Abstract
Background:
Although it has been more than a century since endometriosis was initially described in the literature, understanding the etiology and natural history of the disease has been challenging. However, the broad utility of murine and rat models of experimental endometriosis has enabled the elucidation of a number of potentially targetable processes which may otherwise promote this disease.
Objective:
To review a variety of studies utilizing rodent models of endometriosis to illustrate their utility in examining mechanisms associated with development and progression of this disease.
Results:
Use of rodent models of endometriosis has provided a much broader understanding of the risk factors for the initial development of endometriosis, the cellular pathology of the disease and the identification of potential therapeutic targets.
Conclusion:
Although there are limitations with any animal model, the variety of experimental endometriosis models that have been developed has enabled investigation into numerous aspects of this disease. Thanks to these models, our under-standing of the early processes of disease development, the role of steroid responsiveness, inflammatory processes and the peritoneal environment has been advanced. More recent models have begun to shed light on how epigenetic alterations con-tribute to the molecular basis of this disease as well as the multiple comorbidities which plague many patients. Continued de-velopments of animal models which aid in unraveling the mechanisms of endometriosis development provide the best oppor-tunity to identify therapeutic strategies to prevent or regress this enigmatic disease.
Keywords: Endometriosis, inflammation, epigenetics, steroid action, co-morbidities, mice, rat
1. INTRODUCTION
Descriptions of endometriosis, defined as the presence of endometrial glands and stroma outside the uterus, can be found in medical literature at least as early as the mid-1800’s [1, 2]; however, it would be several decades later before the name “endometriosis” was coined. In his landmark paper, Dr. John Sampson [3] suggested development of endometriosis was due to ectopic implantation of refluxed menstrual tissue [3]. Although research studies continue to support the “retrograde menstruation” theory as one mechanism by which endometriosis can develop [4-6], deposition of menstrual tissue within the peritoneal cavity cannot account for all incidences of disease. Additionally, retrograde menstruation is common among reproductive age women [7-9]; however, only about 10% of women develop endometriosis, suggesting other mechanisms are also involved. Alternative explanations for the occurrence of endometriosis include coelomic metaplasia [10-12] and peritoneal activation of embryonic cell rests [13, 14]. The latter theory likely explains the unusual and rare occurrence of endometriosis in men [15, 16]. Recently, ectopic endometrial tissue has been described in human fetuses which has been theorized to develop as a consequence of ectopic localization of primitive endometrial tissue during organogenesis [17, 18]. Other factors likely affecting an individual’s risk for development of endometriosis include: genetic predisposition, immune dysregulation and/or a history of environmental toxicant exposure (reviewed by [19]). More recently, Brosens and colleagues have postulated a role for neonatal menstruation, as a consequence of early onset steroid responsiveness, in developing endometriosis as an adolescent [20].
While generally considered a benign condition, endometriosis exhibits cancer-like features and can spread throughout the peritoneal cavity and to distal sites. Endometriosis is frequently physically debilitating, as patients often suffer from chronic pelvic pain, dyspareunia, dysmenorrhea and subfertility. Unfortunately, most women with this disease also exhibit one or more co-morbidities including adenomyosis, adhesive disease and inflammatory conditions such as interstitial cystitis and inflammatory bowel disease [21-23]. However, understanding the natural history of endometriosis, as well as the myriad of equally poorly understood co-morbidities, has proven elusive, despite extensive research in each disease area. Given our lack of understanding of the etiology of endometriosis, treatment options for women with this disease remain limited and generally involve a combination of hormonal manipulation and surgery to remove diseased tissue. Unfortunately, the side effects of hormonal therapy for endometriosis cause many women to abandon this treatment; nevertheless, surgical treatment alone is frequently non-curative and many women suffer recurrence [24-32]. Thus, identifying better diagnostic and treatment strategies for this disease is a major focus of many endometriosis research laboratories.
Endometriosis is rare in non-menstruating species, suggesting that shedding of endometrial tissue may be a causative factor as originally proposed by Sampson [3]. Indeed, numerous studies have demonstrated that the transfer of endometrial tissue to the peritoneal cavity can lead to the development of ectopic endometrial lesions. For example, in the mid-1950s, Sampson’s retrograde menstruation theory was experimentally examined in women via intraperitoneal injection of their own menstrual tissue several months prior to a scheduled surgery for fibroids. This study identified ectopic peritoneal lesions in 2 of 13 women (15%), providing support for the retrograde menstruation theory [4]. Given the obvious limitations and ethical considerations of human experimentation, current endometriosis researchers rely heavily on non-human primate or rodent models in order to investigate elements of disease pathophysiology.
2. The models
Among various animals utilized for experimental endometriosis, rodent models have a number of advantages relative to other species. They are cost effective due to their small size and large litters while their short gestation enables transgenerational analysis. Additionally, the wide availability of genetic knock-out mice, antibodies against murine and rat proteins and knowledge of the murine genome make these animals extremely useful for the study of many diseases, including endometriosis. As shown in Table 1, many different model systems have been developed in mice and rats, each with unique features that are valuable for targeted studies. Importantly, rodent models of endometriosis are often utilized for preclinical testing in an attempt to identify new therapeutic agents. The use of experimental endometriosis models for therapeutic testing has recently been reviewed [33, 34]; thus, these studies will not be extensively considered here. Rather, in this review, we will highlight the most commonly used rodent models of endometriosis (Table 1) with an emphasis on studies exploring disease-related mechanisms. We will also explore the use of animal models that have provided insight into the development of comorbidities common among women with endometriosis. Significantly, using appropriate experimental endometriosis models for identifying therapeutic targets is a critical step prior to the examination of a treatment regimen aimed at any particular pathway or protein.
Table 1.
Rodent models of experimental endometriosis.
| Model | Features | Benefits | Limitations | Examples |
|---|---|---|---|---|
| Immunocompromised Models | ||||
|
Athymic Nude Spontaneous mutation of Foxn1 (Chromosome 11) |
Lack T cells, compromised B cell function | Accepts human xenografts; useful for early lesion establishment studies | Age-related compensatory immunity | [36, 41] |
|
Congenic SCID Spontaneous autosomal recessive mutation of Prkdc (DNA repair enzyme) (Chromosome 16) |
Lack B and T cells | Accepts human xenografts; fertility preserved; useful for early lesion establishment studies | Age-related compensatory immunity; high susceptibility to opportunistic infections | [45] |
|
Rag2γ(c) targeted double mutation obtained by crossing gamma c knockout and Rag2 deficient mice (C57Bl/6 background) |
Lack B, T and NK cells | Lack of age-related compensatory immunity allows for longer duration of studies; ability to graft human immune cells allows for natural experimental model | Animals with more aggressive behavior | [38, 46, 47] |
| Autologous Models | ||||
| Rat surgical model | Uterine tissues sewn to arteries of small intestine | Animal maintains normal immune function | Require animal undergo surgery | [48-50] |
| Injection Models | Minced uterine tissues are injected IP or SC | Peritoneal injections more closely mimic retrograde menstruation; either donor or recipient can be treated allowing for large permutation of experimental setups | Ectopic tissue can be difficult to locate; variable success rates in disease establishment | [51] |
| C57bl/6 Green Fluorescent Protein | Transgenic mice that express GFP are used as endometrial donors | Lesions visualized by fluorescence | [52] | |
|
Transgenic knock-out models -PRKO -ERKO |
Mice transgenic for loss of steroid receptor expression. | Role of specific genes can be examined | [51, 53] | |
|
EDC-Exposure Models -TCDD -BPA |
Female mice exhibit uterine progesterone resistance; subfertility Ectopic disease |
Useful to examine the uterine phenotype associated with endometriosis | Unidentified effects of toxicant exposure may additionally impact disease processes. | [54-57] |
| Multigenerational Models | ||||
|
Rat Surgical Model Toxicant Exposed Model |
Daughters fertility impacted Daughters and sons fertility impacted |
Useful for epigenetic studies and comorbidity analysis Useful for epigenetic studies and comorbidity analysis |
[58] [54, 59] |
|
2.1. Experimental Endometriosis in Immunocompromised Mice
Examination of early events associated with endometriosis (ie, endometrial characteristics which promote ectopic implantation and survival), requires an appropriate model system. Therefore, several laboratories, including ours, have utilized chimeric mouse models of endometriosis in which human endometrial tissue can be introduced into immunocompromised mice (for example, [35-38]). Immunocompromised mice lack a fully competent immune system; therefore, these animals do not mount an immune response against human tissue xenografts; therefore, these mice are valuable for examining multiple cellular pathways associated with development of endometriosis. Immunocompromised mouse strains include athymic (nude) mice, Severe Combined Immunodeficient (SCID) mice and rag2γ(c) (Recombinant Activating Gene 2/common cytokine receptor γ chain (γc) double null) mice. The capacity to examine human endometrial cells and tissues growing in vivo make chimeric models of experimental endometriosis particularly useful for pre-clinical testing, which is critical to developing better therapeutic agents. Importantly, experimental endometriosis can be established using tissues obtained from women with and without endometriosis via endometrial biopsy, collection of menstrual effluent or from surgical specimens (for example, [36, 39-41]). By utilizing tissues acquired from women with and without endometriosis, phenotypic differences between control and disease-altered eutopic endometrium can be incorporated into the experimental design. For example, a therapeutic treatment directed at the endometrial tissue phenotype of an endometriosis patient can be initiated in vitro prior to introduction into mice, enabling targeting of specific cellular processes which may be involved in early lesion establishment.
2.1.1. Experimental Endometriosis in Nude Mice
Athymic nude mice have a spontaneous mutation in the forkhead box N1 (Foxn1) gene resulting in an underdeveloped thymus and severely compromised number of T cells [42-44]. Although homozygous nude mice have a normal complement of B cells, the lack of T cells prevents complete B cell maturation. These features render nude mice suitable for receiving transplantation of human xenografts and, not surprisingly, these animals have been widely used for cancer studies. Zamah and colleagues were the first to report experimental endometriosis in nude mice [41]. For their study, human endometrial tissues (eutopic and ectopic) were minced and injected into the peritoneal space of nude mice. Some mice were treated with estrogen, allowing examination of the impact of this steroid on lesion growth. In this study, the majority of animals developed a disease resembling endometriosis; however, the most extensive disease was noted in mice receiving both estrogen and ectopic tissues from women with endometriosis.
Since this first report, the nude mouse model of experimental endometriosis has been used extensively by our group as well as by many other investigators to examine aspects of establishment and progression of this disease within the peritoneal cavity. For our model of experimental endometriosis using nude mice [36], human endometrial tissue is obtained by biopsy during the proliferative phase of the menstrual cycle and initially maintained as an organ culture. Following overnight incubation in media containing estradiol, the minced endometrial tissue fragments are washed in physiological saline to remove residual blood and mucous and tissues are subsequently injected into mice either intraperitoneally or subcutaneously along the ventral midline. Recipient mice can be left intact or ovariectomized and implanted with slow-release estradiol capsules thereby mimicking the growth-promoting environment of the human proliferative phase. As shown in Fig. (1), ectopic peritoneal lesions which establish in mice are similar in appearance (both gross and microscopic) to the spontaneous disease occurring in women.
