Abstract
Autoimmune pulmonary alveolar proteinosis (aPAP) is a rare parenchymal lung disease characterized by accumulation of surfactant in the airways with high levels of granulocyte-macrophage colony stimulating factor (GM-CSF) antibodies in blood. Disease leads to hypoxemic respiratory failure. Whole lung lavage (WLL) is considered the first line therapy, but procedure can be quite demanding, specifically for children. Recently alternative treatment options with inhaled GM-CSF have been described but no consensus about the standard treatment exists. We here describe a unique case of a 14-year-old patient who was successfully treated with WLL and subsequent inhalations with molgramostim – new recombinant human GM-CSF (rhGM-CSF).
Keywords: Pulmonary alveolar proteinosis, Granulocyte-macrophage colony-stimulating factor, GM-SCF, Molgramostim, Inhalation therapy
1. Introduction
Pulmonary alveolar proteinosis (PAP) is a rare parenchymal lung disease characterized by accumulation of surfactant in the airways that leads to hypoxemic respiratory failure [[1], [2], [3]]. There are mainly three types: hereditary, secondary and autoimmune PAP [1], where autoimmune PAP (aPAP) in adults accounts for more than 90% of the cases. The literature regarding aPAP in children is sparse and the treatment algorithms are not well documented. Whole lung lavage (WLL) is considered the golden standard [4], though alternative treatment options with inhaled granulocyte-macrophage colony stimulating factor (GM-CSF) have been described [[5], [6], [7]]. We here describe a unique case of a 14-year-old patient who was successfully treated with WLL and subsequent inhalations with molgramostim – recombinant human GM-CSF (rhGM-CSF).
2. Case
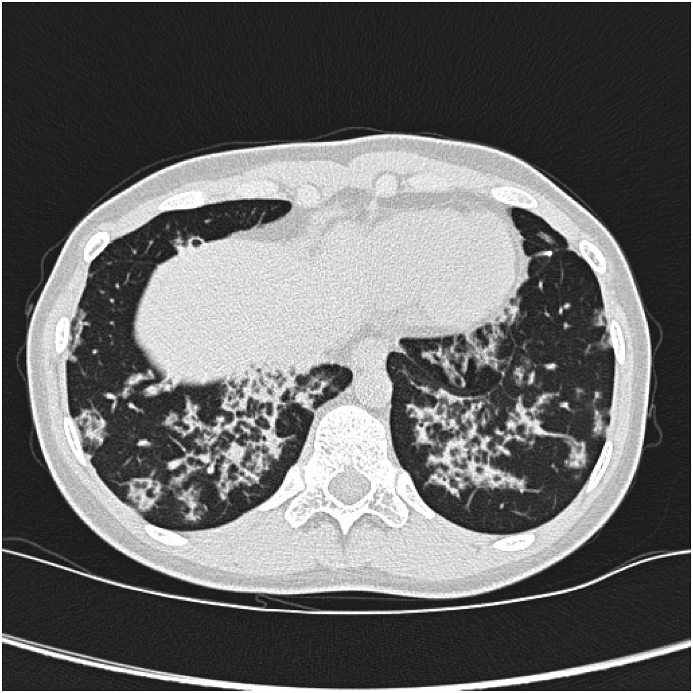
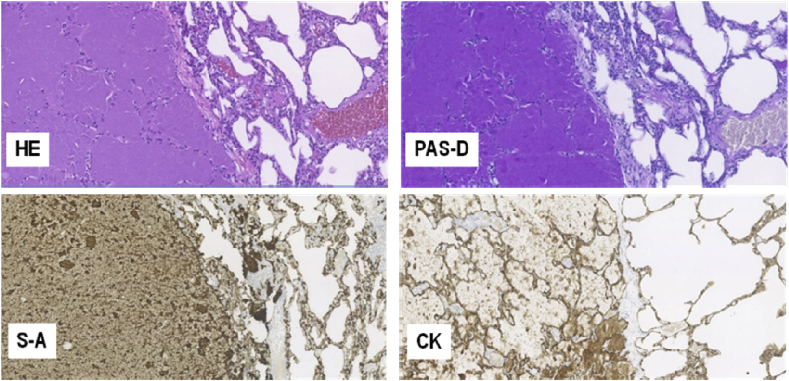
A 14-year-old girl was referred to the Danish Center of Interstitial Lung Disease, Aarhus University Hospital for specialized treatment due to a newly diagnosed aPAP. She complained of a constant cough and dyspnea on exertion starting a year before referral. Neither antibiotics nor inhaled corticosteroids improved the patient's condition and further investigations were performed at another hospital. High resolution computed tomography (HRCT) showed bilateral changes typical for PAP with not a classical crazy paving pattern (Fig. 1). A surgical biopsy revealed alveoli filled with a granular proteinaceous material, strongly eosinophilic on hematoxylin-and eosin staining (HE) and positive with the periodic acid-Schiff stain and diastase-resistant (PAS + D), which is considered characteristic for PAP (Fig. 2). Blood assays showed elevated high levels of GM-CSF antibodies. There was no suspicion of rheumatological or hematological diseases. Based on these results a diagnosis of aPAP was confirmed and the patient was referred for treatment.
