Abstract
Alpha synuclein (α-syn) belongs to a class of proteins which are commonly considered to play a detrimental role in neuronal survival. This assumption is based on the occurrence of a severe neuronal degeneration in patients carrying a multiplication of the α-syn gene (SNCA) and in a variety of experi-mental models, where overexpression of α-syn leads to cell death and neurological impairment. In these conditions, a higher amount of normally structured α-syn produces a damage, which is even worse com-pared with that produced by α-syn owning an abnormal structure (as occurring following point gene muta-tions). In line with this, knocking out the expression of α-syn is reported to protect from specific neurotox-ins such as 1-methyl, 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP). In the present review we briefly dis-cuss these well-known detrimental effects but we focus on findings showing that, in specific conditions α-syn is beneficial for cell survival. This occurs during methamphetamine intoxication which is counteracted by endogenous α-syn. Similarly, the dysfunction of the chaperone cysteine-string protein-alpha leads to cell pathology which is counteracted by over-expressing α-syn. In line with this, an increased expression of α-syn protects against oxidative damage produced by dopamine. Remarkably, when the lack of α-syn is combined with a depletion of β- and γ- synucleins, alterations in brain structure and function occur. This review tries to balance the evidence showing a beneficial effect with the bulk of data reporting a detri-mental effect of endogenous α-syn. The specific role of α-syn as a chaperone protein is discussed to ex-plain such a dual effect.
Keywords: Alpha synuclein, synucleinopathies, alpha synuclein aggregates, loss-of-function, co-chaperonine, neurodegeneration, neuroprotection
1. INTRODUCTION
Alpha synuclein (α-syn) is a small (14.5 kDa) pre-synaptic protein consisting of 140 amino acids and it was originally isolated from synaptic vesicles and nuclear envelopes of the electric organ of Torpedo californica [1-3]. It belongs to the synuclein family, which includes two other proteins that are highly conserved in vertebrates, namely β- and γ-synucleins [1, 4-7]. In particular, α-syn is ubiquitously and heterogeneously expressed in both central and peripheral nervous system, encompassing a wide number of brain regions (isocortex, hippocampus, olfactory bulb, striatum, thalamus and cerebellum) [6, 8]. The protein is mostly found within synaptic terminals, where it often co-localizes with β- and γ-synucleins. A little amount is present in the cell body, dendrites or extra-synaptic sites along the axons [1, 8-11].
Our understanding of the biology of α-syn has significantly increased under the impulse generated by the discovery, in 1997, of a single point mutation in α-syn (SNCA) gene, which may per se determine genetic Parkinsonism [12]. This gene mutation, which induces an altered primary structure of the native protein, was identified in an Italian kindred (the Contursi family) and in three non-related Greek kindreds, and it was associated with autosomal dominant inherited Parkinson’s disease (PD) [12].
From that groundbreaking discovery, several SNCA alterations, which may contribute to the pathogenesis of PD have been identified. These include several point (missense) mutations (i.e. A30P, E46K, H50Q, G51D, A53E, A53T) which are classified as PARK1 type, while genomic duplications and triplications of SNCA are classified as PARK4 genetic parkinsonism [13-22]. These large gene rearrangements produce a higher amount of normally structured α-syn, which produces a dose-dependent damage, which in any case, is worse compared with that caused by point mutations (with an exception of the point mutation G51D which causes a devastating disorder overlapping with Multiple System Atrophy). Thus, in most cases, when normally structured α-syn is present in large amounts, it causes a brain damage, which is even worse than that induced by altered protein structure that is unique among all genetic forms of PD. In fact, SNCA multiplication produces a lethal form of PD, which significantly impairs life expectancy depending on the number of copies in excess. Thus, normally structured α-syn may be really deleterious for cell survival. In line with this, according to the autosomal dominant inheritance of α-syn point mutations, a gain of function is supposed to underlie the brain damage caused by the mutated protein in PARK1 patients. This is confirmed by experimental data from the last couple of decades regarding such a deleterious effect, as in the case of MPTP - [6, 23, 24], 6OHDA- [25], and rotenone-induced [26-28] parkinsonism. Therefore, it may be inferred that α-syn plays just a detrimental role for cell survival.
Despite α-syn represents the culprit of a variety of degenerative disorders named synucleinopathies, it still remains to be elucidated why, albeit its detrimental effects, evolution has preserved α-syn in the cell to serve any kind of functions, which may be beneficial instead. Noteworthy, in certain experimental conditions α-syn plays an opposite role which is critical to counteract cell damage. This is the case of methamphetamine-induced toxicity, where a depletion of α-syn dramatically exacerbates the nigrostriatal damage [6]. Similarly, the overexpression of α-syn may counteract the brain damage induced by a depletion of the chaperone cysteine-string protein-α (CSP- α) [29], or may prevent oxidative damage induced by dopamine (DA) self-oxidation products and parkin depletion [30]. Remarkably, its predominant expression at pre-synaptic terminals accounts for a physiological function in regulating synaptic vesicles dynamics, such as synapse maintenance, neurotransmitter release, and neuronal physiology and plasticity [31-33]. In line with this, α-syn associates with cell membranes and it preferentially binds to anionic lipids and high-curvature lipid vesicles, mostly small uni-lamellar vesicles (SUVs) and large uni-lamellar vesicles (LUVs) [34-38].
In the present review, we will focus on the multi-faceted roles of α-syn analyzing in detail its detrimental and beneficial effects and trying to balance these opposite outcomes in cell homeostasis. This analysis is aimed to provide a deeper insight on the significance of this protein for neuronal physiology and specifically in neurodegeneration. Furthermore, a critical analysis of the dual roles of α-syn will be implemented with its activity as co-chaperonine, which potentially operates within the cell or may be shared within a multicellular context. This may disclose the evolutionary drive leading to protein spreading, which should probably be reconsidered as a natural phenomenon which does not necessarily spread disease but may also help cell survival and sustain plasticity.
2. Alpha-synuclein related neuropathology
In human patients, the mutation of α-syn generates the degeneration of mesencephalic neurons belonging to the substantia nigra pars compacta (SNpc), mostly in the ventral tier of this nucleus, which mainly projects to the basal ganglia [39, 40]. Spared neurons, despite being viable, feature neuronal inclusions which stain specifically for α-syn [41, 42]. Remarkably, if one considers the neuropathology of the SNpc in most PD patients lacking any kind of mutations in SNCA, the occurrence of α-syn positive neuronal inclusions represents the hallmark for the pathological diagnosis of PD [43]. In most cases of PD, α-syn positive inclusions (roughly corresponding to what was once defined Lewy bodies) are not limited to DA neurons of the SNpc but they extend to a variable degree to other brainstem nuclei [44-49]. In fact, the presence of α-syn positive inclusions within spared neurons of the noradrenergic (NA) nucleus of Locus Coeruleus (LC) is now considered to be a key point in the diagnosis of PD [47, 50, 51]. This is in line with the consistently massive involvement of LC in PD, which may surpass the damage of SNpc neurons [39, 47, 52-55]. Apart from these two catecholamine-containing nuclei, α-syn aggregates may be present to a variable extent in a constellation of brainstem reticular nuclei, among which, the most frequently affected are the dorsal motor nucleus of the vagus, the nucleus of the solitary tract, the dorsal raphe nucleus, and the pedunculopontine nucleus. Recently, a clinical-pathological correlation, in which the severity of PD symptoms associates with the number of brainstem nuclei affected by α-syn pathology, has been hypothesized [48, 51]. Such a consideration led to re-define PD as a Monoamine Brainstem Disorder (MBD) in which an innumerous pattern of affected nuclei may justify the high variability of clinical symptoms occurring in multiple PD isotypes [47, 51]. In this way, the widespread α-syn pathology within the brainstem resembles the constellation of affected brainstem nuclei in von Economo’s encephalitis lethargica [56, 57]. In fact, just like it was described for these patients, it is now well recognized that even in PD a number of symptoms affecting non-motor domains may be present. Among these non-motor symptoms (NMS), sleep disturbances occur very frequently [47, 58-60]. Sometimes REM sleep behavioral disorders (RBD) may even anticipate the onset of extra-pyramidal motor symptoms in a way that is reminiscent of what happened after the 1918’s flu pandemia [61, 62]. These overlapping disorders are fascinating and they suggest that similar targets are shared in all PD-related disorders independently from the specific etiology. The presence of pathological α-syn accumulation within cells is not confined to the brainstem but it may extend far away to involve a variety of brain areas [42] and even the spinal cord [55, 63-65]. When spreading massively to cortical regions, α-syn produces a degenerative dementia associated with a parkinsonian movement disorder, known as Dementia with Lewy Bodies (DLB) [55, 66-69]. In this case, α-syn positive inclusions are present in a variety of neurons exceeding the monoamine systems [55, 69-74]. In other pathological conditions, the presence of α-syn aggregates occurs in glial cells as well, thus involving multiple brain areas. In this case, the substantia nigra, the striatum, the cerebellum, and pre-ganglionic sympathetic neurons are markedly affected along with surrounding astrocytes. When this process involves oligodendrocytes and produces a de-myelinization in the affected areas, a pathological condition known as Multiple System Atrophy (MSA) is described. Among synucleinopathies also Pure Autonomic Failure (PAF, according to the Bannister classification) should be considered [75]. This consists in a pure orthosympathetic autonomic dysfunction in the absence of any movement disorders. This pathological overview, which is generally accepted when classifying synulceinopathies, is rather simplistic. In fact, the definition of synucleinopathies consists in multiple disorders of the CNS, where some neuronal systems are affected by α-syn aggregates. In keeping with this, other pathological conditions should be taken into account by using an extended definition of synucleinopathies. This is the case of Huntigton disease (HD), where despite being huntingtin the aberrant protein, large vacuoles accumulating within neurons (mostly in the neostriatum) feature α-syn aggregates along with huntingtin aggregates. Again, a number of disorders characterized by protein accumulation feature α-syn aggregates as well. This is the case of Alzheimer’s disease (AD) [76-78], Frontotemporal Dementia (FTD) [79, 80], Progressive Supranuclear Palsy (PSP) [81], in which α-syn aggregates occur along with Tau accumulation. This should not surprise, since in the presence of α-syn mutations, inherited parkinsonism is also featured by Tau accumulation [82-85]. Apart from protein aggregates, the neuronal pathology related to α-syn is characterized by neuronal loss which happens in all the brain areas that we just described. In addition, when multiple copies of normally structured α-syn are present, a massive neuronal degeneration with a severe cell loss is described in the hippocampal region and other allocortical areas [86]. When analyzing classic α-syn positive inclusions, as they were originally described in PD patients, these are proteinaceous aggregates with no external limiting membrane where treatment with protease does not cleave α-syn [87, 88]. In this way, just like the pathological prion protein, aggregated α-syn is resistant to proteinase K and sarkosyl [89]. In addition, just like prion disorders, a multiple band of the protein is detected at SDS page immunoblotting. This is due to the formation of advanced glycation end-products (AGEs), where specific sugars covalently bind to α-syn. In these aggregates, α-syn often co-exists with ubiquitin, which justifies the previous description by McKeith of Lewy Bodies as ubiquitin-positive inclusions (this happened before the discovery of α-syn in PD) [90]. Indeed, Fritz Lewy provided the definition of Lewy Bodies (LB) in 1912, long time before the use of immunostaining. Thus, the original definition of LB corresponds to pale, eosinophilic cytoplasmic inclusions [42]. It sounds odd that we often treat these pathological hallmarks as a single pathological entity, since many α-syn positive inclusions do not stain for ubiquitin and some of them are not pale, eosinophilic structures. Thus, the staining with α-syn antibodies has modified the amount and the entity of what was once defined as LB, which may create some confusion despite being a guide for the perplex scientists. In fact, the present definition of LBs states that they are α-syn positive aggregates and that α-syn is the best marker to detect LBs thanks to its high sensitivity. However, if one considers the original definition of LB or the classification by McKeith, there is no reason to assume that all α-syn positive aggregates correspond to LBs. We feel that it is rather more correct to state that α-syn stains LBs and other proteinaceous aggregates which were previously unclassified. It is likely that even the molecular mechanisms leading specifically to LB formation, may or may not overlap with α-syn aggregates. In fact, the presence of ubiquitin witnesses for a tagging of a substrate (α-syn itself) to be delivered either to proteasome [87, 91] autophagy or autophagoproteasome [92-95] degradation. Instead, if one considers α-syn-positive/ubiquitin-negative proteinaceous material, there is no reason to assume that this will be eventually delivered for degradation. This is even more distant from the original Lewy’s definition. In fact, the presence of pale eosinophilic cytoplasmic regions generally refers to a uniform variation of the cytosol architecture leaving unknown the specific nature of their content. Thus, we feel that it may be inappropriate to state that α-syn stains very selectively LBs; we rather consider more adherent to facts the definition that α-syn stains very many protein aggregates within PD brains.
