Abstract
Primary localized endobronchial amyloidosis is a rare entity, as pulmonary amyloidosis most commonly occurs as a part of systemic AL amyloidosis. It can be asymptomatic or can present with nonspecific symptoms such as progressive dyspnea, cough, wheezing and rarely respiratory failure. It is frequently misdiagnosed as asthma, COPD or pneumonia. Solitary endobronchial amyloidosis having a nodular appearance can mimic endobronchial tumor. The diagnosis is usually delayed by 8–37 months. The average life expectancy for primary tracheobronchial amyloidosis is approximately 9 years, so the early diagnosis of this disease is very critical to improve the prognosis of patients. We are presenting a case of 65 year old male which was initially diagnosed and treated as asthma exacerbation with minimal improvement. Further workup was done with CT chest, bronchoscopy and biopsy because of persistent shortness of breath, which revealed primary localized solitary endobronchial amyloidosis.
Keywords: Primary endobronchial amyloidosis, Endobronchial tumor, Wavy path sign
1. Introduction
Primary tracheobronchial amyloidosis is a rare disease with extracellular amyloid deposits. With the advancements in fiber optic bronchoscopic techniques and improvement in imaging modalities more and more cases are being reported. Presenting symptoms may vary from mild shortness of breath to significant limitation in activity due to respiratory compromise. Many patients are misdiagnosed and managed with alternative diagnoses until definitive diagnosis is ascertained with bronchoscopic findings and specific histopathological verdicts. According to a recent systematic review, progressive dyspnea, cough and sputum production were the commonest symptoms. The disease becomes clinically relevant when in its systemic form is affecting most of the vital organs or in cases of limited form causes a mass effect (amyloidoma). The diagnostic challenge become even more difficult in cases when amyloidosis is co-existent with other systemic multi-organ diseases. We present a case of a middle-aged man who was treated as for poorly controlled asthma for an extended period until he underwent bronchoscopic evaluation and biopsy.