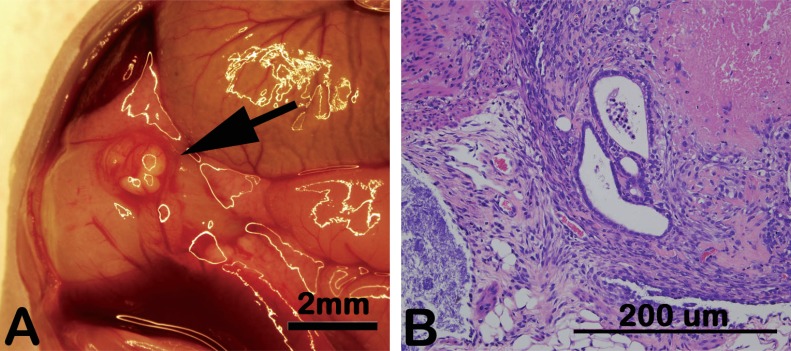
Fig. (1).
Experimental human endometriosis in a nude mouse. Proliferative phase human endometrial tissue readily establishes ectopic lesions in a nude mouse. Prior to injection of endometrial fragments, mice are ovariectomized and provided a slow-release estradiol capsule to mimic the human proliferative phase. Lesions developing in mice are markedly similar to human disease both macroscopically (A) and microscopically following hematoxylin and eosin staining (B). Results are representative of numerous (N>25) experiments using different human samples.
2.1.2. Experimental Endometriosis in SCID Mice
Severe Combined Immunodeficiency (SCID) is observed in humans as well as many other species [60]. This genetic disorder results in dysfunctional T and B lymphocytes leading to an inability of the adaptive immune system to mount or sustain an appropriate immune response. In mice, the condition results from a rare, recessive mutation leading to limited activity of a DNA repair enzyme (Prkdc or “protein kinase, DNA activated, catalytic polypeptide”). Loss of this enzyme is associated with a failure of humoral and cellular immune systems to mature. The mutation was first described in BALB/c-Ighb mice in 1980 by Bosma and associates at Fox Chase Cancer Center [61]. As summarized in Table 1, SCID mice exhibit an impaired ability to make T or B lymphocytes and cannot either efficiently fight infections or reject tumors. For these reasons, SCID mice are also suitable models for establishing experimental endometriosis using human endometrial tissues. The earliest study using SCID mice for endometriosis research was Aoki et al. [45]. These investigators established experimental human endometriosis in both SCID and nude mice; and reported the latter to be a superior model system due to a significantly greater survival rate of human tissues in the SCID animals. However, these animals are more prone to disease and opportunistic infections compared to nude mice.
2.1.3. Experimental Endometriosis in Rag2γ(c) Mice
Significantly, both SCID mice and nude mice develop extrathymic immunity over time [62, 63]; thus, experimental endometriosis studies utilizing these animals must be completed before 3 months of age, severely limiting the utility of these animals for studies over extended periods of time. For this reason, studies of prolonged duration require a different model. Recombinant Activating Gene 2/common cytokine receptor γ chain (γc) double null mice (rag2γ(c)) are more completely immunosuppressed compared to nude or SCID mice, demonstrating an absence of B cells, T cells, NK cell activity and all lymphocytes (Table 1). These mice were first described by Mazurier et al. [47] who crossed the common gamma c knockout mouse (developed by [64]) with the recombinase activating gene-2 (rag2) deficient mouse (developed by [65]). Rag2γ(c) mice do not demonstrate the same age-related compensatory immunity as other immunocompromised mice and, surprisingly, these animals are more disease resistant than SCID mice. Thus, xenographic studies conducted in rag2γ(c) mice can be of much longer duration than studies conducted in nude or SCID mice. Greenberg and Slayden [38] were the first to report the xenotransplantation of endometrial tissues into rag2γ(c) mice. In their study, human endometrium was introduced into the peritoneal cavity of mice which were then subjected to multiple artificial menstrual cycles. Their study revealed that endometriotic-like disease was present in the majority of mice even after four artificial 28 day cycles and provided strong support for the use of these mice for long-term endometriosis studies.
Equally significant, rag2γ(c) mice also accept human immune cells without rejection, allowing these cells to be examined in conjunction with experimental endometriosis [46]. As will be discussed in detail below, the ability to additionally assess the role of human immune cells in establishment or prevention of experimental disease is an important advantage of studies using rag2γ(c) mice compared to studies conducted with either the nude or SCID mouse.
2.1.4. Kidney Capsule Model
Although experimental endometriosis using chimeric murine models is most commonly conducted with intraperitoneal injection of human endometrial tissues, other approaches also have been utilized to an advantage. An important adaptation arising from chimeric mouse models has been the establishment of renal grafts in which human tissue, including reproductive tissues, are placed under the kidney capsule [66, 67]. Endometrial tissue growing under the kidney capsule allows easier identification and excision compared to the random sites of attachment following intraperitoneal injection. An additional advantage of the kidney capsule is its high vascularity, which enhances survival of ectopic tissues. The kidney capsule lends itself not only to insertion of whole tissues (explants), but also enables isolation and recombination of endometrial cells within a collagen matrix. [67]. Renal grafting of various combinations of endometrial cells allows a more precise examination of each endometrial cell type during growth and differentiation in vivo. Importantly, we have found that control human endometrial tissues growing at this site in mice remain responsive to ovarian steroids and exhibit classic orphological appearance and biochemical differentiation in response to progesterone treatments (Fig. 2).
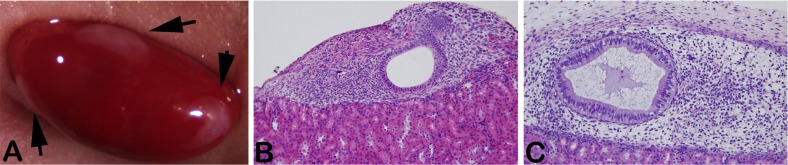
Fig. (2).
Human endometrium growing under the kidney capsule of nude mice. Human endometrial stromal and epithelial cells are isolated and recombined in collagen and placed under the kidney capsule. Tissues are readily visible on gross examination (arrows) (A). Following treatment of the animal with estradiol (B) or estradiol plus medroxyprogesterone acetate (C) lesions exhibit proliferative or secretory phenotypes, respectively. Results are representative of multiple (N=5) experiments utilizing different human tissues.
2.2. Immunocompetent Rodent Models
Endometriosis is recognized to be a steroid-dependent disease largely occurring during the reproductive years of a woman’s life; however, defects within the immune system are also a critical component of disease pathophysiology (reviewed by [68]). Although long considered an autoimmune disease, recent research indicates that cell-mediated immunity is also disrupted in endometriosis. Thus, a major disadvantage of experimental endometriosis models established in immunocompromised mice is the limited ability to examine the role of the immune system in development and progression of disease. Despite the ability of rag2γ(C) mice to accept both human tissue and immune cells, the short-lived nature of circulating human immune cells in these animals remains an important drawback of immunological studies using this experimental model. Therefore, immunocompetent rodent models remain essential to examining the influence of the immune system on development and progression of experimental endometriosis.
2.2.1. Rat Surgical Model
One of the earliest in vivo experimental endometriosis models developed was the rat surgical model, first reported by Vernon and Wilson [50]. In this experimental model, the investigators removed one uterine horn and dissected the endometrial tissue into 2-mm squares. Four uterine squares were subsequently autotransplanted to the arterial cascades of the small intestine of the same animal. Although not useful for examining early events of endometriosis development due to the need for surgical induction of ectopic growth, the rat surgical model has provided significant insight into endometriosis-associated infertility (reviewed by [49]).
2.2.2. Syngeneic Mouse Model
More recently, a number of groups have utilized syngeneic mouse models of endometriosis, in which the uterus of one animal is removed during estrus (natural or induced), minced and injected intraperitoneally into one or two recipient mice [51, 69-72], an approach that is similar to the studies noted above in immunocompromised mice. Syngeneic murine models have several potential advantages over the rat surgical model. First, peritoneal seeding of uterine fragments is more similar to retrograde menstruation in women. Second, either the donor or recipient animal can receive therapeutic intervention or be otherwise manipulated prior to induction of disease. Finally, the availability of an extensive number of transgenic mice, in which specific genes can be either eliminated or overexpressed, make these animals ideal for studying the role of specific pathways in the development and progression of endometriosis and other diseases.
2.3. Toxicant Exposure Models
Although the role of the environment in the development of reproductive dysfunction continues to be debated, endometriosis researchers have long considered the likelihood that environmental toxicants may be one trigger for disease development. As a consequence of industrialization, humans and other animals are exposed to a wide array of manmade toxicants, many which act as endocrine disrupting chemicals (EDCs) [73, 74]. Among human exposures to various EDCs, interest in exposure to TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin, commonly known as dioxin) arose from a primate study published by Rier et al. [75], which suggested that exposure to this chemical family may increase the risk for developing endometriosis. TCDD along with a number of dioxin-like PCBs (polychlorinated byphenols) bind the aryl hydrocarbon receptor (AhR), an orphan nuclear receptor that has been suggested to play a role in normal uterine function [76, 77]. Inappropriate activation of this receptor by environmental toxicants not only disrupts steroid action but also promotes inflammatory processes that may be linked to endometriosis [78, 79]. Nevertheless, although numerous epidemiological studies have been undertaken in order to assess the adult body burden of TCDD or various PCBs in relation to the presence of absence of endometriosis; these studies reach conflicting conclusions. For example, although several epidemiological studies suggested that blood and/or tissue levels of various environmental endocrine disruptors, correlated to the presence of endometriosis in women (for example, [80-84]; other studies failed to find an association (for example, [85-89]). At this juncture, the difficulty in definitively determining a causative role of toxicant body burden and endometriosis is perhaps related to both the variable toxicity of a given agent as well as the significant difficulty in identifying the presence or absence of endometriosis within a study population [90-92]. Therefore, a number of researchers have utilized rodent models of toxicant exposure to explore the potential role of these compounds in the development of endometriosis.