5. Challenging the classic concept of synucleinopathies
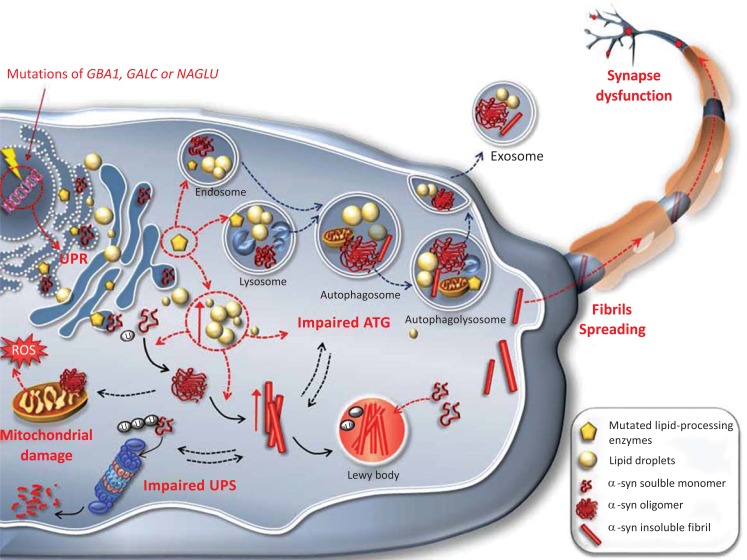
In keeping with real observations and consistent definitions, the term synucleinopathy is rather misused. In fact, the original definition by Martì and coworkers [96] states that a synucleinopathy is a disorder in which α-syn aggregates are found within affected neurons. On the other hand, clinical nosography lists only a few disorders as synucleinopathies, which correspond to: PD, MSA, DLB and PAF. However, a fair pathological view-point includes other disorders in which α-syn aggregates occur consistently within affected neurons. This is the case of lipid storage disorders such as Gaucher’s and Krabbe’s diseases and Sanfilippo Syndrome, as well as HD, AD and Amyotrophic Lateral Sclerosis (ALS) [97-102]. Thus, the classic concept of synucleinopathies, which initially included only PD, MSA, DLB and PAF [96, 103, 104], should be rather considered as a continuously evolving spectrum of disorders which is indeed intermingled with the evolving concept of PD as a disease spectrum. New insights into the pathophysiological mechanisms behind neurodegenerative diseases, in the latest years have shown that disorders such as Krabbe’s and Gaucher’s diseases may themselves constitute a risk factor for developing PD [100, 102]. In detail, Krabbe’s and Gaucher’s diseases feature primary lysosomal dysfunction and marked glycolipids accumulation caused by a decreased glucocerebrosidase activity. The neuropathological evaluation of brains from Gaucher’s and Krabbe’s patients carrying GBA1 and GALC gene mutations respectively, has revealed classical α-syn-positive, ubiquitinated Lewy inclusions [102, 105, 106]. To date, the carrier status of a heterozygous GBA1 mutation is considered the most common genetic risk factor for α-syn aggregation-associated disorder in the brain [100]. The decreased glucocerebrosidase activity affects lipid raft function by interfering with the sorting and trafficking of proteins and lipids associated with the rafts. Thus, it has been postulated that glucosylceramides are responsible for directly stimulating the accumulation and promoting the oligomerization of α-syn [107], which is localized to these lipid rafts in neuronal cells [108]. Consequently, the buildup of α-syn would then fuel further impairment of the lysosomal and proteasomal degradative machineries [109, 110], eventually leading to a more generalized dysfunction and increased neuronal vulnerability to stressors (Fig. 1). α-Syn has also been shown to bind specifically to gangliosides such as GM1 [111]. Mutations at the level of GM1 cause a deficiency of beta-galactosidase, with resulting abnormal storage of acidic lipid materials in cells of the central and peripheral nervous systems, but particularly in the nerve cells. This leads to a perturbation of membrane dynamics due to the imbalance of psychosine degradation, which accumulates within lipid rafts of the membrane, disrupting their architecture [112]. This mechanism is likely to induce localization of α-syn within pre-synaptic terminals and promote its aggregation [108]. α-Syn aggregates are found in Sanfilippo Syndrome as well, a storage disorder caused by α-N-acetylglucosaminidase (NAGLU) gene mutations. Similarly to what occurs in Gaucher’s and Krabbe’s diseases, a general impairment in the heparan sulfate clearance pathway is thought to cause α-syn pathological accumulation in this case [98].
Fig. (1).
Alpha synuclein aggregation in lipid storage diseases.
This cartoon depicts the cascade of the primary molecular events occurring in lipid storage diseases. This provides an example of how an impaired cell metabolism may directly promote α-syn aggregation through a mechanism of “loss of function”. Specific mutations in GBA1, GALC or NAGLU genes in Gaucher’s, Krabbes and Sanfilippo disease, respectively, cause a deficiency in the specific lipid-processing enzymes. This results in an abnormal production of acidic lipid material, which may accumulate in the endoplasmic reticulum and Golgi network thus contributing to an early impairment in the Unfolded Protein Response. An excess of lipids within lysosomes and cytoplasm leads to an impairment of the autophagy (ATG) machinery, and promotes oligomerization and fibrillization of α-syn in these compartments. The buildup of α-syn up to the formation of insoluble Lewy bodies (LBs), further impairs the ATG and proteasome (UPS) degradative machineries and contributes to mitochondrial disruption and release of reactive species of oxygen (ROS). Normally soluble α-syn may be trapped into LBs, resulting in a loss of α-syn function. Thus, a feed-forward vicious circle, while increasing the overall neuronal vulnerability, promotes the spreading of pathological α-syn, which can either move towards pre-synaptic terminals or be released outside the cell by exosomes.
In Huntington disease (HD) the striatum is filled with α-syn aggregates which associate with huntingtin aggregates [97]. Phosphorylated α-syn aggregates co-localizing with superoxide dismutase 1 (SOD1) have been detected in an autopsy case of familial ALS [99]. This exemplifies another case where α-syn aggregation is suggested to be triggered by the interaction of mutant SOD1 protein with α-syn across axo-somatic synapses [99].
α-Syn aggregates have been reported to co-exist with Tau aggregates in classic Tauopathies such as AD and Lewy body variant of AD (LBVAD) [76-78, 113, 114]. Several genetic studies associate Microtubule-Associate Protein Tau (MAPT) H1 haplotype or MAPT variants (i.e. p.A152T) to an increased risk for developing PD and MSA [115-118]. However, other studies suggest that even wild-type Tau promotes in vivo α-syn aggregation and toxicity [119, 120]. This is consistent with evidence demonstrating that over-expressed Tau decreases proteasome activity by inhibiting histone deacetylase 6 (HDAC6), which in turn leads to an increased amount of α-syn aggregates [121].
This indicates a number of disorders in which α-syn aggregates may be found. Most of these disorders are not classified as synucleinopathies although we cannot see why. In fact, even in classic synucleinopathies the aggregation of α-syn may be not a primary event but rather the consequence of an altered cell metabolism (Fig. 1).
Then, it comes natural to challenge the classic concept of “synucleinopathies” when referring to these kind of diseases. While figuring out that the classic definition does not always properly fit the real pathological issue, it becomes mandatory to scrutinize the biological significance of such an intriguing protein. This is actually a less discussed matter, which addresses the alternative though equally valid hypothesis that the aggregation of a-syn results in neurotoxicity through a loss-of-function mechanism, following sequestration of functional soluble a-syn into aggregates. In the next paragraph, the biological and beneficial face of a-syn will be portrayed.
