Abstract
Acquiring resistance to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) is inevitable. Transformation to small cell lung cancer (SCLC) is reported as a possible mechanism of this acquired resistance. We describe the case of a 35-year-old man with lung adenocarcinoma harboring EGFR exon 19 deletion. After 7 months of successful treatment with afatinib, he experienced relapse and rebiopsy revealed SCLC with EGFR exon 19 deletion. Tumor marker tests at this point showed normal levels of serum neuron-specific enolase and pro-gastrin releasing peptide. Our case highlights the importance of rebiopsy for revealing SCLC transformation, a potential mechanism of acquired resistance to afatinib as with other EGFR-TKIs, and normal-range values of tumor markers for SCLC cannot exclude the possibility of SCLC transformation.
Keywords: Transformation, Small cell lung cancer, Adenocarcinoma, Rebiopsy, Acquired resistance, Afatinib
1. Introduction
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) play a key role in the treatment of activating EGFR-mutated advanced non-small cell lung cancer (NSCLC). However, the emergence of acquired resistance to EGFR-TKIs is inevitable. Although transformation to small cell lung cancer (SCLC) has been reported as a possible mechanism of acquired resistance to EGFR-TKIs [[1], [2], [3]], only limited information is available about the transformation to SCLC as a mechanism of acquired resistance to afatinib [4], which is a second-generation EGFR-TKI and an irreversible EGFR inhibitor. Here, we report a case of SCLC transformation after first-line afatinib treatment in a patient with lung adenocarcinoma.
2. Case report
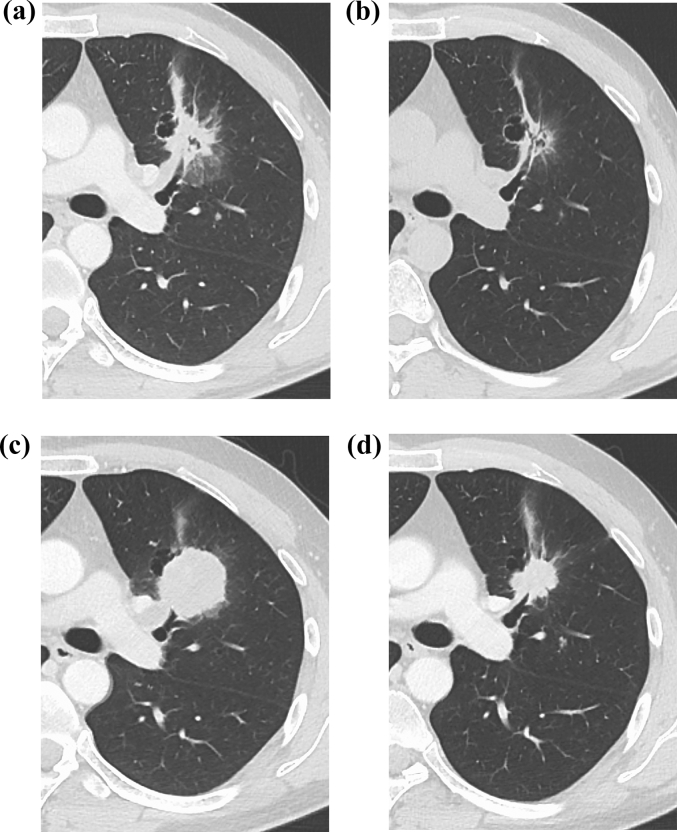
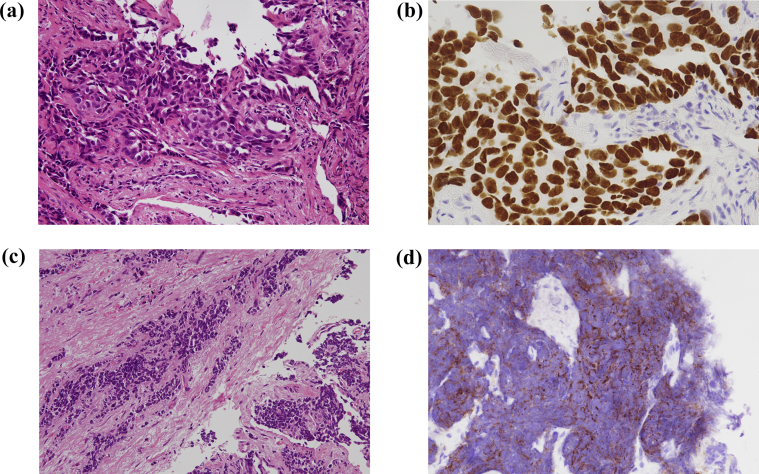
A 35-year-old man with a 15-pack-year smoking history presented to our hospital because of a chest x-ray abnormality at a medical checkup. Chest computed tomography (CT) revealed a 20-mm solitary tumor in the left upper lobe (Fig. 1a) and single left hilar lymph node enlargement and small bilateral pulmonary nodules. Head magnetic resonance imaging (MRI) revealed multiple small brain metastatic lesions. Regarding tumor markers, serum carcinoembryonic antigen (CEA) levels were slightly increased (5.7 ng/mL; normal range, 0–5.0 ng/mL), while neuron-specific enolase (NSE) and pro-gastrin releasing peptide (proGRP) levels were normal. Bronchoscopic biopsy was performed targeting the mass in the left upper lobe, and he was histologically diagnosed with lung adenocarcinoma (cT1bN1M1a, stage IV) (Fig. 2a and b). This biopsy sample was genotyped and an EGFR exon 19 deletion was identified using the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp method (LSI Medience Corporation, Tokyo, Japan).