2.3.1. Adult Toxicant Exposure Models
In a series of studies, Birnbaum and colleagues explored the influence of an adult exposure to TCDD and other EDCs on the progression of ectopic lesions using an experimental endometriosis model in mice similar to the rat surgical model described above. Initially, this group compared the rat and mouse surgical models with regard to growth and progression of experimental disease in response to TCDD exposure [93]. For these studies, adult animals (Sprague-Dawley rats and B6C3F1 mice) were treated with TCDD prior to surgical induction of endometriosis as well as following surgery. Although TCDD treatment negatively impacted disease and reproductive endpoints in both animal models, the enhanced impact of TCDD on immune function in mice led the authors to conclude that these animals may be more appropriate for future toxicity studies. A follow-up study from this group similarly examined other polyhalogenated aromatic hydrocarbons on endometriotic proliferation using the same mouse model. Their second study found that lower doses of TCDD (1 and 3 ug/kg bw) and 4-PeCDF (100 ug/kg bw) significantly enhanced the growth of experimental endometriosis while PCB 126 had no impact on lesion growth. Interestingly, they also found that the highest dose of TCDD had no impact on lesion growth, which the authors suspected was due to ovarian toxicity. Finally, nondioxin-like compounds, PCB 153 and 1,3,6,8-TCDD had no impact of experimental disease, supporting the hypothesis that TCDD and dioxin-like PCBs primarily promote endometriosis via AhR binding [94].
Although human and murine responses to these toxicants are not identical, our group demonstrated that an acute exposure of human endometrial tissue to TCDD subsequently promoted ectopic lesion development in our nude mouse model of experimental endometriosis [95]. As will be discussed later, direct, in vitro toxicant exposure models using adult human reproductive tract cells and tissues can be useful to identify cellular pathways disrupted by EDC exposures in addition to rodent models.
Nevertheless, attempting to link the presence or absence of endometriosis to acute or chronic EDC exposures during adulthood misses the potential role of early life toxicant exposures to the risk of adult onset diseases, including endometriosis [59, 74]. Compared to adult exposure, toxic effects of EDCs are greater when exposures occur earlier in life, before the onset of puberty while exposures occurring during the neonatal period are recognized as being the most damaging [96].
2.3.2. Developmental Exposure Models
As stated above, the negative effects of EDCs may be more severe when exposures occur during early life, either in utero or prior to puberty. Therefore, it is critical that appropriate developmental exposure models be employed in order to fully understand the impact of TCDD or other toxicants on adult development of endometriosis and related co-morbidities. For example, the Birnbaum laboratory exposed both pregnant rats and mice to TCDD on embryonic day 8 (E8) and then surgically induced endometriosis in adult offspring followed by a second exposure to TCDD. This study confirmed their previous studies demonstrating murine models of experimental endometriosis exhibit a higher level of sensitivity to TCDD compared to rats. More importantly, this study revealed that a prior developmental exposure to TCDD enhanced the effect of a secondary adult exposure to this same toxicant [55].
In our model system of developmental toxicant exposure, pregnant C57BL/6 mice are exposed to a single TCDD dose (10μg/kg) by gavage on E15.5, when organogenesis is complete. In contrast to the Birnbaum group, we initially did not attempt to induce experimental endometriosis, rather our goal was to examine the uterine phenotype of the adult offspring following early life toxicant exposure. We found that adult female offspring (F1 mice) of mice exposed to TCDD during development exhibit reduced uterine progesterone responsiveness, a phenotype associated with endometriosis in women [97]. These mice, like women with endometriosis, exhibit subfertility and an increased risk of delivering preterm [54, 98]. Thus, these animals exhibiting an “endometriosis-like” uterine phenotype have been very valuable in examining co-morbidities associated with this disease, which will be discussed below. More recently, we have used these animals to examine development of experimental endometriosis using the syngeneic mouse model described previously.
In addition to the dioxin-like EDCs, other toxicants have been shown to affect the development of endometriosis. For example, Signorile and colleagues exposed balbc mice to the EDC bisphenol A (BPA) during pre- and perinatal development [57]. Specifically, animals were treated with a high or low dose of BPA daily throughout pregnancy and until PND7. At 3 months of age, mice were euthanized and the peritoneal cavity and selected tissues examined, revealing endometriosis-like structures within the abdominal fat of a subset of BPA exposed mice. Significantly, histological examination revealed these lesions contained both glands and stroma. Since mice do not menstruate, these findings provide preliminary evidence, and a potential model system, to investigate activation of embryonic cell rests or stem cells by EDCs as biologically feasible pathway leading to the development of endometriosis.
3. The mechanisms
In large part, current research efforts in the field of endometriosis are focused on the development of new and better clinical therapies for women with this condition. However, designing more effective therapeutic strategies requires that we develop a deeper understanding of the trigger mechanisms driving the development and progression of endometriosis. Detailed below are examples of mechanistic studies, utilizing the models described above, which attempt to identify the pathogenic mechanisms associated with development of endometriosis.
3.1. Early Lesion Establishment and Vascularization
Successful establishment of endometriosis is a multi-step process requiring the rapid occurrence of peritoneal attachment, extracellular matrix degradation and neovascularization. In order to examine early events associated with ectopic attachment of endometrial tissues, our laboratory utilized the chimeric human/mouse model system. Using this model, we explored the potential role of matrix metalloproteinases (MMPs) in the invasive establishment of experimental endometriosis in nude mice [36, 99]. The MMPs are highly regulated enzymes that are necessary for normal and pathologic tissue remodeling, including the cellular migration and matrix restructuring associated with wound-healing or tumor metastasis [100]. Our studies demonstrated that blocking endometrial MMP expression or action prevented the establishment of human endometrial growth at ectopic sites within the peritoneal cavity of nude mice [36].
Following the physical attachment of endometrial tissues to ectopic sites, survival of lesions requires the rapid establishment of a vascular supply and, in a collaborative study, we demonstrated that blocking angiogenesis is effective in preventing lesion development [101]. In a follow-up study focused on very early disease, we demonstrated that vascular development is apparent by 24 hrs with extensive vascularization occurring about 5 days post-injection [102]. Importantly, we noted that peritoneal attachment and acquisition of a vascular supply occurs more rapidly following experimental disease establishment with eutopic endometrial tissues acquired from women with endometriosis, suggesting that the endometrial phenotype associated with endometriosis patients exhibits a greater innate capacity to stimulate peritoneal blood vessel growth in our model [79]. Furthermore, acute exposure of disease-free control endometrial tissue to TCDD, prior to injection into the peritoneal cavity of recipient mice, leads to a similar host vascular response within the peritoneal cavity as observed with tissue acquired from endometriosis patients [79]. Thus, in addition to stimulating MMP expression in endometrial fragments [103], TCDD exposure also promotes a vascular response at the site of endometrial invasion into the murine peritoneal wall, suggesting that this toxicant may promote lesion development via multiple mechanisms.
In order to specifically examine the individual cellular responses within the human endometrium to TCDD, we have begun to utilize organ-on-chip models [104]. These microscaled models enable compartmentalized, heterogeneous cell culture systems that better recapitulate in vivo anatomy and allow the direct evaluation of paracrine and endocrine crosstalk among cell types. Using such a system, in conjunction with the in vivo models described herein, should provide detailed information with regard to the responses of individual cells to TCDD as well as the whole tissue response within the peritoneal cavity.
Although the phenotype of endometrial tissue fragments significantly contributes to disease pathogenesis, recent studies provide evidence that the peritoneal phenotype also plays a critical role in endometriosis. Moreover, recent studies suggest that the eutopic endometrial phenotype works in concert with peritoneal inflammation, synergistically promoting ectopic endometrial growth. For example, in response to retrograde menstruation, there is a large influx of immune cells into the peritoneal cavity, which in healthy women would clear the dead and dying menstrual debris. However, in women with endometriosis, both heavier menstrual bleeding and immune cell dysfunction may reduce the capacity of the innate immune system to scavenge refluxed tissues. Equally important, inflammatory mediators produced at inappropriate levels likely impact the invasive and neoangiogenic processes that are necessary for establishment of endometriosis [68, 105]. Menstrual debris may also stimulate pro-inflammatory cytokine production as a consequence of inflammasome activation.
The inflammasome is a family of multiprotein complexes that are expressed by macrophages and other immune cells. These components act as immune system receptors and sensors and regulate the inflammatory response associated with both infectious microbes and damaged-associated host proteins. To date, four inflammasome complexes have been identified; each having a distinct protein composition which are formed in a stimulus-dependent manner (reviewed [106]). Among these, the NLRP3 (NLR Family Pyrin Domain Containing 3) complex is the most well-studied and is present in a variety of normal and disease states [107]. All of the inflammasome complexes cleave pro-IL-1β, leading to its activation and promoting an inflammatory cascade. Activation of this system in the context of endometriosis may inadvertently promote tissue repair rather than tissue clearing [108]. This acute inflammatory response system can also activate the local vasculature, further promoting lesion survival. To this end, using the nude mouse model of experimental endometriosis, we found that vessels present within lesions revealed anastomosis had occurred between human and murine endothelial cells [101], suggesting that the human tissue produces chemoattractants that promote growth of the murine vasculature. Supporting these findings, chimeric human/murine vessels were also identified by Alvarez-Gonzalez et al. in a SCID mouse model of human experimental endometriosis in [109]. This latter study confirmed that the chimeric vessels were functional, with a circulating blood supply established by 3 weeks. Clearly, development of a vascular supply is essential for ectopic endometrial survival. Therefore, a large number of studies have utilized murine models to examine the potential therapeutic value of angiogenesis inhibitors [101, 110-117]. Collectively, these studies indicate that both natural and pharmaceutical agents that inhibit angiogenesis can impede maintenance and growth of experimental endometriosis and support investigation of angiostatic agents as potential therapeutic agents for women with this disease.
3.2. Steroid Action in Endometriosis
Normal endometrial function is largely dependent on the sequential action of the ovarian steroids estrogen and progesterone [118]. Following menstruation, rising levels of estradiol act to induce endometrial regrowth and estrogen levels remain high even after ovulation when progesterone levels increase to induce endometrial maturation. Progesterone not only acts on the endometrium to promote differentiation in preparation for pregnancy, but is also a potent anti-inflammatory steroid. If nidation does not occur, progesterone levels will begin to decline, resulting in a loss of endometrial steroid support and culminating in the acute inflammatory process of menstruation. Discussing the multiple cellular processes within the endometrium that are controlled by ovarian steroids is beyond the scope of this review examining experimental models of endometriosis. Nevertheless, researchers interested in the pathogenesis of endometriosis have long recognized the critical roles of the sex steroids in endometrial function whether within the uterus or at ectopic sites of growth. Exposure to estrogen is one of the principal endocrine risk factors for developing endometriosis [19], largely as result of this steroid’s ability to promote rapid endometrial proliferation.
In contrast to estrogen action, progesterone exposure (ie, pregnancy or combined oral contraceptives) acts to reduce proliferation and induce cellular maturation, thus serving as a negative risk factor for development of endometriosis [19, 119]. However, numerous studies have demonstrated that women with endometriosis exhibit altered expression of a variety of genes and proteins that are normally regulated by the sex steroids [120, 121] revealing potential therapeutic targets [122, 123]. Importantly, both steroids act via their respective receptors, primarily progesterone receptor-B (PR-B) and estrogen receptor-α (ESR1). Additionally, PR-A, a truncated isoform of PR-B, can act as a dominant repressor of PR-B [124, 125] and has been found to be overexpressed in endometriosis [103, 126, 127]. Similarly, estrogen receptor-β (ESR2) is overexpressed in women with endometriosis and appears to play a role in disease development [119, 128, 129].
