Abstract
Nowadays we have novel equipment for lung cancer diagnosis, however; due to lack of symptoms, lung cancer is still diagnosed at a late stage. Currently we have the following therapies for non-small cell lung cancer: a) non-specific cytotoxic agents, b) targeted therapies and c) immunotherapy. Each therapy has its own advantages and adverse effects. In the current case we will present a rare case of psoriacic arthritis that was presented after two cycles of nivolumab administration and we will also present a review of the literature.
Keywords: Lung cancer, Adenocarcinoma, Convex endobronchial ultrasound, PD-L1, Targeted therapy
1. Introduction
Non-small cell lung cancer treatment (NSCLC) can be treated with non-specific cytotoxic agents, targeted therapy and immunotherapy [1]. Targeted therapy according to the latest guidelines is focused on the following gene expression: a) epidermal growth factor receptor (EGFR), b) anaplastic lymphoma kinase (ALK), c) proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B (BRAF) and proto-oncogene tyrosine-protein kinase ROS (ROS-1). Based on the positive expression of these genes tyrosine inhibitors (TKIs) are administered to the patient. Unfortunately it has been observed that lung cancer has mechanisms which in time differentiate the first gene expression and a mutation is observed and therefore second or third line TKIs can be administered or treatment can be switched to non-specific chemotherapy or immunotherapy. The time to administer a new TKI is based on