2. Case report
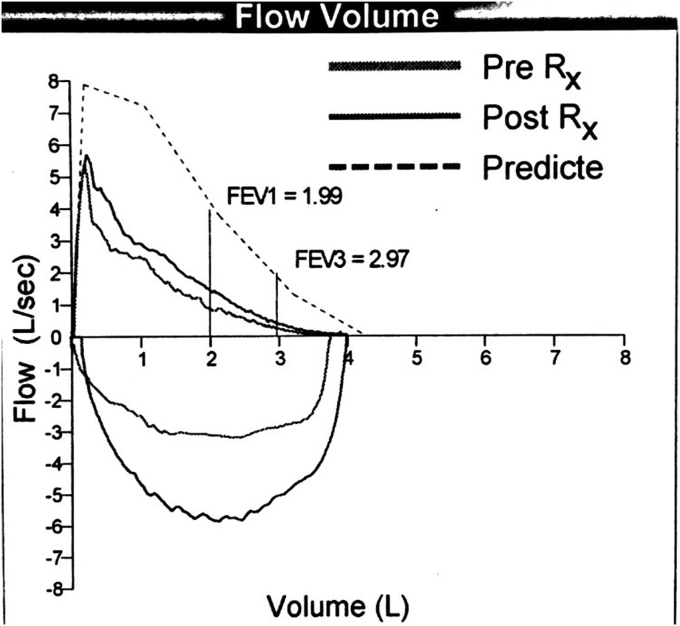
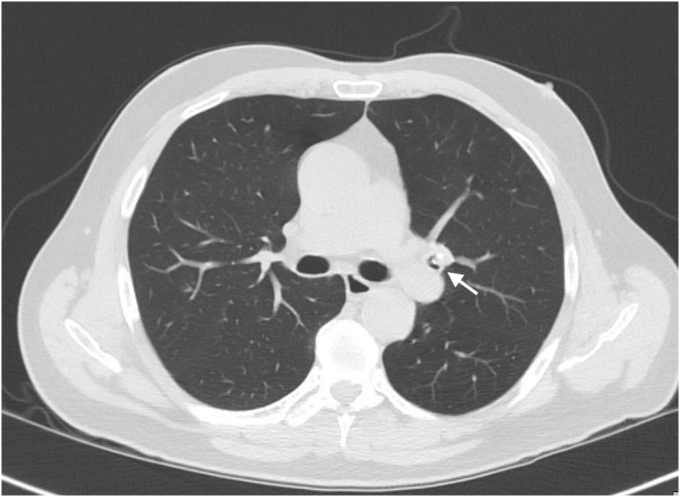
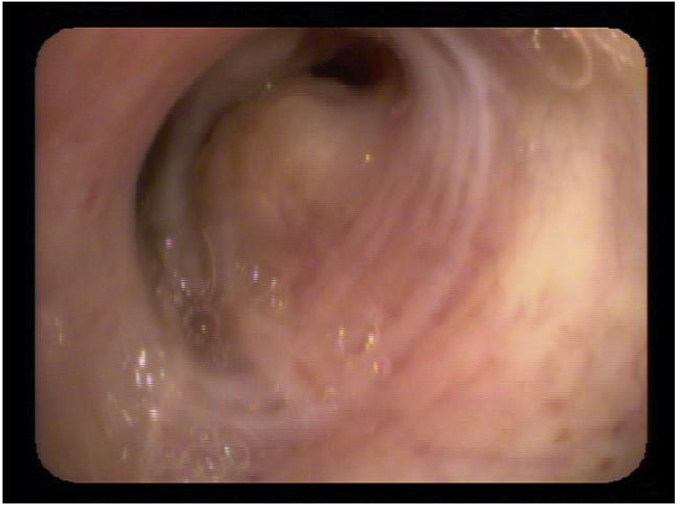
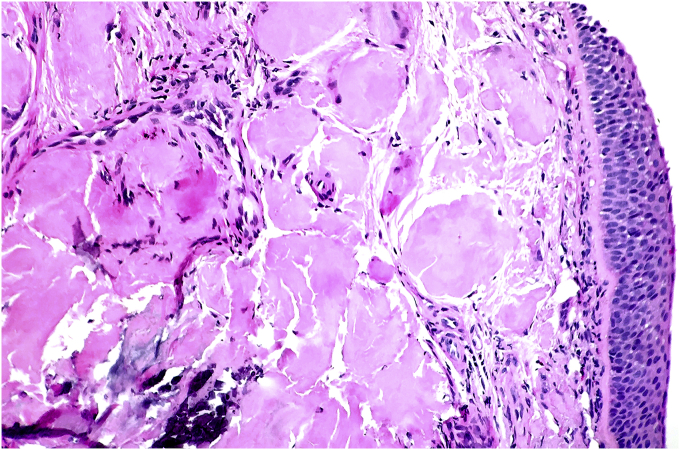
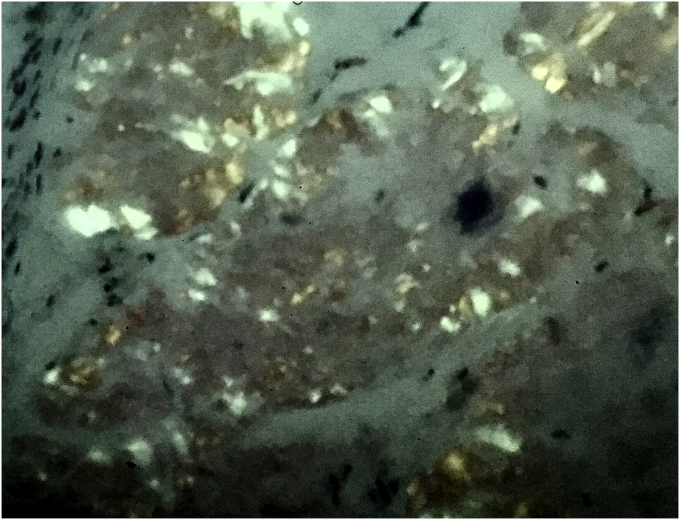
A 65 year old male presented with complain of shortness of breath. He was diagnosed with bronchial asthma since childhood, which was well controlled on inhaled bronchodilators and inhaled steroids. He was a former smoker and had quit smoking 20 years back, with a cumulative smoking history of 20 pack years. Patient worked as a construction worker in past but had been on disability for past 22 years due to chronic back pain. He was having persistent worsening of shortness of breath and rhonchi for last two years. He received multiple courses of systemic steroids apart from constantly being on inhaled corticosteroid and inhaled bronchodilator, with minimal improvement. Radioallergosorbent test (RAST) was normal with no allergens identified and immunoglobulin E levels (IgE) were within normal limits. There was no eosinophilia. Pulmonary function tests done were indicative of mild obstructive physiology with no significant change in volumes or flow after bronchodilator administration. Prebronchodilator spirometric values are as follows: % FEV1/FVC - 76%, % FEV1 - 74%, % FVC - 97%, % FEF25-75 – 38%, % FEF50 – 40%, % FEF75 – 31%, % PEFR – 74%. Flow – volume loop has been shown in Fig. 1. As there was persistent rhonchi and shortness of breath despite optimal treatment for asthma exacerbation, CT chest was done which revealed left upper lobe bronchial wall thickening and calcification with moderate stenosis (Fig. 2). In view of CT chest findings, fiberoptic bronchoscopy was done which showed yellowish color, nodular tumor like lesion in left upper lobe bronchus (Fig. 3). Biopsy of the lesion revealed benign bronchial mucosa and underlying stoma with pink amorphous material deposits showing orange red by congo red stain and green birefringence under polarized light, suggesting amyloidosis (Fig. 4, Fig. 5). Serum and Urine electrophoresis were done to look for systemic amyloidosis, which revealed normal pattern. Serum protein electrophoresis showed total serum protein 6.9g/dl, albumin 