Abstract
Carcinosarcoma is a rare histological type of non-small cell carcinoma (NSCLC), and its prognosis has been reported to be worse compared with other NSCLCs. Nanoparticle albumin-bound paclitaxel (nab-PTX) + carboplatin (CBDCA) achieves a favorable response rate in patients with non-small cell lung cancer (NSCLC). We administered nab-PTX + CBDCA to a 68-year-old man with postoperative recurrent carcinosarcoma with interstitial lung disease (ILD). A partial response was evident after four cycles of chemotherapy. To the best of our knowledge, the present study is the first to report the safety and efficacy of nab-PTX + CBDCA for treating carcinosarcoma with ILD.
Keywords: Carcinosarcoma, Chemotherapy, Nab-paclitaxel, Carboplatin, Interstitial lung disease
1. Introduction
It has been reported that carcinomas with a histological sarcomatous component is rare and more aggressive than other types of lung cancer. In 2015, the World Health Organization classified carcinomas with a sarcomatous-like component with a spindle cell or giant cell appearance, or those associated with an occasional heterogeneous sarcomatous component, as sarcomatoid carcinomas. There are five subtypes: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma and pulmonary blastoma [1]. Sarcomatoid carcinomas may arise in the lung where they account for approximately 0.4% of non-small cell lung cancers (NSCLCs) [2]. Carcinosarcoma, a subtype of sarcomatoid carcimomas, was first defined by Kita et al., in 1908 as a poorly differentiated non-small cell lung carcinoma containing a component with sarcoma or sarcoma-like features [3].