the clinical disease relapse of the patient under first line targeted treatment and with the diagnosis of novel mutations either with liquid biopsy or tissue biopsy [2,3]. Immunotherapy can be administered either as first line with pembrolizumab in the case of programmed death-ligand 1 (PD-L1) ≥50% for extensive NSCLC and ≥2% as second line treatment. Nivolumab can be administered as second line treatment indifferent of the PD-L1 expression [4,5]. Treatment is definitely based on the molecular profile of the patient. Each treatment has its own adverse effects, immunotherapy although is currently administered for more than a year, we still do not have observed all them. In several patients the immunogenic system is differentiated after immunotherapy treatment and an adverse effect can be observed. Such is the case of our patient were psoriatic arthritis was observed after nivolumab administration.
2. Case presentation
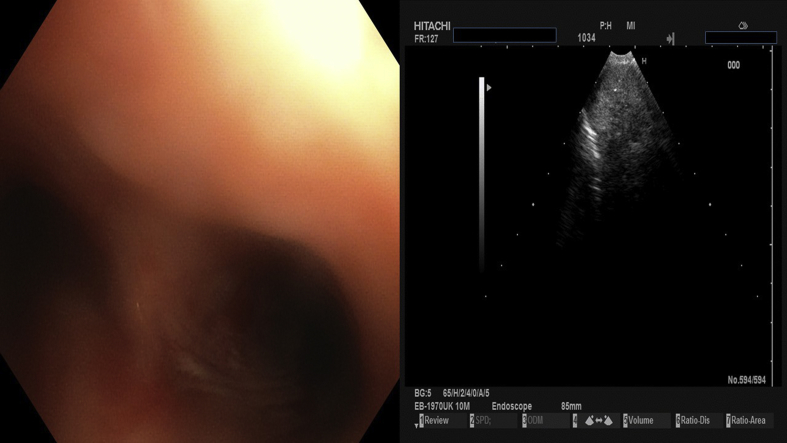
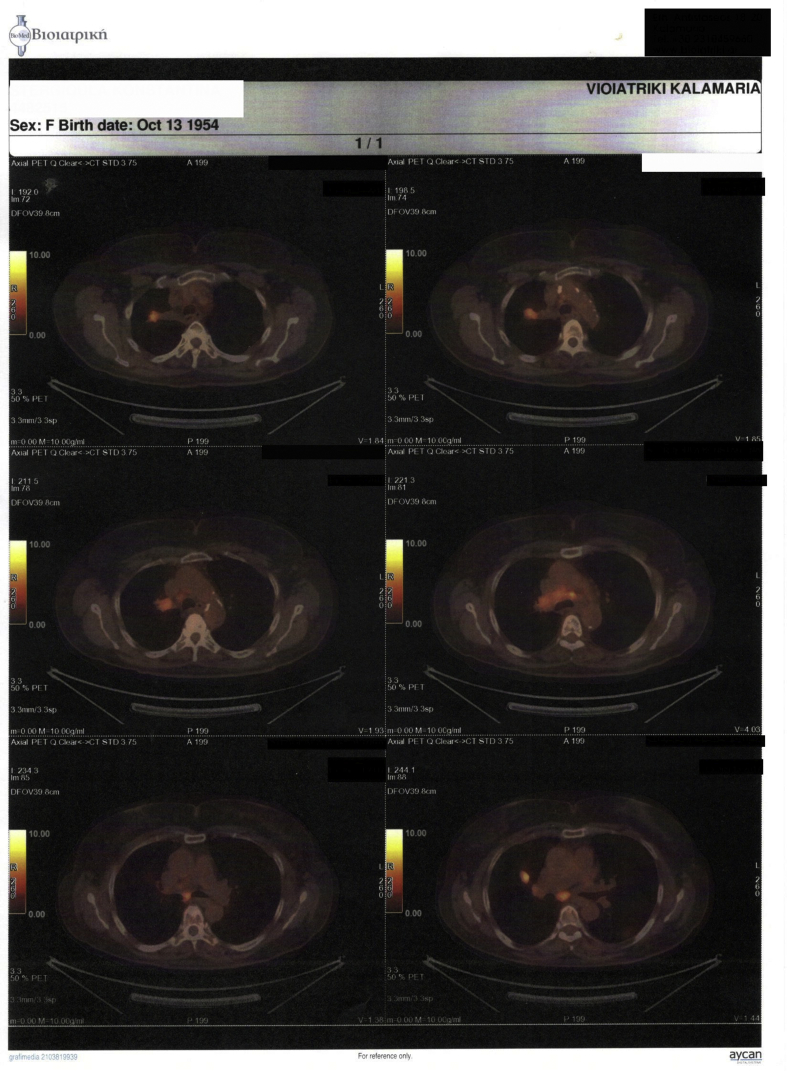
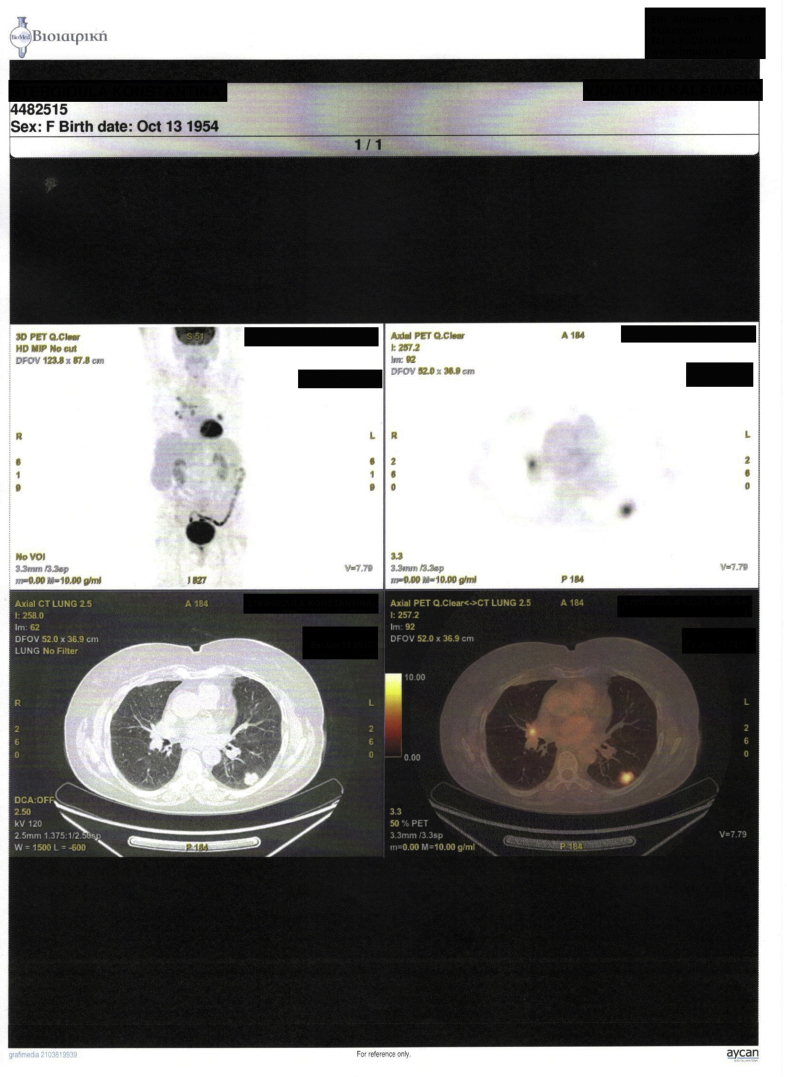
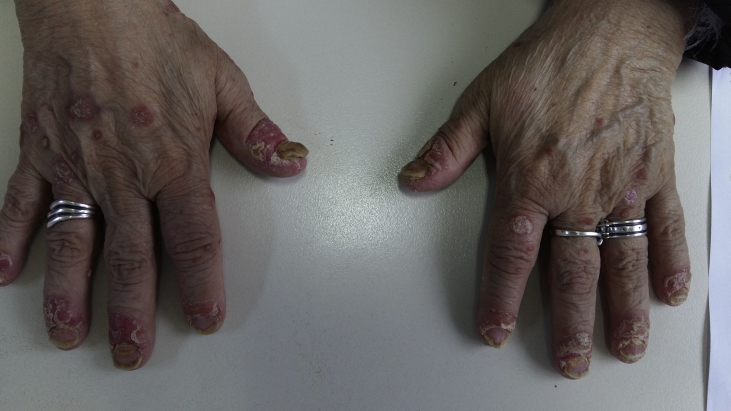
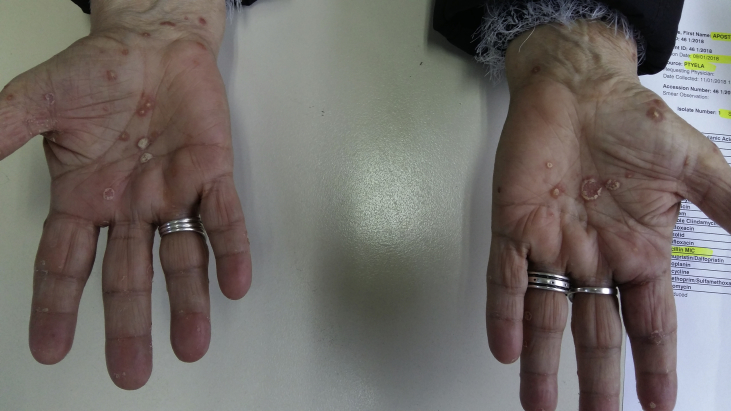
We present a case of a 64 year old female diagnosed with convex probe endobronchial ultrasound (EBUS) from lymphnode number 7 with adenocarcinoma almost a year ago (right lung mass) (Fig. 1). She has diametes mellitus diagnosed since 12 years with pill treatment. She also had a thyroid surgery six years ago. And she receives 20mg/p.o of T4 daily. The patient was negative for EGFR and ALK