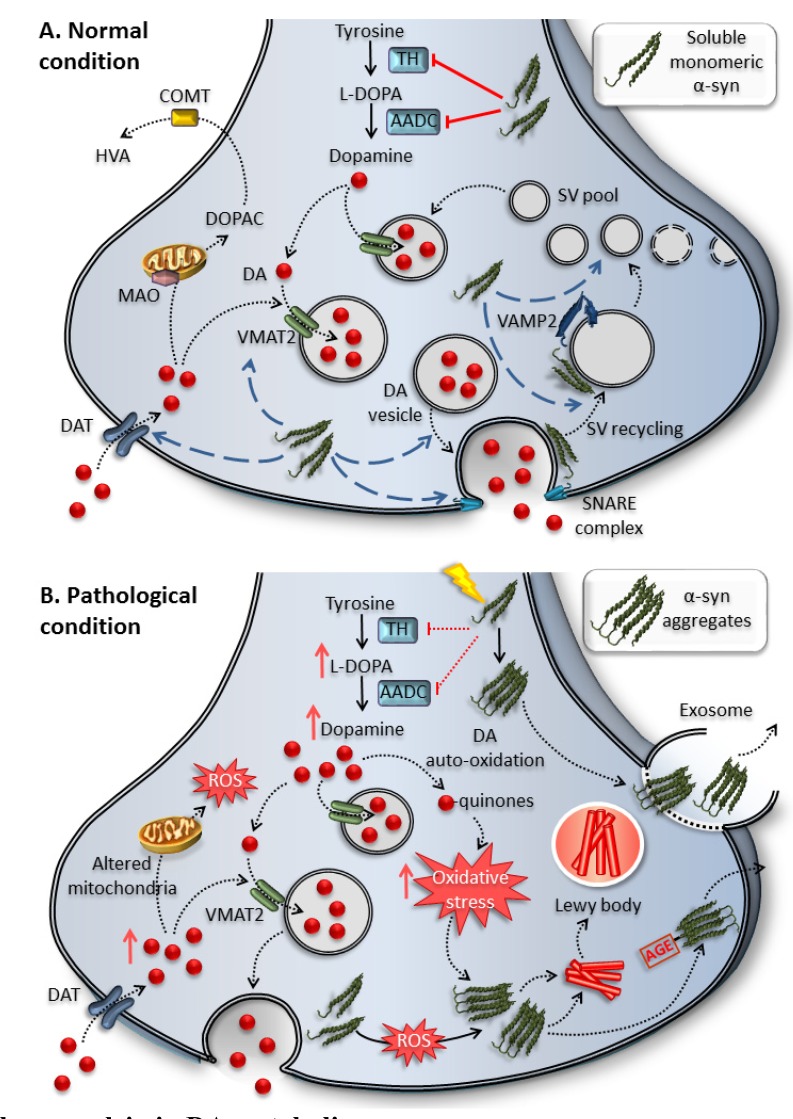
(A) α-Syn has been reported to regulate a variety of physiological processes and properties of synaptic membranes such as the size of the vesicular pool, synaptic vesicles’ trafficking, docking and recycling, as well as neurotransmitter release. In fact, α-syn promotes membrane bending by interacting with a variety of proteins such as synaptobrevin-2/VAMP2. Moreover, α-syn is reported to control neurotransmitter release by co-chaperoning SNARE-complex assembly. For instance, α-syn is involved in the regulation of DA biosynthesis and handling. Under normal conditions, α-syn inhibits the activity of TH and AADC and increases DAT and VMAT2.
(B) Upon either specific genetic mutations or environmental factors (low pH, oxidative stress, iron), α-syn is modified and sequestered into aggregates with a “loss of function”. Thus, a loss of TH inhibition increases DA synthesis, reduces the uptake of DA and impairs synaptic vesicle trafficking and recycling. An increased DA synthesis in the absence of vesicle storage, augments unsequestered cytosolic DA which readily auto-oxidizes to form DA-quinone and reactive oxygen species (ROS), which contribute to the formation of α-syn aggregates. This vicious cycle of α-syn aggregates and oxidative stress may contribute to DA neurons degeneration. Similarly, these aggregates may be transmitted intercellularly as AGE-α-syn aggregates and/or exosomes.
4. Physiological and beneficial role of Α-syn
A growing body of evidence reported in literature, converges in validating the implication of α-syn in a variety of physiological processes encompassing regulation of synaptic vesicles’ trafficking, neurotransmitter release, gene expression and stress response (by counteracting oxidative stress and several toxic insults) (Fig. 2). In this paragraph, while revising the above-mentioned aspects, we report some salient findings where accordingly to its physiological role, a-syn behaves as a neuroprotective agent.
Fig. (2).
Physiological role of alpha synuclein in DA metabolism.
4.1. Membrane Curvature and Synaptic Vesicles’ Homeostasis
α-Syn's astonishing conformational plasticity and it’s affinity for binding membranes, especially highly curved ones [122, 123], as well as its ability to actively alter their shape/curvature [124], confers to α-syn a crucial role in synaptic vesicle homeostasis and endo-/exocytosis. For instance, α-syn is involved in the regulation of several processes and properties of synaptic membranes such as the size of the vesicular pool, vesicular trafficking to- and docking with the presynaptic membrane and clathrin-associated formation of synaptic vesicles [125] (Fig. 2). In detail, experimental evidence demonstrates that synucleins are key in maintaining the functional endocytic rate of synaptic vesicles. Biochemical and ultrastructural analyses revealed that synucleins are involved in early stage clathrin-mediated endocytosis, before clathrin and dynamins action, and they act by promoting membrane bending [126]. This is in line with the findings that α-syn can sense and generate membrane curvature during endocytosis, similarly but less efficiently to endophilin [124, 127, 128]. In vitro membrane tubulation assay showed that these properties are shared between all the members of the synuclein family. Interestingly, the authors demonstrated that α-syn concentration at synaptic level (2-5 µM), is in the range needed to promote tubulation. This is expected to generate a robust stimulus to sustain membrane curvature since membrane tubulation increases linearly for low doses of α-syn up to 7 µM [128]. This activity is shared by all synucleins including β and γ [128], which allows a wide compensatory range when α-syn is not working.
Moreover, α-syn is reported to participate in the control of neurotransmitter release via interactions with members of the SNARE family. More specifically, it was suggested to promote SNARE-complex assembly through a non-enzymatic mechanism, by binding to phospholipids via its N-terminal and to synaptobrevin-2 via its C-terminal [129]. Despite α-syn’s role in SNARE-complex assembly still remains controversial, several studies focused on the role of α-syn in co-chaperoning SNARE when acting in combination with other proteins. For instance, α-syn strongly interacts with CSPα, a synaptic vesicle protein with a co-chaperone activity. This pre-synaptic co-chaperone is essential for neurotransmitter release and neuronal survival [130, 131]. Deletion of CSPα in mice results in a loss of the proper SNARE complex assembly and produces neurodegeneration, thus resulting in a lethal phenotype [132, 133]. Remarkably, the deletion of α- and β-synucleins exacerbates CSPα KO lethal phenotype, which is consistently reversed by a transgenic overexpression of α-syn [29]. In detail, α-syn overexpression prevents the neurodegeneration caused by CSPα deficiency, such as spinal cord gliosis, neuronal cell death, and decreased neuromuscular junction average size. Moreover, overexpression of α-syn rescues locomotor deficits and motor behavior and increases CSPα KO mice survival [29]. Again, overexpression of α-syn rescues altered synaptic structure and function yielded by the complete deletion of all endogenous synucleins (α-, β- and γ-) [134]. In line with this, singularly transfecting synuclein null cultures of hippocampal neurons with each of synucleins (either α-, β- or γ-) prevents impaired synaptic vesicles endocytosis, thus supporting the functional redundancy and the mutual compensatory effect of synucleins in synaptic homeostasis [126].
All this evidence strongly suggests a role as a co-chaperone protein similar to CSPα for all synucleins, which may work within each cell and across a cell population [135]. Notably, α-syn’s cell-to-cell transmission, from the donor towards the acceptor cell, either between neurons or from neurons to glial cells [135, 136], occurs without any sign of cell membrane damage and leakage nor cell death [135].
4.2. Handling Dopamine Release
Accumulating attention has been paid to the potential role of α-syn as a regulator of dopamine (DA) biosynthesis and handling (Fig. 2). Several in vivo studies have adopted synuclein’s deletions to investigate the impaired homeostatic regulation of DA levels. An extended deletion encompassing α- and β-synucleins turned out to reduce striatal DA terminals in the nigrostriatal system [136, 137]. In line with this, the striatal monoamines quantification by HPLC and coulometry performed in the dorsal striatum from synuclein deficient mice revealed a decrease in DA levels compared with controls [137]. In addition, the triple synuclein deletion furtherly reduces striatal DA terminals while altering brain structure [138]. Even the single α-syn KO may produce degeneration, as it was demonstrated in mice lacking α-syn, which displayed an enhanced damage of DA nigrostriatal terminals induced by methamphetamine (METH). Compared to wild-type littermates, α-syn KO mice resulted more sensitive to METH neurotoxicity and they exhibited a threefold loss of DA amount [6].
In keeping with a role as a co-chaperone, a loss of a-syn function has been postulated to disinhibit TH and AADC enzymes [33, 139-141], which triggers increased DA synthesis, reduction of the amount of VMAT2 on synaptic vesicles [142] and impaired synaptic vesicle trafficking and recycling [35, 126, 129, 143-145]. These events result in a net increase of cytosolic DA which auto-oxidizes to produce toxic adducts such as DA-quinone and ROS [146]. This may be furtherly accompanied by the sequestration of normally soluble α-syn into growing aggregates, resulting in the definitive loss of α-syn function and of its regulated-proteins [147]. In fact, an overexpression of α-syn prevents DA self-oxidation and the accumulation of toxic DA metabolites induced by a loss of the co-chaperone parkin [30], which strengthens the evidence for a physiological role of α-syn in DA homestasis.
4.3. Counteracting Oxidative Stress and Toxic Insults
The scenario of a beneficial and protective role of a-syn in decreasing cell vulnerability in response to several neurotoxic insults, has been continuously amplifying as reported by several studies [148-151]. For instance, α-syn protects against oxidative stress [151, 152], apoptotic stimuli [148], and various environmental toxic agents such as the pesticides paraquat and rotenone [149, 150].
