Abstract
Chiral ionic liquids with a focus on their applications in asymmetric Michael additions and related reactions were reviewed. The examples were classified on the basis of the mode of asymmetric induction (e.g., external induction/non-covalent interaction or internal induction/covalent bond formation), the roles in reactions (as a solvent or catalyst), and their structural features (e.g., imidazolium-based chiral cations, other chiral oniums; proline derivatives). Most of the reactions with high chiral induction are Michael addition of ketones or aldehydes to chalcones or nitrostyrenes where proline-derived chiral ionic liquids catalyze the reaction through enamine/ iminium formation. Many reports demonstrate the recyclability of ionic liquid-tagged pyrrolidines.
Keywords: Michael addition, imidazole ionic liquid, Chiral ionic liquid, asymmetric reaction, chiral solvent, catalysts
1. INTRODUCTION
In 1999, Seddon’s group reported the first example of a chiral ionic liquid, 1-butyl-3-methylimidazolium lactate [1]. Since then, the syntheses of various chiral ionic liquids and their applications to organic synthesis have been reported [2-6]. Unlike the first example where chirality was induced by the lactate anion, most of the chiral ionic liquids reported thus far consist of chiral cations and achiral anions. Many of the reported chiral ionic liquids have been used as chiral agents for asymmetric organic synthesis, e.g., aldol reaction, Baylis-Hillman reaction, and Michael addition [2-8]. This review provides an overview of the asymmetric Michael addition mediated by chiral ionic liquids. Michael addition is one of the most powerful, efficient methods for the formation of carbon-carbon bonds, and its asymmetric version is beneficial in organic synthesis. In the following sections, chiral ionic liquids and their applications in Michael addition were classified based on the structural features of the ionic liquids and the manner in which they cause asymmetric induction.
2. External asymmetric induction by chiral cations THROUGH NON-COVALENT INTERACTIONs
2.1. Chiral Ionic Liquids as Solvents
Bao and co-workers have reported the application of imidazolium-based chiral ionic liquids to the enantioselective Michael addition (Scheme 1) [9]. A majority of the chiral ionic liquids reported in previous studies other than Bao’s study, especially those from the chiral pool, contain positively charged atoms adjacent to chiral centers, possibly causing the racemization of the chiral ionic liquids [10]. In view of this drawback, Bao and co-workers synthesized chiral ionic liquids with “better configurational stability” compared to natural acid derivatives. Imidazolium salts 1a, and 2a are synthesized from L-(+)-diethyl tartrate or ethyl (S)-lactate in five steps, and the anion exchange affords hexafluorophosphates 1b, 2b, and tetrafluoroborate 2c. Chiral imidazoliums 1b, 2a-c was applied to the enantioselective Michael addition of diethyl malonate to chalcone as the chiral reaction media. Co-solvents were used to ensure that the reaction mixture could be stirred smoothly, because low temperature was required to enhance the chiral induction and the melting points of 1b, 2a-c were greater than 40 °C. In the reaction using 2c, better enantioselectivity (24% ee) is obtained in toluene compared to DMSO (17% ee) and DMF (16% ee). The chiral ionic liquid with C2 symmetry 1b exhibits lower asymmetric induction than 2a-c. Enantioselectivity was possibly induced by molecular interactions, e.g., electrostatic attractions between the imidazolium and malonate anion. N-heterocyclic carbenes are expected to be produced from ionic liquids under basic conditions to mediate the reaction. However, that is unlikely considering that the reported pKa values of the imidazolium salts range from 19.7 to 24 in DMSO [11-13] and from 19.8 to 25.4 in water [14, 15], whereas a pKa value of the conjugate acid of potassium carbonate is ca. ~10.
Scheme 1.
In addition, Ou and Huang have reported the same type of enantioselective Michael reaction using imidazolium-based chiral ionic liquids prepared from L-amino alcohols and 1-(2,4-dinitrophenyl)-3-methylimidazolium chloride in one or two steps (Scheme 2) [16] using acetonitrile as the co-solvent. In the presence of potassium carbonate, the addition of diethyl malonate to chalcone proceeds in moderate-to-good yields. A majority of their chiral ionic liquids exhibited enantioselectivity up to 15% ee.
Scheme 2.
Suzuki’s group has reported the synthesis of four structural types of chiral ionic liquids and their applications (Scheme 3) [17]: monoimidazolium salts bearing an asymmetric carbon atom α to the nitrogen atom on a N-substituent (6); monoimidazolium salts bearing an asymmetric carbon atom β to the nitrogen atom on an N-substituent (2a) (for structure, see Scheme 1); C2-symmetric bisimidazolium salts bearing asymmetric carbon atoms α to the nitrogen atoms of imidazoles (7); and C2-symmetric bisimidazolium salts bearing asymmetric carbon atoms β to the nitrogen atoms of imidazoles (8, 9). They also performed Michael addition between chalcone derivatives and malonates using them as solvents to evaluate their chiral induction abilities. Bisimidazoliums 7-9 exerted subtle, but steady, induction effects.
Scheme 3.
2.2. Chiral Ionic Liquids as Catalysts
Vo-Thanh and Trung have synthesized chiral ionic liquids 10-13 in two or three steps from (−)-ephedrine and applied them for the asymmetric Michael addition of diethyl acetylaminomalonate to chalcone derivatives (Scheme 4) [18]. A solvent-free microwave-assisted methodology was utilized to prepare 10−13. The Michael addition proceeded in moderate-to-good yields and enantioselectivities (up to 80% yield and 70% ee, respectively). Particularly, the best ee values are obtained using 10a and 11a. Besides the steric effect, one possible explanation for the higher selectivity of this reaction compared with that observed for the above-mentioned examples in Scheme 1 (Section 1.1) and the present and following examples in this section is the hydrogen bonding between the catalysts and substrates. The substrate diethyl acetylaminomalonate comprises a hydrogen-bond donor, i.e., amide hydrogen. Particularly, the pyridine moieties of 10, and 11 can act as hydrogen-bond acceptors.