Estrogen’s ability to promote endometrial proliferation in both eutopic [118] and ectopic [19] sites of growth has made this steroid an important target of investigation. For example, using a syngeneic mouse model of endometriosis, [51] the Korach laboratory demonstrated estradiol-mediated signaling was required in the development of endometriosis-like lesions. Specifically, this group utilized estrogen receptor knockout (αERKO and βERKO) mice to explore the influence of expression or absence of each receptor in the ectopic lesions as well as within the recipient animal. Their studies demonstrated that, compared to wild-type donors, the number of successful lesions was dramatically reduced in mice receiving minced uterine tissue from either αERKO or βERKO mice, which they surmise was related to an inability of αERKO uteri to acquire a vasculature in response to estradiol. Interestingly, the ER status of the recipient animal had little impact on lesion establishment.
Unlike estrogen, progesterone exposure (ie, pregnancy) is known to reduce the risk of endometriosis. For this reason, experimental models of endometriosis have been useful for preclinical studies examining the potential benefit of various progestins. A potential confounding issue with progestin-based therapy for endometriosis is that many patients with this disease exhibit sensitivity to progesterone [97, 130]. As noted earlier, our studies more than a decade ago demonstrated that the reduced response to progesterone action in endometrial tissue acquired from endometriosis patients is associated with increased expression of MMPs and an enhanced capacity to establish experimental endometriosis [130]. A later study revealed that although endometrial tissues from women with endometriosis established as experimental disease in nude mice were resistant to regression by progesterone, treatment with tanaproget, a selective PR-B agonist, effectively reduced disease burden [131]. Additionally, experimental endometriosis using transgenic mice in which one of the major steroid receptors has been deleted further underscores the important and complex relationship between estrogen and progesterone action and endometriosis. Using a syngeneic endometriosis model with wild-type and progesterone receptor knockout (PRKO) mice, Bulun and colleagues demonstrated that the presence of PR in ectopic uterine tissue was essential to prevent estrogen-mediated proliferation and survival of lesions. The authors concluded that resistance to progesterone of human endometriosis may be a consequence of reduced PR expression or function [53]. Taken together, these data suggest that normalizing progesterone action within the endometrium would effectively reduce a woman’s risk for developing endometriosis.
3.3. Inflammatory Response in Women with and Without Endometriosis
The presence of menstrual debris within the peritoneal cavity is generally accepted as a mechanical factor in a woman’s risk for the development of endometriosis. Nevertheless, an important, but, unanswered question is why only certain women develop this disease despite retrograde menstruation being a common phenomenon among reproductive age women [9] One explanation may be the intrinsic differences in progesterone responses noted between endometrial tissues of patients compared to women without endometriosis. However, as reviewed by Brosens et al. [132], alterations within the eutopic endometrium of women with endometriosis are not limited to reduced progesterone responsiveness. Indeed, alterations in expression of angiogenic factors, identification of nerve fibers and expression of inflammatory cytokines have been demonstrated to be altered in patients compared to disease-free women. Clearly, these differences likely promote either the development of endometriosis or play a role in co-morbidities associated with this disease.
In our laboratory, we identified elevated expression of IL-1α and IL-1β in endometrial tissues acquired from women with endometriosis which was associated with an enhanced ability to establish experimental disease in our chimeric nude mouse model [129]. Indeed, an important advantage of immunocompromised mice is the ability to compare endometrial tissues from women with and without endometriosis in an in vivo system. Our published data using this model, in which human endometrial fragments are introduced into the peritoneal cavity of immunocompromised mice, demonstrated an invasive advantage of eutopic endometrium obtained from women with endometriosis [37, 79]. Specifically, although endometrial tissues from women with and without endometriosis are equally capable of establishing ectopic disease, tissues from women with endometriosis establish larger lesions that are more quickly able to establish a vasculature [102]. Significantly, we found that peripheral immune cells obtained from control women limited the growth of experimental endometriosis [46]; however, in contrast, a similar immune cell preparation obtained from women with endometriosis enhanced the development of experimental disease (Fig. 3 and Table 2). These data suggest an important phenotypic difference in immune cells, in addition to endometrial tissue, obtained from women with and without endometriosis.
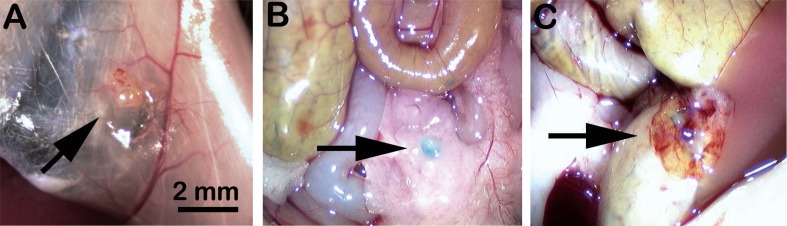
Fig. (3).
Experimental human endometriosis in rag2γ(c) mice. Proliferative phase human endometrial tissue growing as ectopic, intraperitoneal lesions in rag2γ(c) mice that were ovariectomized and implanted with slow release estradiol capsules (A-C). Mice received no immune cells (A), immune cells from a disease-free donor (B) or cells from an endometriosis patient (C). Prior to injection, immune cells were incubated with CarboxyFluoroscein Succinimidyl Ester (CSFE), resulting a blue-green appearance. Representative results of multiple (N=3) experiments using different human tissues are shown.
Table 2.
Impact of immune cell phenotype on experimental endometriosis.
| Mouse Treatment | Tissue Type/Cell Type | Percent w/ Disease | Avg Total Vol Lesions/Animal |
|---|---|---|---|
| E | Normal/None | 100% | 2.9 |
| E | Normal/Normal | 44% | 0.3 |
| E | Normal/Endometriosis | 100% | 3.3 |
The hyperinflammatory peritoneal environment observed in association with endometriosis in women has prompted a number of studies in rodents examining the efficacy of anti-inflammatory agents. Many of these studies, targeting specific inflammatory cytokines, were recently reviewed by Nothnick and Alali [105] and will not be detailed here. However, in contrast to targeting a specific inflammatory cytokine, a number of recent studies have explored the utility of dietary modulators of inflammation for the treatment of endometriosis. For example, using the rat surgical model of endometriosis, Akyol et al. [133] demonstrated that omega-3 fatty acids (found in fish oil) reduced experimental disease burden in association with a reduction in peritoneal levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and vascular endothelial growth factor (VEGF). Using a similar approach, İlhan et al. [134] treated rats with surgically induced endometriosis with a mixture of sea buckthorn and St. John's wort oils. These compounds have been used for many years in folk medicine for the treatment of a wide array of inflammatory disorders [135-138], but had not previously been examined with regard to endometriosis. In their study, İlhan et al. (2016) found that following 4 weeks of treatment with the oil mixture, rats in the treated group exhibited significantly fewer endometriotic lesions and adhesions compared to vehicle treated rats. Reduction in disease burden was associated with decreased peritoneal levels of IL-6, VEGF and TNF-α. Given the side effects of traditional pharmacologic approaches for the treatment of endometriosis, these studies support the exploration of anti-inflammatory diets for women with this disease.
4. Drivers of Co-Morbidities Associated with Endometriosis
As stated above, women with endometriosis frequently exhibit serious co-morbidities, but the drivers of these disease associations remain unclear. However, experimental rodent models of endometriosis have begun to reveal that inflammation may be contributing to epigenetic changes that play an important role in understanding the relationship between endometriosis and other diseases/conditions.
4.1. Epigenetics
Epigenetic marking of DNA, unlike genetic mutations, alters gene expression without changing the DNA sequence. Epigenetic modification is one mechanism by which DNA accessibility is controlled, altering the ability of the cellular machinery to activate transcription. The most well-studied epigenetic mechanisms are methylation and acetylation of DNA and histones [139]. Importantly, although these marks can be reversible, they are stable and, when occurring within the germline, these changes can be inherited [140-142]. During development, epigenetic reprogramming of individual cells results in cellular differentiation specific for each organ system, despite each cell having the same DNA. Thus, it is the epigenetic marks that ultimately determine each cell’s fate and allows the formation of a complex, multi-organ animal from the same genetic blueprint. Additionally, epigenetic modifications accumulate as we age, largely in response to our own choices (diet, activity level) or where we live (environmental exposures). These accumulated epigenetic marks are primarily why we become susceptible to age-related diseases. Additionally, environmental factors that affect epigenetic modification of the germ cells have the potential to contribute to an offspring’s phenotype [141]. Significantly, the presence of aberrant epigenetic patterns can cause developmental abnormalities and are associated with the etiology of certain human diseases [143]. As will be discussed below, recent research suggests that epigenetic changes, perhaps driven by inflammation related to environmental toxicant exposure, may promote the development of endometriosis and/or associated comorbidities.
4.2. Infertility
Infertility impacts up to 40% of women with endometriosis [19], but whether infertility is a function of inflammation that is associated with the disease or a consequence of intrinsic endometrial defects or both remains unclear. In an effort to address this question, Stilley et al. [58] used the rat surgical model of endometriosis to examine the impact of ectopic disease on fertility. Perhaps not surprisingly, fertility in animals with surgically induced endometriosis was significantly compromised, while fertility was not compromised in sham operated rats (hemi-hysterectomy, but no ectopic lesions). Furthermore, this study identified a number of ovarian alterations (fewer ovarian follicles and corpora lutea with luteinized unruptured follicles), poor preimplantation embryo development and spontaneous abortion in rats bearing endometriosis-like lesions compared the sham rats. Finally, this study also identified the same reproductive abnormalities in the daughters of rats with endometriosis-like lesions, suggesting that exposure to the inflammatory environment of this disease during in utero development may lead to permanent epigenetic changes in the offspring.
Using our developmental toxicant exposure model [54, 98], we initially reported that exposure of pregnant C57bl/6 mice to TCDD led to adult daughters (F1 females) with a uterine phenotype similar to that of women with endometriosis [98]. More specifically, we found that F1 females exhibit reduced uterine PR expression and loss of progesterone-sensitive TGF-β2 (transforming growth factor β) expression, proteins essential for establishment and maintenance of pregnancy. Thus, we were not surprised to find that subfertility was also common among F1 females mated to control breeder males. Approximately 50% of female F1 mice failed to exhibit signs of pregnancy (weight gain/nipple prominence) despite multiple matings and observation of vaginal plugs (4+). In addition to subfertility, F1 females which achieved pregnancy also exhibited a high rate of spontaneous preterm birth [54], a pregnancy outcome that has recently been linked to endometriosis [132]. Importantly, F2-F4 female mice continued to exhibit an endometrial phenotype similar to women with endometriosis, even though these animals were not subjected to additional toxicant exposure. Specifically, although the animals in our study did not have experimental endometriosis, we identified both multi-generational (F1-F2) and transgenerational (F3-F4) occurrence of reproductive disorders that are similar to those encountered by endometriosis patients [54].