Prognosis is poor for patients with sarcomatoid carcinoma, including those with carcinosarcoma, compared with other NSCLCs because of the high rate of resistance to conventional first-line chemotherapy [4]. Chemotherapy for lung cancer occasionally exacerbates preexisting interstitial lung disease (ILD), especially idiopathic pulmonary fibrosis (IPF), which can be fatal [5]. Several studies report that the efficacy and safety of paclitaxel (PTX) and carboplatin (CBDCA) doublet chemotherapy is a suitable regimen for patients with NSCLC with ILD or IPF [[6], [7], [8], [9]]. Nanoparticle (130-nm) albumin-bound paclitaxel (nab-PTX) exhibits higher activity and causes reduced toxicity compared with PTX. A phase 3 study found that administration of nab-PTX achieved a higher overall response rate and less neuropathy [10].
To our knowledge, there are no studies that address the use of this regimen for treating sarcomatoid carcinomas, particularly pulmonary carcinosarcoma with ILD. Here we describe a patient with pulmonary carcinosarcoma with ILD who responded to first-line treatment with nab-PTX + CBDCA without experiencing adverse effects within 28 days after last administration of chemotherapy.
2. Case report
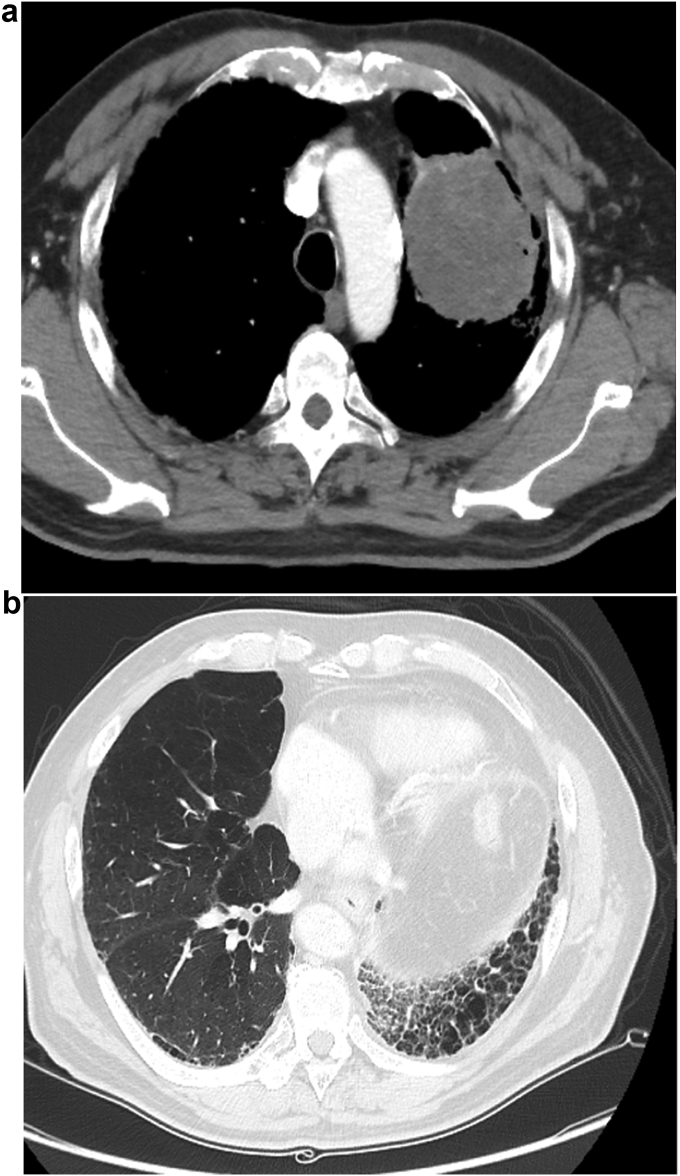
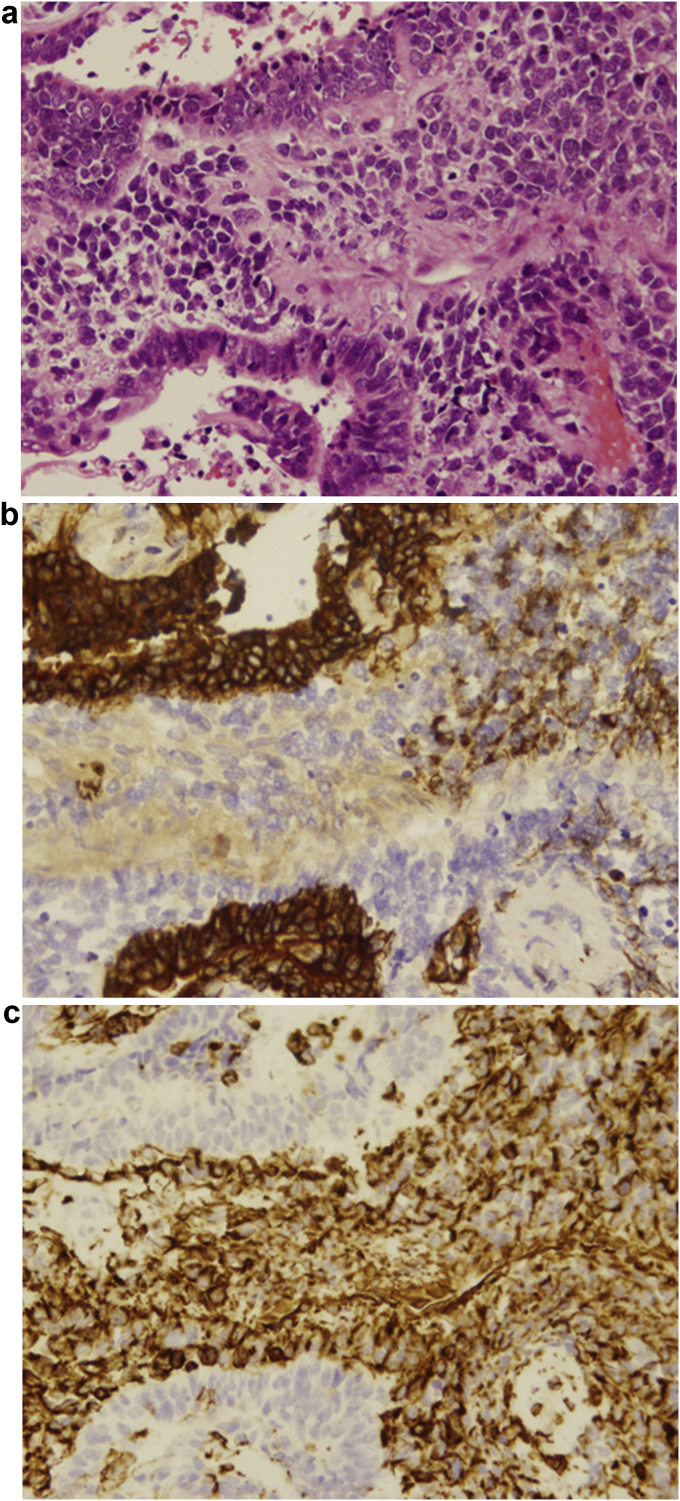