Fig. 1.
Computed tomography (CT) scan of the left lung. (a) CT on admission, showing a 20-mm lung tumor in the left lower lobe. (b) The lung tumor had shrunk remarkably after 4 weeks of afatinib treatment. (c) CT scan showed the re-growing lung tumor in the left upper lobe when disease progression was confirmed after afatinib treatment. (d) The lung tumor had shrunk remarkably after 4 cycles of cisplatin and irinotecan treatment.
Fig. 2.
Histopathological findings of the lung specimens. (a) Hematoxylin and eosin staining of the lung tissue obtained by bronchoscopic biopsy, showing histopathology of adenocarcinoma. (b) Immunohistochemical staining of thyroid transcription factor 1, demonstrating strong nuclear expression in tumor cells. (c) Post-afatinib, hematoxylin and eosin staining of lung tissue showed small cell lung cancer transformation. (d) Post-afatinib, immunohistochemical staining of lung tissue showed strong staining for CD56.
Thereafter, afatinib 40 mg once daily was started. One month later, CT and MRI showed partial remission of pulmonary tumors (Fig. 1b) and disappearance of brain metastases. However, after starting afatinib treatment for 7 months, CT and MRI demonstrated recurrence of the primary tumor in the left upper lobe (Fig. 1c) and multiple brain metastases. At this point, tumor marker tests showed normal levels of serum CEA, NSE, and proGRP.
Bronchoscopic rebiopsy was performed for the re-growing tumor at the left upper lobe, which was the same site of the first biopsy. Histological assessment indicated SCLC (Fig. 2c and d). Repeat EGFR mutation analysis using both tissue samples and plasma samples revealed the same exon 19 deletion without additional mutations, such as T790 M mutation (the cobas® EGFR Mutation Test v2, Roche Molecular Systems, Pleasanton, California, USA). Therefore, he was considered to have acquired resistance to EGFR-TKI and histological transformation to SCLC.
Afatinib was discontinued and chemotherapy was administered with a cisplatin and irinotecan regimen. During the 1 month from rebiopsy to chemotherapy, tumor markers showed elevation, including NSE (34.6 ng/mL; normal range, 0–12.0 ng/mL) and ProGRP (116.4 ng/mL; normal range, 0–80.9 pg/mL). After 4 cycles of chemotherapy, the patient showed shrinkage of the lung tumor (Fig. 1d) and tumor markers (NSE and ProGRP) became normal. Currently, he is completing 4 cycles of chemotherapy with cisplatin and irinotecan and is being followed-up in the outpatient setting without disease progression.
