.S. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil. Steril. 1999;71(1):56–60. doi: 10.1016/s0015-0282(98)00400-2. [DOI] [PubMed] [Google Scholar]
- 93.Hughesdon P.E. The structure of endometrial cysts of the ovary. J. Obstet. Gynaecol. Br. Emp. 1957;64(4):481–487. doi: 10.1111/j.1471-0528.1957.tb06276.x. [DOI] [PubMed] [Google Scholar]
- 94.Brosens I.A., Puttemans P.J., Deprest J. The endoscopic localization of endometrial implants in the ovarian chocolate cyst. Fertil. Steril. 1994;61(6):1034–1038. doi: 10.1016/s0015-0282(16)56752-1. [DOI] [PubMed] [Google Scholar]
- 95.Sampson J. Perforating haemorrhagic (chocolate) cysts of the ovary. Arch. Surg. 1921;3:245–323. [Google Scholar]
- 96.Nezhat F., Nezhat C., Allan C.J., Metzger D.A., Sears D.L. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J. Reprod. Med. 1992;37(9):771–776. [PubMed] [Google Scholar]
- 97.Jain S., Dalton M.E. Chocolate cysts from ovarian follicles. Fertil. Steril. 1999;72(5):852–856. doi: 10.1016/s0015-0282(99)00367-2. [DOI] [PubMed] [Google Scholar]
- 98.Matsuura K., Ohtake H., Katabuchi H., Okamura H. Coelomic metaplasia theory of endometriosis: evidence from in vivo studies and an in vitro experimental model. Gynecol. Obstet. Invest. 1999;47(Suppl. 1):18–20. doi: 10.1159/000052855. [DOI] [PubMed] [Google Scholar]
- 99.Koninckx P.R., Martin D.C. Deep endometriosis: a consequence of infiltration or retraction or possibly adenomyosis externa? Fertil. Steril. 1992;58(5):924–928. doi: 10.1016/s0015-0282(16)55436-3. [DOI] [PubMed] [Google Scholar]
- 100.Vercellini P., Aimi G., Panazza S., Vicentini S., Pisacreta A., Crosignani P.G. Deep endometriosis conundrum: evidence in favor of a peritoneal origin. Fertil. Steril. 2000;73(5):1043–1046. doi: 10.1016/s0015-0282(00)00420-9. [DOI] [PubMed] [Google Scholar]
- 101.Tosti C., Pinzauti S., Santulli P., Chapron C., Petraglia F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod. Sci. 2015;22(9):1053–1059. doi: 10.1177/1933719115592713. [DOI] [PubMed] [Google Scholar]
- 102.Braun D.P., Gebel H., Rana N., Dmowski W.P. Cytolysis of eutopic and ectopic endometrial cells by peripheral blood monocytes and peritoneal macrophages in women with endometriosis. Fertil. Steril. 1998;69(6):1103–1108. doi: 10.1016/s0015-0282(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 103.Jones R.K., Bulmer J.N., Searle R.F. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum. Reprod. Update. 1998;4(5):702–709. doi: 10.1093/humupd/4.5.702. [DOI] [PubMed] [Google Scholar]
- 104.Monsanto S.P., Edwards A.K., Zhou J., et al. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil. Steril. 2016;105(4):968–977. doi: 10.1016/j.fertnstert.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harada T., Iwabe T., Terakawa N. Role of cytokines in endometriosis. Fertil. Steril. 2001;76(1):1–10. doi: 10.1016/s0015-0282(01)01816-7. [DOI] [PubMed] [Google Scholar]
- 106.Lebovic D.I., Mueller M.D., Taylor R.N. Immunobiology of endometriosis. Fertil. Steril. 2001;75(1):1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 107.Kyama C.M., Debrock S., Mwenda J.M., D’Hooghe T.M. Potential involvement of the immune system in the development of endometriosis. Reprod. Biol. Endocrinol. 2003;1(1):123. doi: 10.1186/1477-7827-1-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laschke M.W., Menger M.D. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am. J. Obstet. Gynecol. 2016;215(1):68.e1–68.e4. doi: 10.1016/j.ajog.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 109.Nothnick W, Alali Z. Recent advances in the understanding of endometriosis:the role of inflammatory mediators in disease pathogenesis and treatment. F1000Res 2016; 5: Pii: F1000 Faculty Rev-186. 2016. [DOI] [PMC free article] [PubMed]
- 110.Acosta A.A., Buttram V.C., Jr, Besch P.K., Malinak L.R., Franklin R.R., Vanderheyden J.D. A proposed classification of pelvic endometriosis. Obstet. Gynecol. 1973;42(1):19–25. [PubMed] [Google Scholar]