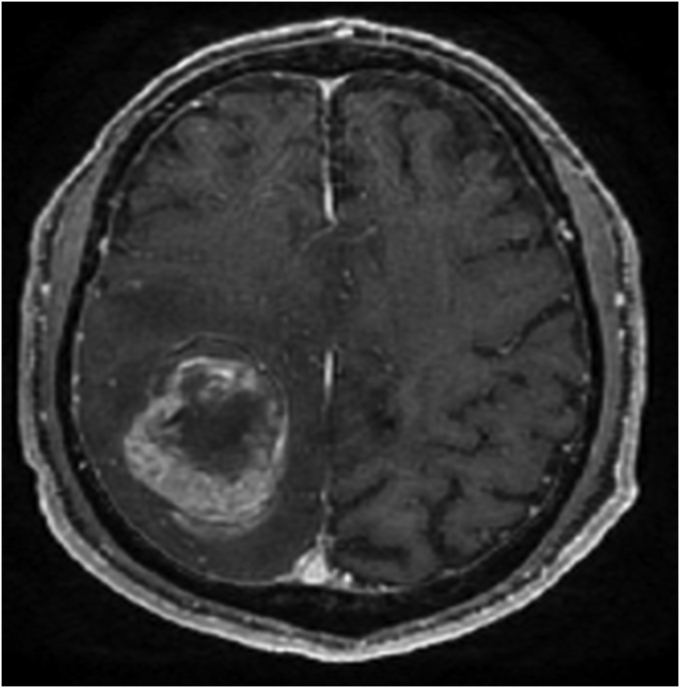
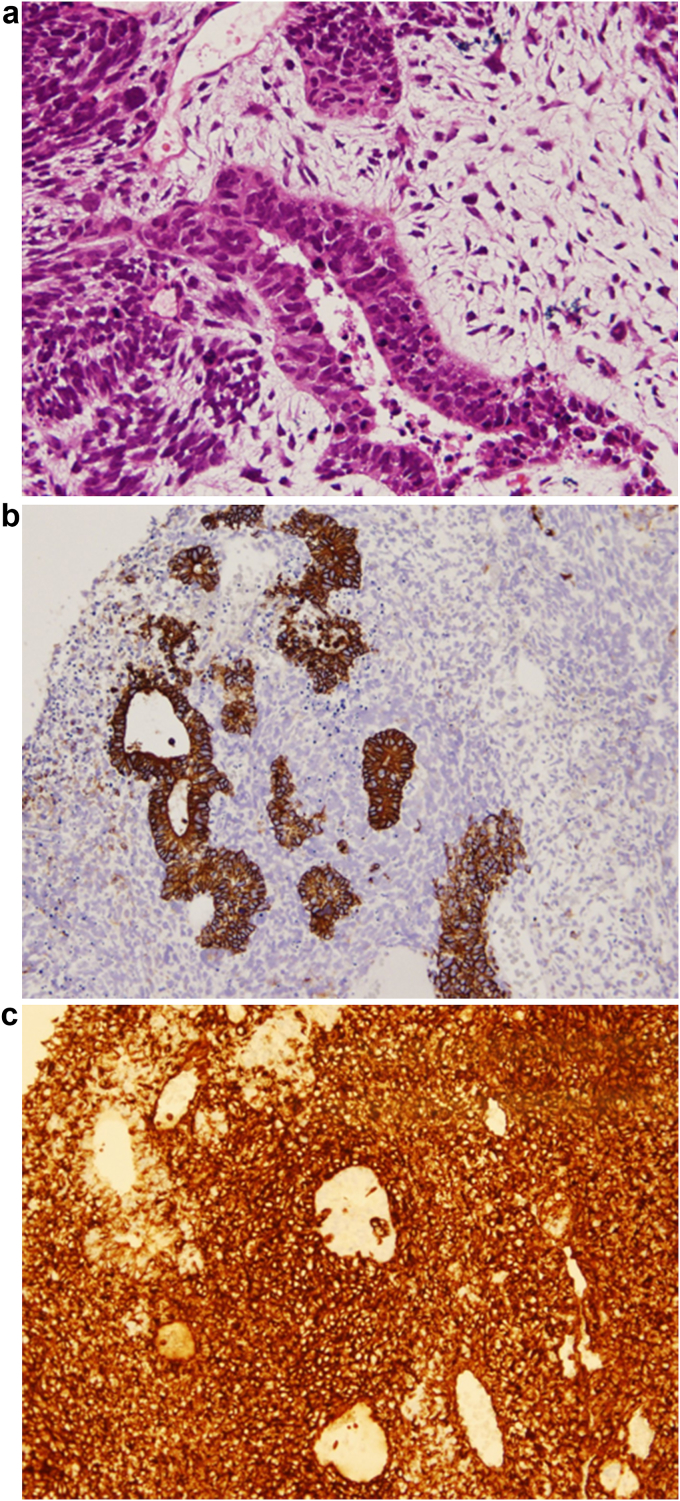
A 68-year-old man, a former smoker (27 pack years), was admitted to another hospital in September 2016 for treatment following X-ray imaging detection of an abnormal shadow during a regular health examination. Computed tomography (CT) revealed a 72-mm irregularity in the upper-left lobe with typical interstitial pneumonia in the inferior lobes (Fig. 1). The patient underwent left upper lobectomy, and histopathology showed diffuse proliferation of pleomorphic tumor cells, including spindle-shaped and giant cells in the stroma, and tubular formation of columnar atypical cells. Immunohistochemical analysis demonstrated that the columnar tumor cells of the tubular component were diffusely positive for cytokeratin AE1/AE3, and partially positive for vimentin. Stromal spindle and giant cells are diffusely positive for vimentin and negative for AE1/AE3. Stromal component does not show specific differentiation such as bone, cartilage nor musculus (see Fig. 2). We considered the tumor consists of adenocarcinoma as the epithelial component, and sarcoma as the mesenchymal component. The patient was diagnosed with carcinosarcoma of the lung, which met the World Health Organization criteria [1]. Two months later, the patient was admitted to our hospital with paralysis of his left arm and leg. Head magnetic resonance imaging showed a brain tumor on right brain hemisphere (Fig. 3). We