4g/dl, alpha1 globulin 0.2g/dl, alpha2 globulin 0.7g/dl, beta1 globulin 0.4g/dl, beta2 globulin 0.2g/dl and gamma globulin 1.3g/dl. Serum Rheumatoid factor (7 IU/ml), CCP Ab (<16Units), CRP (0.23mg/dl), myeloperoxidase Ab (<1.0 AI) , proteinase 3 Ab (<1.0), SSA Ab (<1.0 U), Anticentromere Ab (<1.0 U) and Scl 70 Ab (<1.0 U) were within normal range. Echocardiogram showed no signs of infiltrative disease and ejection fraction was normal. Based on the above findings and clinical presentation patient was diagnosed with primary localized endobronchial amyloidosis.
Fig. 1.
Flow – volume loop of pulmonary function test.
Fig. 2.
Axial High Resolution CT of Thorax (Lung Window) showing a nodular opacity with foci of calcification within the lumen of left upper lobe bronchus along with adjacent bronchial wall thickening (white arrow).
Fig. 3.
Fiberoptic bronchoscopy showing yellowish color, nodular tumor like lesion in left upper lobe bronchus.
Fig. 4.
Section shows bronchial mucosa with extra cellular deposition of amorphous pink material (H&E, 10×).
Fig. 5.
Section shows apple green birefringent staining of amyloid under polarized microscope. (Congo red stain, 20×).
3. Discussion
Amyloidosis is a spectrum of diseases of unclear etiology, which are characterized by the deposition of abnormal proteins in fibrillar form, termed as amyloid in extracellular tissues resulting in disruption of tissue architecture and impairment of organ function. These protein deposits were first described by Rokitansky in 1842 [1]. However, the term amyloid was given by Virchow in 1854, because on treatment with iodine they stained similar to starch [1]. Amyloidosis can be hereditary or acquired, localized or systemic. The major types of amyloid deposits are of AL (immunoglobulin light chain) and AA (amyloid associated). AL amyloid is made of immunoglobulin light chain proteins derived from plasma cells and AA amyloid are composed of immunoglobulin protein derived from liver. AL is associated with primary systemic amyloidosis, myeloma-associated amyloid, and most localized forms of amyloid. AA is associated with secondary amyloidosis [2]. Amyloid deposits commonly affects males (2:1) of middle age group (50–60 years) [3].