Scheme 4.
Gortor and co-workers have synthesized optically active ionic liquids 14 derived from cyclohexenone and imidazole by lipase-catalyzed kinetic resolution and applied them to organocatalysis (Scheme 5) [19]. Eighteen optically active salts with (1R,3R)-trans, (1R,3S)-cis, or (1S,3R)-cis configuration were used as asymmetric phase-transfer catalysts in the reaction between chalcone and diethyl malonate. The catalyst with the octyl group exhibits higher catalytic activity than those comprising benzyl or butyl groups. (R)- and (S)-products were preferentially produced using cis and trans catalysts, respectively, albeit with low selectivities.
Scheme 5.
As another example, chiral ionic liquid 15 (Fig. 1) was synthesized from chiral bicycle [3.3.1] nonane-2,6-dione in three steps, which was used in the reaction between chalcone and diethyl malonate [20]. The reaction was carried out at room temperature for 12 h using potassium carbonate as the base, affording the (S)-adduct with 57% ee.
Fig. (1).
Imidazole ionic liquid from Chiral bicyclo[3.3.1]nonane-2,6-dione.
Zlotin and co-workers have recently reported tertiary amine-derived ionic liquid-supported squaramides 16, 17a,b and ent-17a (enantiomer of 17a) as organocatalysts for asymmetric Michael additions of β-dicarbonyl compounds to nitroolefins (Scheme 6) [21]. The catalysts were prepared via chiral tertiary amines as key precursors, which were derived from CBz-protected cyclohexane-1,2-diamine. Both 16 and 17a perform well as catalysts at ambient temperature in various organic solvents, affording the adduct (Scheme 6, R = Ph) with the (R)-configuration in high yields. The enantiomeric enrichment of the product is higher (up to 96% ee in organic solvent) with 17a, especially when pure water is used as solvent (98% ee). Catalyst 16 exhibits inferior activity and stereoselectivity under aqueous conditions. In contrast to hydrophobic hexafluorophospate 17a, water-soluble bromide 17b affords nearly racemic product in the same reaction. The catalyst loading of 17a can be reduced to 0.1 mol%, however, longer reaction time is required and a slightly lower enantioselectivity is observed. In addition, 17a is readily recoverable and reusable at least 30 times without a significant decrease of catalytic activity and enantioselectivity. It is suggested that in the presence of water, the amphiphilic catalyst 17a is located in the frontier zone between the organic phase and aqueous phase, which accelerates the catalytic transformation through a hydrophobic solvation effect.
Scheme 6.
3. Internal asymmetric induction via enamine or iminium formation by chiral cationS
3.1. Imidazole Ionic Liquids Bearing a 2-Pyrrolidinyl-Methyl Group as a N-Substituent
Cheng’s group has reported asymmetric organocatalysts 18-21 for the Michael addition to nitroolefins, which have a proline-derived chiral pyrrolidine unit covalently tethered to the imidazolium moiety (Scheme 7) [22]. The pyrrolidine unit was designed as the catalytic site, and the imidazolium moiety served as the phase tag and chiral-induction group.
Scheme 7.
The chiral ionic-liquid-catalyzed Michael addition of cyclohexanone to nitrostyrene was conducted in neat mixtures using TFA as the co-catalyst. Most of the catalysts exhibit higher activity than those reported previously for chiral pyrrolidine catalysis in ionic liquids [23]. Amongst these catalysts, bromide 19a and tetrafluoroborate 19b are the best chiral catalysts, with nearly quantitative yields and high diastereoselectivity (syn/anti = 99:1) and enantioselectivity (98% ee), whereas 40 mol% of L-proline is required to perform the same reaction in ionic liquids for 60 h with a 75% yield (d.r. = 95:5 and 75% ee for the syn diastereomer) [23]. The catalysts with the imidazolium core are superior to those with the 2-methylimidazolium core, and the protic group in the side chain of the cation (as in 21a,b) decreases the catalytic activity and selectivity. Catalysts 19a,b were also applied to the Michael addition between other acceptors (nitroolefins) and donors (aldehydes and ketones) to afford the products shown in Fig. (2).
Fig. (2).
Various Michael addition products produced in the presence of 19a and 19b.
The catalysts are easily recycled by precipitation with diethyl ether. Recycled 19b is used in the second run of the reaction between cyclohexanone and nitrostyrene, with identical activities and slightly decreased selectivities. The loss of activity is observed in the third and fourth reuse, but excellent yields and ee values are achieved with a long reaction time.
The high diastereo- and enantioselectivities can be explained by the concept of an acyclic synclinal transition state [24]. That is, the ionic-liquid moiety would shield the Si face of the double bond of the enamine formed from the pyrrolidine and ketone, and the reaction would proceed through a Re-Re approach (Fig. 3). Zhang’s group has conducted a theoretical investigation to elucidate the stereoselectivity of the Michael addition to olefins catalyzed by this type of pyrrolidine-based imidazolium ionic liquids [25, 26].
Fig. (3).

Proposed transition state for the reaction between cyclohexanones and nitroolefins.
Luo, Cheng, and their co-workers have applied the chiral ionic-liquid-catalyzed Michael addition to the desymmetrization of prochiral ketones (Scheme 8) [27]. During the screening of 19d, 21-25 for their catalytic activities in the reaction between 4-methylcyclohexanones and nitrostyrene, benzimidazolium 22 is found as the optimal catalyst. Thiazolidine-containing cation 23 and bis-cation 24 are inactive in the reaction. In addition, the effects of side chains of the imidazole core are observed. Specifically, a protic group (21a) and longer chain (25) lead to decreased product yields.