In keeping with neuroprotective properties, a very recent study shows that α-syn overexpression ameliorates phenotype and neuromuscular junction pathology in the Smn2B/- mouse model of Spinal Muscular Atrophy (SMA) [153]. The decreased disease severity, accompanied by increased weight gain and extended life span, suggest that α-syn may act as a motor neuron disease phenotype modifier exerting a neuroprotective effect in motor neuron pathology. In line with this, transgenic mice for mutated α-syn are reported to develop a motor neuron disorder [154].
Despite conflicting data still exist, all this evidence about α-syn neuroprotective effects, which extend beyond PD and synucleinopathies, calls for further investigation regarding the physiological role of this protein.
4.4. Genetic and Epigenetic Modulation
α-Syn has been found to also have nuclear localization [155-157], which suggests it may play further yet undiscovered roles related to the regulation of gene expression. Epigenetic-mediated alterations, such as abnormal methylation or miRNA dysregulation, have been reported to affect the expression and/or the aggregation of α-syn, and in turn, abnormal spreading of α-syn into the nucleus may influence the epigenetic and genetic landscape by interacting with chromatin, histones and DNA regions [158]. In nigral dopaminergic neurons of SNCA transgenic mice the levels of protein kinase C delta (PKC δ) and of p300 were found down-regulated suggesting that α-syn may be protective in this experimental model [159]. Moreover, α-syn expression has been reported to drastically decrease the apoptotic response of neuronal cells by down-regulating p53 expression and transcriptional activity, and this effect gets lost when soluble α-syn becomes sequestered in aggregates [160].
5. The fate in the structure
In the context of this multi-faceted behavioral mechanism, detailing not only the physiologic role of a-syn, but even peculiar features in its structure, may be essential in providing potential explanations about its susceptibility to pathological misfolding and aggregation. For instance, studies aimed to unravel the structural properties of native brain α-syn, provide a clear demonstration on how fate in the structure may be closely predictive of spontaneous misfolding and aggregation properties. Studies so far reported in literature have postulated two main theories about the nature of endogenous α-syn. The vast majority define it as a natively unfolded monomer of about 14 kDa acquiring an α-helical secondary structure only upon binding to lipid vesicles [1, 2, 161-163]. In contrast, in 2011 Bartels and coworkers [164] reported that endogenous α-syn occurs mainly as a stably folded tetramer of about 58 kDa. Analytical ultracentrifugation, electron microscopy and in vitro cell crosslinking under non-denaturing conditions from neuronal and non-neuronal cell lines, brain tissue and living human cells, revealed that native α-syn possesses α-helical structure without lipid addition but it owns a greater lipid-binding capacity than recombinant α-syn. Differently from recombinant monomers, these native human tetramers underwent little or no amyloid-like aggregation in vitro. Thus, they proposed that α-syn misfolding and aggregation event is actually preceded by the destabilization of the helically folded tetramers [164]. In 2013 instead, the monomeric state of native brain α-syn was reconfirmed by Burré and coworkers [165]. They demonstrated that native α-syn, directly purified from mouse brain, is a largely unstructured monomer, which aggregates time-dependently. The authors detected a molecular mass of ~63 kDa, which is close to that predicted for a folded tetramer, but Filtration Gel migration and Size-Exclusion Chromatography coupled with Multi-Angle Laser-Light Scattering (SEC-MALS) argued against a folded multimeric conformation [165]. These results sustain the theory following which a labile rather than a stable conformational state, is what renders α-syn much more prone to aggregation. In this intrinsically disordered unfolded state, a plethora of dynamic changes, especially significant post-translational modifications, occur at the level of a-syn, thus playing a crucial role in regulating its function and conformation (Fig. 3). The C-terminus and the N-terminus, which contain a large number of charged residues, have been identified as the two main regions being particularly susceptible to acetylation, phosphorylation, oxidation, nitration, ubiquitination and truncation [166-170]. Certain conditions have been reported to favor conformational modifications and drive a pathological cascade of conversion from α-syn self-assembly up to insoluble fibrils and oligomers formation [171, 172]. These range from environmental factors such as low pH [173], oxidative stress [174] and iron [175, 176], to genetic mutations [177, 178], and eventually to binding or interacting molecular partners [179, 180], including the same DA [181]. Nonetheless, in this context, oligomers or protofibrils may accelerate the conversion of physiological α-syn into aggregates by acting as seeds [182]. In line with this, several recent studies in mice have shown that stereotaxically injected aggregates (but not monomers) of α-syn, can spread to neuroanatomically connected regions and initiate de novo aggregation [183-185]. A recent study where a new Proximity-Ligation-Assay technique was set up for detecting oligomeric α-syn in human PD post-mortem brain tissues, demonstrated that disease-associated α-syn species show an intermediate proteinase K sensitivity, suggesting they are oligomers in a very early stage of aggregation [186]. They clearly appeared to be folded differently than physiological presynaptic α-syn but had not yet acquired a highly compact structure resistant to prolonged digestion by proteinase K [186]. These observations fit the hypothesis following which interacting α-syn molecules are an early and necessary step allowing the misfolded species to transfer their pathogenic folding to newly recruited units in a prion-like route [187].
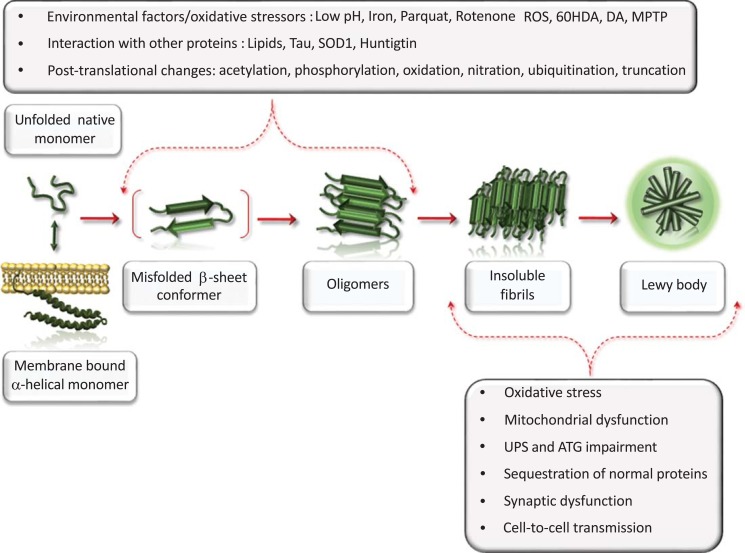
Fig. (3).
Disruption of alpha synuclein conformational equilibrium: causes and consequences.
These data suggest that events triggering misfolding of α-syn can be in part drawn back at the level of its intrinsic structural features, which in certain conditions may consequently contribute to an aberrant conformation (Fig. 3).
CONCLUSION
The dynamic and plastic chameleon-like structure of α-syn coupled with its potential in performing so many critical physiological functions configure it as a double edged sward, which better exemplifies the intriguing paradigm of this multi-faced protein. When analyzed from this point of view it becomes easier to envision how the biological background and the complex interaction with so many other molecular factors, may affect the amount, conformation and localization of normal α-syn, leading to a loss of its biologic function up to a gain in neurotoxic properties. Remarkably, as we revised here, there are many studies providing evidence about a neuroprotective role of α-syn in several experimental settings. On the other hand, when mutated or simply up-regulated, α-syn may target further membranous structures, regulatory molecules or even whole molecular pathways, and this presumably accounts for its toxicity. This is why continuous studies and overall reappraisals of the biological background where these interactions take place are essential to shed light on α-syn toxicity. Most notably, this implies the need for compelling treatment options, which while battling aggregated pathological forms of a-syn, should also preserve its physiological function. In line with this, in the latest years, natural compounds such as curcumin and its analogs [188] are emerging as potential drugs able to inhibit a-synuclein fibrillization and to stabilize it in a non-toxic state.
This cartoon provides a schematic summary about the mechanisms affecting α-syn conformation, and those resulting from a loss of α-syn function. α-Syn is a natively unfolded monomer that acquires α-helical secondary structure upon binding to lipid membranes. In a physiological state, a dynamic equilibrium exists between these two conformations. Several environmental factors, oxidative stressors, post-translational changes and interactions with other proteins may directly favor α-syn misfolding and drive a pathological cascade of conversion from self-assembly up to insoluble fibrils formation. In this aberrant conformational state α-syn may propel a generalized impairment of cell homeostasis consisting in synaptic dysfunction, oxidative stress, mitochondrial disruption, impairment of cell-clearing mechanism, sequestration of normal proteins into insoluble LBs, and cell-to-cell spreading of misfolded and aggregated α-syn conformers.
ACKNOWLEDGEMENTS
LR wrote the paper and made artwork; CLB provided a critical review of the literature; FL contributed to write the paper and made artwork; FB contributed to review literature; SG provided a review of the literature; FF wrote the paper and provided a critical review of the whole manuscript.