Pulmonary amyloidosis most commonly occurs as a part of systemic AL amyloidosis, which accounts for up to 80% of pulmonary amyloid lesions [4]. AA pulmonary amyloidosis has been reported as well in a case of tracheobronchial amyloidosis complicating mediastinal fibrosis [5]. Localized AL amyloidosis is most often identified in the upper respiratory, urogenital and gastrointestinal tracts, the skin and the orbit [6]. Primary localized respiratory amyloidosis involving lower respiratory tract, either lung parenchyma or tracheobronchial tree is extremely uncommon and it was first described by Lesser in 1877 [7]. Berk et al. reported three patterns of airway involvement in primary localized respiratory amyloidosis that is proximal, mid or main bronchial, and distal disease [8]. Primary respiratory amyloidosis can present in three characteristic forms: diffuse interstitial amyloid deposits, single or multiple pulmonary nodules (amyloidomas), and focal or diffuse submucosal tracheobronchial deposits [9]. Primary tracheobronchial amyloidosis, is the most common localized form of respiratory amyloidosis, but still it is a rare disorder with only around 150 cases reported in the literature [7]. Primary endobronchial amyloidosis is characterized by submucosal plaques or nodules, of amyloid deposits which may be localized, diffuse, solitary or multifocal [6,7]. Solitary tracheobronchial amyloid deposits are unusual [10,11]. Primary endobronchial amyloidosis can also present as solitary nodular tumor, mimicking an endobronchial neoplasm which is extremely uncommon as in our case [3]. The endobronchial amyloidosis is not associated with systemic amyloidosis [6,12]. It is unusual to have both interstitial and tracheobronchial involvement in the same patient. Tracheobronchopathia osteochondroplastica which is characterized by the deposition of calcified or cartilaginous submucosal nodules in the airways have been found in association with tracheobronchial amyloidosis [13].
Tracheobronchial amyloidosis (TBA) can be asymptomatic or can present with nonspecific symptoms, mimicking common respiratory conditions. The diagnosis is usually delayed by 8–37 months and is frequently misdiagnosed as asthma, COPD or pneumonia [14]. Out of all symptomatic tracheobronchial lesions, only 0.5% are amyloid lesions. The location and the amount of amyloid deposition determine the presenting symptoms [15]. Common presenting symptoms are progressive dyspnea, cough, and wheezing that can be mistaken with asthma. Serraj et al. reported a case of TBA involving trachea that was misdiagnosed as asthma [6]. In a case reported by Kang et al. initially misdiagnosed as asthma, it was found to have multiple endobronchial nodules involving right upper, left upper and left lower lobe [2]. In our case also, there was a delay in diagnosis due the masquerading symptoms of poorly controlled asthma exacerbations for two years, after which he which found to have solitary endobronchial amyloid nodule in left upper lobe as a result of workup for persistent dyspnea and wheezing. Rarely endobronchial amyloidosis can present with hemoptysis. Zhang et al. reported a case of primary localized TBA which presented with recurrent hemoptysis [15]. Massive hemoptysis is an important cause resulting in death in localized pulmonary amyloidosis patients. Recurrent pneumonia, bronchiectasis, atelectasis and progressive respiratory failure can also occur due to progressive airway narrowing and obstruction. Solitary endobronchial amyloidosis having a nodular appearance is present in 14% of patients and can mimic endobronchial tumor [16]. In our case also, solitary nodular tumor was identified in left upper lobe mimicking an endobronchial neoplasm, which was later diagnosed as endobronchial amyloidosis.