Scheme 8.
Among several Brønsted acids (e.g., acetic acid, benzoic acid, chloroacetic acid, salicylic acid, trifluoroacetic acid (TFA), trifluoromethanesulfonic acid) screened as additives, salicylic acid affords optimal results in terms of both activity and stereoselectivity. Using 22 and salicylic acid, the Michael addition of other 4-substituted cyclohexanones and nitrostyrene derivatives was examined. The reaction of 4-ethyl, 4-t-butyl, 4-phenyl, 4-azido, and 4-acetylmercapto-cyclohexanone affords the Michael adducts in 61-99% yields with excellent enantioselectivities (93-99% ee) and diastereoselectivities ranging from 4:1 to 12:1. In contrast, the reaction does not proceed with the use of 4-hydroxy, 4-bromo, and 4-cyanocyclohexanone.
Ionic liquid 22 is recycled by precipitation with diethyl ether and reused four times with a similar degree of stereoselectivities. However, decreased activity (prolonged reaction time and decreased yield) is observed in the third and fourth reuse.
The Michael addition of cyclohexane to nitrostyrene catalyzed by the same class of chiral ionic liquids 26a-g, comprising either/both alternative side chains on the imidazolium core and/or bis(trifluoromethylsulfonyl)imide as the counter anion, has also been reported by another group (Fig. 4) [28]. There are also reports on a similar type of pyrrolidine-based chiral imidazolium ionic liquids with higher reusability, i.e., polymer-immobilized chiral ionic liquids 27a-c and silica-gel-supported chiral ionic liquids 28, 29 (Fig. 4). Both ionic liquids are simply recovered by filtration. For the asymmetric Michael addition to nitrostyrenes, 27a can be recycled up to eight times without the loss of activity and enantioselectivity [29]. Silica-gel-supported chiral ionic liquids 28, 29 can be recycled at least five times [30].
Fig. (4).
Other imidazole ionic liquids bearing a 2-pyrrolidinylmethyl group.
3.2. Imidazole Ionic Liquids Tethered to a Pyrrolidinylmethyltriazole Moiety
Chiral ionic liquids 30, and 31 (Fig. 5) containing a triazole moiety between the imidazole core and pyrrolidine were synthesized by “click chemistry,” and they were applied to the enantioselective Michael addition of ketones to nitroolefins [31, 32]. In the reaction using 30, the addition of TFA significantly enhances the reaction rate and enantioselectivity. Catalyst 30 is easily recycled by precipitation via the addition of diethyl ether into the reaction mixture, and reused without additional TFA four times with no significant decrease in yields and enantioselectivities [31]. Ionic liquid 31a could be recycled in a similar manner by precipitation and reused at least eight times without the loss of its catalytic activity [32].
Fig. (5).
Imidazole ionic liquids tehtered to a pyrrolidinylmethyltriazole moiety.
3.3. Imidazole Ionic Liquids Bearing a N-(Pyrrolidin-2-ylmethyl)sulfonamide Moiety
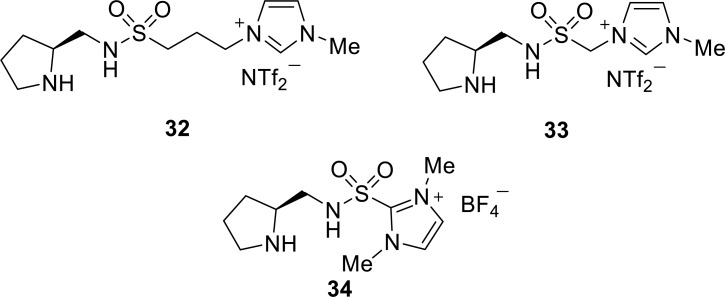
Headley and co-workers have developed a series of pyrrolidine-based chiral imidazolium ionic liquids containing a sulfonamide moiety as a protic functionality (Fig. 6) [33-35]. They first reported imidazolium 32 as a recyclable organocatalyst for the asymmetric Michael addition of aldehydes and cyclohexanone to nitrostyrenes (Scheme 9 and 10) [33]. The acidic N-H plays an important role in the selectivity of the reaction via the formation of hydrogen bonds to the nitrostyrene substrate. In the addition of aldehyde, C−C bond formation would occur by the preferential addition of the enamine to the less hindered Si-face of nitrostyrene, affording a product with 2R,3S configuration (Fig. 7A) [34]. In contrast to cyclohexanone, the ketone-derived enamine attacks nitrostyrene from the less hindered Re-face, affording a product with the 2S,3R configuration (Fig. 7B).
Fig. (6).
Imidazole ionic liquids bearing a n-(pyrrolidin-2ylmethyl)sulfonamide moiety.
Scheme 9.
Scheme 10.
Fig. (7).
Mechanism for Michael addition in the presence of imidazole ionic liquid bearing a N-(pyrrolidin-2ylmethyl)sulfonamide moiety.
Considering the stabilization effect of the acidic N-H on the transition state via a hydrogen bond, ionic liquids with a shorter methylene chain between the sulfonyl group and imidazolium unit than that of 32 are designed as a more efficient catalyst [34]. Ionic liquid 33 contains a more acidic N-H and sterically bulky groups near the catalytic site. In combination with TFA, 33 has been reported to be more effective than 32 for the Michael addition of α,α-disubstituted aldehydes (Scheme 9, R1 = R2) and nitrostyrenes, enantioselectively affording the Michael adducts with one quaternary carbon center. In contrast, 32 produces superior enantioselectivities for reactions involving α-monosubstituted aldehydes (R1 = H, R2 = alkyl), whereas 33 is more reactive, affording products in a higher yield. Catalysts 32 and 33 are recovered by precipitation with ether and are reused five times without any significant loss in activity.