LIST OF ABBREVIATIONS
- AADC
Aromatic Amino Acid Decarboxylase
- AD
Alzheimer’s Disease
- AGEs
Advanced Glycation End-products
- ALS
Amyotrophic Lateral Sclerosis
- ATG
Autophagy
- COMT
Catechol-O methyl Transferase
- CSP- α
Cysteine-string Protein-α
- DA
Dopamine
- DAT
Dopamine Transporter
- DLB
Dementia with Lewy Bodies
- DOPAC
3,4-Dihydroxyphenylacetic Acid
- FTD
Frontotemporal Dementia
- GALC
Galactocerebrosidase
- GBA
Glucocerebrosidase Beta
- HD
Huntigton Disease
- HDAC6
Histone Deacetylase 6
- HVA
Homovanilic Acid
- LBs
Lewy Bodies
- LBVAD
Lewy body Variant of AD
- MAO
Monoamine Oxidase
- MAPT
Microtubule-Associate Protein Tau
- MBD
Monoamine Brainstem Disorders
- METH
Methamphetamine
- MPTP
1-Methyl, 4-phenyl 1,2,3,6-tetrahydropyridine
- MSA
Multiple System Atrophy
- NAGLU
α-N-acetylglucosaminidase
- PAF
Pure Autonomic Failure
- PD
Parkinson’s Disease
- PKC δ
Protein Kinase C Delta
- PSP
Progressive Supranuclear Palsy
- RBD
REM Sleep Behavioral Disorders
- ROS
Reactive Oxygen Species
- SMA
Spinal Muscular Atrophy
- SNpc
Substantia Nigra Pars Compacta
- SOD1
Superoxide Dismutase 1
- SV
Synaptic Vesicles
- TH
Tyrosine Hydroxylase
- UPS
Ubiquitin Proteasome System
- VAMP2
Vesicle-associated Membrane Protein 2
- VMAT2
Vesicular Monoamine Transporter 2
Consent for Publication
Not applicable.
conflict of interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Jakes R., Spillantini M.G., Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345(1):27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 2.Maroteaux L., Campanelli J.T., Scheller R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recchia A., Debetto P., Negro A., Guidolin D., Skaper S.D., Giusti P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004;18(6):617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Divane A., Goedert M. Assignment of human alpha-synuclein (SNCA) and beta-synuclein (SNCB) genes to chromosomes 4q21 and 5q35. Genomics. 1995;27(2):379–381. doi: 10.1006/geno.1995.1063. [DOI] [PubMed] [Google Scholar]
- 5.George J.M. The synucleins. Genome Biol. 2002;3(2) doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlüter O.M., Fornai F., Alessandrí M.G., Takamori S., Geppert M., Jahn R., Südhof T.C. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118(4):985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 7.Chandra S. Synucleins. In: Squire L., editor. The new encyclopedia of neuroscience. New York, Oxford: Academic; 2009. pp. 833–837. [Google Scholar]
- 8.Iwai A., Masliah E., Yoshimoto M., Ge N., Flanagan L., de Silva H.A., Kittel A., Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 9.George J.M., Jin H., Woods W.S., Clayton D.F. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15(2):361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 10.Mori F., Tanji K., Yoshimoto M., Takahashi H., Wakabayashi K. Immunohistochemical comparison of alpha- and beta-synuclein in adult rat central nervous system. Brain Res. 2002;941(1-2):118–126. doi: 10.1016/s0006-8993(02)02643-4. [DOI] [PubMed] [Google Scholar]
- 11.Quilty M.C., Gai W.P., Pountney D.L., West A.K., Vickers J.C. Localization of alpha-, beta-, and gammasynuclein during neuronal development and alterations associated with the neuronal response to axonal trauma. Exp. Neurol. 2003;182(1):195–207. doi: 10.1016/s0014-4886(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 12.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di Iorio G., Golbe L.I., Nussbaum R.L. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 13.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M.R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 14.Chartier-Harlin M. Causal relation between a-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1169–1171. [Google Scholar]
- 15.Ibáñez P., Bonnet A.M., Débarges B., Lohmann E., Tison F., Pollak P., Agid Y., Dürr A., Brice A. Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 16.Zarranz J.J., Alegre J., Gómez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D.G., de Yebenes J.G. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 17.Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B., Weir D., Thompson C., Szu-Tu C., Trinh J., Aasly J.O., Rajput A., Rajput A.H., Jon Stoessl A., Farrer M.J. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson disease. Mov. Disord. 2013;28(6):811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 18.Lesage S., Anheim M., Letournel F., Bousset L., Honoré A., Rozas N., Pieri L., Madiona K., Dürr A., Melki R., Verny C., Brice A., French Parkinson’s Disease Genetics Study Group G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 2013;73(4):459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 19.Kasten M., Klein C. The many faces of alpha-synuclein mutations. Mov. Disord. 2013;28(6):697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- 20.Proukakis C., Dudzik C.G., Brier T., MacKay D.S., Cooper J.M., Millhauser G.L., Houlden H., Schapira A.H. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013;80(11):1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., Tienari P.J., Pöyhönen M., Paetau A. Novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. 2014. [DOI] [PubMed]
- 22.Ferese R., Modugno N., Campopiano R., Santilli M., Zampatti S., Giardina E., Nardone A., Postorivo D., Fornai F., Novelli G., Romoli E., Ruggieri S., Gambardella S. Four copies of SNCA responsible for autosomal dominant Parkinson’s disease in two Italian siblings. Parkinsons Dis. 2015;2015:546462. doi: 10.1155/2015/546462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dauer W., Kholodilov N., Vila M., Trillat A.C., Goodchild R., Larsen K.E., Staal R., Tieu K., Schmitz Y., Yuan C.A., Rocha M., Jackson-Lewis V., Hersch S., Sulzer D., Przedborski S., Burke R., Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl. Acad. Sci. USA. 2002;99(22):14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fornai F., Schlüter O.M., Lenzi P., Gesi M., Ruffoli R., Ferrucci M., Lazzeri G., Busceti C.L., Pontarelli F., Battaglia G., Pellegrini A., Nicoletti F., Ruggieri S., Paparelli A., Südhof T.C. Parkinson-like syndrome induced by continuous MPTPinfusion: Convergent roles of the ubiquitin-proteasome system andalpha-synuclein. Proc. Natl. Acad. Sci. USA. 2005;102(9):3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez-Fischer D., Henze C., Strenzke C., Westrich J., Ferger B., Höglinger G.U., Oertel W.H., Hartmann A. Characterization of the striatal 6-OHDA model of Parkinson’s disease in wild type and alpha-synuclein-deleted mice. Exp. Neurol. 2008;210(1):182–193. doi: 10.1016/j.expneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Orth M., Tabrizi S.J., Schapira A.H., Cooper J.M. Alpha-synuclein expression in HEK293 cells enhances the mitochondrial sensitivity to rotenone. Neurosci. Lett. 2003;351(1):29–32. doi: 10.1016/s0304-3940(03)00941-8. [DOI] [PubMed] [Google Scholar]
- 27.Betarbet R., Canet-Aviles R.M., Sherer T.B., Mastroberardino P.G., McLendon C., Kim J.H., Lund S., Na H.M., Taylor G., Bence N.F., Kopito R., Seo B.B., Yagi T., Yagi A., Klinefelter G., Cookson M.R., Greenamyre J.T. Intersecting pathways to neurodegeneration in Parkinson’s disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006;22(2):404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y., Liang Z.H., Wang T., Qiao X., Liu H.J., Sun S.G. alpha-Synuclein redistributed and aggregated in rotenone-induced Parkinson’s disease rats. Neurosci. Bull. 2006;22(5):288–293. [PubMed] [Google Scholar]
- 29.Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O.M., Südhof T.C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Machida Y., Chiba T., Takayanagi A., Tanaka Y., Asanuma M., Ogawa N., Koyama A., Iwatsubo T., Ito S., Jansen P.H., Shimizu N., Tanaka K., Mizuno Y., Hattori N. Common anti-apoptotic roles of parkin and alpha-synuclein in human dopaminergic cells. Biochem. Biophys. Res. Commun. 2005;332(1):233–240. doi: 10.1016/j.bbrc.2005.04.124. [DOI] [PubMed] [Google Scholar]
- 31.Jensen P.H., Nielsen M.S., Jakes R., Dotti C.G., Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J. Biol. Chem. 1998;273(41):26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 32.Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., Hynes M., Phillips H., Sulzer D., Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 33.Murphy D.D., Rueter S.M., Trojanowski J.Q., Lee V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000;20(9):3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson W.S., Jonas A., Clayton D.F., George J.M. Stabilization of α-Synuclein in secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 35.Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M.R., Südhof T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferkorn C.M., Jiang Z., Lee J.C. Biophysics of α-synuclein membrane interactions. Biochim. Biophys. Acta. 2012;1818(2):162–171. doi: 10.1016/j.bbamem.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guardia-Laguarta C., Area-Gomez E., Rüb C., Liu Y., Magrané J., Becker D., Voos W., Schon E.A., Przedborski S. α-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 2014;34(1):249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middleton E.R., Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys. J. 2010;99(7):2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tretiakoff C. Contributions a l’etude de l’anatomie pathologique du locus niger de soemmering avec quelques deductions relatives a la pathogenie des troubles de tonus musculaire et de la maladie de Parkinson. 1919. [Google Scholar]
- 40.Forno L.S., Alvord E.C. Depigmentation in the nerve cells of the substantia nigra and locus ceruleus in Parkinsonism. Adv. Neurol. 1974;5:195–202. [PubMed] [Google Scholar]
- 41.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shults C.W. Lewy bodies. Proc. Natl. Acad. Sci. USA. 2006;103(6):1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doherty K.M., Silveira-Moriyama L., Parkkinen L., Healy D.G., Farrell M., Mencacci N.E., Ahmed Z., Brett F.M., Hardy J., Quinn N., Counihan T.J., Lynch T., Fox Z.V., Revesz T., Lees A.J., Holton J.L. Parkin disease: A clinicopathologic entity? JAMA Neurol. 2013;70(5):571–579. doi: 10.1001/jamaneurol.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soldani P., Fornai F. The functional anatomy of noradrenergic neurons in Parkinson’s disease. Funct. Neurol. 1999;14(2):97–109. [PubMed] [Google Scholar]
- 45.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 46.Braak H., Bohl J.R., Müller C.M., Rüb U., de Vos R.A., Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov. Disord. 2006;21(12):2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 47.Fornai F., Frati A., Gesi M., Fulceri F., Paparelli S., Falleni A., Ruggieri S. Neurobiology and neuroanatomy of psychiatric symptoms in parkinsonism. Arch. Ital. Biol. 2013;151(4):179–191. [PubMed] [Google Scholar]
- 48.Fornai F., Ruggieri S. Re-defining Parkinson’s disease. Arch. Ital. Biol. 2013;151(4):137–142. [PubMed] [Google Scholar]