Fig. 1.
High Resolution computed tomography at diagnosis showing pattern with interlobular thickening and ground glass opacities, and not a classical crazy paving pattern.
Fig. 2.
Light microscopy of the surgical biopsy from the left lung's lingula shows lung parenchyma containing large areas where the alveoli are totally filled with a granular proteinaceous material that is strongly eosinophilic on hematoxylin-and eosin staining (HE). The alveolar walls display diffuse moderate infiltration of lympho-histiocytic cells. The intraalveolar material is also positive with the periodic acid-Schiff stain and diastase-resistant (PAS + D), as well as strongly immunohistochemically positive for surfactant-A protein (S–A) and to a less extent for surfactant-B (not shown), consistent with its derivation from surfactant phospholipids and protein components. Accumulation of surfactant-A is also seen in the hyperplastic pneumocytes covering the alveolar walls and in the intraalveolar macrophages. Cytokeratin staining (CK) highlights the hyperplastic type-II pneumocytes on the alveolar walls and the proteinaceous cell debris of exfoliated pneumocytes in the intraalveolar material. (All figures, 100X).
At the time of referral to our department the patient reported regular cough and dyspnea upon physical exertion. She had no weight loss, fever or recurrent infections. She had no family history of lung disease and was a never-smoker. Physical examination was normal. Pulmonary function tests revealed a forced expiratory volume in one second (FEV1) of 2.23 L (62%), forced vital capacity (FVC) of 2.64 L (70%), total lung capacity (TLC) of 72% and carbon monoxide diffusing capacity (DLCO) of 54% of predicted. Arterial gas analysis showed pO2 within a normal range of 14.3 kPa. According to the classification proposed by Inoue et al. her disease severity score (DSS) was 2 (symptomatic and PaO2 over 70 mmHg) [2]. After evaluation of the patient's history she was treated with WLL without improvement. After the third WLL, supporting treatment with inhaled GM-SCF was therefore initiated. Firstly, sargramostim (Leukine; Sanofi-Aventis; 250 μg x 2 per week) was used for a short period, but when molgramostim became available in a named patient program, it was decided to change the inhalations to rhGM-CSF – molgramostim (Molgradex; Savara Pharmaceuticals). We chose the following treatment protocol: 300 μg daily in seven days repeated with seven days of pause. After seven months, the therapy was intensified to 300 μg daily. HRCT one year after the treatment did not reveal significant regression of the crazy-paving pattern. Nevertheless, there was a significant clinical improvement with remission of all her symptoms, as well as in her pulmonary function, which almost normalized. After 17 months of molgramostim inhalations her FEV1 was 2.58 (79%), FVC 3.05 (79%), TLC 77% and DLCO 74% of predicted. No adverse effects of the molgramostim inhalations were observed. The patient is now stable, and the inhalations have been discontinued.