- 111.Kistner R.W., Siegler A.M., Behrman S.J. Suggested classification for endometriosis: relationship to infertility. Fertil. Steril. 1977;28(9):1008–1010. [PubMed] [Google Scholar]
- 112.AFS Classification of endometriosis. American Fertility Society. 1979;32(6):633–634. [PubMed] [Google Scholar]
- 113.rAFS. Revised American Fertility Society classification of endometriosis: 1985. Fertil. Steril. 1985;43(3):351–352. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 114.rASRM. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997;67(5):817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 115.Rock J.A. The revised American Fertility Society classification of endometriosis: reproducibility of scoring. ZOLADEX Endometriosis Study Group. Fertil. Steril. 1995;63(5):1108–1110. doi: 10.1016/s0015-0282(16)57556-6. [DOI] [PubMed] [Google Scholar]
- 116.Hornstein M.D., Gleason R.E., Orav J., et al. The reproducibility of the revised American Fertility Society classification of endometriosis. Fertil. Steril. 1993;59(5):1015–1021. [PubMed] [Google Scholar]
- 117.Guzick D.S., Silliman N.P., Adamson G.D., et al. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine’s revised classification of endometriosis. Fertil. Steril. 1997;67(5):822–829. doi: 10.1016/s0015-0282(97)81392-1. [DOI] [PubMed] [Google Scholar]
- 118.Rock J., Guzick D., Sengos C., Schweditsch M., Sapp K., Jones H.J. The conservative surgical treatment of endometriosis: evaluation of pregnancy success with respect to the extent of disease as categorized using contemporary classification systems. Fertil. Steril. 1981;35:131–137. doi: 10.1016/s0015-0282(16)45311-2. [DOI] [PubMed] [Google Scholar]
- 119.Adamson G.D., Pasta D.J. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil. Steril. 2010;94(5):1609–1615. doi: 10.1016/j.fertnstert.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 120.Tuttlies F., Keckstein J., Ulrich U., et al. Zentralbl. Gynakol. 2005;127(5):275–281. doi: 10.1055/s-2005-836904. [ENZIAN-score, a classification of deep infiltrating endometriosis]. [DOI] [PubMed] [Google Scholar]
- 121.Haas D., Chvatal R., Habelsberger A., Wurm P., Schimetta W., Oppelt P. Comparison of revised American Fertility Society and ENZIAN staging: a critical evaluation of classifications of endometriosis on the basis of our patient population. Fertil. Steril. 2011;95(5):1574–1578. doi: 10.1016/j.fertnstert.2011.01.135. [DOI] [PubMed] [Google Scholar]
- 122.Haas D., Chvatal R., Habelsberger A., et al. Preoperative planning of surgery for deeply infiltrating endometriosis using the ENZIAN classification. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;166(1):99–103. doi: 10.1016/j.ejogrb.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 123.Haas D., Oppelt P., Shebl O., Shamiyeh A., Schimetta W., Mayer R. Enzian classification: does it correlate with clinical symptoms and the rASRM score? Acta Obstet. Gynecol. Scand. 2013;92(5):562–566. doi: 10.1111/aogs.12118. [DOI] [PubMed] [Google Scholar]
- 124.Haas D., Shebl O., Shamiyeh A., Oppelt P. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet. Gynecol. Scand. 2013;92(1):3–7. doi: 10.1111/aogs.12026. [DOI] [PubMed] [Google Scholar]
- 125.Haas D., Wurm P., Shamiyeh A., Shebl O., Chvatal R., Oppelt P. Efficacy of the revised Enzian classification: a retrospective analysis. Does the revised Enzian classification solve the problem of duplicate classification in rASRM and Enzian? Arch. Gynecol. Obstet. 2013;287(5):941–945. doi: 10.1007/s00404-012-2647-1. [DOI] [PubMed] [Google Scholar]
- 126.Zeitvogel A., Baumann R., Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 2001;159(5):1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bartley J., Jülicher A., Hotz B., Mechsner S., Hotz H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2013;289(4):871–881. doi: 10.1007/s00404-013-3040-4. [DOI] [PubMed] [Google Scholar]