- 49.Jellinger K.A. The pathomechanisms underlying Parkinson’s disease. Expert Rev. Neurother. 2014;14(2):199–215. doi: 10.1586/14737175.2014.877842. [DOI] [PubMed] [Google Scholar]
- 50.Forno L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Gambardella S., Ferese R., Biagioni F., Busceti C.L., Campopiano R., Griguoli A.M.P., Limanaqi F., Novelli G., Storto M., Fornai F. The monoamine brainstem reticular formation as a paradigm for re-defining various phenotypes of Parkinson’s disease owing genetic and anatomical specificity. Front. Cell. Neurosci. 2017;11:102. doi: 10.3389/fncel.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornykiewicz O. The tropical localization and content of noradrenalin and dopamine (3-hydroxytyramine) in the substantia nigra of normal persons and patients with Parkinson’s disease. Wien. Klin. Wochenschr. 1963;75:309–312. [PubMed] [Google Scholar]
- 53.Gesi M., Soldani P., Giorgi F.S., Santinami A., Bonaccorsi I., Fornai F. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci. Biobehav. Rev. 2000;24(6):655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- 54.Zarow C., Lyness S.A., Mortimer J.A., Chui H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003;60(3):337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 55.Ferrucci M., Giorgi F.S., Bartalucci A., Busceti C.L., Fornai F. The effects of locus coeruleus and norepinephrine in methamphetamine toxicity. Curr. Neuropharmacol. 2013;11(1):80–94. doi: 10.2174/157015913804999522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Von Economo C. Sleep as a problem of localization. J. Nerv. Ment. Dis. 1930;71(3):249–259. [Google Scholar]
- 57.Haraguchi T., Ishizu H., Terada S., Takehisa Y., Tanabe Y., Nishinaka T., Kawai K., Kuroda S., Komoto Y., Namba M. An autopsy case of postencephalitic parkinsonism of von Economo type: Some new observations concerning neurofibrillary tangles and astrocytic tangles. Neuropathology. 2000;20(2):143–148. doi: 10.1046/j.1440-1789.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhuri K.R. Nocturnal symptom complex in PD and its management. Neurology. 2003;61(6):S17–S23. doi: 10.1212/wnl.61.6_suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 59.Sauerbier A., Jenner P., Todorova A., Chaudhuri K.R. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat. Disord. 2016;22(1):S41–S46. doi: 10.1016/j.parkreldis.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18(7):435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 61.Kristensson K. Avian influenza and the brain--comments on the occasion of resurrection of the Spanish flu virus. Brain Res. Bull. 2006;68(6):406–413. doi: 10.1016/j.brainresbull.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz J.R., Roth T. Neurophysiology of sleep and wakefulness: Basic science and clinical implications. Curr. Neuropharmacol. 2008;6(4):367–378. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vivacqua G., Yin J.J., Casini A., Li X., Li Y.H., D’Este L., Chan P., Renda T.G., Yu S. Immunolocalization of alpha-synuclein in the rat spinal cord by two novel monoclonal antibodies. Neuroscience. 2009;158(4):1478–1487. doi: 10.1016/j.neuroscience.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Del Tredici K., Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124(5):643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 65.Vivacqua G., Biagioni F., Yu S., Casini A., Bucci D., D’Este L., Fornai F. Loss of spinal motor neurons and alteration of alpha-synuclein immunostaining in MPTP induced Parkinsonism in mice. J. Chem. Neuroanat. 2012;44(2):76–85. doi: 10.1016/j.jchemneu.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Koller W.C., Langston J.W., Hubble J.P., Irwin I., Zack M., Golbe L., Forno L., Ellenberg J., Kurland L., Ruttenber A.J. Does a long preclinical period occur in Parkinson’s disease? Neurology. 1991;41(2):8–13. [PubMed] [Google Scholar]
- 67.Bloch A., Probst A., Bissig H., Adams H., Tolnay M. a-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol. Appl. Neurobiol. 2006;32(3):284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 68.Orimo S., Uchihara T., Nakamura A., Mori F., Kakita A., Wakabayashi K., Takahashi H. Axonal alphasynuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131(Pt 3):642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 69.Del Tredici K., Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov. Disord. 2012;27(5):597–607. doi: 10.1002/mds.24921. [DOI] [PubMed] [Google Scholar]
- 70.Braak H., Rüb U., Gai W.P., Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 2003;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 71.Fornai F., Ruffoli R., Soldani P., Ruggieri S., Paparelli A. The “Parkinsonian heart”: From novel vistas to advanced therapeutic approaches in Parkinson’s disease. Curr. Med. Chem. 2007;14(23):2421–2428. doi: 10.2174/092986707782023631. [DOI] [PubMed] [Google Scholar]
- 72.Ruffoli R., Soldani P., Pasquali L., Ruggieri S., Paparelli A., Fornai F. Methamphetamine fails to alter the noradrenergic integrity of the heart. Ann. N. Y. Acad. Sci. 2008;1139:337–344. doi: 10.1196/annals.1432.017. [DOI] [PubMed] [Google Scholar]
- 73.Phillips R.J., Walter G.C., Wilder S.L., Baronowsky E.A., Powley T.L. Alpha-synuclein immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153(3):733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Natale G., Pasquali L., Paparelli A., Fornai F. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol. Motil. 2011;23(12):1056–1065. doi: 10.1111/j.1365-2982.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 75.Orimo S., Oka T., Miura H., Tsuchiya K., Mori F., Wakabayashi K., Nagao T., Yokochi M. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2002;73(6):776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton R.L. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10(3):378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crews L., Tsigelny I., Hashimoto M., Masliah E. Role of synucleins in Alzheimer’s disease. Neurotox. Res. 2009;16(3):306–317. doi: 10.1007/s12640-009-9073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clinton L.K., Blurton-Jones M., Myczek K., Trojanowski J.Q., LaFerla F.M. Synergistic interactions between Abeta, tau, and alpha-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010;30(21):7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Popescu A., Lippa C.F., Lee V.M-Y., Trojanowski J.Q. Lewy bodies in the amygdala: Increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch. Neurol. 2004;61(12):1915–1919. doi: 10.1001/archneur.61.12.1915. [DOI] [PubMed] [Google Scholar]
- 80.Yancopoulou D., Xuereb J.H., Crowther R.A., Hodges J.R., Spillantini M.G. Tau and alpha-synuclein inclusions in a case of familial frontotemporal dementia and progressive aphasia. J. Neuropathol. Exp. Neurol. 2005;64(3):245–253. doi: 10.1093/jnen/64.3.245. [DOI] [PubMed] [Google Scholar]
- 81.Tong J., Wong H., Guttman M., Ang L.C., Forno L.S., Shimadzu M., Rajput A.H., Muenter M.D., Kish S.J., Hornykiewicz O., Furukawa Y. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: A comparative investigation. Brain. 2010;133(Pt 1):172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- 82.Duda J.E., Giasson B.I., Mabon M.E., Miller D.C., Golbe L.I., Lee V.M., Trojanowski J.Q. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104(1):7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 83.Kotzbauer P.T., Giasson B.I., Kravitz A.V., Golbe L.I., Mark M.H., Trojanowski J.Q., Lee V.M. Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp. Neurol. 2004;187(2):279–288. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Yamaguchi K., Cochran E.J., Murrell J.R., Polymeropoulos M.H., Shannon K.M., Crowther R.A., Goedert M., Ghetti B. Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta Neuropathol. 2005;110(3):298–305. doi: 10.1007/s00401-005-1042-4. [DOI] [PubMed] [Google Scholar]
- 85.Fujioka S., Ogaki K., Tacik P.M., Uitti R.J., Ross O.A., Wszolek Z.K. Update on novel familial forms of Parkinson’s disease and multiple system atrophy. Parkinsonism Relat. Disord. 2014;20(1):S29–S34. doi: 10.1016/S1353-8020(13)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Farrer M., Kachergus J., Forno L., Lincoln S., Wang D.S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J.W. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann. Neurol. 2004;55(2):174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 87.Fornai F., Lenzi P., Gesi M., Ferrucci M., Lazzeri G., Busceti C.L., Ruffoli R., Soldani P., Ruggieri S., Alessandri M.G., Paparelli A. Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J. Neurosci. 2003;23(26):8955–8966. doi: 10.1523/JNEUROSCI.23-26-08955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fornai F., Lenzi P., Gesi M., Soldani P., Ferrucci M., Lazzeri G., Capobianco L., Battaglia G., De Blasi A., Nicoletti F., Paparelli A. Methamphetamine produces neuronal inclusions in the nigrostriatal system and in PC12 cells. J. Neurochem. 2004;88(1):114–123. doi: 10.1046/j.1471-4159.2003.02137.x. [DOI] [PubMed] [Google Scholar]
- 89.Conway K.A., Harper J.D., Lansbury P.T. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39(10):2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 90.McKeith I.G., Galasko D., Kosaka K., Perry E.K., Dickson D.W., Hansen L.A., Salmon D.P., Lowe J., Mirra S.S., Byrne E.J., Lennox G., Quinn N.P., Edwardson J.A., Ince P.G., Bergeron C., Burns A., Miller B.L., Lovestone S., Collerton D., Jansen E.N., Ballard C., de Vos R.A., Wilcock G.K., Jellinger K.A., Perry R.H. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 91.Sawada H., Kohno R., Kihara T., Izumi Y., Sakka N., Ibi M., Nakanishi M., Nakamizo T., Yamakawa K., Shibasaki H., Yamamoto N., Akaike A., Inden M., Kitamura Y., Taniguchi T., Shimohama S. Proteasome mediates dopaminergic neuronal degeneration, and its inhibition causes alpha-synuclein inclusions. J. Biol. Chem. 2004;279(11):10710–10719. doi: 10.1074/jbc.M308434200. [DOI] [PubMed] [Google Scholar]
- 92.Lee H.J., Khoshaghideh F., Patel S., Lee S.J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 2004;24(8):1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castino R., Lazzeri G., Lenzi P., Bellio N., Follo C., Ferrucci M., Fornai F., Isidoro C. Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. J. Neurochem. 2008;106(3):1426–1439. doi: 10.1111/j.1471-4159.2008.05488.x. [DOI] [PubMed] [Google Scholar]
- 94.Pasquali L., Ruggieri S., Murri L., Paparelli A., Fornai F. Does autophagy worsen or improve the survival of dopaminergic neurons? Parkinsonism Relat. Disord. 2009;15(4):S24–S27. doi: 10.1016/S1353-8020(09)70830-2. [DOI] [PubMed] [Google Scholar]