3. Discussion
PAP was first described by Rosen et al., in 1958 [8]. Further investigations revealed the complex nature of the disease. In neonates and children surfactant production disorders are most common and can lead to alveolar wall distortion and different degrees of accumulation of dysfunctional surfactant. The described mutations in the genes of the surfactant protein are for surfactant protein-B (SFTPB), surfactant protein-C (SFTPC), member A3 of the ATP-binding cassette family of transporters (ABCA3) and thyroid transcription factor 1 (NKX2–1) [3,9,10]. Mutations in the genes encoding the GM-CSF receptor (CSF2RA and CSF2RB) are also known [11,12], suggesting that PAP can be familiar.
Autoimmune PAP occurs less often in children. No epidemiological data exists and only five case reports are published [6,7,[13], [14], [15]]. The treatment approach is the same for children as for adults. In 1964 Ramirez-Rivera successfully performed WLL [16] and the procedure became first line therapy for PAP patients. Different techniques are used according to specialized center preferences. Until now the procedure has not been standardized. Regardless, it always requires a skilled team, including anesthesiologists, physiotherapists and pulmonologists as well as careful planning and execution to optimize its safety and efficacy [4]. Campo et al. provides data that determine WLL to be safe and associated with a low rate of procedure-related morbidity: fever (18%), hypoxemia (14%), wheezing (6%), pneumonia (5%) and fluid leakage (4%), pleural effusion (3.1%), and pneumothorax (0.8%) [17]. Nevertheless, alternative treatment options are needed in refractory cases. Recent investigations showed good results of inhaled therapy with recombinant GM-CSF. The first successful treatment with an inhaled GM-CSF was provided by Compa and colleagues in an adult patient with secondary PAP [18]. Price at al [6]. describe a case of a 13-year-old girl with aPAP who was successfully treated with inhaled GM-SCF (sargramostim) after clinically and radiographic deterioration, despite WLL-therapy. Yamamoto et al. [7] treated a 9-year-old girl with subsequent therapy of WLL and inhaled GM-SCF (sargramostim). This case was initially refractory to the inhalation therapy probably due to inefficient access of GM-CSF to the alveolar spaces because of densely accumulated surfactant. After induction of a partial remission by WLL, continued inhalation of GM-CSF consolidated and maintained the remission of PAP. In 2012, Leth et al. suggested an algorithm of treatment of aPAP [19]. Patients with symptoms and DSS 2 should be considered for inhalation therapy. They propose to initially perform WLL once, maybe twice on each affected lung, depending on the response. WLL serves primarily to cure the patient, and secondly to remove as much of the PAS-positive material as possible. Theoretically, this could be speculated to allow the following treatment with inhaled GM-CSF to exert its effect in more peripheral lung areas. There is no consensus on the length of inhaled GM-CSF as well as the choice of inhaled GM-SCF. Molgramostim is a non-glycosylated recombinant human GM-CSF expressed in E. coli., whereas sargramostim is recombinant human GM-CSF expressed in yeast and is therefore glycosylated. The amino acid sequence of molgramostim is identical to human endogenous GM-CSF as compared to sargramostim, which differs from the native protein with a substitution of leucine at position 23. Although the specific biological activity (in vitro potency) is higher for molgramostim than sargramostim, the two compounds have been used interchangeably in aPAP [20,21].
There is no approved pharmacological therapy for aPAP but inhaled GM-CSF is widely used. Molgramostim is a new recombinant GM-CSF that in the present case was beneficial and without any side effects. The current case adds to the clinical experience of inhaled molgramostim [[21], [22], [23]], now also expanding it into the pediatric age group.
Declarations of interest
None.