The symptoms are usually nonspecific, resulting in being misdiagnosed as asthma, recurrent pneumonia, tracheobronchitis, or COPD. The average life expectancy for PTBA is approximately 9 years, and the overall survival rate at 4–6 years is only 31–43% [17]. Due to airway obstruction, patients often develops post-obstructive pneumonia and respiratory failure [17]. Therefore, early diagnosis of this disease is critical to improve the prognosis of patients. So, for patients with asthma, chronic cough, COPD or recurrent pneumonia who do not respond to treatment, we should do complete workup for PTBA with CT chest, bronchoscopy and biopsy. In our case, which was diagnosed as asthma exacerbation, we did a CT chest as patient was having persistent wheezing and shortness of breath despite treatment. The chest CT-transverse section may show tracheobronchial wall circumferential thickening with irregular calcifications, luminal narrowing, atelectasis and/or localized bronchiectasis [18]. These findings represent amyloid protein deposition and infiltration. However CT chest transverse section may be normal in 50% of patients with PTBA [17]. CT Chest coronal section may show diffuse multiple polypoid nodules in the trachea and main bronchi, termed as “wavy path sign”, which might precede those of the transverse section findings and may be more characteristic [17]. Chest radiography is normal in up to 70% of patients [19]. In our case as CT Chest revealed left upper lobe bronchial wall thickening and calcification with moderate stenosis, we performed bronchoscopy. Bronchoscopy is the cornerstone in the diagnosis of TBA that demonstrates the lesions and allows us to obtain biopsy sample for definitive diagnosis. Bronchoscopy may reveal diffuse submucosal plaques, which is the most common lesion. Other lesions are local tumor-like masses or localized solitary nodule or tumor like mass. Solitary localized amyloid nodule or tumor is rare, present in only 14% of the PTBA cases and can mimic endobronchial malignancy. Typically, these lesions have a yellowish color. The diagnosis of amyloidosis requires histological confirmation. These amyloid lesions on congo red staining demonstrates green birefringence under polarized light [6]. Systemic amyloidosis should be ruled out by absence of monoclonal protein on serum and urine protein electrophoresis [2]. Echocardiogram is also obtained to rule out restrictive cardiomyopathy secondary to amyloid deposition. He was found to have ejection fraction of 55–60% with normal diastolic function. Tracheobronchial amyloidosis must be included among the differential diagnoses of tracheal lesions such as tracheal neoplasms.
There is no established treatment for endobronchial amyloidosis. There is no randomized clinical trial and treatment depends upon the patient's symptoms and degree of obstruction. For asymptomatic patient, conservative management is recommended with close monitoring [14]. For symptomatic patients with significant airway obstruction, bronchoscopic debulking and laser ablation are the mainstay of treatment [7]. Cazalets et al. reported two cases managed with bronchoscopic resection producing good result [20]. Silicon stent can be placed to maintain the patency of airway after resection as reported by Yang et al. Amyloid deposition tends to recur, requiring repeated bronchoscopic resections [21].
Endobronchial ablation by Neodymium: Yttrium Aluminum Garnet (Nd:YAG) or Carbon Dioxide (CO2) laser therapy can be done in patients with localized endobronchial obstruction due to amyloidosis. Amyloid lesion are quite sensitive to laser photocoagulation. CO2 laser is usually used for supraglottic, glottic, and some subglottic lesions, while Nd:YAG laser for subglottic and tracheobronchial amyloid deposits [22]. Herman et al. reported 13 cases, while Madden et al. reported 2 cases of tracheobronchial amyloidosis, which were successfully treated using Nd: YAG laser with good outcome [22,23]. Piazza et al. also reported 30 cases of tracheobronchial amyloid successfully treated with laser therapy [24].
External beam radiotherapy (EBRT) has been used in few cases of endobronchial amyloidosis successfully. Plasma cells producing amyloidogenic protein seems to be photosensitive. Neben – Wittich et al. reported 7 patients of endobronchial amyloidosis treated successfully with External beam radiation therapy [25]. EBRT can be used in those cases of endobronchial amyloidosis, who are not suitable for bronchoscopic interventions [25]. Use of Cryotherapy has been reported by Maiwand et al. for treatment of a case of tracheobronchial amyloidosis with successful outcome [26].
Oral Colchicine is usually used for treatment of systemic amyloidosis along with prednisone and melphalan. However, Morales et al. reported a case of tracheobronchial amyloidosis treated with colchicine with good outcome [14]. There are some other cases reportedly treated with colchicine, with variable results.
After treatment, there should be follow up evaluation with CT chest and serial pulmonary function test as tracheobronchial amyloidosis often reoccurs [7].