To increase the N-H acidity for the formation of the strong hydrogen bonds in the transition state, a new type of a pyrrolidine sulfonamide catalyst 34 was designed (Fig. 6) [35]. Catalyst 34 contains a sulfonyl group directly connected to the electronegative C-2 position of the imidazolium cation, which serves as the electron-withdrawing group. In addition, improved stereoselectivity was expected because of the close proximity of the imidazole core to the catalytic center.
Even with a lower catalyst loading (10 mol%) of 34, the Michael addition of six-membered cyclic ketones and i-butylaldehydes to nitroolefins afforded the products in good yields at room temperature with high stereoselectivities (ee up to 99%; syn/anti up to 99/1) (Scheme 11). In contrast, the reaction of cyclopentanone to nitrostyrene using 34 affords the product in low yield, and the reaction of acetone with nitrostyrene affords low enantioselectivity. The catalyst is readily recovered and reused at least five times without any significant loss of catalytic activity and stereoselectivity.
Scheme 11.
The Michael addition of cyclohexanone to styrenes catalyzed by a similar pyrrolidine-based chiral imidazolium ionic liquid 35 containing a sulfonamide moiety has also been reported by another research group Fig. (8) [36].
Fig. (8).
Imidazole ionic liquid bearing a (N-(pyrrolidin-2ylmethyl)sulfamoyl)benzyl Group.
3.4. Imidazole and Triazole Ionic Liquids Tethered to a Prolinol Moiety
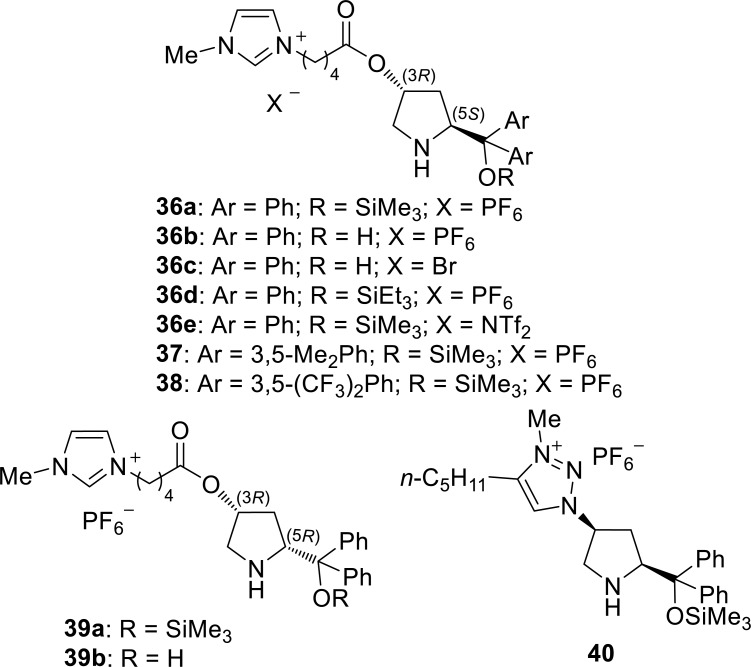
A series of chiral imidazolium ionic liquids bearing an α,α-diphenylprolinol unit have been developed as recoverable organocatalysts 36 a-d, 37-40 for the asymmetric Michael addition by Zlotin and co-workers (Fig. 9) [37-41].
Fig. (9).
Ionic liquids tethered to a prolinol moiety.
Proline-derived compounds 36a-c were used in the reaction between trans-cinnamaldehydes and malonates (Scheme 12) [37]. The reactions with 10 mol% of 36a proceed under mild conditions (4 °C) in high yield (up to 98%) with high enantioselectivities (up to 96% ee), whereas the presence of the free hydroxyl groups in 36b,c decreases the conversion (less than or equal to 26%). Catalyst 36a could be used four times without any decrease in the activity and enantioselectivity.
Scheme 12.
The catalysts with the S configuration at position 5 of the pyrrolidine (36 a,b,d) and their diastereomers with the R configuration (39 a,b) were used in a reaction between α,β-enals and nitromethane derivatives (Scheme 13, Scheme 14) [38]. O-trimethylsilylated compounds 36a,39a exhibit high catalytic performance with various substrates. In contrast, O-triethylsilylated analog 36d is nearly inactive. Compounds with a free hydroxyl group 36b,39b exhibit lower selectivity than their O-trimethylsilylated counterparts 36a,39a. (Scheme 13) Although 36 a,b and 39 a,b are not enantiomers, the reactions with 36a,b and with 39a,b afford (S)-products and (R)-products, respectively. The existence of water in the reaction medium is favorable. That is, the reaction conversion in the presence of catalyst 36b was >99% in 96% MeOH, whereas it was 66% in dry MeOH. Although the catalytic activity decreases after the third use, catalyst 36a, b and 39a could be used five times without any decrease in the product yield and ee values.
Scheme 13.
Scheme 14.
The Michael addition of acetaldehyde derivatives with nitroolefins catalyzed by the same class of chiral ionic liquid 36e, which affords functionalized 4-nitrobutanals, has also been reported (Scheme 15) [42].
Scheme 15.
The domino reaction between α,β-enals and hydroxylamines in the presence of the chiral imidazolium ionic liquids has also been reported by Zlotin’s research group (Fig. 9, Scheme 16) [39-41]. The products of this domino reaction are chiral 3-aryl-5-hydroxyisoxazolidines, which are formed by the Michael addition followed by spontaneous intramolecular acetalization. Catalyst 36a exhibits activity greater than those of 36b, 37, 38, and 40 in the reaction between trans-cinnamaldehyde and N-benzyloxycarbonyl-protected hydroxylamine, selectively affording (S)-product [40]. In contrast, 39a preferentially affords the (R)-product.