References
- 1.Ben-Dov I., Segel M.J. Autoimmune pulmonary alveolar proteinosis: clinical course and diagnostic criteria. Autoimmun. Rev. 2014 Apr-May;13(4–5):513–517. doi: 10.1016/j.autrev.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Inoue Y., Trapnell B.C., Tazawa R., Arai T., Takada T., Hizawa N. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. [Internet] 2008 Apr 1;177(7):752–762. doi: 10.1164/rccm.200708-1271OC. http://www.atsjournals.org/doi/abs/10.1164/rccm.200708-1271OC [cited 2017 Sep 7] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki T., Trapnell B.C. Pulmonary alveolar proteinosis syndrome. Clin. Chest Med. [Internet] 2016 Sep;37(3):431–440. doi: 10.1016/j.ccm.2016.04.006. http://linkinghub.elsevier.com/retrieve/pii/S0272523116300430 [cited 2017 Jul 27] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awab A., Khan M.S., Youness H.A. Whole lung lavage—technical details, challenges and management of complications. J. Thorac. Dis. [Internet] 2017 Jun;9(6):1697–1706. doi: 10.21037/jtd.2017.04.10. http://jtd.amegroups.com/article/view/13803/11597 [cited 2017 Jul 27] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wylam M.E., Ten R., Prakash U.B.S., Nadrous H.F., Clawson M.L., Anderson P.M. Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur. Respir. J. [Internet] 2006 Mar 1;27(3):585–593. doi: 10.1183/09031936.06.00058305. http://erj.ersjournals.com/cgi/doi/10.1183/09031936.06.00058305 [cited 2017 Sep 7] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Price A., Manson D., Cutz E., Dell S. Pulmonary alveolar proteinosis associated with anti-GM-CSF antibodies in a child: successful treatment with inhaled GM-CSF. Pediatr. Pulmonol. [Internet] 2006 Apr;41(4):367–370. doi: 10.1002/ppul.20347. http://doi.wiley.com/10.1002/ppul.20347 [cited 2017 Aug 15] Available from: [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H., Yamaguchi E., Agata H., Kandatsu N., Komatsu T., Kawai S. A combination therapy of whole lung lavage and GM-CSF inhalation in pulmonary alveolar proteinosis. Pediatr. Pulmonol. [Internet] 2008 Aug;43(8):828–830. doi: 10.1002/ppul.20856. http://www.ncbi.nlm.nih.gov/pubmed/18618617 [cited 2017 Aug 15] Available from. [DOI] [PubMed] [Google Scholar]
- 8.ROSEN S.H., CASTLEMAN B., LIEBOW A.A., Enzinger F.M., Hunt R.T.N. Pulmonary alveolar proteinosis. N. Engl. J. Med. [Internet] 1958 Jun 5;258(23):1123–1142. doi: 10.1056/NEJM195806052582301. http://www.nejm.org/doi/abs/10.1056/NEJM195806052582301 [cited 2017 Sep 16] Available from: [DOI] [PubMed] [Google Scholar]
- 9.Whitsett J.A., Wert S.E., Trapnell B.C. Genetic disorders influencing lung formation and function at birth. Hum. Mol. Genet. [Internet] 2004 Oct 1;13(suppl_2):R207–R215. doi: 10.1093/hmg/ddh252. https://academic.oup.com/hmg/article-lookup/doi/10.1093/hmg/ddh252 Spec No. [cited 2017 Sep 16] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Alavuk Kundović S., Popović L. Congenital pulmonary alveolar proteinosis: from birth to ten-years of age. Indian J. Pediatr. 2017 Sep;89(9):721–723. doi: 10.1007/s12098-017-2365-6. http://link.springer.com/10.1007/s12098-017-2365-6 Available from. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T., Sakagami T., Rubin B.K., Nogee L.M., Wood R.E., Zimmerman S.L. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med. [Internet] 2008 Nov 24;205(12):2703–2710. doi: 10.1084/jem.20080990. http://www.jem.org/lookup/doi/10.1084/jem.20080990 [cited 2017 Sep 16] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirksen U., Nishinakamura R., Groneck P., Hattenhorst U., Nogee L., Murray R. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J. Clin. Invest. [Internet] 1997 Nov 1;100(9):2211–2217. doi: 10.1172/JCI119758. http://www.jci.org/articles/view/119758 [cited 2017 Sep 16] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trukalj M., Perica M., Ferenčić Ž., Erceg D., Navratil M., Redžepi G. Successful treatment of autoimmune pulmonary alveolar proteinosis in a pediatric patient. Am. J. Case Rep. [Internet] 2016 Sep 5;17:641–645. doi: 10.12659/AJCR.897868. http://www.ncbi.nlm.nih.gov/pubmed/27592713 [cited 2017 Sep 16] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sideris G.A., Josephson M. Pulmonary alveolar proteinosis and Niemann Pick disease type B: an unexpected combination. Respir. Med. Case Rep. [Internet] 2016;19:37–39. doi: 10.1016/j.rmcr.2016.06.009. http://linkinghub.elsevier.com/retrieve/pii/S2213007116300533 [cited 2017 Sep 16] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson T.E., Trapnell B.C., Goris M.L., Quittell L.M., Cornfield D.N. Quantitative analysis of longitudinal response to aerosolized granulocyte-macrophage colony-stimulating factor in two adolescents with autoimmune pulmonary alveolar proteinosis. Chest [Internet] 2009 Mar;135(3):842–848. doi: 10.1378/chest.08-1317. http://www.ncbi.nlm.nih.gov/pubmed/19265094 [cited 2017 Sep 16] Available from. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez J., Kieffer R.F., Ball W.C. Bronchopulmonary lavage in man. Ann. Intern. Med. [Internet] 1965 Nov;63(5):819–828. doi: 10.7326/0003-4819-63-5-819. http://www.ncbi.nlm.nih.gov/pubmed/5848630 [cited 2017 Sep 19] Available from. [DOI] [PubMed] [Google Scholar]
- 17.Campo I., Luisetti M., Griese M., Trapnell B.C., Bonella F., Grutters J. Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet. J. Rare Dis. [Internet] 2016 Aug 31;11(1):115. doi: 10.1186/s13023-016-0497-9. http://ojrd.biomedcentral.com/articles/10.1186/s13023-016-0497-9 [cited 2017 Sep 19] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compa D.R., Judson M.A., Beegle S.H. Granulomatosis and polyangitis followed by alveolar proteinosis in a 32-year-old woman. Chest [Internet] 2012 May;141(5):1359–1360. doi: 10.1378/chest.11-2002. http://linkinghub.elsevier.com/retrieve/pii/S0012369212603018 [cited 2018 Feb 16] Available from. [DOI] [PubMed] [Google Scholar]
- 19.Leth S., Bendstrup E., Vestergaard H., Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology [Internet] 2013 Jan;18(1):82–91. doi: 10.1111/j.1440-1843.2012.02274.x. http://doi.wiley.com/10.1111/j.1440-1843.2012.02274.x [cited 2017 Aug 15] Available from: [DOI] [PubMed] [Google Scholar]
- 20.Seymour J.F., Presneill J.J., Schoch O.D., Downie G.H., Moore P.E., Doyle I.R. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am. J. Respir. Crit. Care Med. Internet. 2001;163:524–531. doi: 10.1164/ajrccm.163.2.2003146. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi K., Sato A., Takada T., Arai T., Nei T., Kasahara Y. Direct evidence that GM-CSF inhalation improves lung clearance in pulmonary alveolar proteinosis. Respir. Med. [Internet] 2012 Feb;106(2):284–293. doi: 10.1016/j.rmed.2011.10.019. http://www.ncbi.nlm.nih.gov/pubmed/22112784 [cited 2017 Jul 27] Available from. [DOI] [PubMed] [Google Scholar]
- 22.Tazawa R., Hamano E., Arai T., Ohta H., Ishimoto O., Uchida K. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2005 May 15;171(10):1142–1149. doi: 10.1164/rccm.200406-716OC. [DOI] [PubMed] [Google Scholar]
- 23.yan Yu H., Sun X feng, Wang Y xun, Xu Z jun, Huang H. Whole lung lavage combined with Granulocyte-macrophage colony stimulating factor inhalation for an adult case of refractory pulmonary alveolar proteinosis. BMC Pulm. Med. 2014 May 19;14:87. doi: 10.1186/1471-2466-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]