- 95.Lenzi P., Lazzeri G., Biagioni F., Busceti C.L., Gambardella S., Salvetti A., Fornai F. The autophagoproteasome a novel cell clearing organelle in baseline and stimulated conditions. Front. Neuroanat. 2016;10:78. doi: 10.3389/fnana.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martí M.J., Tolosa E., Campdelacreu J. Clinical overview of the synucleinopathies. Mov. Disord. 2003;18(6):S21–S27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- 97.Charles V., Mezey E., Reddy P.H., Dehejia A., Young T.A., Polymeropoulos M.H., Brownstein M.J., Tagle D.A. Alpha-synuclein immunoreactivity of huntingtin polyglutamine aggregates in striatum and cortex of Huntington’s disease patients and transgenic mouse models. Neurosci. Lett. 2000;289(1):29–32. doi: 10.1016/s0304-3940(00)01247-7. [DOI] [PubMed] [Google Scholar]
- 98.Winder-Rhodes S.E., Garcia-Reitböck P., Ban M., Evans J.R., Jacques T.S., Kemppinen A., Foltynie T., Williams-Gray C.H., Chinnery P.F., Hudson G., Burn D.J., Allcock L.M., Sawcer S.J., Barker R.A., Spillantini M.G. Genetic and pathological links between Parkinson’s disease and the lysosomal disorder Sanfilippo syndrome. Mov. Disord. 2012;27(2):312–315. doi: 10.1002/mds.24029. [DOI] [PubMed] [Google Scholar]
- 99.Takei Y., Oguchi K., Koshihara H., Hineno A., Nakamura A., Ohara S. α-Synuclein coaggregation in familial amyotrophic lateral sclerosis with SOD1 gene mutation. Hum. Pathol. 2013;44(6):1171–1176. doi: 10.1016/j.humpath.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 100.Sardi S.P., Cheng S.H., Shihabuddin L.S. Gaucher-related synucleinopathies: The examination of sporadic neurodegeneration from a rare (disease) angle. Prog. Neurobiol. 2015;125:47–62. doi: 10.1016/j.pneurobio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Barker R.A., Williams-Gray C.H. Review: The spectrum of clinical features seen with alpha synuclein pathology. Neuropathol. Appl. Neurobiol. 2016;42(1):6–19. doi: 10.1111/nan.12303. [DOI] [PubMed] [Google Scholar]
- 102.Marshall M.S., Bongarzone E.R. Beyond Krabbe’s disease: The potential contribution of galactosylceramidase deficiency to neuronal vulnerability in late-onset synucleinopathies. J. Neurosci. Res. 2016;94(11):1328–1332. doi: 10.1002/jnr.23751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCann H., Stevens C.H., Cartwright H., Halliday G.M. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014;20(1):S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 104.Thaisetthawatkul P. Pure Autonomic Failure. Curr. Neurol. Neurosci. Rep. 2016;16(8):74. doi: 10.1007/s11910-016-0673-2. [DOI] [PubMed] [Google Scholar]
- 105.Neumann J., Bras J., Deas E., O’Sullivan S.S., Parkkinen L., Lachmann R.H., Li A., Holton J., Guerreiro R., Paudel R., Segarane B., Singleton A., Lees A., Hardy J., Houlden H., Revesz T., Wood N.W. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong K., Sidransky E., Verma A., Mixon T., Sandberg G.D., Wakefield L.K., Morrison A., Lwin A., Colegial C., Allman J.M., Schiffmann R. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 2004;82(3):192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 107.Mazzulli J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fortin D.L., Troyer M.D., Nakamura K., Kubo S., Anthony M.D., Edwards R.H. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. 2004;24(30):6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lindersson E., Beedholm R., Højrup P., Moos T., Gai W., Hendil K.B., Jensen P.H. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J. Biol. Chem. 2004;279(13):12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A.C., Mazzulli J., Mosharov E.V., Hodara R., Fredenburg R., Wu D.C., Follenzi A., Dauer W., Przedborski S., Ischiropoulos H., Lansbury P.T., Sulzer D., Cuervo A.M. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 2008;118(2):777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinez Z., Zhu M., Han S., Fink A.L. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46(7):1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 112.White A.B., Givogri M.I., Lopez-Rosas A., Cao H., van Breemen R., Thinakaran G., Bongarzone E.R. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci. 2009;29(19):6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lippa C.F., Schmidt M.L., Lee V.M., Trojanowski J.Q. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann. Neurol. 1999;45(3):353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 114.Muntane G., Dalfo E., Martinez A., Ferrer I. Phosphorylation of tau and alpha-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related alpha-synucleinopathies. Neuroscience. 2008;152(4):913–923. doi: 10.1016/j.neuroscience.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 115.Zabetian C.P., Hutter C.M., Factor S.A., Nutt J.G., Higgins D.S., Griffith A., Roberts J.W., Leis B.C., Kay D.M., Yearout D., Montimurro J.S., Edwards K.L., Samii A., Payami H. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson’s disease. Ann. Neurol. 2007;62(2):137–144. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vilarino-Guell C., Soto-Ortolaza A.I., Rajput A., Mash D.C., Papapetropoulos S., Pahwa R., Lyons K.E., Uitti R.J., Wszolek Z.K., Dickson D.W., Farrer M.J., Ross O.A. MAPT H1 haplotype is a risk factor for essential tremor and multiple system atrophy. Neurology. 2011;76(7):670–672. doi: 10.1212/WNL.0b013e31820c30c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Colom-Cadena M., Gelpi E., Marti M.J., Charif S., Dols-Icardo O., Blesa R., Clarimon J., Lleo A. MAPT H1 haplotype is associated with enhanced alpha-synuclein deposition in dementia with Lewy bodies. Neurobiol. Aging. 2013;34(3):936–942. doi: 10.1016/j.neurobiolaging.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 118.Labbé C., Ogaki K., Lorenzo-Betancor O., Soto-Ortolaza A.I., Walton R.L., Rayaprolu S., Fujioka S., Murray M.E., Heckman M.G., Puschmann A., McCarthy A., Lynch T., Siuda J., Opala G., Rudzinska M., Krygowska-Wajs A., Barcikowska M., Czyzewski K., Sanotsky Y., Rektorová I., McLean P.J., Rademakers R., Ertekin-Taner N., Hassan A., Ahlskog J.E., Boeve B.F., Petersen R.C., Maraganore D.M., Adler C.H., Ferman T.J., Parisi J.E., Graff-Radford N.R., Uitti R.J., Wszolek Z.K., Dickson D.W., Ross O.A. Role for the microtubule-associated protein tau variant p.A152T in risk of α-synucleinopathies. Neurology. 2015;85(19):1680–1686. doi: 10.1212/WNL.0000000000001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Badiola N., de Oliveira R.M., Herrera F., Guardia-Laguarta C., Gonçalves S.A., Pera M., Suárez-Calvet M., Clarimon J., Outeiro T.F., Lleó A. Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS One. 2011;6(10):e26609. doi: 10.1371/journal.pone.0026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khandelwal P.J., Dumanis S.B., Herman A.M., Rebeck G.W., Moussa C.E. Wild type and P301L mutant tau promote neuro-inflammation and alpha-synuclein accumulation in lentiviral gene delivery models. Mol. Cell. Neurosci. 2012;49(1):44–53. doi: 10.1016/j.mcn.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perez M., Santa-Maria I., Gomez de Barreda E., Zhu X., Cuadros R., Cabrero J.R., Sanchez-Madrid F., Dawson H.N., Vitek M.P., Perry G., Smith M.A., Avila J. Tau—an inhibitor of deacetylase HDAC6 function. J. Neurochem. 2009;109(6):1756–1766. doi: 10.1111/j.1471-4159.2009.06102.x. [DOI] [PubMed] [Google Scholar]
- 122.Pranke I.M., Morello V., Bigay J., Gibson K., Verbavatz J.M., Antonny B., Jackson C.L. Alpha-synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J. Cell Biol. 2011;194(1):89–103. doi: 10.1083/jcb.201011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jain N., Bhasne K., Hemaswasthi M., Mukhopadhyay S. Structural and dynamical insights into the membrane-bound α-synuclein. PLoS One. 2013;8(12):e83752. doi: 10.1371/journal.pone.0083752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varkey J., Isas J.M., Mizuno N., Jensen M.B., Bhatia V.K., Jao C.C., Petrlova J., Voss J.C., Stamou D.G., Steven A.C., Langen R. Membrane curvature induction and tabulation are common features of synucleins and apolipoproteins. J. Biol. Chem. 2010;285(42):32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bellucci A., Zaltieri M., Navarria L., Grigoletto J., Missale C., Spano P. From α-synuclein to synaptic dysfunctions: New insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012;1476:183–202. doi: 10.1016/j.brainres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 126.Vargas K.J., Makani S., Davis T., Westphal C.H., Castillo P.E., Chandra S.S. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J. Neurosci. 2014;34(28):9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pandey A.P., Haque F., Rochet J.C., Hovis J.S. α-Synuclein induced tubule formation in lipid bilayers. J. Phys. Chem. B. 2011;115(19):5886–5893. doi: 10.1021/jp1121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westphal C.H., Chandra S.S. Monomeric synucleins generate membrane curvature. J. Biol. Chem. 2013;288(3):1829–1840. doi: 10.1074/jbc.M112.418871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burré J., Sharma M., Sudhof T.C. Systematic mutagenesis of a-synuclein reveals distinct sequence requirements for physiological and pathological activities. J. Neurosci. 2012;32(43):15227–15242. doi: 10.1523/JNEUROSCI.3545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma M., Burre J., Sudhof T.C. CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat. Cell Biol. 2011;13(1):30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- 131.Sharma M., Burre J., Bronk P., Zhang Y., Xu W., Sudhof T.C. CSPalpha knockout causes neurodegeneration by impairing SNAP-25 function. EMBO J. 2012;31(4):829–841. doi: 10.1038/emboj.2011.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zinsmaier K.E., Eberle K.K., Buchner E., Walter N., Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263(5149):977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]