Scheme 16.
3.5. Imidazole Ionic Liquids Tethered to a Chiral Primary Amine
Zlotin’s research group has reported chiral primary amine-derived ionic liquids 41, 42 as reusable organocatalysts for the asymmetric Michael addition reactions (Scheme 17) [41, 43]. The catalytic properties of 41 and 42 were evaluated in the reaction of 4-hydroxycoumarin with benzylideneacetone at room temperature using THF as the solvent. With 20 mol% of 41, the (R)-enantiomer-enriched product (10% ee) is obtained in 60% yield, whereas the reaction with 42 preferentially affords the (S)-enantiomer in 73% yield with 68% ee. The addition of acetic acid to this reaction using 42 leads to improved product yield (up to 80%) and enantioselectivity (up to 80% ee). The reactions of 4-hydroxycoumarin and its sulfur-containing analog with various benzylideneacetones or cyclohexenone in the presence of 42 also affords (S)-adducts in up to 77% ee. The catalytic efficacy of 42 progressively declines after the third cycle plausibly because of its deactivation by the irreversible reaction with the Michael adduct. To suppress this Brønsted-acid-sensitive undesired off-cycle reaction, structurally-modified catalyst 43 was designed [44]. In the presence of 43, the Michael adducts are obtained in high yields (up to 97%) and enantioselectivities (up to 90% ee). The catalyst can be recycled five times with minor decreases in yield and enantioselectivity.
Scheme 17.
3.6. Pyridinium and Ammonium Ionic Liquids Bearing a 2-Pyrrolidinylmethyl Group as the N-Substituent
Studies related to the synthesis of proline-derived chiral ionic liquids with onium moieties other than imidazolium and their use in asymmetric Michael addition reactions have been published. 1-(Pyrrolidin-2-ylmethyl)pyridinium ionic liquid 44 has been reported by Headley and his co-workers (Scheme 18) [45], and alkyl ammonium ionic liquids bearing N-pyrrolidin-2-ylmethyl group 45 and 46 have been reported by Wang’s group (Scheme 19) [46] and Chen’s group (Scheme 20) [47], respectively.
Scheme 18.
Scheme 19.
Scheme 20.
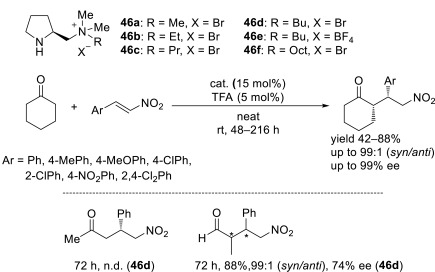
All these ionic liquids were used in the Michael addition of ketones and aldehydes to nitroolefins as organocatalysts. In general, the reaction of cyclic ketones (except for cycloheptanone) using 44-46 afforded the adducts in good yields with high diastereo- and enantioselectivities. Similar to the examples of proline-derived imidazolium ionic liquids in Scheme 6 and Fig. 2 (Section 2.1), and in Schemes 8-10 (Section 2.3), (3R)-adducts are selectively obtained in the reaction of ketones, and (3S)-products are preferentially generated in the reaction of aldehydes. Catalyst 44b could be recycled two times with a slightly decreased yield, with decreased selectivity in the third cycle (Scheme 18). Among catalyst 45a-c, catalyst 45b is considered as the catalyst of choice, and the optimal reaction solvent is [Bmim][BF4] (Scheme 19). The catalyst remains in the solvent after the reaction. Thus, the catalyst and solvent are easily recovered in greater than 90% yield, and it can be reused at least six times without any loss of stereoselectivity and a slightly decreased activity. A good performance is observed for catalyst 46a-f, but a longer time is required in the third cycle of the reaction, i.e., 480 h for a product yield of 70%, whereas in the first cycle, the product yield is 80% after a 72-h reaction (Scheme 20).
Hirose and co-workers have used chiral ammonium ionic liquids containing a prolinamide moiety as the catalytic site in the asymmetric Michael addition of aldehydes to nitroolefins (Scheme 21) [48]. Catalyst 47 was synthesized from commercially available (+)-cis-2-benzamidocyclohexanecarboxylic acid and N-protected proline. Chiral ammonium ionic liquid 47b with a longer alkyl group is found to be a catalyst superior to 47a in the reaction between aldehydes and nitroolefins. Recyclability was tested for both catalysts. Although fairly constant enantioselectivities are observed, the catalytic activities decrease in the third run.
Scheme 21.
4. Asymmetric induction by L-prolinate and L-prolinium
Ohno’s research group has reported the synthesis and the properties of ionic liquids containing natural amino-acid-derived anions [49]. Twenty chiral ionic liquids are prepared by the neutralization of 1-ethyl-3-methylimidazolium hydroxide with a series of natural amino acids. Wang’s research group has used one of these amino acid ionic liquids, imidazolium L-prolinate 48, as the catalyst for the asymmetric Michael addition of cyclohexanone to chalcones (Scheme 22) [50]. In the reactions catalyzed by 48, the solvent-dependent inversion of the enantioselectivity is observed. In particular, the reaction in methanol, ethanol, toluene, THF, dichloromethane, or in ionic liquids afforded (2S,3R)-enantiomer-enriched products. In contrast, the reaction in DMSO preferentially afforded (2R, 3S)-products.
Scheme 22.