Finally, we have also examined whether the male offspring of mice exposed to TCDD during pregnancy could transfer the endometriosis phenotype to his female progeny. Similar to our findings in F1 females, F1 males also exhibit a hyper-inflammatory phenotype and reduced fertility [144]. Importantly, the daughters of F1 males (F2P females) exhibited the same reproductive abnormalities identified in F1 females and their offspring, including reduced PR expression, subfertility and increased risk of spontaneous PTB (Table 3). Our animal studies, taken with the studies by other groups described above, strongly support a developmental origin of endometriosis. Equally important, our findings suggest that the environmental exposure history of either parent can lead to a transgenerational risk for the development of endometriosis. Understanding the potential role of toxicant-mediated inflammation may provide important insight enabling targeted therapies that reduce disease risk.
Table 3.
Impact of paternal TCDD exposure on pregnancy outcomes in his adult daughter (F2P).
| Mouse History | Pregnancy |
Pregnancy
Full-Term |
Outcome
Preterm |
|---|---|---|---|
| Control | 15/15 (100%) | 100% | 0% |
| F2P Female | 7/19 (37%) | 71% | 29% |
4.3. Adenomyosis
Adenomyosis, the presence of endometrial glands and stroma embedded within the uterine muscle, is frequently identified in women undergoing hysterectomy as a surgical treatment for endometriosis [145]. Adenomyosis, like endometriosis, has been associated with reduced fertility, pelvic pain, heavy menstrual bleeding and dysmenorrhea [22, 146]. The causes of adenomyosis are currently unknown, although both human and animal studies have suggested a role of inflammatory processes in the development of this disease [59, 147-149]. Furthermore, the occurrence of adenomyosis as a co-morbidity in women with endometriosis, fibroids and menorrhagia, supports a potential role of estrogen action and inflammation in the pathogenesis of this disease as well.
In our TCDD exposure model, we recently reported the transgenerational occurrence of adenomyosis in mice exhibiting the endometriosis-like uterine phenotype as a consequence of developmental toxicant exposure of F1 animals [147]. Within this recent study, we conducted a retrospective analysis of uteri from TCDD exposed F1 female mice and two generations of their offspring to determine whether histological evidence of adenomyosis was present. Although none of the control mice examined exhibited adenomyosis, we identified deep, adenomyotic lesions in the majority of mice with a history of direct (F1-F2) or indirect (F3) TCDD exposure. Since we have demonstrated that F1-F4 mice exhibit a “hyperinflammatory” systemic phenotype, the occurrence of adenomyosis in these animals provides additional support for inflammatory mechanisms in the promotion of this disease.
As stated above, in addition to inflammation, several studies have reported the promotion of adenomyosis by estrogen. For example, Koike et al. [150] exposed pregnant mice (ICR/Jcl, CLEA) to 0.01 mg ethinyl estradiol (EE2)/kg per day or vehicle (olive oil) by gavage from day 11 to 17 of gestation. Adult female offspring (F1 mice) were either treated with the same dose EE2 or to vehicle twice a week until 20 weeks of age. The control female offspring were also exposed to either vehicle of 0.01 mg EE2. All mice were euthanized at 7 months. Adenomyosis was common in in the EE2 -exposed uteri, and incidence of ectopic glands and serous cysts were significantly increased in the prenatally EE2 -exposed ovaries as compared with respective controls. This study suggests that continuous EE2 exposure can promote the development of adenomyosis. Using a very different model, Otto et al., [151] also demonstrated an important role of estrogen in the development of adenomyosis. This study examined the influence of the estrogen modulating compounds Faslodex and cetrorelix in SHN mice. SHN mice have been found to develop adenomyosis after pituitary grafting, which leads to an altered endocrine profile. The GnRH antagonist cetrorelix and the estrogen receptor antagonist Faslodex, which negatively interfered with estrogen-mediated signaling, completely inhibited development of adenoymosis, whereas danazol, was slightly less effective in inhibiting disease in SHN mice [151].
4.4. Adhesive Disease
Abdominal adhesions are fibrous bands of scar tissue that fuse two or more abdominal organs to each other and/or the peritoneum. Although adhesions most commonly occur as a complication of abdominal surgery; de novo formation may also occur in the absence of surgery as a consequence of inflammatory conditions within the abdomen [152]. For this reason, it is perhaps not surprising that compared to the general population; women with endometriosis are at a higher risk of both surgery-associated adhesions and spontaneous development of adhesive disease [153]. The basic pathophysiology of postsurgical adhesion development is known to involve inflammatory processes that occur during normal wound healing. Certainly, macrophages and neutrophils play key roles in the initiation of inflammation related to wound healing by releasing both proinflammatory cytokines and proangiogenic factors. Women with endometriosis are known to exhibit dysregulated immune cell function, which likely contributes to an enhanced inflammatory response and increased risk of developing adhesive disease [154]. In our chimeric nude mouse model, we examined the development of adhesive disease in association with experimental endometriosis [154, 155]. As reported in our published studies, we noted a cooperative effect of the presence of endometrial tissue fragments on the development of post-surgical adhesions when tissue fragments were introduced into the peritoneum within 16 hours of ovariectomy. Sham surgery (removal of the fat pad surrounding ovary, but not the ovary) or saline injection in the absence of human tissue was not associated with increased adhesive disease [154, 155]. Thus, within the peritoneal cavity, immune cell responses to injury, similar to infection or radiation therapy, may promote formation of adhesions [156]. Interestingly, we found that adhesions did not form when an identical experiment was conducted in rag2γ(c) mice (Table 4), animals which are more severely immunocompromised compared to nude mice. Nude mice exhibit loss of T cell function, but other immune cells are functional. In contrast, rag2γ(c) mice lack functional receptors for multiple interleukins, including IL-2, IL-4, IL-7, IL-9, and IL-15. As a consequence, B and T cell maturation are both compromised. Further, rag2γ(c) mice lack functional NK cells. An absence of adhesions in these animals suggests an additional role of one or more of these cytokines and/or immune cell types in post-surgical adhesion development. Interestingly, enhanced expression of IL-4 and IL-15 has been observed in patients with endometriosis [157, 158], raising the possibility that these cytokines might contribute to de novo adhesions in these women.
Table 4.
Influence of mouse genotype on endometriosis-associated adhesions.
| Mouse Genotype | N | Percent w/ Lesions | Avg Total Vol Lesions/Animal | Percent w/Adhesions | Adhesion Score |
|---|---|---|---|---|---|
| Nude | 16 | 91% | 2.5 | 81% | 3.2 |
| Rag2γ(c) | 12 | 100% | 2.7 | 0 | -- |
In studies using our developmental TCDD exposure model, we made the incidental observation that adhesions were rare in control mice at necropsy, but commonly observed F1-F3 mice. Therefore, in order to prospectively determine whether developmental TCDD exposure promotes the development of endometriosis-associated adhesive disease, we established a syngeneic endometriosis model in control and F1 mice within 16 hours of a surgical ovariectomy similar to the approach used with the chimeric adhesion model described above [154, 155]. These studies revealed that 100% of F1 mice developed adhesive disease following ovariectomy and injection of uterine fragments, regardless of the origin of the tissue (control or F1 uterus). In contrast, only 30% of control mice subjected to ovariectomy and uterine tissue injection (control or F1 tissue) developed adhesions [59]. These data further suggest that alterations in inflammatory responses within the peritoneal environment may be the most important driving force in the development of post-surgical adhesive disease.
4.5. Pain
Endometriosis-associated pain is a significant contributor to morbidity; however, studies correlating pain to extent or location of disease has not yielded advances in treatment (reviewed by [159]). Significantly, recent studies have illustrated that women with endometriosis exhibit “central sensitization”, a phenomenon by which pain itself promotes sensitivity to subsequent painful stimuli. Over time, a patient develops significant sensitivity to any stimuli, feeling more pain with less stimulation. Ultimately, even stimuli which should not be painful (i.e. a light touch) can trigger pain as a consequence of central sensitization [160, 161]. Central sensitization is particularly refractory to treatment; thus, animal models which can advance our understanding of this condition are desperately needed.
Using the rat surgical model described above, Berkley and colleagues have demonstrated that ectopic endometrial tissues become innervated and lead to vaginal hyperalgesia [48]. Significantly, inflammation appears to be a key player in the development of innervated lesions [160]; thus several groups are actively using various rodent models to test whether blocking the actions of the proinflammatory agent prostaglandin E2 can reduce experimental disease as well as associated pain [34, 162]. For example, using the chimeric rag2γ(c) mouse model, Arosh et al. [162] demonstrated that selective inhibition of EP2/EP4 not only inhibited survival of ectopic lesions, but also inhibited innervation of lesions and suppressed inflammation of dorsal root ganglia neurons. Significantly, following treatment with the EP2/EP4 inhibitors, animals in this study exhibited a reduction in pelvic pain, as assessed by stimulating the pelvic floor with von-Frey filaments (a standard nociception assay [163]).
Conclusion
Despite a century of research, the pathogenesis of endometriosis remains poorly understood. Nevertheless, the use of a variety of rodent models of experimental endometriosis has enabled significant advancement in our understanding of disease processes necessary for development of this disease. Although these models have limitations and cannot completely recapitulate the human disease process, studies described herein and in other recent reviews [33, 34, 164, 165] demonstrate the significant contribution of rodent models in providing a broader understanding of the complex and interactive roles of the endometrial phenotype, the peritoneal microenvironment, host angiogenic responses and the competency of the immune system which collectively determine an individual’s risk of developing endometriosis. Examination of inflammatory events within the peritoneal cavity in concert with identifying genetic and epigenetic alterations associated with endometriosis is expected to further enhance our understanding of why only certain women develop this condition. Clearly, developing new and more effective therapies will require a more complete understanding of the mechanisms which promote this disease. Furthermore, identifying specific factors (phenotypic, genetic and/or epigenetic) which predispose an at risk individual to the development of endometriosis is critical to ultimately preventing the development of this disease as well as its many comorbidities.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
Author contributions are as follows: KBT and KO designed the original studies and wrote the manuscript. Studies presented herein were conducted by KBT, TD, SM and JLH. We also acknowledge and appreciate the editorial assistance of Ms. Hailey Fox during the preparation of this manuscript. Original studies presented herein were supported in part by VA BX002583, NIEHS ES14942 and NICHD HD052012.
CONFLICT OF INTEREST
None of the authors have any conflicts of interests pertaining to the content of this manuscript.