- 128.McCluggage W.G., Sumathi V.P., Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39(3):273–278. doi: 10.1046/j.1365-2559.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 129.Sumathi V.P., McCluggage W.G. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J. Clin. Pathol. 2002;55(5):391–392. doi: 10.1136/jcp.55.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Onda T., Ban S., Shimizu M. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J. Clin. Pathol. 2003;56(1):79. doi: 10.1136/jcp.56.1.79-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aplin J.D., Charlton A.K., Ayad S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988;253(1):231–240. doi: 10.1007/BF00221758. [DOI] [PubMed] [Google Scholar]
- 132.Beliard A., Donnez J., Nisolle M., Foidart J.M. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil. Steril. 1997;67(2):266–272. doi: 10.1016/S0015-0282(97)81909-7. [DOI] [PubMed] [Google Scholar]
- 133.Harrington D.J., Lessey B.A., Rai V., et al. Tenascin is differentially expressed in endometrium and endometriosis. J. Pathol. 1999;187(2):242–248. doi: 10.1002/(SICI)1096-9896(199901)187:2<242::AID-PATH221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 134.Gazvani R., Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123(2):217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 135.Hull M.L., Escareno C.R., Godsland J.M., et al. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am. J. Pathol. 2008;173(3):700–715. doi: 10.2353/ajpath.2008.071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kasvandik S., Samuel K., Peters M., et al. Deep quantitative proteomics reveals extensive metabolic reprogramming and cancer-like changes of ectopic endometriotic stromal cells. J. Proteome Res. 2016;15(2):572–584. doi: 10.1021/acs.jproteome.5b00965. [DOI] [PubMed] [Google Scholar]
- 137.Guo S-W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009;15(10):587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 138.Nasu K., Kawano Y., Kai K., et al. 2014. Aberrant histone modification in endometriosis. [DOI] [PubMed] [Google Scholar]
- 139.Teague EMCO. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009;23(2):265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Z.Z., Croft L., Nyholt D.R., et al. Evaluation of polymorphisms in predicted target sites for micro RNAs differentially expressed in endometriosis. Mol. Hum. Reprod. 2011;17(2):92–103. doi: 10.1093/molehr/gaq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saare M., Rekker K., Laisk-Podar T., et al. High-throughput sequencing approach uncovers the miRNome of peritoneal endometriotic lesions and adjacent healthy tissues. PLoS One. 2014;9(11):e112630. doi: 10.1371/journal.pone.0112630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu Y., Strawn E., Basir Z., Halverson G., Halverson G., Guo S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- 143.Xue Q., Lin Z., Cheng Y.H., et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol. Reprod. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 144.Bouquet De Joliniere J., Ayoubi J.M., Gianaroli L., Dubuisson J.B., Gogusev J., Feki A. Endometriosis: a new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity. Front. Surg. 2014;1:16. doi: 10.3389/fsurg.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forte A., Cipollaro M., Galderisi U. Genetic, epigenetic and stem cell alterations in endometriosis: new insights and potential therapeutic perspectives. Clin. Sci. (Lond.) 2014;126(2):123–138. doi: 10.1042/CS20130099. [DOI] [PubMed] [Google Scholar]