3. Discussion
We have reported a case of SCLC transformation after second-generation irreversible EGFR-TKI afatinib treatment for lung adenocarcinoma. In a recent report of rebiopsy results from 42 patients who had acquired resistance to afatinib, there were no cases in which SCLC transformation was the acquired resistance mechanism [5]. In fact, most previous reports of SCLC transformation as an acquired resistance mechanism are for first- or third-generation EGFR-TKIs [[1], [2], [3],[6], [7], [8]], and there are only a few published reports of SCLC transformation for second-generation EGFR-TKIs [4]. Our case is different from previous report [4] in that rebiopsy was performed for the primary lung tumor, which was the same site of the first biopsy. Considering our case and the prior literature, clinicians should be aware that SCLC transformation can occur for all EGFR-TKI generations. Serum NSE levels may be useful for detecting early SCLC transformation [9]; however, tests for tumor markers showed normal levels of serum NSE and proGRP in the current case.
Although NSCLC and SCLC are generally thought to be different diseases in terms of their biologic and clinical features, the phenomenon of transformation to SCLC from adenocarcinoma suggests that these tumors originate from a common cell type. Lee et al. [10] recently reported that EGFR-TKI-resistant SCLCs branch out from adenocarcinoma clones early in disease development, because of complete inactivation of RB1 and TP53. They suggested it may be useful to test RB1 and TP53 status in patients with lung adenocarcinoma before starting chemotherapy.
Loss of the tumor suppressors TP53 and RB1 is needed for SCLC pathogenesis [11], and the loss of the function of these genes is also important for small cell transformation from adenocarcinoma [2,12]. However, loss of these genes alone is insufficient for SCLC transformation [13]; other changes also need to occur. Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC) mutagenesis has been reported to be important for tissue transformation, and APOBEC-induced mutational process can be hyperactivated during transformation into SCLC [10,14]. Nevertheless, the APOBEC profile may result from different processes that contribute to SCLC transformation, and the exact biological mechanism of the transformation is therefore unclear.
In our case, we cannot completely rule out the possibility that EGFR-mutant SCLC was present before afatinib treatment. However, from a clinical point of view, it seems unlikely that an SCLC component was present at initial diagnosis because SCLC progresses rapidly without effective treatment, and because the patient originally responded to EGFR-targeted therapy for 7 months. Additionally, the transformed SCLC tumor specimen retained its original EGFR-activating mutation, suggesting tumor transformation was a mechanism of resistance to afatinib treatment.
In summary, clinicians should remember that SCLC transformation can occur at any stage during chemotherapy. Although measurement of serum NSE and ProGRP level may help to identify small cell transformation, normal-range values cannot exclude the possibility of SCLC transformation. To rule out SCLC transformation and select appropriate treatment, repeat biopsy is recommended for patients with NSCLC and rapid clinical deterioration.
Funding
None.
Conflicts of interest
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sequist L.V., Waltman B.A., Dias-Santagata D. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002003. 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oser M.G., Niederst M.J., Sequist L.V., Engelman J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H.A., Arcila M.E., Rekhtman N. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Canc. Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manca P., Russano M., Pantano F., Tonini G., Santini D. Change from lung adenocarcinoma to small cell lung cancer as a mechanism of resistance to afatinib. Oncotarget. 2017;8:59986–59990. doi: 10.18632/oncotarget.17607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S.G., Liu Y.N., Tsai M.F. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7:12404–12413. doi: 10.18632/oncotarget.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ham J.S., Kim S., Kim H.K. Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment. J. Thorac. Oncol. 2016;11:e1–4. doi: 10.1016/j.jtho.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Li L., Wang H., Li C. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: a case report. Oncotarget. 2017;8:18609–18614. doi: 10.18632/oncotarget.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piotrowska Z., Niederst M.J., Karlovich C.A. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Canc. Discov. 2015;5:713–722. doi: 10.1158/2159-8290.CD-15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L., He J., Xia J. Resistance to epithelial growth factor receptor tyrosine kinase inhibitors in a patient with transformation from lung adenocarcinoma to small cell lung cancer: a case report. Oncol Lett. 2017;14:593–598. doi: 10.3892/ol.2017.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.K., Lee J., Kim S. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. 2017;35:3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 11.George J., Lim J.S., Jang S.J. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederst M.J., Sequist L.V., Poirier J.T. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 2015;6:6377. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meder L., König K., Ozretić L. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int. J. Canc. 2016;138:927–938. doi: 10.1002/ijc.29835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal-Hanjani M., Wilson G.A., McGranahan N. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]