REFERENCES
- 1.Benagiano G., Brosens I. The history of endometriosis: identifying the disease. Hum. Reprod. 1991;6(7):963–968. doi: 10.1093/oxfordjournals.humrep.a137470. [DOI] [PubMed] [Google Scholar]
- 2.Diesterweg A. Ein Fall Von Cystofibroma uteri verum. Z. Geburtshilfe. 1883;9:191–195. [Google Scholar]
- 3.Samspon J.A. Peritoneal endometriosis due to menstrual dissemenation of endometrial tissues into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927;14:422–469. [Google Scholar]
- 4.Ridley J.H., Edwards I.K. Experimental endometriosis in the human. Am. J. Obstet. Gynecol. 1958;76(4):783–789. doi: 10.1016/0002-9378(58)90011-5. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen S.S., Andersen L.F., Lose G. Endometriosis by implantation: a complication of endometrial ablation. Lancet. 1994;343(8907):1226. doi: 10.1016/s0140-6736(94)92435-x. [DOI] [PubMed] [Google Scholar]
- 6.Te Linde R.W., Scott R.B. Experimental endometriosis. Am. J. Obstet. Gynecol. 1950;60(5):1147–1173. doi: 10.1016/0002-9378(50)90517-5. [DOI] [PubMed] [Google Scholar]
- 7.Blumenkrantz M.J., Gallagher N., Bashore R.A., Tenckhoff H. Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstet. Gynecol. 1981;57(5):667–670. [PubMed] [Google Scholar]
- 8.Eskenazi B., Warner M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. North Am. 1997;24(2):235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 9.Halme J., Hammond M.G., Hulka J.F., Raj S.G., Talbert L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 10.El-Mahgoub S., Yaseen S. A positive proof for the theory of coelomic metaplasia. Am. J. Obstet. Gynecol. 1980;137(1):137–140. doi: 10.1016/0002-9378(80)90399-3. [DOI] [PubMed] [Google Scholar]
- 11.Meyer R. Uber Adenomatose. Wucherung der serosa in einer Banchuabe. Zeit Geburt Gynak. 1903;49:32–38. [Google Scholar]
- 12.Nakamura M., Katabuchi H., Tohya T., et al. Scanning electron microscopic and immunohistochemical studies of pelvic endometriosis. Hum. Reprod. 1993;8(12):2218–2226. doi: 10.1093/oxfordjournals.humrep.a138006. [DOI] [PubMed] [Google Scholar]
- 13.Batt R.E., Smith R.A. Embryologic theory of histogenesis of endometriosis in peritoneal pockets. Obstet. Gynecol. Clin. North Am. 1989;16(1):15–28. [PubMed] [Google Scholar]
- 14.Batt R.E., Smith R.A., Buck G.M., Severino M.F., Naples J.D. Mullerianosis. Prog. Clin. Biol. Res. 1990;323:413–426. [PubMed] [Google Scholar]
- 15.Schrodt G.R., Alcorn M.O., Ibanez J. Endometriosis of the male urinary system: a case report. J. Urol. 1980;124(5):722–723. doi: 10.1016/s0022-5347(17)55627-x. [DOI] [PubMed] [Google Scholar]
- 16.Witz C.A. Current concepts in the pathogenesis of endometriosis. Clin. Obstet. Gynecol. 1999;42(3):566–585. doi: 10.1097/00003081-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Signorile P.G., Baldi F., Bussani R., et al. New evidence of the presence of endometriosis in the human fetus. Reprod. Biomed. Online. 2010;21(1):142–147. doi: 10.1016/j.rbmo.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Signorile P.G., Baldi F., Bussani R., et al. Embryologic origin of endometriosis: analysis of 101 human female fetuses. J. Cell. Physiol. 2012;227(4):1653–1656. doi: 10.1002/jcp.22888. [DOI] [PubMed] [Google Scholar]
- 19.Bulun S.E. Endometriosis. N. Engl. J. Med. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 20.Brosens I., Curcic A., Vejnovic T., et al. The perinatal origins of major reproductive disorders in the adolescent: Research avenues. Placenta. 2015;36(4):341–344. doi: 10.1016/j.placenta.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Sinaii N., Cleary S.D., Ballweg M.L., Nieman L.K., Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod. 2002;17(10):2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 22.Struble J., Reid S., Bedaiwy M.A. Adenomyosis: A clinical review of a challenging gynecologic condition. J. Minim. Invasive Gynecol. 2016;23(2):164–185. doi: 10.1016/j.jmig.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Tirlapur S.A., Kuhrt K., Chaliha C., et al. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int. J. Surg. 2013;11(3):233–237. doi: 10.1016/j.ijsu.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Abbott J.A., Hawe J., Clayton R.D., Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2-5 year follow-up. Hum. Reprod. 2003;18(9):1922–1927. doi: 10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- 25.Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. 2002. [DOI] [PubMed]
- 26.D’Hooghe T.M. Immunomodulators and aromatase inhibitors: are they the next generation of treatment for endometriosis? Curr. Opin. Obstet. Gynecol. 2003;15(3):243–249. doi: 10.1097/01.gco.0000072859.73466.e3. [DOI] [PubMed] [Google Scholar]
- 27.Donnez J., Pirard C., Smets M., Jadoul P., Squifflet J. Pre- and post-surgical management of endometriosis. Semin. Reprod. Med. 2003;21(2):235–242. doi: 10.1055/s-2003-41329. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar C.M. Extracts from the “clinical evidence”. Endometriosis. BMJ. 2000;320(7247):1449–1452. doi: 10.1136/bmj.320.7247.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison R.F., Barry-Kinsella C. Efficacy of medroxyprogesterone treatment in infertile women with endometriosis: a prospective, randomized, placebo-controlled study. Fertil. Steril. 2000;74(1):24–30. doi: 10.1016/s0015-0282(00)00577-x. [DOI] [PubMed] [Google Scholar]
- 30.Howard F.M. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet. Gynecol. Surv. 1993;48(6):357–387. doi: 10.1097/00006254-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Hughes E.G., Fedorkow D.M., Collins J.A. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil. Steril. 1993;59(5):963–970. [PubMed] [Google Scholar]
- 32.Kettel L.M., Murphy A.A. Combination medical and surgical therapy for infertile patients with endometriosis. Obstet. Gynecol. Clin. North Am. 1989;16(1):167–177. [PubMed] [Google Scholar]
- 33.Edwards A.K., Nakamura D.S., Virani S., Wessels J.M., Tayade C. Animal models for anti-angiogenic therapy in endometriosis. J. Reprod. Immunol. 2013;97(1):85–94. doi: 10.1016/j.jri.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Greaves E., Critchley H.O.D., Horne A.W., Saunders P.T.K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet. Gynecol. Scand. 2017;96(6):644–658. doi: 10.1111/aogs.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awwad J.T., Sayegh R.A., Tao X.J., et al. The SCID mouse: an experimental model for endometriosis. Hum. Reprod. 1999;14(12):3107–3111. doi: 10.1093/humrep/14.12.3107. [DOI] [PubMed] [Google Scholar]
- 36.Bruner K.L., Matrisian L.M., Rodgers W.H., Gorstein F., Osteen K.G. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J. Clin. Invest. 1997;99(12):2851–2857. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. 2002. [DOI] [PubMed]
- 38.Greenberg LH, Slayden OD. 2004.
- 39.Bergqvist A., Jeppsson S., Kullander S., Ljungberg O. Human uterine endometrium and endometriotic tissue transplanted into nude mice. Morphologic effects of various steroid hormones. Am. J. Pathol. 1985;121(2):337–341. [PMC free article] [PubMed] [Google Scholar]
- 40.Nisolle M., Casanas-Roux F., Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil. Steril. 2000;74(2):306–312. doi: 10.1016/s0015-0282(00)00601-4. [DOI] [PubMed] [Google Scholar]
- 41.Zamah N.M., Dodson M.G., Stephens L.C., et al. Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am. J. Obstet. Gynecol. 1984;149(6):591–597. doi: 10.1016/0002-9378(84)90240-0. [DOI] [PubMed] [Google Scholar]
- 42.Flanagan S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 43.Pantelouris E.M. Absence of thymus in a mouse mutant. Nature. 1968;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- 44.Rygaard J. Thymus and self. Immunobiology of the mouse mutant nude. London, New York: John Wiley & Sons; 1973. p. 194. [Google Scholar]
- 45.Aoki D., Katsuki Y., Shimizu A., Kakinuma C., Nozawa S. Successful heterotransplantation of human endometrium in SCID mice. Obstet. Gynecol. 1994;83(2):220–228. [PubMed] [Google Scholar]
- 46.Bruner-Tran K.L., Carvalho-Macedo A.C., Duleba A.J., Crispens M.A., Osteen K.G. Experimental endometriosis in immunocompromised mice after adoptive transfer of human leukocytes. Fertil. Steril. 2010;93(8):2519–2524. doi: 10.1016/j.fertnstert.2009.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazurier F., Fontanellas A., Salesse S., et al. A novel immunodeficient mouse model--RAG2 x common cytokine receptor gamma chain double mutants--requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J. Interferon Cytokine Res. 1999;19(5):533–541. doi: 10.1089/107999099313983. [DOI] [PubMed] [Google Scholar]
- 48.McAllister S.L., Giourgas B.K., Faircloth E.K., et al. Prostaglandin levels, vaginal innervation, and cyst innervation as peripheral contributors to endometriosis-associated vaginal hyperalgesia in rodents. Mol. Cell. Endocrinol. 2016;437:120–129. doi: 10.1016/j.mce.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. 2002. [DOI] [PubMed]
- 50.Vernon M.W., Wilson E.A. Studies on the surgical induction of endometriosis in the rat. Fertil. Steril. 1985;44(5):684–694. [PubMed] [Google Scholar]
- 51.Burns K.A., Rodriguez K.F., Hewitt S.C., et al. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153(8):3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirata T., Osuga Y., Yoshino O., et al. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum. Reprod. 2005;20(8):2092–2096. doi: 10.1093/humrep/dei012. [DOI] [PubMed] [Google Scholar]
- 53.Fang Z., Yang S., Lydon J.P., et al. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil. Steril. 2004;82(3):673–678. doi: 10.1016/j.fertnstert.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 54.Bruner-Tran K.L., Osteen K.G. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 2011;31(3):344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cummings A.M., Hedge J.M., Birnbaum L.S. Effect of prenatal exposure to TCDD on the promotion of endometriotic lesion growth by TCDD in adult female rats and mice. Toxicol. Sci. 1999;52(1):45–49. doi: 10.1093/toxsci/52.1.45. [DOI] [PubMed] [Google Scholar]