Pernak and co-workers synthesized ammonium L-prolinate 49 and applied it to the asymmetric Michael addition of cyclohexanone to nitrostyrene (Scheme 23) [51]. They concluded that the catalytic activity and selectivity of 49 are better than those of L-proline in [bmim]BF4 [22] and worse than functionalized ionic liquid 17b (Scheme 6 in Section 2.1).
Scheme 23.
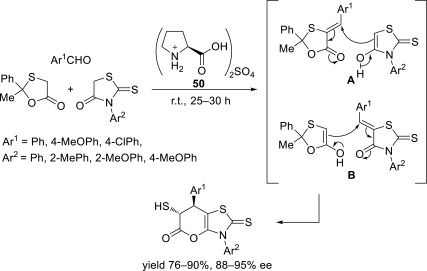
Yadav’s group has reported stereocontrolled multicomponent reactions using a chiral ionic liquid, L-prolinium sulfate 50 [52], as both solvent and catalyst to obtain mercaptopyranothiazoles [53] (Scheme 24). In the postulated reaction mechanism, the products are obtained by the Michael addition to arylidenes, which are generated as intermediates. In general, high yields (up to 90%) and good selectivity (up to 95% ee) are achieved.
Scheme 24.
CONCLUSION
Examples of the asymmetric Michael addition reaction using chiral ionic liquids as solvents or catalysts were reviewed. A majority of the reported chiral ionic liquids applied in the Michael addition are pyrrolidine-based salts originating from proline. In addition, most of the chiral ionic liquids, which afforded products with high yields and high selectivities in the asymmetric Michael addition, were derived from proline, and these catalysts were developed as recyclable catalysts using the pyrrolidinyl groups as the catalytic sites and the cationic moieties as functionalities for immobilization.
Ionic liquids are widely used as reaction solvents in organic synthesis. Although successful syntheses through asymmetric induction by chiral solvents are rare, chiral ionic liquids demonstrate potential as viable chiral solvents for effectively transferring their chiralities to reaction products because of their recyclability, ease of synthesis, and high degree of organization.
Consent for Publication
Not applicable.
ACKNOWLEDGeMENTS
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Earle M.J., McCormac P.B., Seddon K.R. Diels-Alder reactions in ionic liquids. A safe recyclable alternative to lithium perchlorate-diethyl ether mixtures. Green Chem. 1999;1(1):23–25. [Google Scholar]
- 2.Baudequin C., Bregeon D., Levillain J., Guillen F., Plaquevent J-C., Gaumont A-C. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry. 2005;16(24):3921–3945. [Google Scholar]
- 3.Ding J., Armstrong D.W. Chiral ionic liquids: Synthesis and applications. Chirality. 2005;17(5):281–292. doi: 10.1002/chir.20153. [DOI] [PubMed] [Google Scholar]
- 4.Headley A.D., Ni B. Chiral imidazolium ionic liquids: Their synthesis and influence on the outcome of organic reactions. Aldrichim Acta. 2007;40(4):107–117. [Google Scholar]
- 5.Chen X., Li X.I., Hu A., Wang F. Advances in chiral ionic liquids derived from natural amino acids. Tetrahedron Asymmetry. 2008;19(1):1–14. [Google Scholar]
- 6.Ni B., Headley A.D. Ionic-Liquid-Supported (ILS) catalysts for asymmetric organic synthesis. Chemistry. 2010;16(15):4426–4436. doi: 10.1002/chem.200902747. [DOI] [PubMed] [Google Scholar]
- 7.Siyutkin D.E., Kucherenko A.S., Zlotin S.G. Ionic liquid organocatalysts. 2013.
- 8.Payagala T., Armstrong D.W. Chiral ionic liquids: A compendium of syntheses and applications (2005-2012). Chirarity. 2012;24(1):17–53. doi: 10.1002/chir.21975. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Wang Q., Zhang Y. Bao. Synthesis of new chiral ionic liquids from natural acids and their applications in enantioselective Michael addition. Tetrahedron Lett. 2005;46(27):4657–4660. [Google Scholar]
- 10.Jodry J.J., Mikami K. New chiral imidazolium ionic liquids: 3D-network of hydrogen bonding. Tetrahedron Lett. 2004;45(23):4429–4431. [Google Scholar]
- 11.Alder R.W., Allen P.R., Williams S.J. Stable carbenes as strong bases. J. Chem. Soc. Chem. Commun. 1995;128(12):1267–1268. [Google Scholar]
- 12.Kim Y-J., Streitwieser A. Basicity of a stable carbene, 1, 3-di-tert-butylimidazol-2-ylidene, in THF. J. Am. Chem. Soc. 2002;124(20):5757–5761. doi: 10.1021/ja025628j. [DOI] [PubMed] [Google Scholar]
- 13.Chu Y., Deng H., Cheng J-P. An acidity scale of 1, 3-dialkyiimidazolium salts in dimethyl sulfoxide solution. J. Org. Chem. 2007;72(20):7790–7793. doi: 10.1021/jo070973i. [DOI] [PubMed] [Google Scholar]
- 14.Higgins E.M., Sherwood J.A., Lindsay A.G., Armstrong J., Massey R.S., Alder R.W., O’Donoghue A.C. pKas of the conjugate acids of N-heterocyclic carbenes in water. Chem. Commun. (Camb.) 2011;47:1559–1561. doi: 10.1039/c0cc03367g. [DOI] [PubMed] [Google Scholar]
- 15.Amyes T.L., Diver S.T., Richard J.P., Rivas F.M., Toth K. Formation and stability of N-heterocyclic carbenes in water: The carbon acid pKa of imidazolium cations in aqueous solution. J. Am. Chem. Soc. 2004;126(13):4366–4374. doi: 10.1021/ja039890j. [DOI] [PubMed] [Google Scholar]