suspected metastasis of the lung cancer to the right parietal lobe of the cerebrum. The tumor was excised, and histopathology revealed that the tumor cells in the brain were similar to those of the left lung (consisting of epithelial and mesenchymal component, and positive for AE1/AE3 and vimentin, respectively) (Fig. 4).
Fig. 1.
Chest computed tomography (CT) before lobectomy. It revealed a 72-mm irregularity in the upper-left lobe (A) with typical interstitial pneumonia in the inferior lobes (B).
Fig. 2.
Histopathological findings obtained from left upper lobectomy. An image of a hematoxylin and eosin-stained section showing the tumor diffuse proliferation of pleomorphic tumor cells. (A) (magnification, ×100) Immunohistochemical analysis showed positive for cytokeratin AE1/AE3 in the epithelial component (B) (magnification, ×100) and vimentin in the stromal component. (C) (magnification, ×100).
Fig. 3.
Head magnetic resonance imaging (MRI) showed a brain tumor on right brain hemisphere.
Fig. 4.
Histopathological findings obtained from a brain tumor resection. An image of a hematoxylin and eosin-stained section showing. (A) (magnification, ×200) Immunohistochemical analysis showed positive for cytokeratin AE1/AE3 in the epithelial component (B) (magnification,×100) and vimentin diffusely . (C) (magnification, ×100).
The patient was diagnosed with pathological T4N1M0 stage ⅢA lung carcinosarcoma with no driver mutation, such as EGFR mutation.