expression. PD-L1 expression was 3% with DAKO. Therefore non-specific cytotoxic agent was initiated with carboplatin and pemetrexed for 6 cycles in total. The patient was stage IIIB upon diagnosis and upon restaging mediastinum lymph nodes remained still active >6SUV although the primary lesion diameter had decreased from 6 cm to 3 cm (Fig. 2.). After 4 months of treatment break immunotherapy was initiated 180 mg every 15 days, since a new lesion was observed in the left lower lobe of 2 cm (Fig. 3). BRAF and ROS-1 were investigated according to the guidelines, however; the gene expression was negative for both [6]. Three days after the second nivolumab administration the patient started to have severe clinical findings of peeling in both upper and lower limbs (Fig. 4, Fig. 5). In the next 7 days the patient was directed to a dermatologist who diagnosed psoriasic arthritis and INN-apremilast was initiated. After the loading dose the patient is receiving 30 mg daily for 2 weeks now and nivolumab administration has stopped. There were mild joint paints which were easily managed with mild doses of paracetamol. The clinical findings are milder however; the patient will not receive nivolumab again at least for the next month, since we wait to observe if psoriasic arthritis will recess. Upon manifestation 1000 mg of methylprednisolone were administered and methylprednisolone 16 mg daily were administered for 15 days afterwards with tapering within the next 30 days. If the disease remains then we will initiate chemotherapy.