- 16.Ou W-H., Huang Z-Z. An efficient and practical synthesis of chiral imidazolium ionic liquids and their application in an enantioselective Michael reaction. Green Chem. 2006;8(8):731–734. [Google Scholar]
- 17.Suzuki Y., Wakatusuki J., Tsubaki M., Sato M. Imidazolium-based chiral ionic liquids: Synthesis and application. Tetrahedron. 2013;69(46):9690–9700. [Google Scholar]
- 18.Truong T-K-T., Vo-Thanh G. Synthesis of functionalized chiral ammonium, imidazolium, and pyridinium-based ionic liquids derived from (-)-ephedrine using solvent-free microwave activation. Applications for the asymmetric Michael addition. Tetrahedron. 2010;66(27):5277–5282. [Google Scholar]
- 19.Paul C.E., Gotor-Fernández V., Lavandera I., Montejo-Bernardo J., García-Granda S., Gotor V. Chemoenzymatic preparation of optically active 3-(1H-imidazol-1-yl)cyclohexanol-based ionic liquids: Application in organocatalysis and toxicity studies. RSC Advances. 2012;2(16):6455–6463. [Google Scholar]
- 20.Bilinska A., Žilinskas A. Synthesis of chiral bicyclo[3.3.1]nonane derivative as ionic liquid and using it for asymmetric Michael’s addition reaction. Chemija. 2012;23(4):301–305. [Google Scholar]
- 21.Tukhvatshin R.S., Kucherenko A.S., Nelyubina Y.V., Zlotin S.G. Tertiary amine-derived ionic liquid-supported squaramide as a recyclable organocatalyst for noncovalent “on water” catalysis. ASC Catal. 2017;7(4):2981–2989. [Google Scholar]
- 22.Luo S., Mi X., Zhang L., Liu S., Xu H., Cheng J-Pi. Functionalized chiral ionic liquids as highly efficient asymmetric organocatalysts for Michael addition to nitroolefins. Angew. Chem. Int. Ed. 2006;45(19):3093–3097. doi: 10.1002/anie.200600048. [DOI] [PubMed] [Google Scholar]
- 23.Rasalkar M.S., Potdar M.K., Mohile S.S., Salunkhe M.M. An ionic liquid influenced l-proline catalysed asymmetric Michael addition of ketones to nitrostyrene. J. Mol. Catal. Chem. 2005;235(1-2):267–270. [Google Scholar]
- 24.Seebach D., Goliński J. Synthesis of open-chain 2, 3-disubstituted 4-nitroketones by diastereoselective Michael-addition of (E)-enamines to (E)-nitroolefins. A topological rule for C, C-bond forming processes between prochiral centres. Preliminary communication. Helv. Chim. Acta. 1981;64(5):1413–1423. [Google Scholar]
- 25.Sun H., Zhang D., Zhang C., Liu C. Theoretical study on the asymmetric Michael addition of cyclohexanone with trans-β-nitrostyrene catalyzed by a pyrrolidine-type chiral ionic liquid. Chirality. 2010;22(9):813–819. doi: 10.1002/chir.20841. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Zhang D. Theoretical elucidation on the functional role of pyrrolidine-type ionic liquids in inducing stereoselectivity of the Michael addition of cyclohexanone with trans-β-nitrostyrene. Chirality. 2011;23(3):260–264. doi: 10.1002/chir.20908. [DOI] [PubMed] [Google Scholar]
- 27.Luo S., Zhang L., Mi X., Qiao Y., Cheng J-P. Functionalized chiral ionic liquid catalyzed enantioselective desymmetrizations of prochiral ketones via asymmetric Michael addition reaction. J. Org. Chem. 2007;72(24):9350–9352. doi: 10.1021/jo7020357. [DOI] [PubMed] [Google Scholar]
- 28.Dąbrowski Z., Wiśniewska A., Kulig-Adamiak A., Kamiński J., Cybulski J. Ionic liquids as the catalysts for asymmetric reactions. Polimery. 2012;57(5):375–381. [Google Scholar]
- 29.Li P., Wang L., Wang M., Zhang Y. Polymer-immobilized pyrrolidine-based chiral ionic liquids as recyclable organocatalysts for asymmetric Michael additions to nitrostyrenes under solvent-free reaction conditions. Eur. J. Org. Chem. 2008;2008(7):1157–1160. [Google Scholar]
- 30.Li P., Wang L., Zhang Y., Wang G. Silica gel supported pyrrolidine-based chiral ionic liquid as recyclable organocatalyst for asymmetric Michael addition to nitrostyrenes. Tetrahedron. 2008;6(32):7633–7638. [Google Scholar]
- 31.Wu L-Y., Yan Z-Y., Xie Y-X., Niu Y-N., Liang Y-M. Ionic-liquid-supported organocatalyst for the enantioselective Michael addition of ketones to nitroolefins. Tetrahedron Asymmetry. 2007;18(17):2086–2090. [Google Scholar]
- 32.Miao T., Li P., Yan J. A highly efficient and recyclable ionic liquid anchored pyrrolidine catalyst for enantioselective Michael additons. Synthesis. 2008;2008(23):3828–3834. [Google Scholar]
- 33.Ni B., Zhang Q., Headley A.D. Functionalized chiral ionic Liquid as recyclable organocatalyst for asymmetric Michael addition to nitrostyrenes. Green Chem. 2007;9(7):737–739. [Google Scholar]
- 34.Zhang Q., Ni B., Headley A.D. Asymmetric Michael addition reactions of aldehydes with nitrostyrenes catalyzed by functionalized chiral ionic liquids. Tetrahedron. 2008;64(22):5091–5097. [Google Scholar]