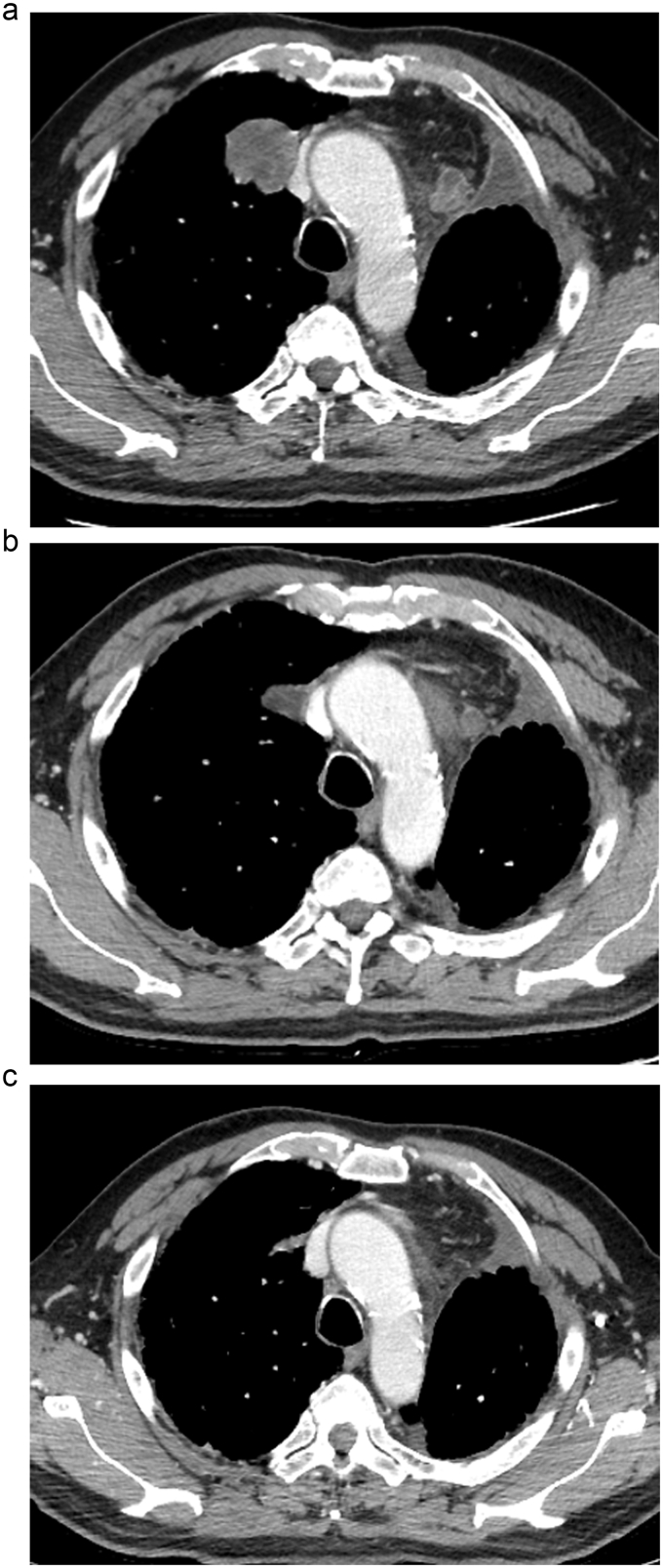
We therefore diagnosed distant recurrence of the lung carcinosarcoma. Moreover, chest CT scans detected new lesions in his right lung and mediastinal lymph nodes (Fig. 5 (A)). After surgery to remove the metastatic tumor from the brain, the patient's performance status improved after one week. He was lucid and walked unattended, although he was short of breath. We evaluated his performance status as 1. The patient's organ function was within normal range except for coincidence of ILD; therefore, we recommended him a first line chemotherapy regimen comprising nab-PTX (100 mg/m2) + CBDCA, area under the curve = 6, every 4 weeks. CT scans of the chest after four cycles of this regimen revealed a 47% reduction in the sum of the diameters of the target lesions compared with baseline, indicating a partial response (PR) (Fig. 5(B)). A reduction in the size of each of the target lesions was detected after 4 cycles of this regimen (Fig. 5(C)). The response was evaluated according to the Response Evaluation Criteria in Solid Tumors, version 1.1 [11]. The toxicity profile, reported as the highest toxicity grades of all cycles of this regimen, was as follows: Hematological toxicity in the form of grade 4 neutropenia, grade 3 leukopenia, and grade 1 thrombocytopenia; and nonhematological toxicity in the form of grade 1 constipation and hiccups. Pulmonary toxicity, including acute exacerbation of ILD, was not observed. The patient was unable to receive nab-PTX on day 15 of each cycle because of neutropenia that was subsequently cured using filgrastim in the first course of this regimen, and we used pegfilgrastim to prevent severe neutropenia on day 9 after the second course. As this patient did not experience serious toxicity, dose reduction was not required. Finally, he was alive 11 months after diagnosis, with stable disease maintenance of 7 months.