- 146.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braicu C., Tomuleasa C., Monroig P., Cucuianu A., Berindan-Neagoe I., Calin G.A. Exosomes as divine messengers: are they the Hermes of modern molecular oncology. Cell Death Differ. 2015;22(1):34–45. doi: 10.1038/cdd.2014.130. [quest]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ng Y.H., Rome S., Jalabert A., et al. Endometrial exosomes/ microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013;8(3):e58502. doi: 10.1371/journal.pone.0058502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Texidó L., Romero C., Vidal A., et al. Ecto-nucleotidases activities in the contents of ovarian endometriomas: Potential biomarkers of endometriosis. Mediators Inflamm. 2014;2014:120673. doi: 10.1155/2014/120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harp D., Driss A., Mehrabi S., et al. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016;365(1):187–196. doi: 10.1007/s00441-016-2358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Braza-Boils A., Salloum-Asfar S., Mari-Alexandre J., et al. Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum. Reprod. 2015;30(10):2292–2302. doi: 10.1093/humrep/dev204. [DOI] [PubMed] [Google Scholar]
- 152.Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. 2002. [DOI] [PubMed]
- 153.Lessey BA. Implantation defects in infertile women with endometriosis. 2002. [DOI] [PubMed]
- 154.Sharpe-Timms K.L. Endometrial Anomalies in Women with Endometriosis. Ann. N. Y. Acad. Sci. 2001;943:131–147. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- 155.Pabona J.M., Simmen F.A., Nikiforov M.A., et al. Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2012;97(3):E376–E392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zelenko Z., Aghajanova L., Irwin J.C., Giudice L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod. Sci. 2012;19(2):152–162. doi: 10.1177/1933719111415546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aghajanova L., Giudice L.C. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod. Sci. 2011;18(3):229–251. doi: 10.1177/1933719110386241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burney R.O., Hamilton A.E., Aghajanova L., et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009;15(10):625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Y., Kajdacsy-Balla A., Strawn E., et al. Transcriptional Characterizations of Differences between Eutopic and Ectopic Endometrium. Endocrinology. 2006;147(1):232–246. doi: 10.1210/en.2005-0426. [DOI] [PubMed] [Google Scholar]
- 160.McKinnon B.D., Kocbek V., Nirgianakis K., Bersinger N.A., Mueller M.D. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum. Reprod. Update. 2016 doi: 10.1093/humupd/dmv060. [DOI] [PubMed] [Google Scholar]
- 161.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Serrano-Gomez S.J., Maziveyi M., Alahari S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiong W., Zhang L., Yu L., et al. Estradiol promotes cells invasion by activating beta-catenin signaling pathway in endometriosis. Reproduction. 2015;150(6):507–516. doi: 10.1530/REP-15-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang L., Xiong W., Xiong Y., Liu H., Liu Y. 17 β-Estradiol Promotes Vascular Endothelial Growth Factor Expression via the Wnt/β-catenin Pathway during the Pathogenesis of Endometriosis. Mol. Hum. Reprod. 2016 doi: 10.1093/molehr/gaw025. [DOI] [PubMed] [Google Scholar]
- 165.de Mattos R.M., Pereira P.R., Barros E.G., et al. Aberrant levels of Wnt/beta-catenin pathway components in a rat model of endometriosis. Electronic; 2016. pp. 1699–5848. [DOI] [PubMed] [Google Scholar]
- 166.Gaetje R., Holtrich U., Karn T., et al. Characterization of WNT7A expression in human endometrium and endometriotic lesions. Fertil. Steril. 2007;88(6):1534–1540. doi: 10.1016/j.fertnstert.2007.01.128. [DOI] [PubMed] [Google Scholar]
- 167.Matsuzaki S., Darcha C. In vitro effects of a small-molecule antagonist of the Tcf/ss-catenin complex on endometrial and endometriotic cells of patients with endometriosis. PLoS One. 2013;8(4):e61690. doi: 10.1371/journal.pone.0061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fuentes R.G., Arai M.A., Ishibashi M. Natural compounds with Wnt signal modulating activity. Nat. Prod. Rep. 2015;32(12):1622–1628. doi: 10.1039/c5np00074b. [DOI] [PubMed] [Google Scholar]