- 35.Ni B., Zhang Q., Dhungana K., Headley A.D. Ionic Liquid-Supported (ILS) (S)-pyrrolidine sulfonamide, a recyclable organocatalyst for the highly enantioselective Michael addition to nitroolefins. Org. Lett. 2009;11(4):1037–1040. doi: 10.1021/ol900003e. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Liu L. Synthesis of new functionalized chiral ionic liquid and its organocatalytic asymmetric Michael addition. Synth. Commun. 2013;43(4):476–485. [Google Scholar]
- 37.Maltsev O.V., Kucherenko A.S., Zlotin S.G. O-TMS-α,α-diphenyl-(S)-prolinol modified with an ionic liquid moiety: A recoverable organocatalyst for the asymmetric Michael reaction between α,β-enals and dialkyl malonates. Eur. J. Org. Chem. 2009;2009(30):5134–5137. [Google Scholar]
- 38.Maltsev O.V., Kucherenko A.S., Beletskaya I.P., Tartakovsky V.A., Zlotin S.G. Chiral ionic liquids bearing O-silylated α,α-diphenyl (S)- or (R)-prolinol units: Recoverable organocatalysits for asymmetric Michael addition of nitroalkanes to α,β -enals. Eur. J. Org. Chem. 2010;2010(5):2927–2933. [Google Scholar]
- 39.Kucherenko A.S., Siyutkin D.E., Maltsev O.V., Kochetkov S.V., Zlotin S.G. Asymmetric organocatalysis: From proline to highly efficient immobilized organocatalysts. Russ. Chem. Bull. Int. Ed. 2012;61(7):1313–1320. [Google Scholar]
- 40.Maltsev O.V., Kucherenko A.S., Chimishkyan A.L., Zlotin S.G. α, α-Diarylprolinol-derived chiral ionic liquids: Recoverable organocatalysts for the domino reaction between α,β-enals and N-protected hydroxylamines. Tetrahedron Asymmetry. 2010;21(21):2659–2670. [Google Scholar]
- 41.Zlotin S.G., Kuherenko A.S., Maltsev O.V., Chizhov A.O. Chiral ionic liquid/ESI-MS methodology as an efficient tool for the study of transformations of supported organocatalysts. Top. Catal. 2013;56(11):923–932. doi: 10.1002/chem.201100388. [DOI] [PubMed] [Google Scholar]
- 42.Šebesta R., Latika A. Enantioselective Michael additions of aldehydes to nitroalkenes catalyzed with ionically tagged organocatalyst. Cent. Eur. J. Chem. 2014;12(3):416–425. [Google Scholar]
- 43.Kucherenko A.S., Siyutkin D.E., Nigmatov A.G., Chizhov A.O., Zlotin S.G. Chiral primary amine tagged to ionic group as reusable organocatalyst for asymmetric Michael reactions of C-nucleophiles with α,β-unsaturated ketones. Adv. Synth. Catal. 2012;354(16):3078–3086. [Google Scholar]
- 44.Kucherenko A.S., Lisnyak V.G., Chizhov A.O., Zlotin S.G. Primary amine attached to an N-(carboxyalkyl)imidazolium cation: A recyclable organocatalyst for the asymmetric Michael reaction. Eur. J. Org. Chem. 2014;2014(18):3808–3813. [Google Scholar]
- 45.Ni B., Zhang Q., Headley A.D. Pyrrolidine-based chiral pyridinium ionic liquids (ILs) as recyclable and highly efficient organocatalysts for the asymmetric Michael addition reactions. Tetrahedron Lett. 2008;49(7):1249–1252. [Google Scholar]
- 46.Xu D-Z., Liu Y., Shi S., Wang Y. Chiral quaternary alkylammonium ionic liquid [Pro-dabco][BF4]: As a recyclable and highly efficient organocatalyst for asymmetric Michael addition reactions. Tetrahedron Asymmetry. 2010;21(20):2530–2534. [Google Scholar]
- 47.Wang G., Sun H., Cao X., Chen L. Pyrrolidine-based chiral quaternary alkylammonium ionic liquids as organocatalysts for asymmetric Michael additions. Catal. Lett. 2011;141(9):1324–1331. [Google Scholar]
- 48.Wang W-H., Wang X-B., Kodama K., Hirose T., Zhang G-Y. Novel chiral ammonium ionic liquids as efficient organocatalysts for asymmetric Michael addition of aldehydes to nitroolefins. Tetrahedron. 2010;66(27-28):4970–4976. [Google Scholar]
- 49.Fukumoto K., Yoshizawa M., Ohno H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005;127(8):2398–2399. doi: 10.1021/ja043451i. [DOI] [PubMed] [Google Scholar]
- 50.Qian Y., Xiao S., Liu L., Wang Y. A mild and efficient procedure for asymmetric Michael additions of cyclohexanone to chalcones catalyzed by an amino acid ionic liquid. Tetrahedron Asymmetry. 2008;19(13):1515–1518. [Google Scholar]
- 51.Cybulski J., Wiśniewska A., Kulig-Adamiak A., Dąbrowski Z., Praczyk T., Michalczyk A., Walkiewicz F., Matema K., Pernak J. Mandelate and prolinate ionic liquids: Synthesis, characterization, catalytic and biological activity. Tetrahedron Lett. 2011;52(12):1325–1328. [Google Scholar]
- 52.Tao G-h., He L., Sun N., Kou Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun. (Camb.) 2005;2005(28):3562–3564. doi: 10.1039/b504256a. [DOI] [PubMed] [Google Scholar]
- 53.Yadav L.D.S., Yadav B.S., Rai V.K. Multicomponent reactions in chiral ionic liquids: A stereocontrolled route to mercaptopyranothiazoles. J. Het. Chem. 2008;45(5):1315–1319. [Google Scholar]