- 56.Ding T., McConaha M., Boyd K.L., Osteen K.G., Bruner-Tran K.L. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod. Toxicol. 2011;31(3):351–358. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Signorile P.G., Spugnini E.P., Mita L., et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen. Comp. Endocrinol. 2010;168(3):318–325. doi: 10.1016/j.ygcen.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 58.Stilley J.A., Woods-Marshall R., Sutovsky M., Sutovsky P., Sharpe-Timms K.L. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 1. Biol. Reprod. 2009;80(4):649–656. doi: 10.1095/biolreprod.108.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruner-Tran K.L., Gnecco J., Ding T., et al. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod. Toxicol. 2017;68:59–71. doi: 10.1016/j.reprotox.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Villartay J.P. V(D)J recombination deficiencies. Adv. Exp. Med. Biol. 2009;650:46–58. doi: 10.1007/978-1-4419-0296-2_4. [DOI] [PubMed] [Google Scholar]
- 61.Bosma G.C., Owen J., Easton G., et al. Concentration of IgG1 and IgG2a allotypes in serum of nude and normal allotype-congenic mice. J. Immunol. 1980;124(2):879–884. [PubMed] [Google Scholar]
- 62.MacDonald H.R., Blanc C., Lees R.K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J. Immunol. 1986;136(12):4337–4339. [PubMed] [Google Scholar]
- 63.Abo T. Extrathymic pathways of T-cell differentiation: a primitive and fundamental immune system. Microbiol. Immunol. 1993;37(4):247–258. doi: 10.1111/j.1348-0421.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 64.Cao X., Kozak C.A., Liu Y.J., et al. Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) gamma chain: chromosomal mapping and tissue specificity of IL-2R gamma chain expression. Proc. Natl. Acad. Sci. USA. 1993;90(18):8464–8468. doi: 10.1073/pnas.90.18.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alt F.W., Rathbun G., Oltz E., Taccioli G., Shinkai Y. Function and control of recombination-activating gene activity. Ann. N. Y. Acad. Sci. 1992;651:277–294. doi: 10.1111/j.1749-6632.1992.tb24626.x. [DOI] [PubMed] [Google Scholar]
- 66.Cunha G.R. Age-dependent loss of sensitivity of female urogenital sinus to androgenic conditions as a function of the epithelia-stromal interaction in mice. Endocrinology. 1975;97(3):665–673. doi: 10.1210/endo-97-3-665. [DOI] [PubMed] [Google Scholar]
- 67.Kurita T., Medina R., Schabel A.B., et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation. 2005;73(6):313–322. doi: 10.1111/j.1432-0436.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 68.Herington J.L., Bruner-Tran K.L., Lucas J.A., Osteen K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011;7(5):611–626. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greaves E., Cousins F.L., Murray A., et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 2014;184(7):1930–1939. doi: 10.1016/j.ajpath.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altan Z.M., Denis D., Kagan D., et al. A long-acting tumor necrosis factor alpha-binding protein demonstrates activity in both in vitro and in vivo models of endometriosis. J. Pharmacol. Exp. Ther. 2010;334(2):460–466. doi: 10.1124/jpet.110.166488. [DOI] [PubMed] [Google Scholar]
- 71.Cummings A.M., Metcalf J.L. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod. Toxicol. 1995;9(3):233–238. doi: 10.1016/0890-6238(95)00004-t. [DOI] [PubMed] [Google Scholar]
- 72.Mizumoto Y., Hirata J., Tokuoka S., et al. Effect of culture supernatants of endometriotic lesions, uterine endometrium and peritoneum from rats with experimental endometriosis on the natural killer activity of spleen cells. Gynecol. Obstet. Invest. 1996;41(2):122–127. doi: 10.1159/000292056. [DOI] [PubMed] [Google Scholar]
- 73.Giulivo M., Lopez de Alda M., Capri E., Barcelo D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Heindel J.J. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin. Reprod. Med. 2006;24(3):168–177. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 75.Rier S.E., Martin D.C., Bowman R.E., Dmowski W.P., Becker J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1993;21(4):433–441. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez-Ochoa I., Karman B.N., Flaws J.A. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem. Pharmacol. 2009;77(4):547–559. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pocar P., Fischer B., Klonisch T., Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129(4):379–389. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- 78.Bruner-Tran K.L., Ding T., Osteen K.G. Dioxin and endometrial progesterone resistance. Semin. Reprod. Med. 2010;28(1):59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruner-Tran K.L., Yeaman G.R., Crispens M.A., Igarashi T.M., Osteen K.G. Dioxin may promote inflammation-related development of endometriosis. Fertil. Steril. 2008;89(5) Suppl.:1287–1298. doi: 10.1016/j.fertnstert.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heilier J.F., Nackers F., Verougstraete V., et al. Increased dioxin-like compounds in the serum of women with peritoneal endometriosis and deep endometriotic (adenomyotic) nodules. Fertil. Steril. 2005;84(2):305–312. doi: 10.1016/j.fertnstert.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Heilier J.F., Verougstraete V., Nackers F., et al. Assessment of cadmium impregnation in women suffering from endometriosis: a preliminary study. Toxicol. Lett. 2004;154(1-2):89–93. doi: 10.1016/j.toxlet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Jirsova S., Masata J.V., Drbohlava P., et al. Ceska Gynekol. 2005;70(4):262–268. [Differences in the polychlorinated biphenyl levels in follicular fluid in individual types of sterility]. [PubMed] [Google Scholar]
- 83.Louis G.M., Weiner J.M., Whitcomb B.W., et al. Environmental PCB exposure and risk of endometriosis. Hum. Reprod. 2005;20(1):279–285. doi: 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- 84.Smarr M.M., Kannan K., Buck Louis G.M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 2016;106(4):959–966. doi: 10.1016/j.fertnstert.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 85.De Felip E., Porpora M.G., di Domenico A., et al. Dioxin-like compounds and endometriosis: a study on Italian and Belgian women of reproductive age. Toxicol. Lett. 2004;150(2):203–209. doi: 10.1016/j.toxlet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 86.Fierens S., Mairesse H., Heilier J.F., et al. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: findings in a population-based study in Belgium. Biomarkers. 2003;8(6):529–534. doi: 10.1080/1354750032000158420. [DOI] [PubMed] [Google Scholar]
- 87.Lebel G., Dodin S., Ayotte P., et al. Organochlorine exposure and the risk of endometriosis. Fertil. Steril. 1998;69(2):221–228. doi: 10.1016/s0015-0282(97)00479-2. [DOI] [PubMed] [Google Scholar]
- 88.Pauwels A., Schepens P.J., D’Hooghe T., et al. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: a case-control study of infertile women. Hum. Reprod. 2001;16(10):2050–2055. doi: 10.1093/humrep/16.10.2050. [DOI] [PubMed] [Google Scholar]
- 89.Tsukino H., Hanaoka T., Sasaki H., et al. Associations between serum levels of selected organochlorine compounds and endometriosis in infertile Japanese women. Environ. Res. 2005;99(1):118–125. doi: 10.1016/j.envres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Anger D.L., Foster W.G. The link between environmental toxicant exposure and endometriosis. Front. Biosci. 2008;13:1578–1593. doi: 10.2741/2782. [DOI] [PubMed] [Google Scholar]
- 91.Heilier J.F., Donnez J., Lison D. Organochlorines and endometriosis: a mini-review. Chemosphere. 2008;71(2):203–210. doi: 10.1016/j.chemosphere.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 92.Rier S., Foster W.G. Environmental dioxins and endometriosis. Semin. Reprod. Med. 2003;21(2):145–154. doi: 10.1055/s-2003-41321. [DOI] [PubMed] [Google Scholar]
- 93.Cummings A.M., Metcalf J.L., Birnbaum L. Promotion of endometriosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats and mice: time-dose dependence and species comparison. Toxicol. Appl. Pharmacol. 1996;138(1):131–139. doi: 10.1006/taap.1996.0106. [DOI] [PubMed] [Google Scholar]
- 94.Johnson K.L., Cummings A.M., Birnbaum L.S. Promotion of endometriosis in mice by polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls. Environ. Health Perspect. 1997;105(7):750–755. doi: 10.1289/ehp.97105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bruner-Tran K.L., Rier S.E., Eisenberg E., Osteen K.G. The potential role of environmental toxins in the pathophysiology of endometriosis. Gynecol. Obstet. Invest. 1999;48(Suppl. 1):45–56. doi: 10.1159/000052868. [DOI] [PubMed] [Google Scholar]
- 96.Mallozzi M., Bordi G., Garo C., Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. C Embryo Today. 2016;108(3):224–242. doi: 10.1002/bdrc.21137. [DOI] [PubMed] [Google Scholar]
- 97.Osteen K.G., Bruner-Tran K.L., Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil. Steril. 2005;83(3):529–537. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 98.Nayyar T., Bruner-Tran K.L., Piestrzeniewicz-Ulanska D., Osteen K.G. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod. Toxicol. 2007;23(3):326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruner K.L., Eisenberg E., Gorstein F., Osteen K.G. Progesterone and transforming growth factor-beta coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids. 1999;64(9):648–653. doi: 10.1016/s0039-128x(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 100.VanSaun M.N., Matrisian L.M. Matrix metalloproteinases and cellular motility in development and disease. Birth Defects Res. C Embryo Today. 2006;78(1):69–79. doi: 10.1002/bdrc.20061. [DOI] [PubMed] [Google Scholar]
- 101.Hull M.L., Charnock-Jones D.S., Chan C.L., et al. Antiangiogenic agents are effective inhibitors of endometriosis. J. Clin. Endocrinol. Metab. 2003;88(6):2889–2899. doi: 10.1210/jc.2002-021912. [DOI] [PubMed] [Google Scholar]
- 102.Bruner-Tran K.L., Osteen K.G., Duleba A.J. Simvastatin protects against the development of endometriosis in a nude mouse model. J. Clin. Endocrinol. Metab. 2009;94(7):2489–2494. doi: 10.1210/jc.2008-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Igarashi T.M., Bruner-Tran K.L., Yeaman G.R., et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil. Steril. 2005;84(1):67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 104.Gnecco J.S., Pensabene V., Li D.J., et al. Compartmentalized Culture of Perivascular Stroma and Endothelial Cells in a Microfluidic Model of the Human Endometrium. Ann. Biomed. Eng. 2017;45(7):1758–1769. doi: 10.1007/s10439-017-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nothnick W, Alali Z. 2016.