Our patient was advised for endobronchial cryotherapy as an option for definitive treatment, at a specialized interventional pulmonology center. However, patient declined any invasive intervention and is currently being managed conservatively with close outpatient follow up and bronchodilator therapy.
4. Conclusion
Tracheobronchial amyloidosis is a rare entity which presents with nonspecific symptoms and often misdiagnosed as asthma, chronic bronchitis, atelectasis or recurrent pneumonia. As the prognosis is poor and it can lead to progressive respiratory failure due to airway obstruction, we should keep high degree of clinical suspicion for primary endobronchial amyloidosis, if the patients with previously mentioned diagnosis are not responding to treatment as anticipated. We should pursue with full workup including CT chest, bronchoscopy and biopsy if required on a case to case basis. We should be aware of CT chest and bronchoscopic findings of endobronchial amyloidosis. Apart from CT chest findings on transverse section, we should look for characteristic wavy path sign on CT chest coronal section. CT chest coronal section findings are more characteristic, appear earlier than transverse section findings and this can help us in diagnosing endobronchial amyloidosis early. We should also be familiar with various bronchoscopic findings including solitary endobronchial tumor which is less common finding and can mimic endobronchial neoplasm. Diagnosis is established by biopsy and congo red staining.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.02.007.
Appendix ASupplementary data
The following is the supplementary data related to this article:
References
- 1.Wang Q., Chen H., Wang S. Laryngo-tracheobronchial amyloidosis: a case report and review of literature. Int. J. Clin. Exp. Pathol. 2014;7:7088–7093. [PMC free article] [PubMed] [Google Scholar]
- 2.Kang H.W., Oh H.J., Park H.Y., Park C.K., Shin H.J., Lim J.H., Kwon Y.S., Oh I.J., Choi Y.D. Endobronchial amyloidosis mimicking bronchial asthma: a case report and review of the literature. Open Med. 2016;11:174–177. doi: 10.1515/med-2016-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanrıverdi E., Özgül M.A., Uzun O., Gül Ş., Çörtük M., Yaşar Z., Acat M., Arda N., Çetinkaya E. Tracheobronchial amyloidosis mimicking tracheal tumor. Case Rep. Med. 2016;2016 doi: 10.1155/2016/1084063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitz M.W., Gibson I.W., Johnston J.B. Isolated pulmonary amyloidosis: case report and review of the literature. Am. J. Hematol. 2006;81:212–213. doi: 10.1002/ajh.20518. [DOI] [PubMed] [Google Scholar]
- 5.Hoag J.B., Yung R.C. An unexpected finding of endobronchial amyloidosis in a patient with mediastinal fibrosis. J. Bronchol. 2008;15:61–63. [Google Scholar]
- 6.Serraj M., Kamaoui I., Znati K., Kouara S., Sahnoune F., Amara B., Biaze M.E., Tizniti S., Amarti A., Benjelloun M.C. Pseudotumoral tracheobronchial amyloidosis mimicking asthma: a case report. J. Med. Case Rep. 2012;6:40. doi: 10.1186/1752-1947-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleiro S., Hespanhol V., Magalhães Adriana. Endobronchial amyloidosis. J. Bronchol. 2008;15:95–99. [Google Scholar]
- 8.Berk J.L., O'Regan A., Skinner M. Pulmonary and tracheobronchial amyloidosis. Semin. Respir. Crit. Care Med. 2002;23:155–165. doi: 10.1055/s-2002-25304. [DOI] [PubMed] [Google Scholar]