Fig. 5.
Chest CT after surgery to remove the metastatic tumor from the brain showed new lesions in his right lung and mediastinal lymph nodes (A), and following 2 (B) and 4 (C) cycles of nab-paclitaxel plus carboplatin combination chemotherapy.
3. Discussion
A retrospective study of a cohort of 97 patients with advanced or recurrent pulmonary sarcomatoid carcinoma who received conventional chemotherapy experienced poor progression-free survival (PFS) and overall survival (OS) (median PFS, 2.0 months; median OS, 6.3 months) [4]. In this study, no cases achieving PR were observed except for a single patient who received platinum-based chemotherapy, and the most common regimen administered was solvent-based paclitaxel (sb-PTX) [4]. It was reported that a sub-population of pleomorphic carcinoma, a subtype of sarcomatoid carcinomas, had an epidermal growth factor receptor (EGFR) mutation and showed response to EGFR tyrocin kinase inhibitors (EGFR-TKIs). However, the duration of the efficacy of EGFR-TKI was obviously shorter than that of non-sarcomatoid NSCLCs, even when the tumors had active EGFR mutation [12]. Immune checkpoint inhibitors such as nivolumab or pembrolizumab are speculated as promising agents against sarcomatous carcinomas, but this has not yet been established [13,14].
The optimal chemotherapy regimen for patients with NSCLC with ILD has not been established yet because of the lack of randomized trials. The combination of CBDCA with sb-PTX is most frequently used as first-line treatment for patients with NSCLC with ILD in practice. For example, the median PFS and OS were 2.5–5.3 months and 7.0–10.6 months, respectively, for patients treated with sb-PTX + CBDCA [7,15,16]. It has been well-known that anti-cancer drugs have some risk of induction of ILD as an adverse event, even in patients without ILD. In particular, the incidence of drug-induced ILD due to molecular targeting drugs is reported to be higher that of other drugs, therefore those drugs are not recommended for patients with ILD [15]. The most important toxicity of chemotherapy in cancer patients with ILD is a drug-induced acute exacerbation, a potentially fatal adverse effect [5]. The incidence of acute exacerbation of ILD was relatively lower in patients treated with sb-PTX + CBDCA [7,16,17] than other chemotherapy regimens.
In a phase III study of patients with advanced NSCLC, which compared nabPTX with sb-PTX in combination with carboplatin, nab-PTX achieved a significantly higher response rate and less neuropathy [10]. We therefore reasoned that nab-PTX + CBDCA might serve as an equally safe regimen for patients with NSCLC with ILD compared with sb-PTX + CBDCA. Further, nab-PTX + CBDCA treatment might be effective for treating sarcomatoid carcinoma, including carcinosarcoma, compared with sb-PTX + CBDCA for treating patients with other NSCLCs [4]. To the best of our knowledge, the present study is the first to demonstrate clinically meaningful antitumor activity of CBDCA + nab-PTX associated with a positive response by a patient with carcinosarcoma with ILD. The one previous case report described the efficacy of nab-PTX + CBDCA for treating a patient with pulmonary spindle cell carcinoma, one of the pulmonary sarcomatoid carcinomas [18]. Additionally, Azuma et al. reported the usefulness of this regimen for a patient with small cell lung carcinoma mixed with a squamous cell carcinoma component and ILD [19].