- 106.Bullon P., Alcocer-Gomez E., Carrion A.M., et al. AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome. Antioxid. Redox Signal. 2016;24(3):157–170. doi: 10.1089/ars.2014.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaidt M.M., Hornung V. Alternative inflammasome activation enables IL-1beta release from living cells. Curr. Opin. Immunol. 2017;44:7–13. doi: 10.1016/j.coi.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bullon P., Navarro J.M. Inflammasome as a Key Pathogenic Mechanism in Endometriosis. Curr. Drug Targets. 2017;18(9):997–1002. doi: 10.2174/1389450117666160709013850. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez Gonzalez M.L., Frankenne F., Galant C., et al. Mixed origin of neovascularization of human endometrial grafts in immunodeficient mouse models. Hum. Reprod. 2009;24(9):2217–2224. doi: 10.1093/humrep/dep203. [DOI] [PubMed] [Google Scholar]
- 110.Bora P.S., Hu Z., Tezel T.H., et al. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc. Natl. Acad. Sci. USA. 2003;100(5):2679–2684. doi: 10.1073/pnas.0438014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu Z., Garen A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc. Natl. Acad. Sci. USA. 2000;97(16):9221–9225. doi: 10.1073/pnas.97.16.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Z., Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc. Natl. Acad. Sci. USA. 2001;98(21):12180–12185. doi: 10.1073/pnas.201420298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu Z., Sun Y., Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc. Natl. Acad. Sci. USA. 1999;96(14):8161–8166. doi: 10.1073/pnas.96.14.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krikun G., Hu Z., Osteen K., et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am. J. Pathol. 2010;176(2):1050–1056. doi: 10.2353/ajpath.2010.090757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nap A.W., Griffioen A.W., Dunselman G.A., et al. Antiangiogenesis therapy for endometriosis. J. Clin. Endocrinol. Metab. 2004;89(3):1089–1095. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- 116.Tezel T.H., Bodek E., Sonmez K., et al. Targeting tissue factor for immunotherapy of choroidal neovascularization by intravitreal delivery of factor VII-Fc chimeric antibody. Ocul. Immunol. Inflamm. 2007;15(1):3–10. doi: 10.1080/09273940601147760. [DOI] [PubMed] [Google Scholar]
- 117.Xu H., Lui W.T., Chu C.Y., et al. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009;24(3):608–618. doi: 10.1093/humrep/den417. [DOI] [PubMed] [Google Scholar]
- 118.King A.E., Critchley H.O. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 2010;120(2-3):116–126. doi: 10.1016/j.jsbmb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Bulun S.E., Monsavais D., Pavone M.E., et al. Role of estrogen receptor-beta in endometriosis. Semin. Reprod. Med. 2012;30(1):39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kao L.C., Germeyer A., Tulac S., et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 121.Kao L.C., Tulac S., Lobo S., et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 122.Han S.J., O’Malley B.W. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum. Reprod. Update. 2014;20(4):467–484. doi: 10.1093/humupd/dmu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lessey B.A., Young S.L. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Semin. Reprod. Med. 2014;32(5):365–375. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 124.Vegeto E., Cocciolo M.G., Raspagliesi F., et al. Regulation of progesterone receptor gene expression. Cancer Res. 1990;50(17):5291–5295. [PubMed] [Google Scholar]
- 125.Vegeto E., Shahbaz M.M., Wen D.X., et al. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 1993;7(10):1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 126.Attia G.R., Zeitoun K., Edwards D., et al. Progesterone receptor isoform A but not B is expressed in endometriosis. J. Clin. Endocrinol. Metab. 2000;85(8):2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 127.Wolfler M.M., Kuppers M., Rath W., et al. Altered expression of progesterone receptor isoforms A and B in human eutopic endometrium in endometriosis patients. Ann. Anat. 2016;206:1–6. doi: 10.1016/j.aanat.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 128.Simmen R.C., Kelley A.S. Reversal of fortune: estrogen receptor-beta in endometriosis. J. Mol. Endocrinol. 2016;57(2):F23–F27. doi: 10.1530/JME-16-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stocks M.M., Crispens M.A., Ding T., et al. Therapeutically targeting the inflammasome product in a chimeric model of endometriosis-related surgical adhesions. Reprod. Sci. 2017;•••:1933719117698584. doi: 10.1177/1933719117698584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bruner-Tran K.L., Eisenberg E., Yeaman G.R., et al. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J. Clin. Endocrinol. Metab. 2002;87(10):4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 131.Bruner-Tran K.L., Zhang Z., Eisenberg E., Winneker R.C., Osteen K.G. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J. Clin. Endocrinol. Metab. 2006;91(4):1554–1560. doi: 10.1210/jc.2005-2024. [DOI] [PubMed] [Google Scholar]
- 132.Brosens I., Brosens J.J., Benagiano G. The eutopic endometrium in endometriosis: are the changes of clinical significance? Reprod. Biomed. Online. 2012;24(5):496–502. doi: 10.1016/j.rbmo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 133.Akyol A., Simsek M., Ilhan R., et al. Efficacies of vitamin D and omega-3 polyunsaturated fatty acids on experimental endometriosis. Taiwan. J. Obstet. Gynecol. 2016;55(6):835–839. doi: 10.1016/j.tjog.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 134.Ilhan M., Suntar I., Demirel M.A., et al. A mixture of St. John’s wort and sea buckthorn oils regresses endometriotic implants and affects the levels of inflammatory mediators in peritoneal fluid of the rat: A surgically induced endometriosis model. Taiwan. J. Obstet. Gynecol. 2016;55(6):786–790. doi: 10.1016/j.tjog.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Li T, Wang L.
- 136.Mazza G., editor. 1998. [Google Scholar]
- 137.Suryakumar G., Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011;138(2):268–278. doi: 10.1016/j.jep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 138.Yesilada E., Honda G., Sezik E., et al. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995;46(3):133–152. doi: 10.1016/0378-8741(95)01241-5. [DOI] [PubMed] [Google Scholar]
- 139.Yesilada E., Honda G., Sezik E., et al. Traditional medicine in Turkey. IV. Folk medicine in the Mediterranean subdivision. J. Ethnopharmacol. 1993;39(1):31–38. doi: 10.1016/0378-8741(93)90048-a. [DOI] [PubMed] [Google Scholar]
- 140.Choo K.B. Epigenetics in disease and cancer. Malays. J. Pathol. 2011;33(2):61–70. [PubMed] [Google Scholar]
- 141.Gabory A., Attig L., Junien C. Epigenetic mechanisms involved in developmental nutritional programming. World J. Diabetes. 2011;2(10):164–175. doi: 10.4239/wjd.v2.i10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guerrero-Bosagna C., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol. Cell. Endocrinol. 2012;354(1-2):3–8. doi: 10.1016/j.mce.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morgan H.D., Santos F., Green K., Dean W., Reik W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005;14(Spec No 1):R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 144.Jiang Y.H., Sahoo T., Michaelis R.C., et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am. J. Med. Genet. A. 2004;131(1):1–10. doi: 10.1002/ajmg.a.30297. [DOI] [PubMed] [Google Scholar]
- 145.Bruner-Tran K.L., Ding T., Yeoman K.B., et al. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS One. 2014;9(8):e105084. doi: 10.1371/journal.pone.0105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vercellini P., Vigano P., Somigliana E., et al. Adenomyosis: epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006;20(4):465–477. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 147.Cockerham A.Z. Adenomyosis: a challenge in clinical gynecology. J. Midwifery Womens Health. 2012;57(3):212–220. doi: 10.1111/j.1542-2011.2011.00117.x. [DOI] [PubMed] [Google Scholar]
- 148.Bruner-Tran K.L., Duleba A.J., Taylor H.S., Osteen K.G. Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol. Reprod. 2016;95(4):73. doi: 10.1095/biolreprod.116.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jichan N., Xishi L., Guo S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010;17(11):995–1005. doi: 10.1177/1933719110377118. [DOI] [PubMed] [Google Scholar]
- 150.Li Y., Zhang S.F., Zou S.E., Xia X., Bao L. Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis. Reprod. Biol. Endocrinol. 2011;9:30. doi: 10.1186/1477-7827-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koike E., Yasuda Y., Shiota M., et al. Exposure to ethinyl estradiol prenatally and/or after sexual maturity induces endometriotic and precancerous lesions in uteri and ovaries of mice. Congenit. Anom. (Kyoto) 2013;53(1):9–17. doi: 10.1111/j.1741-4520.2012.00383.x. [DOI] [PubMed] [Google Scholar]
- 152.Otto C., Schkoldow J., Krahl E., Fuchs I., Ulbrich H.F. Use of a murine endometriosis interna model for the characterization of compounds that effectively treat human endometriosis. Exp. Ther. Med. 2012;3(3):410–414. doi: 10.3892/etm.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tabibian N., Swehli E., Boyd A., Umbreen A., Tabibian J.H. Abdominal adhesions: A practical review of an often overlooked entity. Ann. Med. Surg. (Lond.) 2017;15:9–13. doi: 10.1016/j.amsu.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fortin C.N., Saed G.M., Diamond M.P. Predisposing factors to post-operative adhesion development. Hum. Reprod. Update. 2015;21(4):536–551. doi: 10.1093/humupd/dmv021. [DOI] [PubMed] [Google Scholar]
- 155.Herington JL, Crispens MA, Carvalho-Macedo AC, et al. Development and prevention of postsurgical adhesions in a chimeric mouse model of experimental endometriosis. 2011. [DOI] [PMC free article] [PubMed]
- 156.Herington J.L., Glore D.R., Lucas J.A., Osteen K.G., Bruner-Tran K.L. Dietary fish oil supplementation inhibits formation of endometriosis-associated adhesions in a chimeric mouse model. Fertil. Steril. 2013;99(2):543–550. doi: 10.1016/j.fertnstert.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Imudia A.N., Kumar S., Saed G.M., Diamond M.P. Pathogenesis of Intra-abdominal and pelvic adhesion development. Semin. Reprod. Med. 2008;26(4):289–297. doi: 10.1055/s-0028-1082387. [DOI] [PubMed] [Google Scholar]
- 158.Arici A., Matalliotakis I., Goumenou A., et al. Increased levels of interleukin-15 in the peritoneal fluid of women with endometriosis: inverse correlation with stage and depth of invasion. Hum. Reprod. 2003;18(2):429–432. doi: 10.1093/humrep/deg083. [DOI] [PubMed] [Google Scholar]
- 159.Hsu C.C., Yang B.C., Wu M.H., Huang K.E. Enhanced interleukin-4 expression in patients with endometriosis. Fertil. Steril. 1997;67(6):1059–1064. doi: 10.1016/s0015-0282(97)81439-2. [DOI] [PubMed] [Google Scholar]
- 160.Stratton P., Berkley K.J. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum. Reprod. Update. 2011;17(3):327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McKinnon B.D., Bertschi D., Bersinger N.A., Mueller M.D. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol. Metab. 2015;26(1):1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 162.Giamberardino M.A., Tana C., Costantini R. Pain thresholds in women with chronic pelvic pain. Curr. Opin. Obstet. Gynecol. 2014;26(4):253–259. doi: 10.1097/GCO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 163.Arosh J.A., Lee J., Balasubbramanian D., et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. USA. 2015;112(31):9716–9721. doi: 10.1073/pnas.1507931112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martinov T., Mack M., Sykes A., Chatterjea D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013;(82):e51212. doi: 10.3791/51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grummer R. Translational animal models to study endometriosis-associated infertility. Semin. Reprod. Med. 2013;31(2):125–132. doi: 10.1055/s-0032-1333477. [DOI] [PubMed] [Google Scholar]