- 133.Fernández-Chacón R.F., Wolfel M., Nishimune H., Tabares L., Schmitz F., Castellano-Munoz M., Rosenmund C., Montesinos M.L., Sanes J.R., Schneggenburger R., Südhof T.C. The synaptic vesicle protein CSPα prevents presynaptic degeneration. Neuron. 2004;42(2):237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- 134.Greten-Harrison B., Polydoro M., Morimoto-Tomita M., Diao L., Williams A.M., Nie E.H., Makani S., Tian N., Castillo P.E., Buchman V.L., Chandraa S.S. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA. 2010;107(45):19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S., Hwang D., Masliah E., Lee S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chandra S., Fornai F., Kwon H.B., Yazdani U., Atasoy D., Liu X., Hammer R.E., Battaglia G., German D.C., Castillo P.E., Sudhof T.C. Double knockout mice for α- and β-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA. 2004;101(41):14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anwar S., Peters O., Millership S., Ninkina N., Doig N., Connor-Robson N., Threlfell S., Kooner G., Deacon R.M., Bannerman D.M., Bolam J.P., Chandra S.S., Cragg S.J., Wade-Martins R., Buchman V.L. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011;31(20):7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perez R.G., Waymire J.C., Lin E., Liu J.J., Guo F.L., Zigmond M.J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002;22(8):3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tehranian R., Montoya S.E., Van Laar A.D., Hastings T.G., Perez R.G. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J. Neurochem. 2006;99(4):1188–1196. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- 141.Alerte T.N.M., Akinfolarin A.A., Friedrich E.E., Mader S.A., Hong C.S., Perez R.G. alpha-synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: Lessons from viral transduction of knockout mice. Neurosci. Lett. 2008;435(1):24–29. doi: 10.1016/j.neulet.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen M.K., Kuwabara H., Zhou Y., Adams R.J., Brasić J.R., McGlothan J.L., Verina T., Burton N.C., Alexander M., Kumar A., Wong D.F., Guilarte T.R. VMAT2 and dopamine neuron loss in a primate model of Parkinson’s disease. J. Neurochem. 2008;105(1):78–90. doi: 10.1111/j.1471-4159.2007.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maroteaux L., Campanelli J.T., Scheller R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cole N.B., Gottschalk W., McIlwain K.L., Orrison B., Chen A., Ellis C.E., Paylor R., Lu B., Nussbaum R.L. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fountaine T.M., Venda L.L., Warrick N., Christian H.C., Brundin P., Channon K.M., Wade-Martins R. The effect of alphasynuclein knockdown on MPP plus toxicity in models of human neurons. Eur. J. Neurosci. 2008;28(12):2459–2473. doi: 10.1111/j.1460-9568.2008.06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Asanuma M., Miyazaki I., Ogawa N. Dopamine- or d-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003;5(3):165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 147.Perez R.G., Hastings T.G. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J. Neurochem. 2004;89(6):1318–1324. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- 148.da Costa C.A., Ancolio K., Checler F. Wild-type but not Parkinson’s disease-related ala-53Ð> Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J. Biol. Chem. 2000;275(31):24065–24069. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 149.Manning-Bog A.B., McCormack A.L., Purisai M.G., Bolin L.M., Di Monte D.A. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J. Neurosci. 2003;23(8):3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y.Y., Zhao H.Y., Zhao C.L., Duan C.L., Lu L.L., Yang H. Overexpression of alpha-synuclein in SH-SY5Y cells partially protected against oxidative stress induced by rotenone. Sheng Li Xue Bao. 2006;58(5):421–428. [PubMed] [Google Scholar]
- 151.Monti B., Polazzi E., Batti L., Crochemore C., Virgili M., Contestabile A. Alpha-synuclein protects cerebellar granule neurons against 6-hydroxydopamine-induced death. J. Neurochem. 2007;103(2):518–530. doi: 10.1111/j.1471-4159.2007.04778.x. [DOI] [PubMed] [Google Scholar]
- 152.Hashimoto M., Hsu L.J., Rockenstein E., Takenouchi T., Mallory M., Masliah E. alpha-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J. Biol. Chem. 2002;277(13):11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- 153.Kline R.A., Kaifer K.A., Osman E.Y., Carella F., Tiberi A., Ross J., Pennetta G., Lorson C.L., Murray L.M. Comparison of independent screens on differentially vulnerable motor neurons reveals alpha-synuclein as a common modifier in motor neuron diseases. PLoS Genet. 2017;13(3):e1006680. doi: 10.1371/journal.pgen.1006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26(1):41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mori F., Tanji K., Yoshimoto M., Takahashi H., Wakabayashi K. Immunohistochemical comparison of alpha- and beta-synuclein in adult rat central nervous system. Brain Res. 2002;941(1-2):118–126. doi: 10.1016/s0006-8993(02)02643-4. [DOI] [PubMed] [Google Scholar]
- 156.Goers J., Manning-Bog A.B., McCormack A.L., Millett I.S., Doniach S., Di Monte D.A., Uversky V.N., Fink A.L. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42(28):8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 157.Specht C.G., Tigaret C.M., Rast G.F., Thalhammer A., Rudhard Y., Schoepfer R. Subcellular localisation of recombinant alpha- and gamma-synuclein. Mol. Cell. Neurosci. 2005;28(2):326–334. doi: 10.1016/j.mcn.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 158.Pavlou M.A.S., Pinho R., Paiva I., Outeiro T.F. The yin and yang of α-synuclein-associated epigenetics in Parkinson’s disease. Brain. 2017;140(4):878–886. doi: 10.1093/brain/aww227. [DOI] [PubMed] [Google Scholar]
- 159.Jin H., Kanthasamy A., Ghosh A., Yang Y., Anantharam V., Kanthasamy A.G. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J. Neurosci. 2011;31(6):2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alves da Costa C., Paitel E., Vincent B. Checler; F. Alpha synuclein lowers p53-dependent apoptotic response of neuronal cells. Abolishment by 6-hydroxydopamine and implication for Parkinson’s disease. J. Biol. Chem. 2002;277(52):50980–50984. doi: 10.1074/jbc.M207825200. [DOI] [PubMed] [Google Scholar]
- 161.Weinreb P.H., Zhen W., Poon A.W., Conway K.A., Lansbury P.T. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35(43):13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 162.Recchia A., Debetto P., Negro A., Guidolin D., Skaper S.D., Giusti P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004;18(6):617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- 163.Fauvet B., Mbefo M.K., Fares M.B., Desobry C., Michael S., Ardah M.T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D.J., Schneider B., Aebischer P., El-Agnaf O.M., Masliah E., Lashuel H.A. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012;287(19):15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bartels T., Choi J.G., Selkoe D.J. a-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burré J., Vivona S., Diao J., Sharma M., Brunger A.T., Südhof T.C. Properties of native brain α-synuclein. Nature. 2013;498(7453):E4–E7. doi: 10.1038/nature12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hasegawa M., Fujiwara H., Nonaka T., Wakabayashi K., Takahashi H., Lee V., Trojanowski J.Q., Mann D., Iwatsubo T. Phosphorylated alpha-synuclein is ubiquitinated in alpha synucleinopathy lesions. J. Biol. Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 167.Oueslati A., Fournier M., Lashuel H.A. Role of post translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog. Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 168.Krumova P., Meulmeester E., Garrido M., Tirard M., Hsiao H.H., Bossis G., Urlaub H., Zweckstetter M., Kügler S., Melchior F., Bähr M., Weishaupt J.H. Sumoylation inhibits alpha-synuclein aggregation and toxicity. J. Cell Biol. 2011;194(1):49–60. doi: 10.1083/jcb.201010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bartels T., Ahlstrom L.S., Leftin A., Kamp F., Haass C., Brown M.F., Beyer K. The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys. J. 2010;99(7):2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iyer A., Roeters S.J., Schilderink N., Hommersom B., Heeren R.M., Woutersen S., Claessens M.M., Subramaniam V. The impact of N-terminal Acetylation of alpha-Synuclein on Phospholipid membrane binding and fibril structure. J. Biol. Chem. 2016;291(40):21110–21122. doi: 10.1074/jbc.M116.726612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Uversky V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002;269(1):2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- 172.Kanaan N.M., Manfredsson F.P. Loss of functional alphasynuclein: A toxic event in Parkinson’s disease? J. Parkinsons Dis. 2012;2(4):249–267. doi: 10.3233/JPD-012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahmad B., Chen Y., Lapidus L.J. Aggregation of alpha synuclein is kinetically controlled by intramolecular diffusion. Proc. Natl. Acad. Sci. USA. 2012;109(7):2336–2341. doi: 10.1073/pnas.1109526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hashimoto M., Hsu L.J., Xia Y., Takeda A., Sisk A., Sundsmo M., Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10(7):717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 175.Bharathi I.S.S., Rao K.S. Copper- and iron-induced differential fibril formation in alpha-synuclein: TEM study. Neurosci. Lett. 2007;424(2):78–82. doi: 10.1016/j.neulet.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 176.Schmidt F., Levin J., Kamp F., Kretzschmar H., Giese A., Botzel K. Single-channel electrophysiology reveals a distinct and uniform pore complex formed by alpha-synuclein oligomers in lipid membranes. PLoS One. 2012;7(8):e42545. doi: 10.1371/journal.pone.0042545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Narhi L., Wood S.J., Steavenson S., Jiang Y., Wu G.M., Anafi D., Kaufman S.A., Martin F., Sitney K., Denis P., Louis J.C., Wypych J., Biere A.L., Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 1999;274(14):9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 178.Fredenburg R.A., Rospigliosi C., Meray R.K., Kessler J.C., Lashuel H.A., Eliezer D., Lansbury P.T.J. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46(24):7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 179.Shtilerman M.D., Ding T.T., Lansbury P.T. Molecular crowding accelerates fibrillization of alpha-synuclein: Could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry. 2002;41(12):3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- 180.Uversky V.N.M., Cooper E., Bower K.S., Li J., Fink A.L. Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett. 2002;515(1-3):99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 181.Duce J.A., Wong B.X., Durham H., Devedjian J.C., Smith D.P., Devos D. Post-translational changes to α -synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol. Neurodegener. 2017;12(1):45. doi: 10.1186/s13024-017-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luk K.C., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., Lee V.M.Y. Exogenous alphasynuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luk K.C., Kehm V.M., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M.Y. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Luk K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M.Y. Pathological a-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., Hasegawa M. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136(Pt 4):1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roberts R.F., Wade-Martins R., Alegre-Abarrategui J. Direct visualization of α-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138(Pt 6):1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brundin P., Melki R., Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010;11(4):301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ahsan N., Mishra S., Jain M.K., Surolia A., Gupta S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant α-Synuclein. Sci. Rep. 2015;5:9862. doi: 10.1038/srep09862. [DOI] [PMC free article] [PubMed] [Google Scholar]