Fig. 1.
Biopsy with convex probe Pentax.
Fig. 2.
PET-CT upon diagnosis.
Fig. 3.
PET-CT after 4 months.
Fig. 4.
Upper hand surface.
Fig. 5.
Lower hand surface.
3. Discussion
The choices that the treating physician has for first line treatment are well defined with the current guidelines. The adverse effects for non-specific cytotoxic agents and targeted therapy are also known since they have been used for years, however; we still have a lot to observe regarding immunotherapy [[7], [8], [9], [10]]. Currently a combination of chemotherapy plus immunotherapy is being investigated for nsclc [11]. Radiotherapy as pretreatment to immunotherapy is another issue under investigation, as we have positive data where radiotherapy can assist in the enhancement of immunotherapy [12,13]. The same concept is being investigated with chemotherapy administration prior to immunotherapy [13]. In any case we have to be ready to handle the adverse effects. In the case of immunotherapy cortisone is the best option in high dosages [14]. However; there is the case such colitis where antibodies can be also co-administered in order to recess the clinical findings of immune related colitis [15]. The association between cardiac manifestations, endocrine manifestations and rheumatoid manifestations have been previously presented [[16], [17], [18]]. Immunotherapy is not prohibited for patients with a known systematic disease however; the patient should be carefully handled as stopping the treatment is necessary upon clinical manifestation of adverse effects. In our case psoriasic arthritis was observed and is still under observation and symptomatic treatment, to our knowledge and after careful evaluation of the patient the rheumatoid manifestation was due to the immunotherapy administration.
Disclosure
The authors declare no conflict of interest.
References
- 1.Domvri K., Zarogoulidis P., Darwiche K., Browning R.F., Li Q., Turner J.F., Kioumis I., Spyratos D., Porpodis K., Papaiwannou A., Tsiouda T., Freitag L., Zarogoulidis K. Molecular targeted drugs and biomarkers in NSCLC, the evolving role of individualized therapy. J. Canc. 2013;4(9):736–754. doi: 10.7150/jca.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarogoulidis P., Rapti A., Sardeli C., Chinelis P., Athanasiadou A., Paraskevaidou K., Kallianos A., Veletza L., Trakada G., Hohenforst-Schmidt W., Huang H. Re-biopsy after relapse of targeted therapy. T790M after epidermal growth factor mutation, where and why based on a case series. Respir. Med. Case Rep. 2017;21:171–175. doi: 10.1016/j.rmcr.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarogoulidis P., Gaga M., Huang H., Darwiche K., Rapti A., Hohenforst-Schmidt W. Tissue is the issue and tissue competition. Re-biopsy for mutation T790: where and why? Clin. Transl. Med. 2017;6(1):6. doi: 10.1186/s40169-017-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M., Rodriguez-Abreu D., Robinson A.G., Hui R., Csoszi T., Fulop A., Gottfried M., Peled N., Tafreshi A., Cuffe S., O'Brien M., Rao S., Hotta K., Leiby M.A., Lubiniecki G.M., Shentu Y., Rangwala R., Brahmer J.R., Investigators K. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Khozin S., Abernethy A.P., Nussbaum N.C., Zhi J., Curtis M.D., Tucker M., Lee S.E., Light D.E., Gossai A., Sorg R.A., Torres A.Z., Patel P., Blumenthal G.M., Pazdur R. Characteristics of real-world metastatic non-small cell lung cancer patients treated with Nivolumab and Pembrolizumab during the year following approval. Oncol. 2018 doi: 10.1634/theoncologist.2017-0353. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed H.Z., Liu Y., O'Connell K., Ahmed M.Z., Cassidy R.J., Gillespie T.W., Patel P., Pillai R.N., Behera M., Steuer C.E., Owonikoko T.K., Ramalingam S.S., Curran W.J., Higgins K.A. Guideline-concordant care improves overall survival for locally advanced non-small-cell lung carcinoma patients: a national cancer database analysis. Clin. Lung Canc. 2017;18(6):706–718. doi: 10.1016/j.cllc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Zarogoulidis P., Chinelis P., Athanasiadou A., Porpodis K., Kallianos A., Rapti A., Trakada G., Velentza L., Huang H., Tsiouda T., Hohenforst-Schmidt W. Liquid elbows" due to afatinib administration. Respir. Med. Case Rep. 2017;22:64–66. doi: 10.1016/j.rmcr.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiala O., Hosek P., Pesek M., Finek J., Racek J., Stehlik P., Sorejs O., Minarik M., Benesova L., Celer A., Nemcova I., Kucera R., Topolcan O. Serum concentration of erlotinib and its correlation with outcome and toxicity in patients with advanced-stage NSCLC. Anticanc. Res. 2017;37(11):6469–6476. doi: 10.21873/anticanres.12102. [DOI] [PubMed] [Google Scholar]
- 9.Zarogoulidis K., Zarogoulidis P., Darwiche K., Boutsikou E., Machairiotis N., Tsakiridis K., Katsikogiannis N., Kougioumtzi I., Karapantzos I., Huang H., Spyratos D. Treatment of non-small cell lung cancer (NSCLC) J. Thorac. Dis. 2013;5(Suppl 4):S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X., Roudi R., Chen S., Fan B., Li H.J., Zhou M., Li X., Li B. Immune-related adverse events associated with PD-1 and PD-L1 inhibitors for nonsmall cell lung cancer: protocol for a systematic review and meta-analysis. Medicine. 2017;96(44):e8407. doi: 10.1097/MD.0000000000008407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco P.C., Gridelli C. An update on the developing mitotic inhibitors for the treatment of non-small cell carcinoma. Expet Opin. Emerg. Drugs. 2017;22(3):213–222. doi: 10.1080/14728214.2017.1369952. [DOI] [PubMed] [Google Scholar]
- 12.Voong K.R., Naidoo J., Ettinger D.S. The next frontier in non-small cell lung cancer: synergizing radiation therapy and immune checkpoint blockade. Clin. Adv. Hematol. Oncol. HO (Hum. Organ.) 2017;15(8):615–625. [PubMed] [Google Scholar]
- 13.Attili I., Passaro A., Pavan A., Conte P., De Marinis F., Bonanno L. Combination immunotherapy strategies in advanced non-small cell lung cancer (NSCLC): does biological rationale meet clinical needs? Crit. Rev. Oncol. Hematol. 2017;119:30–39. doi: 10.1016/j.critrevonc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Villadolid J., Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl. Lung Canc. Res. 2015;4(5):560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postow M.A. American Society of Clinical Oncology; 2015. Managing Immune Checkpoint-blocking Antibody Side Effects, American Society of Clinical Oncology Educational Book; pp. 76–83. Meeting. [DOI] [PubMed] [Google Scholar]
- 16.Varricchi G., Galdiero M.R., Marone G., Criscuolo G., Triassi M., Bonaduce D., Marone G., Tocchetti C.G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2(4) doi: 10.1136/esmoopen-2017-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidar M., Giat E., Garelick D., Horowitz Y., Amital H., Steinberg-Silman Y., Shachter J., Shapira-Frommer R., Markel G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun. Rev. 2018 doi: 10.1016/j.autrev.2018.01.003. [ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Cukier P., Santini F.C., Scaranti M., Hoff A.O. Endocrine side effects of cancer immunotherapy. Endocr. Relat. Canc. 2017;24(12):T331–T347. doi: 10.1530/ERC-17-0358. [DOI] [PubMed] [Google Scholar]