There were several limitations in this case study. First, we could not exclude the possibility that the new lesions in the right lung after brain surgery originated from another primary lung cancer. Although we did not perform histopathology, we assumed that the new legions were contralateral lung metastasis, because we were unable to detect tumors in the right lung in CT scans acquired before left-upper lobectomy. Therefore, we believe that it is reasonable to conclude that nab-PTX + CBDCA is a useful option for the treatment of patients with pulmonary sarcomatoid carcinomas with ILD. Second, we could not exclude the increase in risk of acute exacerbation of ILD. Further studies of the efficacy and safety of this regimen for treating pulmonary sarcomatoid carcinomas, including carcinosarcomas with ILD, are required.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.01.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Antoine M., Vieira T., Fallet V. Pulmonary sarcomatoid carcinoma. Ann. Pathol. 2016;36(1):44–54. doi: 10.1016/j.annpat.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Yendamuri S., Caty L., Pine M. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery. 2012;152(3):397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Kakos G.S., Williams T.E., Jr., Assor D. Pulmonary carcinosarcoma. Etiologic, therapeutic, and prognostic considerations. J. Thorac. Cardiovasc. Surg. 1971;61(5):777–783. [PubMed] [Google Scholar]
- 4.Vieira T., Girard N., Ung M. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J. Thorac. Oncol. 2013;8(12):1574–1577. doi: 10.1097/01.JTO.0000437008.00554.90. [DOI] [PubMed] [Google Scholar]
- 5.Kudoh S., Kato H., Nishiwaki Y. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am. J. Respir. Crit. Care Med. 2008;177(12):1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 6.Minegishi Y., Takenaka K., Mizutani H. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern. Med. 2009;48(9):665–672. doi: 10.2169/internalmedicine.48.1650. [DOI] [PubMed] [Google Scholar]
- 7.Shukuya T., Ishiwata T., Hara M. Carboplatin plus weekly paclitaxel treatment in non-small cell lung cancer patients with interstitial lung disease. Anticancer Res. 2010;30(10):4357–4361. [PubMed] [Google Scholar]
- 8.Kenmotsu H., Naito T., Kimura M. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J. Thorac. Oncol. 2011;6(7):12426. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 9.Kenmotsu H., Naito T., Mori K. Effect of platinum-based chemotherapy for nonsmall cell lung cancer patients with interstitial lung disease. Cancer Chemother. Pharmacol. 2015;75(3):521–526. doi: 10.1007/s00280-014-2670-y. [DOI] [PubMed] [Google Scholar]
- 10.Socinski M.A., Bondarenko I., Karaseva N.A. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as firstline therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J. Clin. Oncol. 2012;30(17):2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kaira K., Horie Y., Ayabe E. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J. Thorac. Oncol. 2010;5(4):460–465. doi: 10.1097/JTO.0b013e3181ce3e3c. [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reck M., Rodríguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. 10. [DOI] [PubMed] [Google Scholar]
- 15.Endo M., Johkoh T., Kimura K. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006;52(2):135–140. doi: 10.1016/j.lungcan.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Minegishi Y., Sudoh J., Kuribayasi H. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71(1):70–74. doi: 10.1016/j.lungcan.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu R., Fujimoto D., Kato R. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non-small cell lung cancer with interstitial lung disease. Cancer Chemother. Pharmacol. 2014;74(6):1159–1166. doi: 10.1007/s00280-014-2590-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji T., Kim Y.H., Ozasa H. Successful treatment with carboplatin and nanoparticle albumin-bound paclitaxel in a patient with pulmonary spindle cell carcinoma. Respir. Med. Case Rep. 2015;15:48–50. doi: 10.1016/j.rmcr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azuma Y., Tamiya M., Shiroyama T. Nanoparticle albumin-bound Paclitaxel+Carboplatin therapy for small cell lung cancer combined with squamous cell carcinoma and interstitial lung disease. Intern. Med. 2015;54(22):2911–2913. doi: 10.2169/internalmedicine.54.3243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.