- 9.O'Regan A., Fenlon H.M., Beamis J.F., Jr., Steele M.P., Skinner M., Berk J.L. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine. 2000;79:69–79. doi: 10.1097/00005792-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Utz J.P., Swensen S.J., Gertz M.A. Pulmonary amyloidosis. The mayo clinic experience from 1980-1993. Ann. Intern. Med. 1996;124:407–413. doi: 10.7326/0003-4819-124-4-199602150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cordier J.F., Loire R., Brune J. Amyloidosis of the lower respiratory tract. Clinical and pathologic features in a series of 21 patients. Chest. 1986;90:827–831. doi: 10.1378/chest.90.6.827. [DOI] [PubMed] [Google Scholar]
- 12.Petermann W., Barth J., Schluter E. Localized amyloidosis of central airways. Eur. J. Respir. Dis. 1987;71:210–212. [PubMed] [Google Scholar]
- 13.Capizzi S.A., Betancourt E., Prakash U.B. Tracheobronchial amyloidosis. Mayo Clin. Proc. 2000;75:1148–1152. doi: 10.4065/75.11.1148. [DOI] [PubMed] [Google Scholar]
- 14.Morales A., Pari M., Lopez-Lisbona R., Cubero N., Dorca J., Rosell A. Colchicine treatment for tracheobronchial amyloidosis. Respiration. 2016;91:251–255. doi: 10.1159/000443669. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L.Q., Zhao Y.C., Wang X.W., Yang J., Lu Z.W., Cheng Y.S. Primary localized tracheobronchial amyloidosis presenting with massive hemoptysis: a case report and literature review. Clin. Res. J. 2017;11:122–125. doi: 10.1111/crj.12301. [DOI] [PubMed] [Google Scholar]
- 16.Usta J., Kanj N. Primary tracheobronchial amyloidosis. J. Bronchol. 2005;12:43–45. [Google Scholar]
- 17.Li D., Shi Z., Wang Y., Thakur A. Primary tracheobronchial amyloidosis: coronal CT scan may provide clues for early diagnosis. J. Postgrad. Med. 2013;59:223–225. doi: 10.4103/0022-3859.118045. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner J., Jacobi V., Kardos P., Kollath J. CT findings in extensive tracheobronchial amyloidosis. Eur. Radiol. 1998;8:352–354. doi: 10.1007/s003300050392. [DOI] [PubMed] [Google Scholar]
- 19.Ding L., Li W., Wang K., Chen Y., Xu H., Wang H., Shen H. Primary tracheobronchial amyloidosis in China: analysis of 64 cases and a review of literature. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010;30:599–603. doi: 10.1007/s11596-010-0549-7. [DOI] [PubMed] [Google Scholar]
- 20.Cazalets C., Belleguic C., Sost G., Caulet-Maugendre S., Kernec J., Droz D., Grosbois B. Tracheobronchial amyloidosis: a propos of 2 cases. Rev. Med. Int. 2002;23:317–321. doi: 10.1016/s0248-8663(01)00557-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang S., Chia S.Y., Chuah K.L., Eng P. Tracheobronchial amyloidosis treated with rigid bronchoscopy and stenting. Surg. Endosc. 2003;17:658–659. doi: 10.1007/s00464-002-4260-z. [DOI] [PubMed] [Google Scholar]
- 22.Madden B.P., Lee M., Paruchuru P. Successful treatment of endobronchial amyloidosis using Nd:YAG laser therapy as an alternative to lobectomy. Monaldi Arch. Chest Dis. 2001;56:27–29. [PubMed] [Google Scholar]
- 23.Herman D.P., Colchen A., Milleron B., Bentata-Pessayre M., Personne C., Akoun G. The treatment of tracheobronchial amyloidosis using a bronchial laser. Apropos of a series of 13 cases. Rev. Mal. Respir. 1985;2:19–23. [PubMed] [Google Scholar]
- 24.Piazza C., Cavaliere S., Foccoli P., Toninelli C., Bolzoni A., Peretti G. Endoscopic management of laryngo-tracheobronchial amyloidosis: a series of 32 patients. Eur. Arch. Oto-Rhino-Laryngol. 2003;269:349–354. doi: 10.1007/s00405-003-0592-0. [DOI] [PubMed] [Google Scholar]
- 25.Neben-Wittich M.A., Foote R.L., Kalra S. External beam radiation therapy for tracheobronchial amyloidosis. Chest. 2007;132:262–267. doi: 10.1378/chest.06-3118. [DOI] [PubMed] [Google Scholar]
- 26.Maitwand M.O., Nath A.R., Kamath B.S.K. Cryosurgery in the treatment of tracheobronchial amyloidosis. J. Bronchol. 2001;8:95–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.